Introduction
Esophageal cancer is the eighth most common cancer
and ranks sixth in cancer mortality worldwide (1). It is generally diagnosed at a late
stage and has a poor prognosis, with a 5-year survival rate of
<10% (2). Esophageal squamous
cell carcinoma (ESCC) comprises a majority of esophageal cancer in
Japan (1). Despite improved
multimodal treatment, prognosis remains unsatisfactory, as
effective systemic therapy has not been established for patients
with advanced ESCC (3). Therefore,
investigations that clarify the mechanisms of carcinogenesis and
tumor progression in ESCC are urgently required to develop targets
for therapy and prognostic biomarkers.
It is well known that carcinogenesis of ESCC can be
induced by external factors such as alcohol and smoking.
Genetically, aberrant expression of tumor suppressor genes and
oncogenes coordinately contribute to carcinogenesis and progression
of ESCC. Epigenetic alterations, including promoter
hypermethylation, play an important role in downregulating
suppressor genes (4–6). In particular, dysfunction of membrane
trafficking proteins is essential for cancer progression as it can
promote cells to be stimulated by various growth factors and to
become chemoresistant (7,8).
Differentially expressed in normal and neoplastic
cells (DENN)-domain proteins regulate Rab GTPases and represent a
newly recognized class of membrane trafficking proteins (9–13). Rab
GTPases are members of the Ras family of small GTPases and almost
70 Rab and Rab-like proteins are encoded by the human genome
(14). Rab family proteins are
important in regulating signal transduction and cellular processes
such as differentiation, proliferation, vesicle transport, nuclear
assembly, and cytoskeleton formation and some Rab proteins have
been reported to be necessary for the adhesion and migration of
cancer cells (15,16). The DENN domain present in members of
the connecdenn family of proteins interacts directly with Rab35 and
functions as a guanine nucleotide exchange factor (GEF) for this
GTPase (17). GEFs activate Rabs by
mediating the exchange of GDP for GTP. The human genome encodes
eight DENND (DENN domain) proteins that form eight families based
on homology and domain structure as follows: DENND1A-1C,
DENND2A-2D, DENND3, DENND4A-4C, DENND5A/5B, DENND6A/6B, MTMR5/13
and DENN/MADD. The DENN domain is located towards the N-terminus,
except in the DENND2 family, where it is located towards the
C-terminus (18–21).
Little is known about the function and expression
patterns of DENND family proteins in malignant tumors, although
they play important roles in intracellular signaling pathways by
integrating the activity of Rab pathways. DENND2D, which is located
on chromosome 1p13.3, encodes a 53-kDa protein, which suppresses
the proliferation and tumorigenicity of non-small cell lung cancer
cells (20,22); however, the role of DENND family
proteins in gastroenterological cancer has not been reported.
Accordingly, we focused on DENND2D and investigated the regulation
of its expression in an attempt to identify a tumor suppressor gene
(TSG) regulated by silencing through promoter hypermethylation in
ESCC.
Materials and methods
Ethics statement
The present study conformed to the ethical
guidelines of the World Medical Association Declaration of
Helsinki-Ethical Principles for Medical Research Involving Human
Subjects. Written informed consent for usage of clinical samples
and data, as required by the institutional review board at Nagoya
University, Japan, was obtained from all patients.
Sample collection
Nine ESCC cell lines (TE1, TE2, TE3, NUEC1, NUEC2,
NUEC3, TT, TTn and WSSC) were obtained from the American Type
Culture Collection (ATCC, Manassas, VA, USA) or from Nagoya
University, Japan, stored at −80°C with cell preservative solution
(Cell Banker®; Mitsubishi Chemical Medience Corporation,
Tokyo, Japan) and cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS) at 37°C in a 5% CO2
atmosphere. Sixty-five primary ESCC tissues and corresponding
non-cancerous tissues were obtained from Nagoya University
Hospital, all during radical esophageal resection between December
2001 and April 2012. The samples were immediately frozen in liquid
nitrogen and then stored at −80°C until analysis. Total RNA was
obtained from these samples using the RNeasy kit (Qiagen, Hilden,
Germany). None of the patients underwent preoperative treatment
including chemotherapy and radiation. Specimens were classified
histologically using the UICC TNM staging system for ESCC (23). Demographics, tobacco and alcohol
consumption, preoperative serum tumor markers, tumor size, and
pathological findings including tumor differentiation, tumor depth,
vascular invasion and lymph node metastasis were obtained from the
database retrospectively. Tobacco consumption was estimated using
the Brinkman index, which is defined as the number of cigarettes
smoked per day × smoking years. Data on alcohol consumption were
obtained by questioning patients; excessive alcohol consumption was
defined as alcohol intake >210 g/week for ≥3 years (24). Median duration of patient follow-up
was 33.5 months (range, 1.5–125 months). Postoperative follow-up
examinations included physical examination and measurement of serum
tumor markers every 3 months, and an enhanced computed tomography
scan (chest and abdominal cavity) every 6 months. Adjuvant
chemotherapy was administered to selected patients based on their
condition and at the discretion of the physician.
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative real-time RT-PCR (qPCR)
The level of DENND2D mRNA expression was analyzed
using RT-PCR and qPCR. Total RNA (10 μg) isolated from each of the
ESCC cell lines listed above, 65 primary ESCC tissues, and
corresponding non-cancerous tissues were used as templates to
generate complementary DNAs (cDNAs). PCR primers for DENND2D were:
sense (S), 5′-CACTGCTCTACCCCTTCAGC-3′, in exon 7, and antisense
(AS), 5′-TTTTTCATCACCAACCGACA-3′, in exon 9–10, which amplified a
204-base pair (bp) product. RT-PCR amplification was performed as
follows: 40 cycles at 94°C for 30 sec, 60°C for 30 sec, and 72°C
for 30 sec after an initial denaturation step at 94°C for 5 min. To
confirm that equal amounts of cDNA were used as templates, RT-PCR
of β-actin was performed. Each RT-PCR product was loaded directly
onto 2% agarose gels, electrophoresed, stained with ethidium
bromide and visualized with ultraviolet light. qPCR reactions were
performed using a SYBR®-Green PCR Core Reagents kit
(Life Technologies, Carlsbad, CA, USA) under the following
conditions: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min,
and 45 cycles at 95°C for 15 sec and at 60°C for 30 sec. Real-time
detection of the SYBR-Green emission was conducted using an ABI
Prism® 7000 Sequence Detection System (Life
Technologies). The primers used were those described above. For
standardization, glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
mRNA (TaqMan®, GAPDH control reagents; Life
Technologies) was amplified in each sample. Nine ESCC cell lines
and 65 clinical samples and negative control reactions without a
template were analyzed. All reactions were performed in triplicate.
The amount of amplified DENND2D DNA in each sample was normalized
to GAPDH. The expression of DENND2D mRNA was defined as
downregulated in the tumor tissue when its level was less than
one-third that of the corresponding non-cancerous tissue.
Expression levels for samples are shown as DENND2D values
standardized to the DENND2D/GAPDH ratio. DENND2D mRNA expression in
tumor tissues and corresponding non-cancerous tissues was compared
and correlated with clinicopathological characteristics and
prognosis.
Analysis of the promoter region of
DENND2D
The nucleotide sequence of the DENND2D promoter
region was analyzed to determine the presence or absence of CpG
islands, which were defined as follows: at least a 200-bp region of
DNA with a high GC content (>50%) and an observed CpG/expected
CpG ratio ≥0.6 (25). We used CpG
Island Searcher software (http://cpgislands.usc.edu/) to determine the location
of CpG islands (26).
Methylation-specific PCR (MSP)
DNA samples from ESCC cell lines, ESCC tissues and
corresponding non-cancerous tissues were treated with bisulfite.
Briefly, 2 μg of DNA was denatured with NaOH, reacted with sodium
bisulfite, and purified using the Wizard® PCR Preps DNA
Purification System resin (Promega, Madison, WI, USA), treated
again with NaOH, precipitated with ethanol and resuspended in
water. The sequences of the unmethylated primer pairs that amplify
a 102-bp product were derived from the DENND2D promoter region
upstream of exon 1 and were: S, 5′-GATATGTGTTTTTGTGGATT-3′ and AS,
5′-ACACA TCCAAAACTAAAC-3′. Primer sequences derived from the
DENND2D promoter region used to detect methylated DNA amplified a
193-bp product and were: S, 5′-AGG TGGCGTCGTTTAGTTTC-3′ and AS,
5′-GCGAATCCG ACACTTTCACT-3′. DNA was amplified as follows: 45
cycles at 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec
after an initial denaturation step at 94°C for 5 min. Each PCR
product was loaded directly onto 2% agarose gels, electrophoresed,
stained with ethidium bromide and visualized with ultraviolet
light.
Bisulfite sequence analysis
Genomic bisulfite-treated DNA from ESCC cell lines
was sequenced to verify the MSP results. The sequence of the primer
pair used to generate a fragment for sequencing, which was derived
from the DENND2D promoter region, was: S, 5′-GGAGGTTAAGGATAGGGG-3′
and AS, 5′-ACACTAACCCCCATAACC-3′, which amplified a 133-bp product.
DNA was amplified as follows: 50 cycles at 94°C for 30 sec, 63°C
for 30 sec, and 72°C for 30 sec following an initial denaturation
step at 94°C for 5 min. PCR products were purified directly using
the QIAquick PCR Purification kit (Qiagen). Purified DNA fragments
were subcloned into the TA cloning vector (Life Technologies). Each
DNA was mixed with 3 μl of specific primer (M13) and 4 μl of Cycle
Sequencing Mix (BigDye® Terminator v1. 1 Cycle
Sequencing kit; Life Technologies, Grand Island, NY, USA).
Sequences were analyzed using an Applied Biosystems ABI 310 Prism
DNA Analyzer, and sequence electropherograms were generated using
ABI Sequence Analysis 3.0 software (Life Technologies).
5-aza-2′-deoxycytidine (5-aza-dC)
treatment
To assess the relationship between promoter
hypermethylation and DENND2D expression, ESCC cells
(1.5×106) were treated with 5-aza-dC (Sigma-Aldrich, St.
Louis, MO, USA) to inhibit DNA methylation and then cultured for 6
days with medium changes on days 1, 3 and 5. RNA was extracted and
RT-PCR was performed as described above.
Immunohistochemistry (IHC)
Expression and distribution of DENND2D protein were
analyzed using IHC in representative cases and compared with mRNA
expression patterns. Formalin-fixed, paraffin-embedded tissues were
dewaxed in xylene twice for 5 min, rehydrated in a graded alcohol
series (100, 90 and 70%), and then in H2O for 2 min each
and subsequently treated with 3% H2O2 to
inhibit endogenous peroxidases, followed by epitope retrieval using
five incubations in 10 mM citrate buffer at 95°C, 5 min each. The
samples were incubated with Histofine SAB-PO® (Nichirei,
Tokyo, Japan) for 5 min to limit non-specific reactivity, and were
then incubated for 1 h at room temperature with a rabbit antibody
against DENND2D (PA5-24032; Thermo Fisher Scientific Inc.,
Rockford, IL, USA) diluted 1:100 in Antibody Diluent (Dako,
Glostrup, Denmark). Samples were then washed with
phosphate-buffered saline, followed by a 10-min incubation with a
biotinylated secondary antibody (Histofine SAB-PO®;
Nichirei). Sections were developed for 2 min using liquid
3,3′-diaminobenzidine as the substrate (Nichirei). Staining
properties were determined using surrounding hepatic veins as
internal controls, and staining patterns were compared between
ESCCs and the corresponding non-cancerous tissues. To avoid
subjectivity, specimens were randomized and coded before analysis
by two independent observers blinded to the status of the samples.
Each observer evaluated all specimens at least twice within a given
time interval to minimize intra-observer variation. The expression
level of DENND2D protein was evaluated both in ESCC tissues and in
corresponding non-cancerous tissues. Tissue sections with ≥10% of
stained cells were qualitatively defined as positive.
Statistical analysis
The relative mRNA expression levels (DENND2D/GAPDH)
between ESCCs and non-cancerous tissues were analyzed using the
Mann-Whitney U test. The Chi-square test was used to analyze the
association between the expression and methylation status of
DENND2D and clinicopathological parameters. Disease-specific and
disease-free survival rates were calculated using the Kaplan-Meier
method, and the difference in survival curves was analyzed using
the log-rank test. We performed multivariate regression analysis to
detect prognostic factors using the Cox proportional hazards model
and variables with a P<0.05 were entered into the final model.
All statistical analysis was performed using JMP® 10
software (SAS Institute Inc., Cary, NC, USA). A P-value of <0.05
was considered to indicate a statistically significant result.
Results
Patient characteristics
The mean age of the 65 patients was 64.1±7.3 years
(mean ± standard deviation; range, 46–80 years). The male-to-female
ratio was 53:12. Fifty patients had a history of excessive alcohol
consumption; 28 patients had a Brinkman index ≥1000. When
classified using the 7th edition of the UICC classification, 5, 20,
30 and 10 patients were in stages I, II, III and IV,
respectively.
DENND2D mRNA expression in ESCC cell
lines and tumor tissues
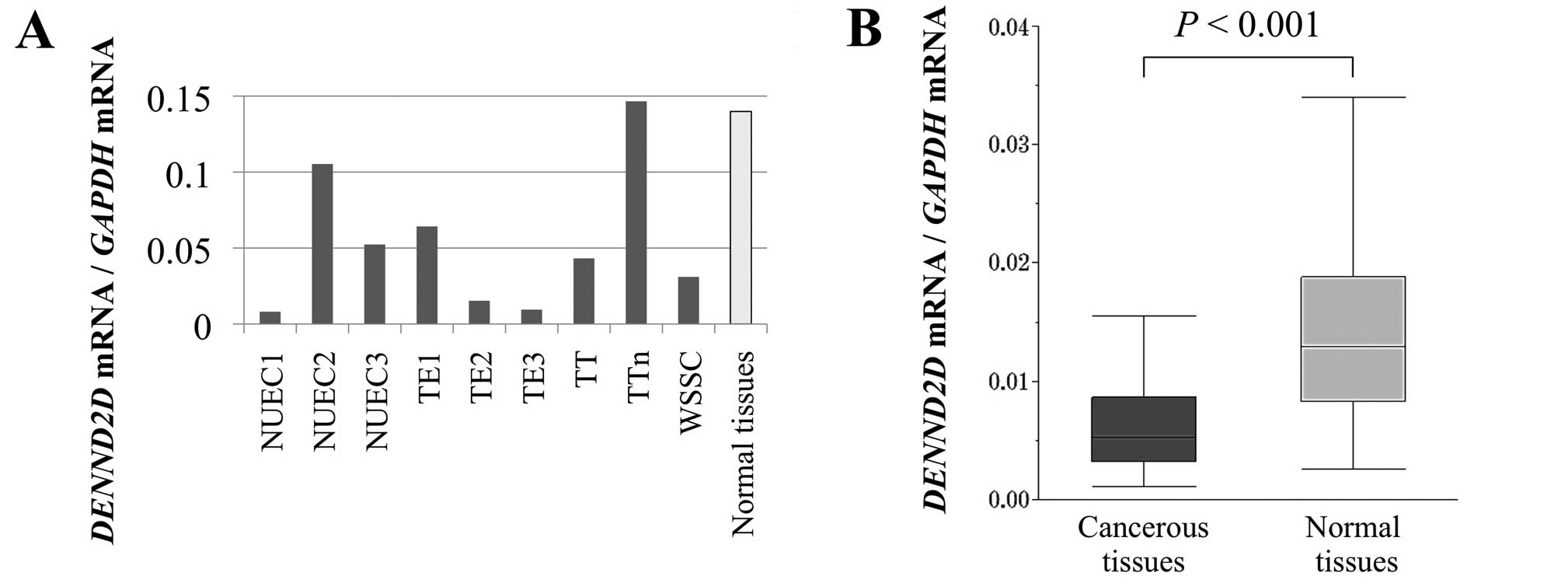
Expression analysis of DENND2D mRNA in ESCC cell
lines was performed to determine whether ESCC involved aberrant
expression of DENND2D or not. The levels of DENND2D mRNA detected
using qPCR were reduced compared with the median level of normal
esophageal tissues in the majority of ESCC cell lines except for
NUEC2 and TTn (Fig. 1A). In
particular, the DENND2D mRNA levels were 10-fold lower in NUEC1,
TE2 and TE3 cells. Based on the results in ESCC cell lines,
expression analysis was expanded to include clinical samples.
Expression of DENND2D mRNA was significantly higher in ESCC tissues
compared to corresponding normal tissues (P<0.001; Fig. 1B).
Identification of a CpG island in the
DENND2D promoter
A CpG island was identified in the DENND2D promoter
region using the CpG Island Searcher. The properties of the CpG
island were as follows: 1214 bp, 68.7% GC and a 0.68 observed
CpG/expected CpG ratio (Fig. 2A).
Therefore, we hypothesized that hypermethylation of the CpG islands
regulated the expression of DENND2D in ESCC tissue.
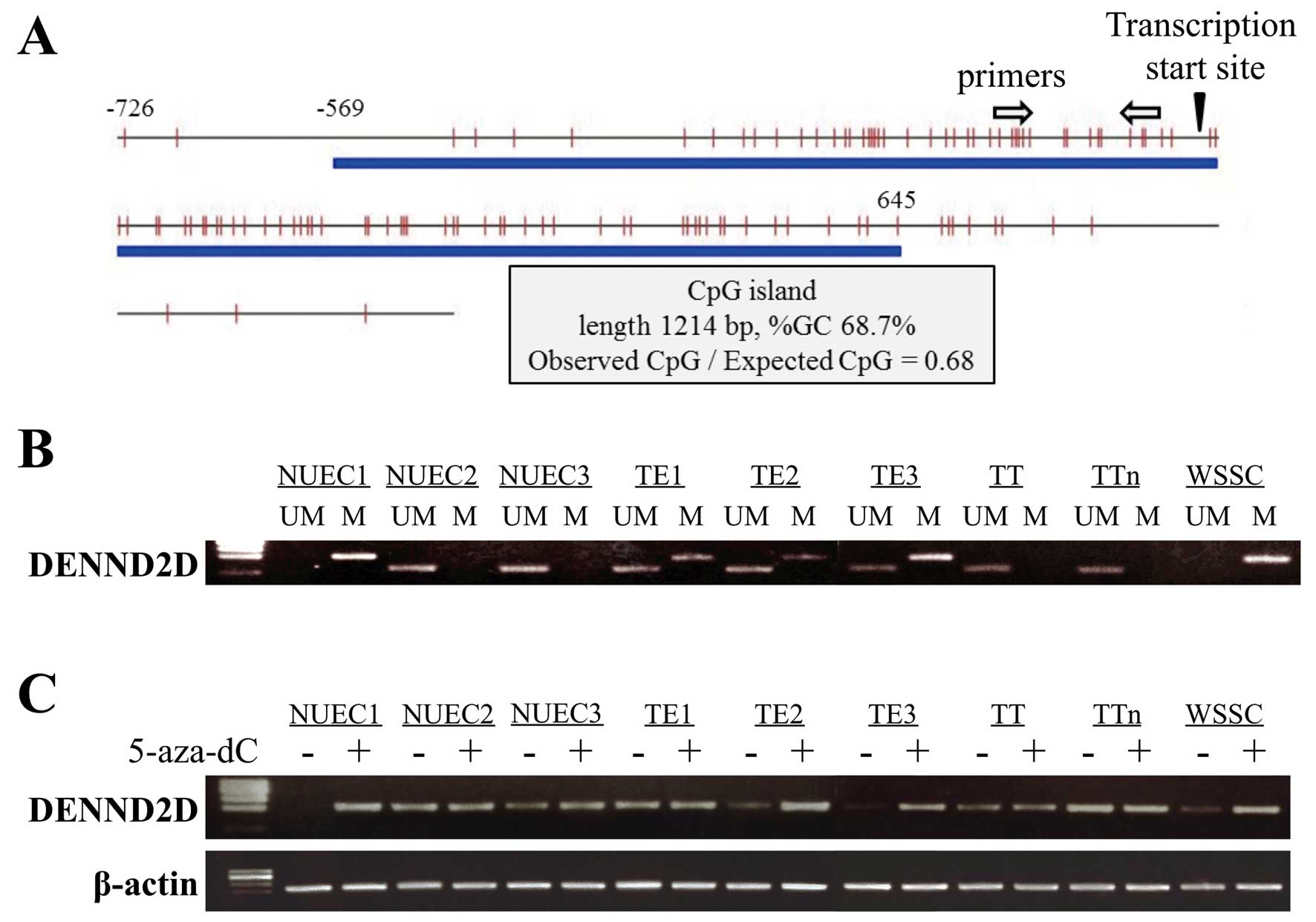 | Figure 2(A) The CpG island, indicated by the
blue line, was centered on the DENND2D transcription initiation
site extending upstream into the promoter region. (B)
Methylation-specific PCR analysis. The DENND2D promoter was
completely methylated in NUEC1 and WSSC; by contrast, methylation
was partial in TE1, TE2 and TE3 cells and was not detected in
NUEC2, NUEC3, TT and TTn cells. (C) RT-PCR analysis before and
after 5-aza-dC treatment. Reactivation or an increase in
methylation was observed if DENND2D expression was detected in
NUEC1, TE2, TE3 and WSSC cells. M, methylated; UM,
unmethylated. |
MSP analysis of ESCC cell lines
MSP was conducted to verify the above hypothesis. We
first determined the methylation status of DENND2D in nine ESCC
cell lines. Bands consistent with methylated DNA were detected in
NUEC1, TE1, TE2, TE3 and WSSC cells (Fig. 2B). We concluded that methylation of
the DENND2D promoter was complete in NUEC1 and WSSC, partial in
TE1, TE2 and TE3, and undetectable in NUEC2, NUEC3, TT and TTn cell
lines.
Transcription of DENND2D in cells treated
with 5-aza-dC
To determine whether promoter hypermethylation led
to the suppression of DENND2D transcription, we analyzed ESCC cell
lines before and after treatment with the DNA methylation
inhibitor, 5-aza-dC. Using semi-quantitative RT-PCR, reactivation
or an increase in DENND2D expression was detected in ESCC cell
lines, which demonstrated positive MSP (Fig. 2C).
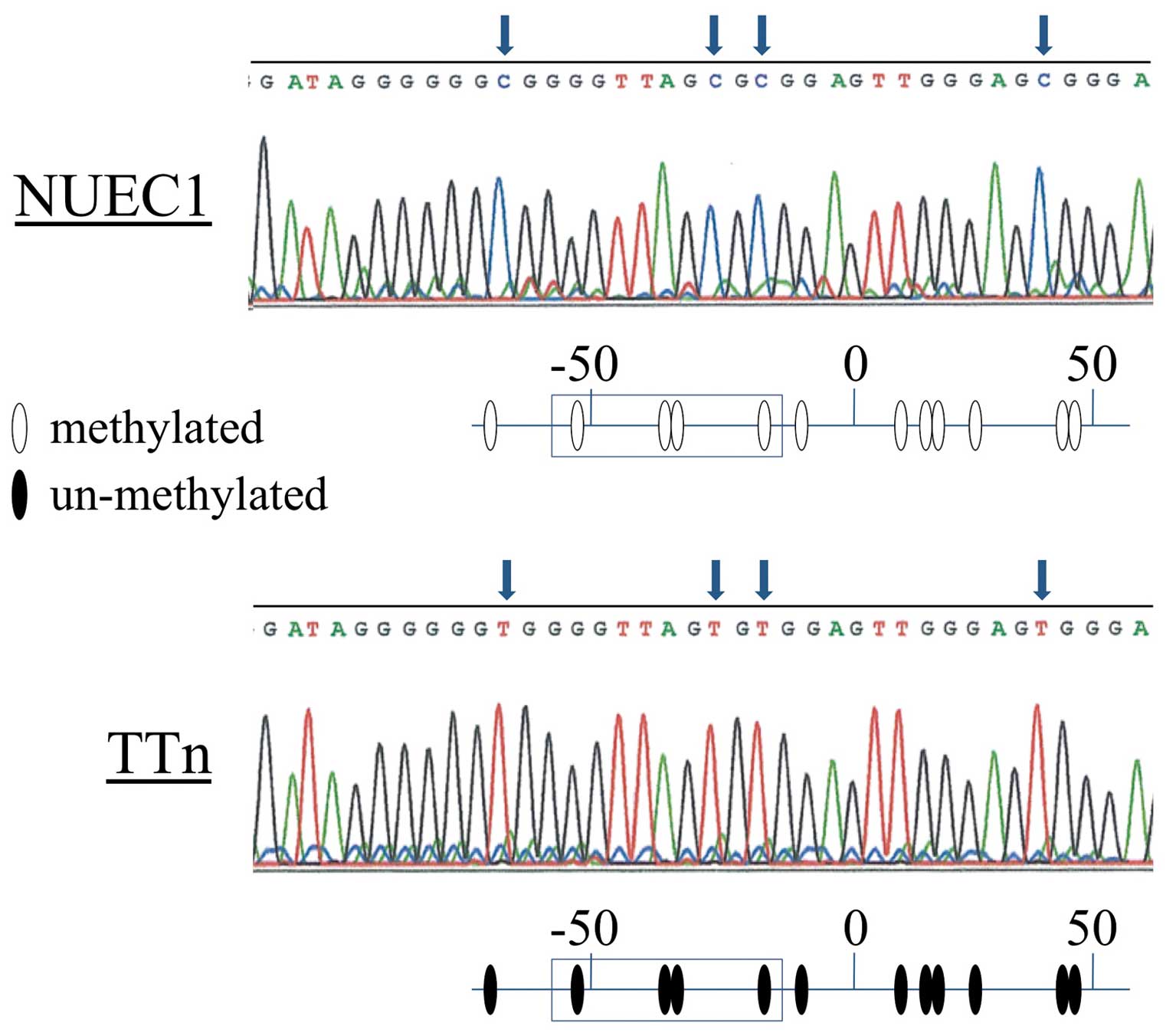
Bisulfite sequence analysis
To confirm the results of the MSP experiments, we
directly sequenced the DENND2D promoter in NUEC1 (complete
methylation) and TTn (undetectable methylation) cells and found
that all CpGs in the NUEC1 fragment were CG, while TG was present
at the corresponding position in TTn cells (Fig. 3), thus, confirming the accuracy of
our MSP data.
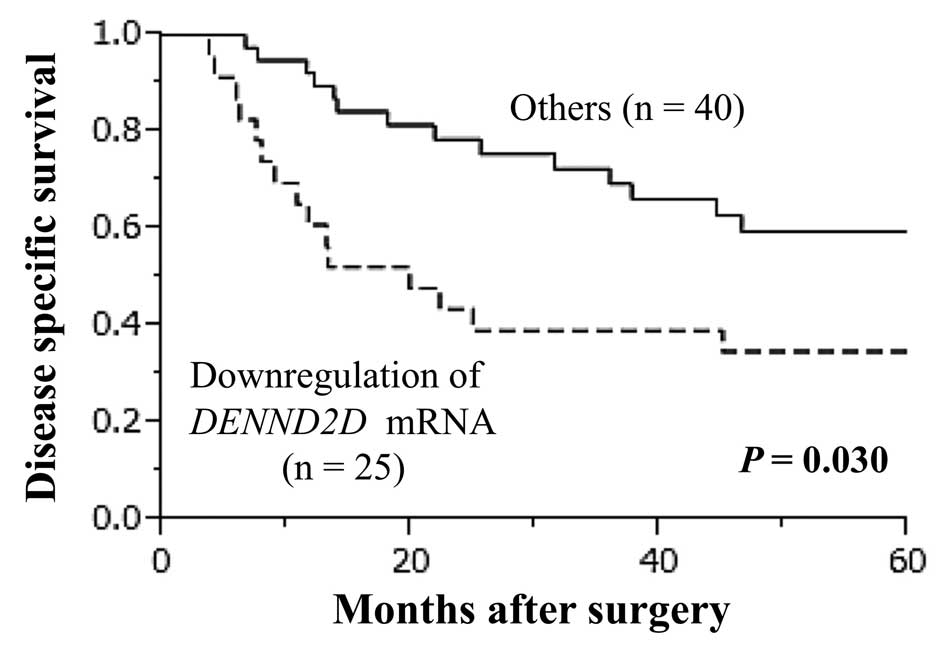
Prognostic value of DENND2D expression in
65 patients with ESCC
Downregulation of DENND2D mRNA was detected in tumor
samples from 25/65 (38.5%) of patients with ESCC. Patients with
reduced expression of DENND2D mRNA had a significantly poorer
prognosis than those without reduced expression (5-year survival
rate 34.8 vs. 59.5%; P=0.030; Fig.
4). Patients were grouped by age, gender, preoperative
symptoms, Brinkman index (≥1000), excessive alcohol consumption,
carcinoembryonic antigen (>5 ng/ml), squamous cell
carcinoma-related antigen (>1.5 ng/ml), tumor size (≥5.0 cm), T
factor (T3–4), tumor differentiation (poor), lymphatic involvement,
vessel invasion, intraepithelial spread, lymph node metastasis and
DENND2D mRNA expression. Of these variables, univariate analysis
identified age (≥65 years), tumor differentiation (poor), lymphatic
involvement and downregulation of DENND2D mRNA as significant
prognostic factors. In multivariate analysis, downregulation of
DENND2D mRNA was identified as an independent prognostic factor
(Hazard ratio, 2.194; P=0.039; Table
I) together with age (≥65 years), tumor differentiation (poor)
and lymphatic involvement. Downregulation of DENND2D mRNA was not
significantly associated with other clinicopathological
parameters.
 | Table IPrognostic factors for
disease-specific survival in 65 patients with squamous cell
carcinoma of the esophagus. |
Table I
Prognostic factors for
disease-specific survival in 65 patients with squamous cell
carcinoma of the esophagus.
| | Univariate
analysis | Multivariate
analysis |
|---|
| |
|
|
|---|
| Variable | N | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (≥65
years) | 28 | 2.14 | 1.05–4.44 | 0.038a | 2.36 | 1.12–5.12 |
0.024a |
| Gender (male) | 53 | 2.13 | 0.83–7.21 | 0.125 | | | |
| Preoperative
symptoms | 53 | 1.12 | 0.49–3.02 | 0.802 | | | |
| Brinkman index
(≥1000) | 28 | 1.85 | 0.91–3.81 | 0.090 | | | |
| Excessive alcohol
consumption | 50 | 0.73 | 0.34–1.75 | 0.456 | | | |
| CEA (>5
ng/ml) | 8 | 1.31 | 0.44–3.14 | 0.595 | | | |
| SCC (>1.5
ng/ml) | 22 | 0.67 | 0.28–1.43 | 0.312 | | | |
| Tumor size (≥5.0
cm) | 39 | 0.65 | 0.32–1.34 | 0.242 | | | |
| UICC T factor
(T3–4) | 48 | 1.63 | 0.74–4.10 | 0.237 | | | |
| Tumor
differentiation (poor) | 11 | 2.47 | 1.03–5.34 | 0.043a | 3.19 | 1.27–7.33 |
0.015a |
| Lymphatic
involvement | 54 | 4.86 | 1.46–30.1 | 0.007a | 5.45 | 1.58–34.3 |
0.004a |
| Vessel
invasion | 26 | 1.14 | 0.55–2.31 | 0.723 | | | |
| Intraepithelial
spread | 33 | 1.26 | 0.63–2.62 | 0.508 | | | |
| Lymph node
metastasis | 44 | 1.78 | 0.83–4.25 | 0.144 | | | |
| Hypermethylation of
DENND2D | 42 | 1.09 | 0.53–2.42 | 0.823 | | | |
| Downregulation of
DENND2D mRNA | 25 | 2.15 | 1.05–4.37 | 0.037a | 2.35 | 1.12–4.89 |
0.024a |
The association between DENND2D mRNA expression and
clinicopathological parameters in the 65 patients is shown in
Table II. Notably, downregulation
of DENND2D mRNA was seen only in male patients and was
significantly associated with T factor (T3–4) and vessel invasion
(P=0.033 and P=0.038, respectively). There was no difference in the
expression level of DENND2D mRNA in non-cancerous tissues between
male and female patients, indicating that DENND2D mRNA was subject
to downregulation in male patients with ESCC.
 | Table IIAssociation between expression of
DENND2D mRNA and clinicopathological parameters in 65
patients with squamous cell carcinoma of the esophagus. |
Table II
Association between expression of
DENND2D mRNA and clinicopathological parameters in 65
patients with squamous cell carcinoma of the esophagus.
| Clinicopathological
parameter | Downregulated
DENND2D expression in tumor tissue (n) | Other (n) | P-value |
|---|
| Age (years) | | | |
| <65 | 13 | 24 | 0.527 |
| ≥65 | 12 | 16 | |
| Gender | | | |
| Male | 25 | 28 |
<0.001a |
| Female | 0 | 12 | |
| Preoperative
symptoms | | | |
| Absent | 2 | 10 | 0.071 |
| Present | 23 | 30 | |
| Brinkman index | | | |
| <1000 | 12 | 25 | 0.251 |
| ≥1000 | 13 | 15 | |
| Excessive alcohol
consumption | | | |
| Absent | 4 | 11 | 0.275 |
| Present | 21 | 29 | |
| CEA (ng/ml) | | | |
| ≤5 | 23 | 34 | 0.391 |
| >5 | 2 | 6 | |
| SCC (ng/ml) | | | |
| ≤1.5 | 14 | 29 | 0.174 |
| >1.5 | 11 | 11 | |
| Tumor size
(cm) | | | |
| <5.0 | 11 | 15 | 0.603 |
| ≥5.0 | 14 | 25 | |
| UICC T factor | | | |
| T1–2 | 3 | 14 |
0.033a |
| T3–4 | 22 | 26 | |
|
Differentiation | | | |
| Moderate to
well | 19 | 35 | 0.235 |
| Poor | 6 | 5 | |
| Lymphatic
involvement | | | |
| Absent | 4 | 7 | 0.875 |
| Present | 21 | 33 | |
| Vessel
invasion | | | |
| Absent | 11 | 28 |
0.038a |
| Present | 14 | 12 | |
| Intraepithelial
spread | | | |
| Absent | 12 | 20 | 0.875 |
| Present | 13 | 20 | |
| Lymph node
metastasis | | | |
| Absent | 5 | 16 | 0.087 |
| Present | 20 | 24 | |
| Hypermethylation of
DENND2D | | | |
| Absent | 4 | 19 |
0.008a |
| Present | 21 | 21 | |
Methylation status of DENND2D in 65
clinical ESCC samples
MSP analysis revealed that 42/65 (64.6%) of ESCC
tissue samples and only 2/65 (3.1%) of the corresponding
non-cancerous tissues showed hypermethylation of the DENND2D
promoter. There was no significant association between
hypermethylation of DENND2D in ESCC tissues and overall or
recurrence-free survival (Table I).
Promoter hypermethylation of DENND2D in ESCCs was significantly
associated with downregulation of DENND2D mRNA expression (P=0.008;
Table II).
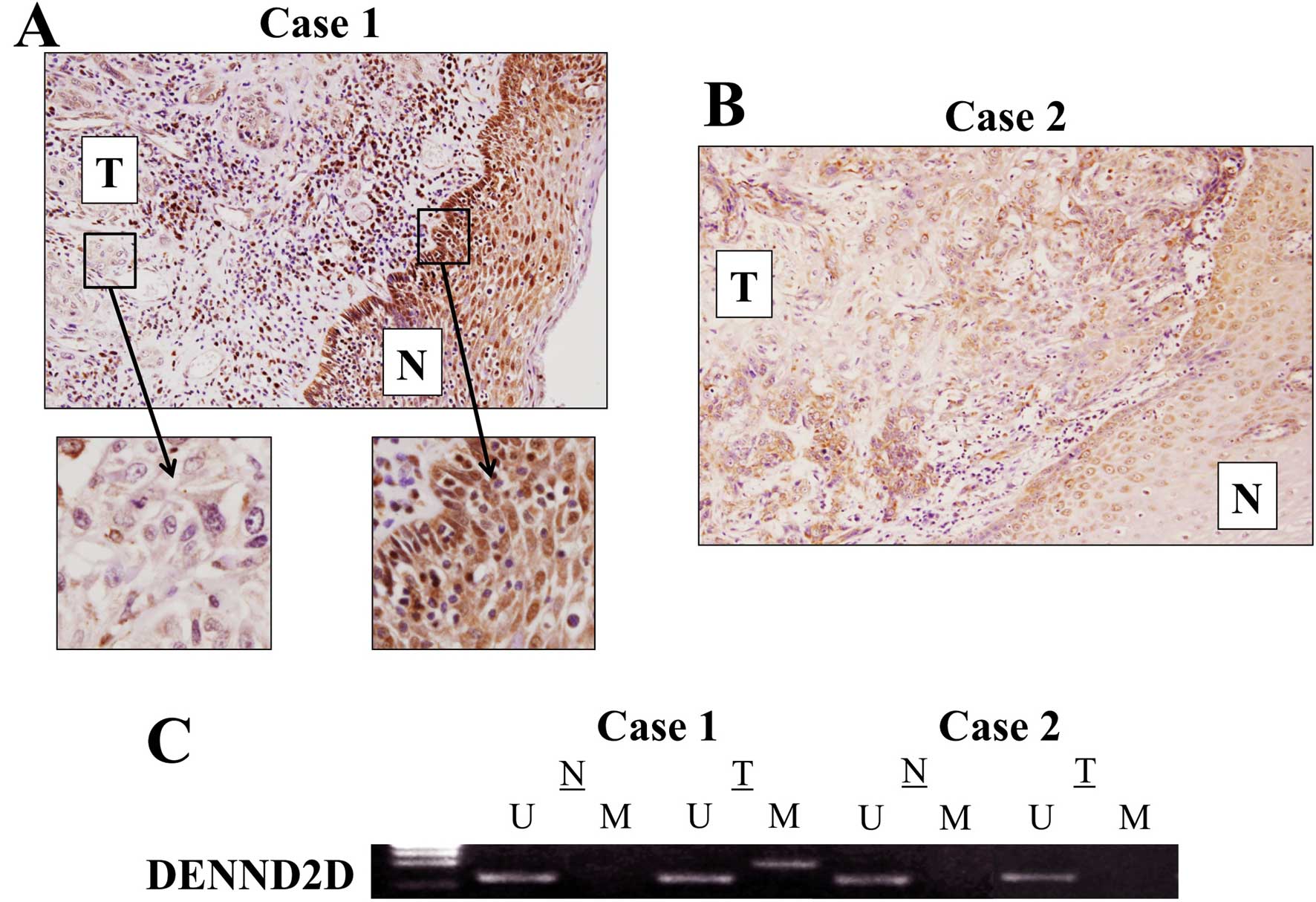
IHC
The expression of DENND2D was determined using IHC
in 30 cases showing relative overexpression, underexpression or
equivalent DENND2D mRNA expression in ESCC tissues compared with
corresponding non-cancerous tissues. A representative case with the
lowest expression level of DENND2D mRNA in ESCC tissues showed
reduced expression of DENND2D in the membrane and cytoplasm of
tumor cells compared with the adjacent non-cancerous tissue
(Fig. 5A). By contrast, equivalent
expression of DENND2D protein in tumor and normal cells was
detected in a representative case without reduced DENND2D mRNA
expression in the ESCC tissue (Fig.
5B). The MSP analysis of these cases is shown in Fig. 5C.
Discussion
Identification of cancer-related genes has provided
an important source from which numerous prognostic biomarkers and
molecular targets have been identified. In the present study,
DENND2D was identified as a candidate TSG that was epigenetically
inactivated in ESCC. We have shown here that the level of DENND2D
mRNA expression was reduced in 8/9 ESCC cell lines and in 59/65
surgical specimens, and the mean expression level was significantly
lower in cancerous tissues than in corresponding normal tissues.
This result indicates that DENND2D may play an important role in
the carcinogenesis of ESCC. Furthermore, the expression pattern of
DENND2D using IHC was consistent with that of its mRNA levels using
qPCR. Moreover, a significant reduction in DENND2D mRNA level in
ESCC tissues was an independent prognostic factor. These findings
suggest that DENND2D acts as a TSG and are consistent with the
results of a study on DENND2D expression in lung cancer (22). Downregulation of DENND2D expression
in ESCC tissues should, therefore, provide a biomarker of ESCC
progression. Another striking finding was that the downregulation
of DENNN2D was observed exclusively in men. The reason for the
differential expression between men and women in the case of
DENNN2D is unknown at this time; however, gender has been reported
to influence gene expression and epigenetics (27,28).
Our data indicates that a unique subset of ESCC exists that is only
observed in men and is associated with poor prognosis, although
confirmation with a greater number of samples is required.
To understand the mechanism of regulation of DENND2D
transcription, we performed methylation analysis of DENND2D after
we identified a CpG island within its promoter. The DENND2D
promoter was hypermethylated in 5/9 ESCC cell lines, and DENND2D
transcription could be reactivated in cells treated with an
inhibitor of methylation. Hypermethylation was frequently detected
(64.6%) in ESCC tissues and significantly associated with
substantial (3 times or more) reduction of DENND2D mRNA levels.
Therefore, we consider promoter hypermethylation as a potent
regulatory factor of DENND2D transcription in ESCC.
The DENND2 family is the only example where the DENN
domain is located within the C-terminal region (20,29).
Each DENND2 protein acts as a GEF for Rab9a/b. DENND2D is composed
of a DENN domain alone (30);
therefore, the DENN domain mediates GEF activity. Rab GTPases
regulate tumorigenesis via trafficking-mediated events and some
function in a trafficking-independent manner (15,31,32).
Additionally, it is known that several members of the Rab GTPase
family affect exosome secretion via the trans-Golgi network or by
inducible vesicular trafficking (33–35).
Exosomes released from cancer cells can be taken up by neighboring
cells and are capable of inducing pathways involved in cancer
initiation and progression (36).
Therefore, it is thought that DENND2D, which is a regulator of Rab
GTPases, plays an important role in carcinogenesis and cancer
progression.
The present study was limited by its lack of
sufficient functional analysis of DENND2D, which tempers the
conclusion that it acts as a tumor suppressor in ESCC. Further
studies are required to clarify the molecular mechanisms underlying
the biological activities and effects that DENND2D has on
chemoresistance in ESCC.
In summary, we propose DENND2D as a candidate TSG
that is inactivated by promoter hypermethylation in ESCC and shows
promise as a novel biomarker of progression of ESCC.
References
|
1
|
Ferlay J, Shin H, Bray F, Forman D,
Mathers C and Parkin D: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
4
|
Kanda M, Nomoto S, Nishikawa Y, Sugimoto
H, Kanazumi N, Takeda S and Nakao A: Correlations of the expression
of vascular endothelial growth factor B and its isoforms in
hepatocellular carcinoma with clinico-pathological parameters. J
Surg Oncol. 98:190–196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanda M, Nomoto S, Okamura Y, Hayashi M,
Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S and Nakao A:
Promoter hypermethylation of fibulin 1 gene is associated with
tumor progression in hepatocellular carcinoma. Mol Carcinog.
50:571–579. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda M, Nomoto S, Okamura Y, Nishikawa Y,
Sugimoto H, Kanazumi N, Takeda S and Nakao A: Detection of
metallothionein 1G as a methylated tumor suppressor gene in human
hepatocellular carcinoma using a novel method of double combination
array analysis. Int J Oncol. 35:477–483. 2009.
|
|
7
|
Wang YN, Yamaguchi H, Hsu JM and Hung MC:
Nuclear trafficking of the epidermal growth factor receptor family
membrane proteins. Oncogene. 29:3997–4006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casas A, Di Venosa G, Hasan T and Al
Batlle: Mechanisms of resistance to photodynamic therapy. Curr Med
Chem. 18:2486–2515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stenmark H: Rab GTPases as coordinators of
vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pawson T and Nash P: Assembly of cell
regulatory systems through protein interaction domains. Science.
300:445–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marat AL and McPherson PS: The connecdenn
family, Rab35 guanine nucleotide exchange factors interfacing with
the clathrin machinery. J Biol Chem. 285:10627–10637. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato M, Sato K, Liou W, Pant S, Harada A
and Grant BD: Regulation of endocytic recycling by C.
elegans Rab35 and its regulator RME-4, a coated-pit protein.
EMBO J. 27:1183–1196. 2008.
|
|
13
|
Levivier E, Goud B, Souchet M, Calmels TP,
Mornon JP and Callebaut I: uDENN, DENN, and dDENN: indissociable
domains in Rab and MAP kinases signaling pathways. Biochem Biophys
Res Commun. 287:688–695. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfeffer S: A model for Rab GTPase
localization. Biochem Soc Trans. 33:627–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subramani D and Alahari SK:
Integrin-mediated function of Rab GTPases in cancer progression.
Mol Cancer. 9:3122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng KW, Lahad JP, Kuo WL, Lapuk A,
Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D,
Gray JW and Mills GB: The RAB25 small GTPase determines
aggressiveness of ovarian and breast cancers. Nat Med.
10:1251–1256. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rink J, Ghigo E, Kalaidzidis Y and Zerial
M: Rab conversion as a mechanism of progression from early to late
endosomes. Cell. 122:735–749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Girard M, Allaire PD, McPherson PS and
Blondeau F: Non-stoichiometric relationship between clathrin heavy
and light chains revealed by quantitative comparative proteomics of
clathrin-coated vesicles from brain and liver. Mol Cell Proteomics.
4:1145–1154. 2005. View Article : Google Scholar
|
|
19
|
Majidi M, Hubbs AE and Lichy JH:
Activation of extracellular signal-regulated kinase 2 by a novel
Abl-binding protein, ST5. J Biol Chem. 273:16608–16614. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marat AL, Dokainish H and McPherson PS:
DENN domain proteins: regulators of Rab GTPases. J Biol Chem.
286:13791–13800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Postel EH, Weiss VH, Beneken J and Kirtane
A: Mutational analysis of NM23-H2/NDP kinase identifies the
structural domains critical to recognition of a c-myc regulatory
element. Proc Natl Acad Sci USA. 93:6892–6897. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling B, Zheng H, Fu G, Yuan J, Shi T, Chen
S, Liu Y, Liu Y, Cao Y, Zheng S, Guo S, Han N, Gao Y, Cheng S and
Zhang K: Suppression of non-small cell lung cancer proliferation
and tumorigenicity by DENND2D. Lung Cancer. 79:104–110. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
International Union Against Cancer. TNM
Classification of Malignant Tumors. 7th edit. Sobin LH,
Gospodarowicz MK and Wittekind C: Wiley-Blackwell; New York:
2009
|
|
24
|
Long MJ, Jiang CQ, Lam TH, Lin JM, Chan
YH, Zhang WS, Jin YL, Liu B, Thomas GN and Cheng KK: Alcohol
consumption and electrocardiographic left ventricular hypertrophy
and mediation by elevated blood pressure in older Chinese men: The
Guangzhou Biobank Cohort Study. Alcohol. 47:473–480. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takai D and Jones PA: Comprehensive
analysis of CpG islands in human chromosomes 21 and 22. Proc Natl
Acad Sci USA. 99:3740–3745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takai D and Jones PA: The CpG island
searcher: a new WWW resource. In Silico Biol. 3:235–240.
2003.PubMed/NCBI
|
|
27
|
Kiyohara C, Wakai K, Mikami H, Sido K,
Ando M and Ohno Y: Risk modification by CYP1A1 and GSTM1
polymorphisms in the association of environmental tobacco smoke and
lung cancer: a case-control study in Japanese nonsmoking women. Int
J Cancer. 107:139–144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mollerup S, Ryberg D, Hewer A, Phillips DH
and Haugen A: Sex differences in lung CYP1A1 expression and DNA
adduct levels among lung cancer patients. Cancer Res. 59:3317–3320.
1999.PubMed/NCBI
|
|
29
|
Bloethner S, Mould A, Stark M and Hayward
NK: Identification of ARHGEF17, DENND2D, FGFR3, and RB1 mutations
in melanoma by inhibition of nonsense-mediated mRNA decay. Genes
Chromosomes Cancer. 47:1076–1085. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshimura S, Gerondopoulos A, Linford A,
Rigden DJ and Barr FA: Family-wide characterization of the DENN
domain Rab GDP-GTP exchange factors. J Cell Biol. 191:367–381.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Croizet-Berger K, Daumerie C, Couvreur M,
Courtoy PJ and van den Hove MF: The endocytic catalysts, Rab5a and
Rab7, are tandem regulators ofthyroid hormone production. Proc Natl
Acad Sci USA. 99:8277–8282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng KW, Lahad JP, Gray JW and Mills GB:
Emerging role of RAB GTPases in cancer and human disease. Cancer
Res. 65:2516–2519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ponnambalam S and Baldwin SA: Constitutive
protein secretion from the trans-Golgi network to the plasma
membrane. Mol Membr Biol. 20:129–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Loomis RJ, Holmes DA, Elms A, Solski PA,
Der CJ and Su L: Citron kinase, a RhoA effector, enhances HIV-1
virion production by modulating exocytosis. Traffic. 7:1643–1653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP,
Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC,
Darchen F, Amigorena S, Moita LF and Thery C: Rab27a and Rab27b
control different steps of the exosome secretion pathway. Nat Cell
Biol. 12:19–30. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henderson MC and Azorsa DO: The genomic
and proteomic content of cancer cell-derived exosomes. Front Oncol.
2:382012. View Article : Google Scholar : PubMed/NCBI
|