Introduction
Mucin 1 (MUC1) is a classic target for cancer
immunotherapy and is under research in many forms in both
preclinical and clinical trials (1,2). MUC1
is a transmembrane glycoprotein normally expressed on the apical
surface of ductal epithelia. A variable number (20–125) of tandem
repeats (VNTRs) of a 20 amino acid sequence was contained in its
extracellular domain. On the apical surface of the normal ductal
epithelia, VNTRs are heavily glycosylated at threonine and serine
residues, up to 70% carbohydrate by weight, while malignant cells
contain underglycosylated VNTR domains, and are overexpressed in
90% of all adenocarcinomas including breast, lung, pancreas,
prostate, stomach, colon and ovary (3). Due to such altered glycosylation, new
epitopes appear on the cell surface that are absent in normal
tissues. These VNTR-derived epitopes are attractive targets in
immunotherapy. Peptide epitopes, containing the PDTR fragment from
VNTR of MUC1 have been found to be immunodominant in T-cell and
B-cell responses (4). A novel
Bacillus Calmette-Guérin-based breast cancer vaccine that
coexpresses multiple tandem repeats of MUC1 and CD80 was reported
to break the immune tolerance and inhibits MUC1-positive breast
cancer growth (5). Mukherjee et
al showed that a novel MUC1-based vaccine in combination with a
cyclooxygenase-2 inhibitor (celecoxib), and low-dose chemotherapy
(gemcitabine) was effective in preventing the progression of
preneoplastic intraepithelial lesions to invasive pancreatic ductal
adenocarcinomas (6).
Research on protein-based vaccines against cancer
has been carried out for many years. However, it is broadly
criticized due to the fact that protein vaccines generally induce
relatively stronger levels of humoral immunity than cellular
immunity, while the latter is considered essential for tumor
immunotherapy. Therefore, effective immunoadjuvant and vaccine
strategies are required for successful induction of cellular immune
responses against the protein vaccine.
Currently, there are many forms of MUC1 vaccine
adjuvants which have already been used in clinical trials, such as
BCG, QS-21, KLH and SB-AS217. Among them, MPL (monophosphoryl lipid
A) activates Toll-like receptor 4 (TLR4) and triggers the
Trif-dependent pathway (7).
Moreover, DDA (dimethyldioctadecylam monium bromide) is a cationic
liposome that is able to enhance antigen uptake and presentation
(8). MPL formulated with cationic
DDA liposomes (DDA/MPL) which was used in the tuberculosis vaccine
has been reported to enhance antigen uptake (9), antigen presentation to T cells and
stimulate DCs through Toll-like receptors (TLRs) (10). In our previous study, DDA/MPL
effectively enhanced the immunity of tumor vaccine targeting
survivin (11). However, little was
known about its function as the adjuvant for the cancer vaccine
targeting MUC1 VNTR. In our study, we used DDA/MPL as the adjuvant
for MUC1 protein vaccine. We investigated the immunity enhancement
of this adjuvant, both the humoral and cellular aspects.
Our previous experiments also showed that
heterologous adenoviral vector prime-recombinant protein and
DDA/MPL boost immunization strategy (termed VPP immune strategy)
presented better antitumor effects than the homologous adjuvant
protein prime-boost immunization (12), which is in accordance with other
reports (13–15). Thus, adenoviral vector
prime-recombinant protein and DDA/MPL boost was conducted for
tumor-suppressive experiments in the present study.
The purpose of our study was to evaluate whether the
9M protein vaccine induces cellular immune responses and antitumor
effects through the optimization of immune adjuvant and
immunization strategies. DDA/MPL was used as immunoadjuvant to
enhance the immune responses. Adenoviral vector prime-DDA/MPL
adjuvant protein boost immune strategy (VPP) was conducted in a
murine melanoma model, and the tumor inhibitory effects and
mechanisms were studied.
Materials and methods
Mice, cell lines and antibody
Female C57BL/6 mice (6–8 weeks) were purchased from
Beijing Huafukang Biology Technology Co. Ltd. Mice were maintained
in microisolator cages under pathogen-free conditions, and animal
care conformed to the Guide for the Care and Use of Laboratory
Animals (National Research Council). Murine C2C12 myoblast cells
and B16 melanoma cells were preserved by the National Engineering
Laboratory of AIDS Vaccine, Jilin University. MUC1 antibody was
purchased from BD Biosciences (550486).
Construction, expression and purification
of recombinant protein
The cDNA fragment containing nine identical peptide
tandem repeats (sequence HGVTSAPDTRPAPGSTAPPA) was synthesized by
Generay Corp. and inserted into pET-26b (Novagen) using the
BamHI and HindIII (Takara) site. Expression of the
recombinant MUC1 VNTR protein (9M) was confirmed by immunoblotting
using a mouse mAb against MUC1 VNTR (BD Pharmingen). The
recombinant His-tagged protein was purified using a HiTrap
chelating HP column (Invitrogen).
Construction of recombinant adenovirus
vector expressing MUC1 VNTRs
The recombinant adenovirus (rAd) vector expressing
MUC1 VNTRs was obtained using the AdMax™ Adenovirus Vector Creation
System (Microbix Biosystems). The 9M fragment with
EcoRI/SalI sites was subcloned into the
EcoRI/SalI sites of the pDC316 shuttle vector. rAd
was produced by homologous recombination between pBHGloxDE1,3Cre,
and pDC316-9M at the loxP or frt position when co-transfected into
HEK293 cells. Plaque formation observed ~10 days post-transfection
indicated successful generation of the rAd. After verification by
PCR or western blot analysis, the rAd containing 9M (AD-9M) was
amplified and purified from cell lysates twice in CsCl density
gradients, as previously described Yu et al (15). Viral products were desalted and
stored at −80°C in PBS containing 10% glycerol (v/v). The titer of
the viral stock was determined using the Reed and Muench method and
expressed as 50% tissue culture infectious doses (TCID50).
qRT-PCR
RNA was extracted by the guanidine isothiocyanate
and chloroform method (Invitrogen) from the C2C12 (a cell line of
mice myoblast), B16 cells and implanted B16 tumors. All RNA samples
were treated with DNase I (Promega). For all samples, 1 μg of total
RNA was used to synthesize first-strand cDNA with reverse
transcriptase (Invitrogen) and random primers. The cDNA synthesis
was performed at 37°C for 60 min after heat inactivation at 95°C
for 10 min. PCR was performed using 1X SYBR Green PCR Master mix
(Transgen) on a real-time PCR system (CFX96; Bio-Rad). MUC1
expression was confirmed by quantitative real-time
reverse-transcriptase (RT) PCR (qRT-PCR) using primers
5′-GTGCCCCCTAGCAGTACCG-3′ and 5′-GACGTGCCCCTACAAGTTGG-3′. Cycling
conditions were: 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15
sec at 95°C, plus 1 min at 60°C. Commercial software (CFX Manager)
was used to calculate relative MUC1 expression normalized to the
β-actin endogenous control (primers 5′-CGTGGACATCCGCAAAGACC-3′ and
5′-GGACTCGTCATACTCCTGCTTGC-3′) for all genes studied using the
2−ΔΔCt method.
Immunization schedule
9M recombinant protein (50 μg), 250 μg DDA (Arcos
Organics) plus 25 μg MPL (Avanti Polar Lipids) were injected
separately or as a mixture subcutaneously in C57BL/6 mice (5 mice
per group) with two week intervals. The mice were vaccinated three
times. Two weeks after the final immunization, all mice were
sacrificed to detect humoral and cellular immune responses.
Humoral responses
Antigen-specific antibodies in mice sera were
estimated by standard enzyme-linked immunosorbent assay (ELISA)
when the mice were sacrificed two weeks after the final
immunization. The ELISA plates were coated with 9M protein at 0.25
μg/well. Sera diluted at 1:100 and horseradish peroxidase
(HRP)-conjugated secondary antibody diluted at 1:10,000 (goat
anti-mouse IgG, Jackson) were used, and the enzyme reaction was
developed with 3,3′,5,5′-tetramethylbenzidine (TMB) for 10–15 min
and stopped with 2 M H2SO4. Optical density
(OD) was determined with a microplate reader at a wavelength of 450
nm.
IFN-γ ELISPOT assay
Releasing of IFN-γ from antigen-specific effecter T
cells was assessed with an ELISPOT kit (BD Biosciences) according
to the manufacturer’s instructions. The reaction was terminated
upon the appearance of dark purple spots, which were quantitated
using the AlphaImager System.
In vitro cytotoxicity assay
In vitro cytotoxicity was determined as
previously reported (16). Briefly,
B16 cells used as target cells were pulsed at a concentration of
1×106 cells/ml with or without 5 μg/ml of the MHC-I
H-2Db restricted MUC1 peptide (SAPDTRPAP) in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) for 2 h at 37°C.
Peptide-loaded B16 cells were then labeled with 5,
6-carboxyfluorescein succinimidylester (CFSE) fluorescent dye (5
μM) in RPMI-1640 medium without FBS for 10 min, while unloaded B16
cells were labeled only with CFSE (0.5 μM). CFSE labeling was
stopped by the addition of an equal volume of cold FBS for 3 min,
and then the cells were washed and counted. CFSEhigh-
and CFSElow-labeled cells were mixed together at a 1:1
ratio, which was confirmed by flow cytometry. Different numbers of
splenocytes from vaccinated mice were then incubated with the B16
cells (peptide-loaded 5×104, unloaded 5×104)
for 6 h at 37°C, after which the co-cultures were analyzed on a
FACS MoFlo XDP (Beckman Coulter, USA) for assessment of the
percentage of CFSE-labeled B16 cells. Specific killing was
calculated as follows: % killing = [1-(peptide - loaded
cells/unloaded cells from immunized group)/(peptide - loaded
cells/unloaded cells from naive group)] ×100.
Tumor immunotherapy in C57BL/6 mice
Tumor-suppressing experiments were carried out in
mice using the following two protocols. C57BL/6 mice (8 per group)
were injected subcutaneously with 1×105 B16 viable cells
per mouse on day 0. Thus, the homologous and heterologous
prime-boost regimen was carried out by different strategies.
Homologous immunity was conducted by immunizing the mice with
9M+DDA/MPL three times on days 3, 10 and 17. While heterologous
immunity was conducted by immunizing the mice with AD-9M
(1×108 pfu) into the tibialis anterior muscles of both
legs (50 μl each) per mouse on day 3 and 9M+DDA/MPL on day 10 and
17. Negative control mice were administered 100 μl PBS. Tumor
diameters were measured in two dimensions every 2 days. Tumor
volumes (mm3) were calculated as follows:
V=(A2xB)/2, where A is the length of the short axis and
B is the length of the long axis. Mice were sacrificed at week 4
after inoculation with B16 cells to remove and weigh the tumors.
Moreover, T cellular immune responses were also detected after the
mice were sacrificed. Survival of mice was monitored for ~60
days.
Cell proliferation
Single cell suspensions of splenocytes at
2×106/ml in PBS were mixed with an equal volume of 20 mM
CFSE in PBS (Molecular Probes). Cells were incubated for 10 min at
room temperature in the dark. Labeling was stopped by addition of
FBS and cells were washed twice with PBS. CFSE-labeled spleen cells
were cultured at 37°C for 5 days and then used for flow cytometry
analysis.
Cytokine detection by multiplex flow
immunoassay
Splenocytes (1×107) from the vaccinated
tumor-bearing mice were stimulated with the 9M protein at a
concentration of 5 μg/ml for 15 h, and then the cell culture
supernatants were collected to measure multiple cytokines. IL-2 and
IL-10 were detected by Bio-Plex pro assays. After the Bio-Plex
beads were incubated with the cell culture supernatants in a
reaction vessel for half an hour at room temperature in the dark,
they were washed and a fluorescent reporter antibody was added to
the reaction mixture. Following the second incubation ~40 min and
wash cycle, the beads were suspended in buffer and passed through a
flow-based detector.
Statistical analyses
All in vivo and in vitro experiments
were performed at least 3 times. Data were analyzed using one-way
ANOVA. Differences between the groups were assessed for statistical
significance using the unpaired T-test. P<0.05 was considered
significant, and P<0.01 was considered highly significant. All
statistical analyses were performed with Graphpad Prism
software.
Results
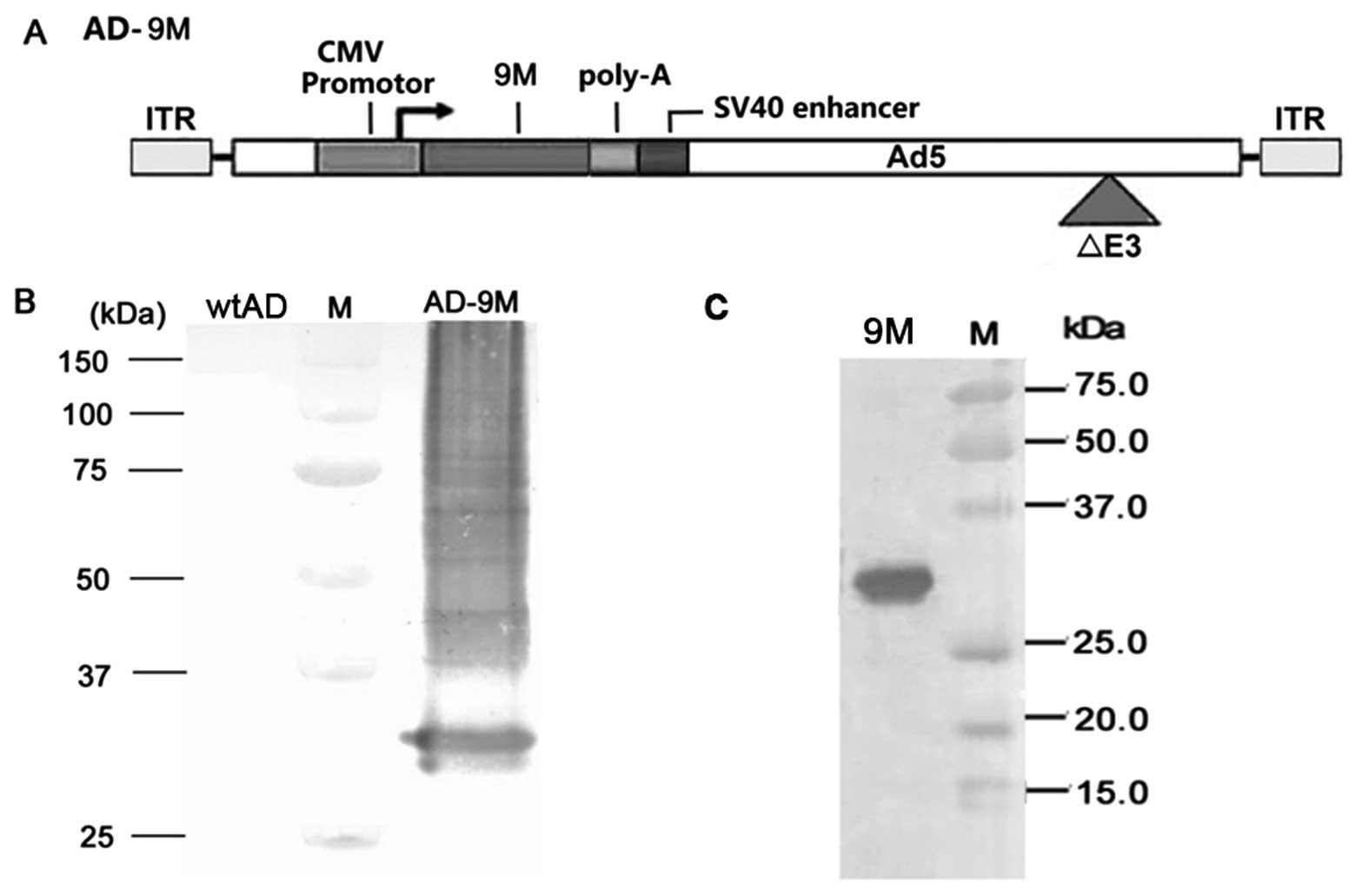
Construction and expression of AD-9M and
9M protein
MUC1 VNTRs were also subcloned into the adenovirus
vector as previously described (Fig.
1A), and the expression of wild-type adenovirus (wtAD) and
recombinant adenovirus expressing MUC1 VNTRs (AD-9M) in HEK293
cells is shown in Fig. 1B. Fig. 1C shows identification of purified
MUC1 VNTR protein by western blotting with anti-MUC1 VNTR mAb.
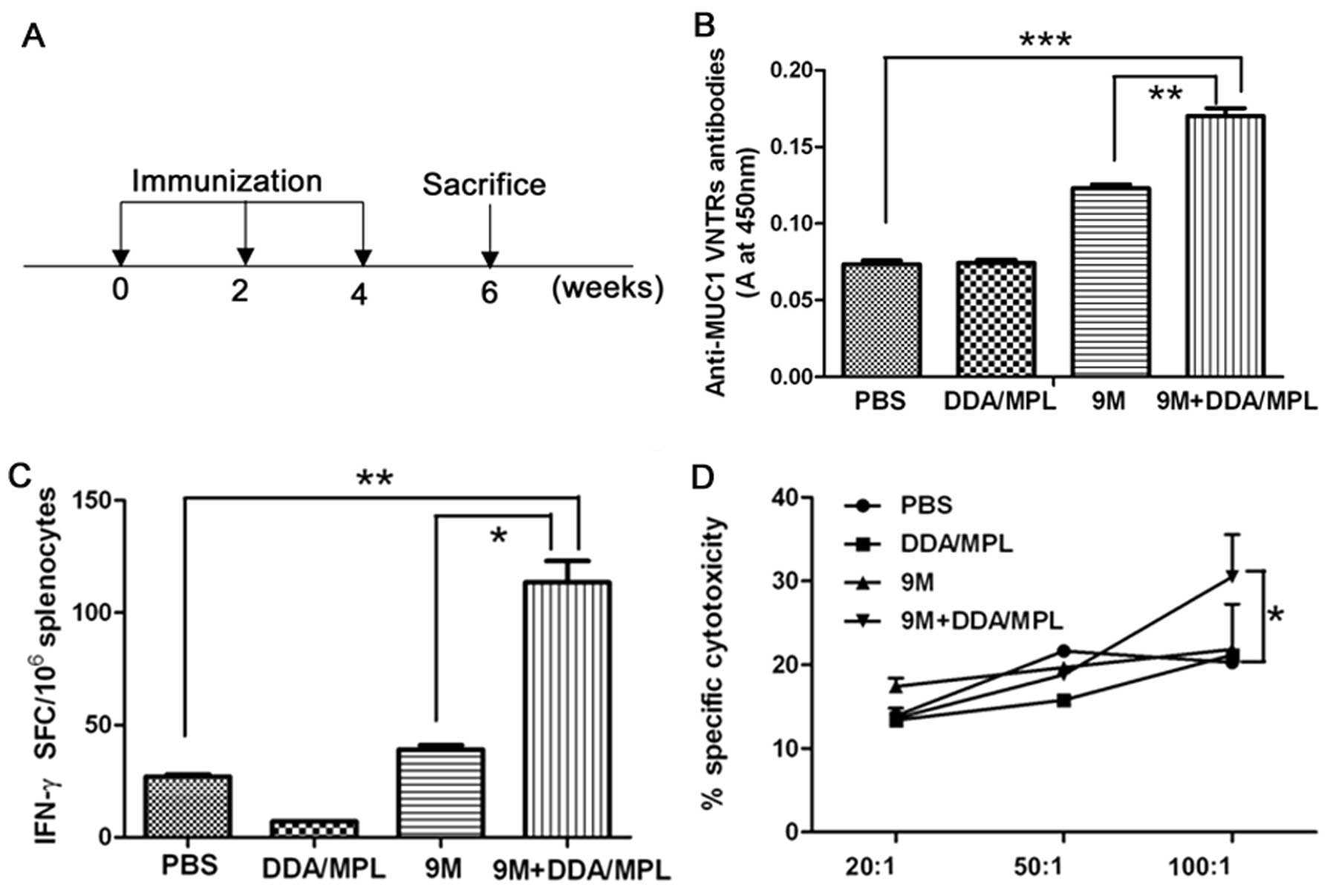
DDA/MPL enhances humoral and cellular
immune response induced by the 9M protein vaccine
To investigate the adjuvant effect of DDA/MPL on 9M
protein vaccine, groups of mice were immunized with 9M+DDA/MPL, the
9M and DDA/MPL as shown in Fig. 2A.
By ELISA, the 9M group showed ~2-fold higher antibody titer than
the PBS and DDA/MPL group (P>0.05); however, serum from the
9M+DDA/MPL group mice had higher antibody titers than the PBS,
DDA/MPL and 9M group (P<0.001; P<0.01, especially),
suggesting that DDA/MPL could substantially enhance the humoral
immunity induced by protein vaccine (Fig. 2B). Moreover, the trend was also
observed in cellular immune response. ELISPOT assay was used to
detect the frequency of antigen-specific IFN-γ-secreting T cells.
The results showed that IFN-γ induced by the 9M protein group was
no more than the PBS group (P>0.05); however, the response in
mice vaccinated with recombinant 9M protein and DDA/MPL was
significantly higher (nearly three times) than that observed for
mice given separately the 9M protein and DDA/MPL (P<0.05)
(Fig. 2C). Specific killing was
measured after incubating the splenocytes for 2 h with
SAPDTRPAP-pulsed CFSE-labeled B16 cells (target cells). The mixture
of 9M and DDA/MPL induced higher specific cytotoxic CD8+
T cell (CTL) response in mice than separately used when the ratio
of effector cells to target cells was 100:1 (Fig. 2D). Therefore, DDA/MPL served as an
effective adjuvant for 9M protein vaccine for enhanced humoral and
cellular immune response. However, DDA/MPL did not enhance tumor
growth inhibition and extend the survival of melanoma-bearing
mice.
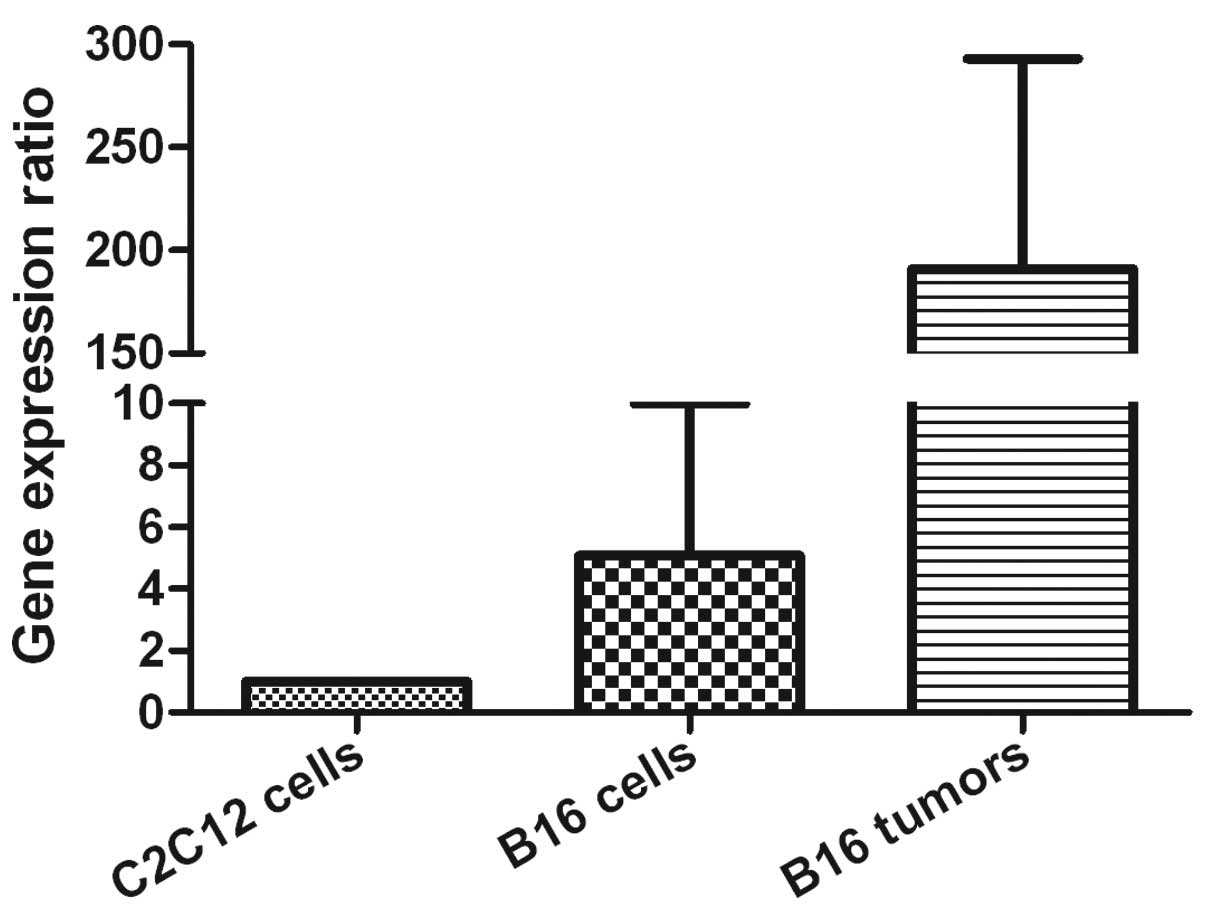
The expression level of MUC1 was evaluated in B16
cells as well as C2C12 cells. The results indicated that B16 cells
and implanted B16 tumors showed nearly 5-fold and 200-fold higher
mRNA levels compared to C2C12 cells separately (Fig. 3), indicating that MUC1 was
overexpressed in melanoma tissues and cells than normal myoblast
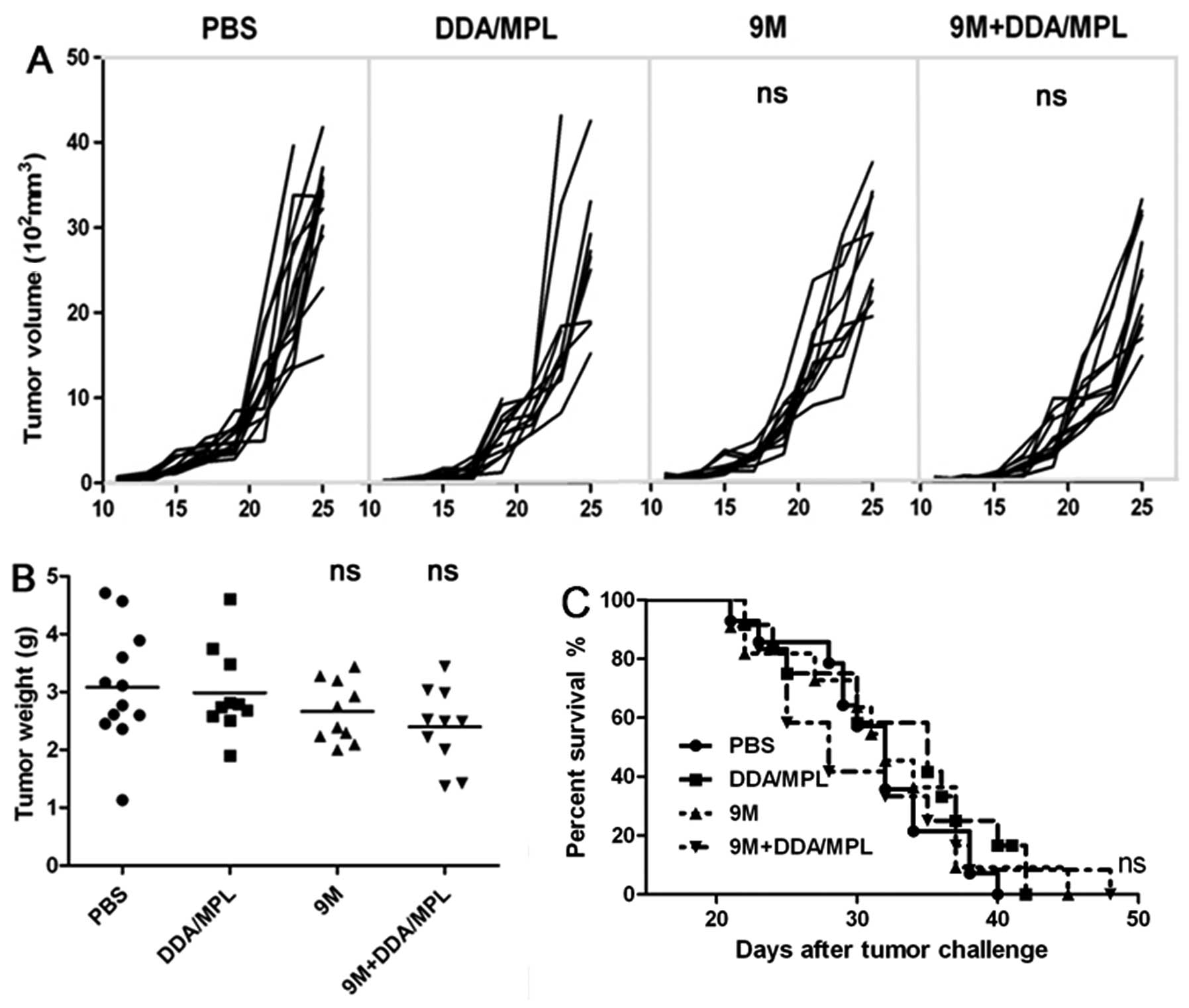
cells. To evaluate whether the 9M+DDA/MPL vaccine had inhibitory
effects on tumor growth, C57BL/6 mice (10 per group) were
inoculated with B16 cells (1×105) on day 0. After tumor
establishment, the mice were immunized with PBS, 9M, DDA/MPL,
9M+DDA/MPL on days 3, 10 and 17. Tumor growth was monitored for 28
days after inoculation. As shown in Fig. 4A–C, 9M+DDA/MPL homologous
immunization strategy did not inhibit tumor growth and extend the
survival of melanoma-bearing mice, and the difference was not
significant (P>0.05).
VPP vaccine displays anti-melanoma effect
in mice
As the adjuvant vaccine did not significantly
enhance the antitumor activity, immunization strategy was studied.
Our previous data showed that recombinant adenovirus prime protein
boost heterologous immunization strategy showed superior antitumor
effects than homologous immunization (11). Thus, an adenoviral prime protein
boost immunization (VPP) was conducted in this study. Mice were
inoculated with 1×105 B16 cells at day 0 into the right
hind leg. After the tumor was established, the mice were immunized
with AD-9M at day 3, and 9M+DDA/MPL at day 10 and 17. Tumor volume
in the subcutaneous B16 melanoma-bearing mice was measured every
other day and the survival of each treated mouse was monitored. As
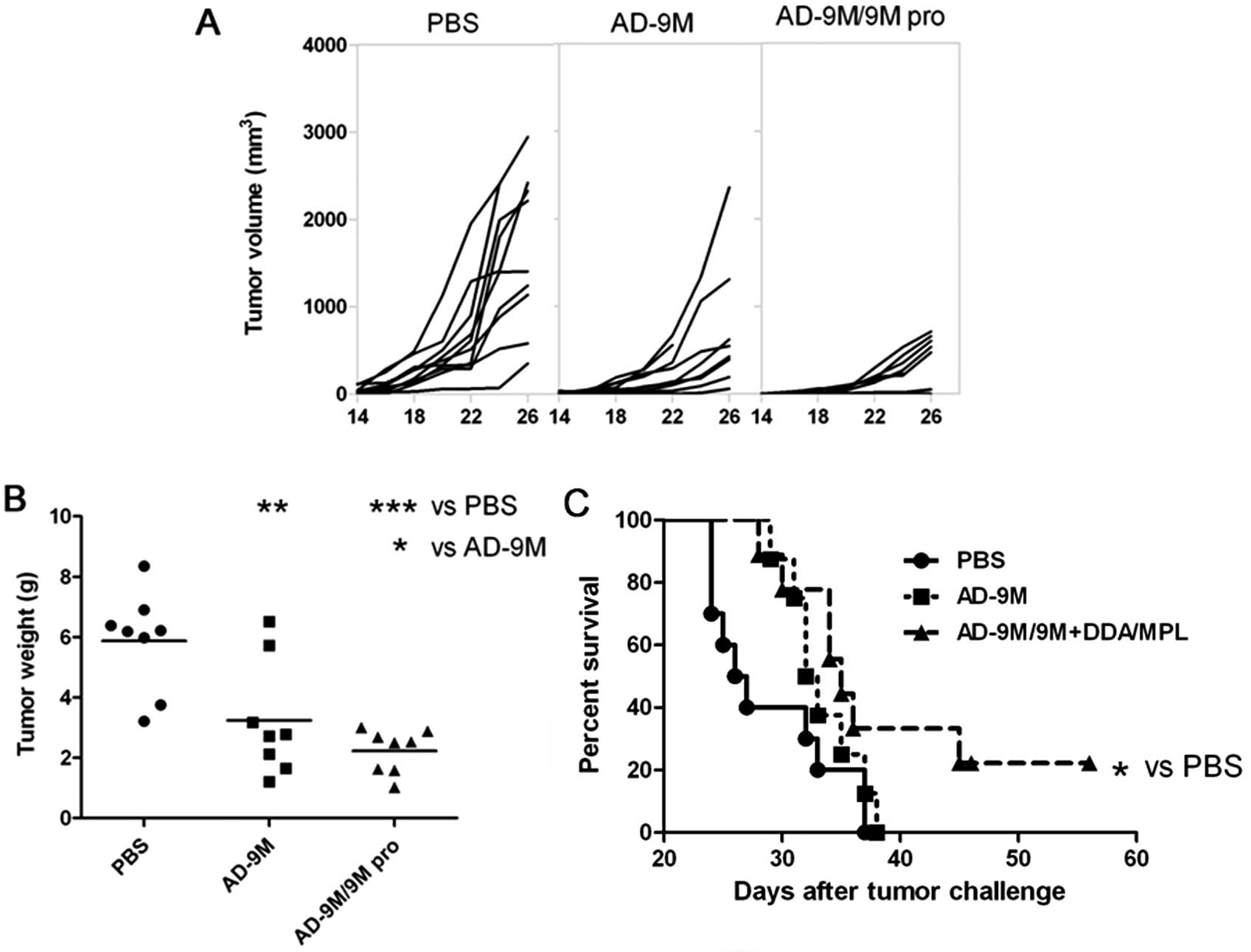
shown in Fig. 5A, both AD-9M and
AD-9M/9M+DDA/MPL VPP vaccine induced growth inhibition of the tumor
(P<0.01; P<0.001) compared with PBS. According to tumor
weight (Fig. 5B), the
AD-9M/9M+DDA/MPL strategy showed a tumor inhibition ratio of 59.45%
to the PBS group (P<0.05). The AD-9M group showed a tumor
inhibition ratio of 39.86% to the PBS group. In parallel, a
significant prolonged survival was recorded in the mice immunized
with AD-9M/9M+DDA/MPL VPP vaccines. As shown in Fig. 5C, by day 36 and 38 after B16
melanoma inoculation, all mice in the PBS and AD-9M groups died.
Until day 50 after tumor challenge, 30% of the mice immunized with
AD-9M/9M+DDA/MPL VPP vaccines survived. The survival rate of
melanoma-bearing mice vaccinated with AD-9M/9M+DDA/MPL was 33.33%
(P<0.05), whereas the survival rate of mice vaccinated with
AD-9M was 22.22% (P>0.05).
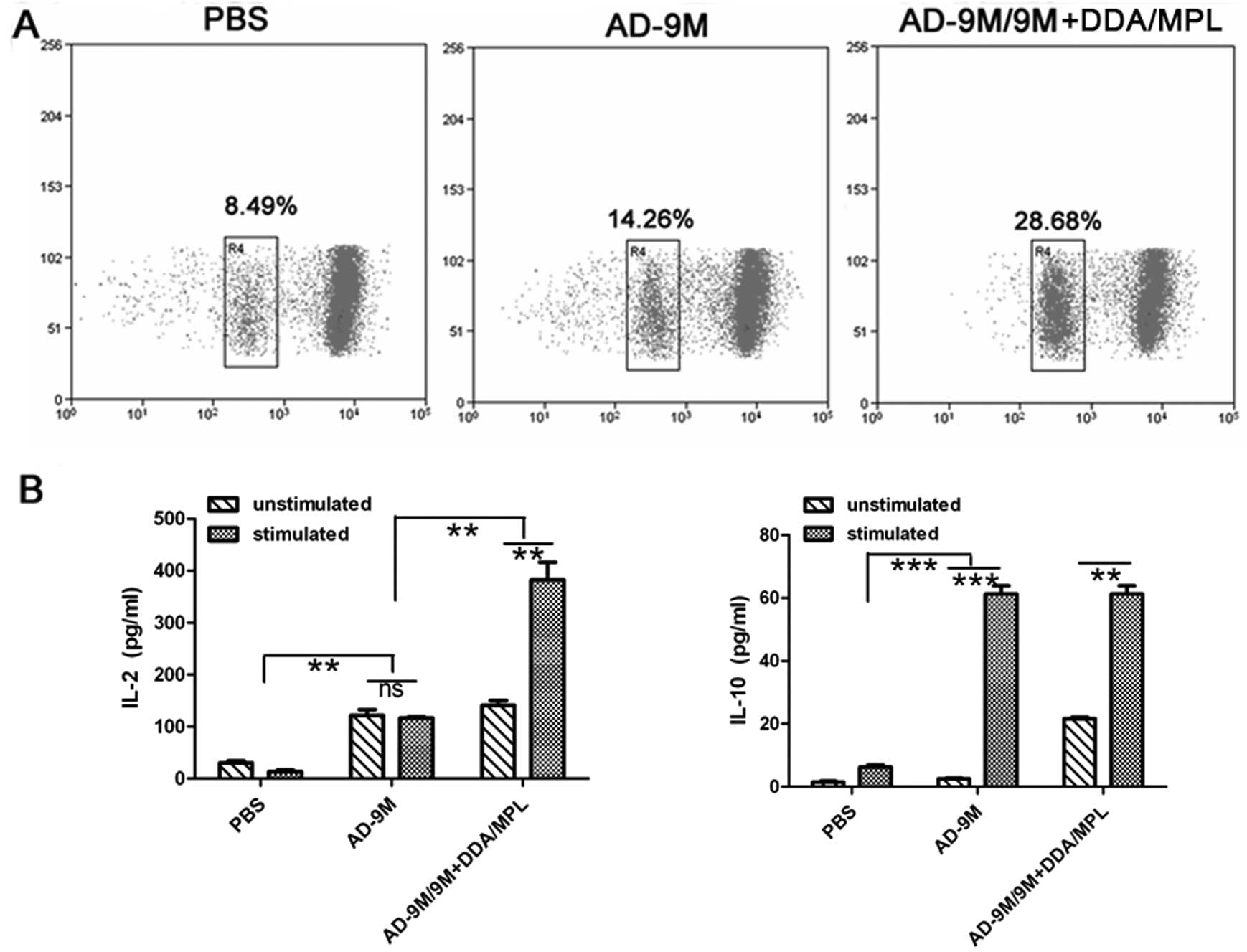
Antitumor mechanism of the vaccine
To investigate the antitumor mechanism of the
vaccine, lymphocyte proliferation and cytokines were detected. It
was shown that the ability of promoting lymphocyte proliferation of
the AD-9M/9M+DDA/MPL group was >3-fold compared to the PBS
group, while that of the AD-9M group was nearly 2-fold compared to
PBS (Fig. 6A). IL-2 and IL-10
concentration (pg/ml) in the culture supernatant after stimulation
with 9M protein were detected simultaneously using the BioPlex™
2200 (Fig. 6B). Both the AD-9M and
AD-9M/9M+DDA/MPL groups elicited ~6-fold higher IL-10 level than
the PBS group (P<0.01), and there was no difference between the
two groups (P>0.05). However, AD-9M enhanced >3-fold IL-2
levels than the PBS group (P<0.01), while the VPP vaccine
enhanced ~13-fold to the PBS group (P<0.001) (Fig. 6B). The VPP vaccine group showed
~2.5-fold IL-2 concentration to the AD-9M group (P<0.001 and
P<0.01, especially) and the AD-9M vaccine group showed ~3-fold
IL-2 concentration to PBS. IL-10 was a Th2-type cytokine and IL-2
was a Th1-type cytokine. It was suggested that the AD-9M and the
VPP vaccine in the tumor-challenged mice may enhance both Th1 and
Th2 type immune response. However, the VPP vaccine enhanced more
Th1 type immune response than Th2 type immune response, which may
be more beneficial to antitumor effects.
Discussion
In the present study, we sought to prepare a
recombinant adenovirus vector expressing MUC1 and to select a
proper adjuvant (DDA/MPL) to the recombinant MUC1 protein vaccine.
Adenoviral prime protein boost immunization induced an efficient
antitumor effect in a murine melanoma model. Moreover, this
antitumor effect might be predominantly mediated by Th1-type immune
response. The data suggested that adenoviral prime protein plus
adjuvant boost immunization strategy may be a potential treatment
against melanoma.
MPL formulated with DDA liposomes (DDA/MPL) is not
approved for clinical trials as an adjuvant, it has been shown to
induce a significant level of protective immunity in Chlamydia and
tuberculosis vaccine research (17,18).
In this study, DDA/MPL was selected as an adjuvant for the MUC1
VNTR-based protein vaccine to evaluate its ability to enhance
immune response in our study. The data showed that DDA/MPL could
effectively enhance the cellular and humoral immunity against MUC1
protein.
Adenovirus (AD) has been clinically evaluated and
proven to be immunogenic and safe (19). As a classic tumor-associated
antigen, MUC1 has been studied for many years both in preclinical
and clinical trials. MUC1 has been prioritized as a cancer vaccine
target antigen (20). In our study,
a recombinant adenovirus expressing MUC1 was constructed and
evaluated for the anti-melanoma effect. The results showed that
less tumor inhibition was induced by the AD-9M than the VPP
vaccine, which might be caused by different Th types.
It is well accepted that multiple immunizations
(i.e. ‘prime-boost’) are crucial for even the most successful
vaccines. In many cases, heterologous prime-boost can be more
immunogenic than homologous prime-boost (21). Our previous data also showed that
heterologous adenovirus prime-protein boost strategy displayed more
suppressive effects of tumor growth. In this study, homologous
immunization strategy could not inhibit tumor growth and extend the
survival of melanoma-bearing mice (Fig.
4A–C). However, AD-9M prime-9M+DDA/MPL boost immunization was
conducted for effect on tumor volume and survival of challenged
C57BL/6 mice. The results showed the VPP vaccine could inhibit
tumor growth of melanoma model mice in the tumor volume and weight
inhibition (Fig. 5A and B).
Moreover, the VPP vaccine could prolong survival time effectively
(Fig. 5C). The survival time of VPP
vaccine increased 33.33% to PBS group (P<0.05), yet AD-9M
increased 22.22% (P>0.05).
To investigate the mechanism of antitumor effects,
splenocyte proliferation and elicited cytokines were evaluated. The
AD-9M/9M+DDA/MPL VPP vaccine group showed 3-fold and 2-fold
proliferation rates to PBS and AD-9M groups (Fig. 6A). The concentration of IL-10 after
stimulation was 60.5 pg/ml, which was similar to the AD-9M group.
The IL-2 concentration of the AD-9M/9M+DDA/MPL VPP vaccine group
after inoculation with 9M protein was 382 pg/ml, which was 2.5-fold
higher than the AD-9M groups (Fig.
6B). Moreover, IL-2 was a Th1 type cytokine and IL-10 was a Th2
type cytokine. We speculated that both Th1 and Th2 type immune
response play an important role in tumor inhibition, especially the
IL-2 biased Th1 type.
In summary, DDA/MPL could significantly enhance the
cellular and humoral immunity based on 9M protein. However, the
homologous protein prime-boost strategy applied vaccine (PPP) could
slightly inhibit the tumor growth and prolong the survival of
tumor-bearing mice, but the difference was not significant
(P>0.05). However, adenoviral vector prime-recombinant protein
and DDA/MPL boost immune strategy (VPP) exerted significant
anti-melanoma effects in mice. It was shown that the MUC1 VPP
vaccine was able to inhibit the growth of B16 melanoma in mice and
to prolong the survival of B16 melanoma-bearing mice (P<0.05).
The data suggested that the VPP strategy combined with DDA/MPL
adjuvant might be a simple and efficient way to produce efficient
MUC1-based vaccines for melanomas or other tumors.
Acknowledgements
This study was supported by the Specialized Research
Fund for the National Natural Science Foundation of China (no.
31300765), the Specialized Research Fund for the Doctoral Program
of Higher Education (New Teachers) (grant no. 20120061120025),
Jilin Province Science and Technology Development Program (no.
20130522006JH), and the National Science and Technology Major
Project of the Ministry of Science and Technology of China (no.
2012ZX10001009-002).
References
|
1
|
Tang CK and Apostolopoulos V: Strategies
used for MUC1 immunotherapy: preclinical studies. Expert Rev
Vaccines. 7:951–962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang CK, Katsara M and Apostolopoulos V:
Strategies used for MUC1 immunotherapy: human clinical studies.
Expert Rev Vaccines. 7:963–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snyder LA, Goletz TJ, Gunn GR, et al: A
MUC1/IL-18 DNA vaccine induces anti-tumor immunity and increased
survival in MUC1 transgenic mice. Vaccine. 24:3340–3352. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tarp MA, Sorensen AL, Mandel U, et al:
Identification of a novel cancer-specific immunodominant
glycopeptide epitope in the MUC1 tandem repeat. Glycobiology.
17:197–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan S, Shi C, Lv Y, Wang T, Wang H and
Han W: A novel Bacillus Calmette-Guérin-based breast cancer vaccine
that coexpresses multiple tandem repeats of MUC1 and CD80 breaks
the immune tolerance and inhibits MUC1-positive breast cancer
growth. Cancer Biother Radiopharm. 24:607–613. 2009.PubMed/NCBI
|
|
6
|
Mukherjee P, Basu GD, Tinder TL, et al:
Progression of pancreatic adenocarcinoma is significantly impeded
with a combination of vaccine and COX-2 inhibition. J Immunol.
182:216–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nicholls EF, Madera L and Hancock RE:
Immunomodulators as adjuvants for vaccines and antimicrobial
therapy. Ann NY Acad Sci. 1213:46–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korsholm KS, Agger EM, Foged C, et al: The
adjuvant mechanism of cationic dimethyldioctadecylammonium
liposomes. Immunology. 121:216–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Persing DH, Coler RN, Lacy MJ, et al:
Taking toll: lipid A mimetics as adjuvants and immunomodulators.
Trends Microbiol. 10:S32–S37. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takeuchi O, Hoshino K, Kawai T, et al:
Differential roles of TLR2 and TLR4 in recognition of gram-negative
and gram-positive bacterial cell wall components. Immunity.
11:443–451. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang YQ, Zhang HH, Liu CL, et al:
Enhancement of survivin-specific anti-tumor immunity by adenovirus
prime protein-boost immunity strategy with DDA/MPL adjuvant in a
murine melanoma model. Int Immunopharmacol. 17:9–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harari A, Bart PA, Stohr W, et al: An
HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces
reliable, polyfunctional, and long-lasting T cell responses. J Exp
Med. 205:63–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Magalhaes I, Sizemore DR, Ahmed RK, et al:
rBCG induces strong antigen-specific T cell responses in rhesus
macaques in a prime-boost setting with an adenovirus 35
tuberculosis vaccine vector. PLoS One. 3:e37902008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia-Hernandez Mde L, Gray A, Hubby B
and Kast WM: In vivo effects of vaccination with six-transmembrane
epithelial antigen of the prostate: a candidate antigen for
treating prostate cancer. Cancer Res. 67:1344–1351. 2007.PubMed/NCBI
|
|
15
|
Yu B, Zhang Y, Zhan Y, et al:
Co-expression of herpes simplex virus thymidine kinase and
Escherichia coli nitroreductase by an hTERT-driven
adenovirus vector in breast cancer cells results in additive
anti-tumor effects. Oncol Rep. 26:255–264. 2011.PubMed/NCBI
|
|
16
|
You Q, Jiang C, Wu Y, et al: Subcutaneous
administration of modified vaccinia virus Ankara expressing an
Ag85B-ESAT6 fusion protein, but not an adenovirus-based vaccine,
protects mice against intravenous challenge with Mycobacterium
tuberculosis. Scand J Immunol. 75:77–84. 2012. View Article : Google Scholar
|
|
17
|
Yu H, Karunakaran KP, Jiang X, Shen C,
Andersen P and Brunham RC: Chlamydia muridarum T cell
antigens and adjuvants that induce protective immunity in mice.
Infect Immun. 80:1510–1518. 2012. View Article : Google Scholar
|
|
18
|
Kolibab K, Parra M, Yang AL, Perera LP,
Derrick SC and Morris SL: A practical in vitro growth inhibition
assay for the evaluation of TB vaccines. Vaccine. 28:317–322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Gruijl TD and van de Ven R: Chapter six
- Adenovirus-based immunotherapy of cancer: promises to keep. Adv
Cancer Res. 115:147–220. 2012.PubMed/NCBI
|
|
20
|
Cheever MA, Allison JP, Ferris AS, et al:
The prioritization of cancer antigens: a national cancer institute
pilot project for the acceleration of translational research. Clin
Cancer Res. 15:5323–5337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu S: Heterologous prime-boost
vaccination. Curr Opin Immunol. 21:346–351. 2009. View Article : Google Scholar
|