Introduction
In recent years, several studies have indicated that
aberrant miRNA levels occur in multiple human malignancies
including lung cancer (1–3). It has been proposed that some miRNAs
are downregulated during tumorigenesis and seem to function as
tumor suppressors, while others are upregulated and may control
proto-oncogenic signals (4,5). miR-145 with tumor suppression gene
function has been described in lung cancer (6). Decreased levels of miR-145 expression
that can control many tumor-associated targets such as c-Myc,
STAT1, YES, lose their tumor suppression function and contribute to
tumorigenesis (7,8). Although miR-145 may potentially alter
complex cellular processes, the molecular mechanisms of
downregulated miR-145 levels in lung cancer cells remain largely
unknown.
Epidermal growth factor receptor (EGFR) is a 170 kDa
type I transmembrane growth factor receptor with tyrosine kinase
(TK) activity and belongs to the HER/erbB family of receptor TKs,
which contains HER1 (EGFR), HER2, HER3 and HER4 (9). With binding to its specific ligands
(such as EGF), homodimerization and/or heterodimerization with
other family members activates the TK, and subsequently leads to
autophosphorylation of the cytoplasmic domain of the receptor and
enables it to interact with adaptor molecules, which couple the
receptors to downstream signaling pathways. It was reported that
all lung carcinomas almost closely related to EGFR ectopic
expression, including overexpression of EGFR protein, EGFR TK
domain mutations or EGFR gene copy amplification, any of which may
result in overactivation of EGFR signaling pathway (10). Previous studies have shown that
activation of the EGFR signaling pathway interacts with miRNAs to
promote tumor formation (11). Due
to the EGFR signaling pathway activation and the downregulation of
miR-145 in lung cancer cells, we inferred that there is some
connection between them. To date, there is no evidence to support
that the activation of EGFR plays a regulatory role in miR-145 in
lung cancer cells.
In the present study, we identified that EGFR
signaling pathway negatively regulated the expression of miR-145 by
ERK1/2 in human lung cancer cell lines. These findings may indicate
that the EGFR with abnormal activation affects the expression of
miRNAs through its downstream signaling molecules, involved in the
occurrence and development of lung cancer.
Materials and methods
Cell culture, reagents and
treatments
Normal human lung epithelial cell line (BEAS-2B),
human lung adenocarcinoma cell line (H1650) with EGFR mutation (del
L747_E749 and A750P), human lung adenocarcinoma cell line (H1975)
with EGFR mutation (L858R), human lung adenocarcinoma cell lines
(A549 and H292) with wild EGFR were obtained from American Type
Culture Collection (Manassas, VA, USA). All cell culture reagents
were purchased from Invitrogen Corporation (Carlsbad, CA, USA).
Briefly, the cells were cultured in RPMI-1640 supplemented with 10%
fetal bovine serum under standard conditions (37°C in a humidified
atmosphere containing 5% CO2)
To evaluate the effect of AG1478 on the EGFR
signaling pathway and miR-145 expression levels, cells were serum
starved for 24 h, incubated in the presence or absence of AG1478 (5
μM; Calbiochem) for 2 h, and then for an additional 24 h in the
presence or absence of EGF (20 ng/ml; Promega). U0126 (Cell
Signaling Technology, Inc., Beverly, MA, USA), a pharmacological
MEK1/2 inhibitor, was dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 10 mM and stored at −20°C until used (10 μM).
LY194002, PI3K kinase inhibitor and AG490, JAK2 inhibitor, were
purchased from Calbiochem and dissolved in DMSO at a recommend
concentration and stored at −20°C.
Western blot analysis
Cells were washed once with PBS and then lysed in
buffer containing 10 mM Tris, pH 7.6, 150 mM NaCl, 5 mM EDTA, pH
8.0, 10 ml/l Triton X-100, 1 mM DTT and 0.1 mM
phenylmethanesulfonyl fluoride (PMSF). After 30 min on ice, lysates
were collected and clarified by centrifugation at 15,000 × g for 5
min at 4°C. Protein concentrations were measured by BCA protein
detection kit (Pierce, Rockford, IL, USA). Equal amounts of protein
(10–30 μg/lane) from whole-cell lysates were separated by gel
electrophoresis on 10% gels, transferred to nitrocellulose
membranes and were probed with specific primary antibody
[phospho-EGFR (Tyr1068) rabbit mAb; phospho-AKT (Ser473) rabbit
mAb; phospho-ERK1/2 (Thr202/Tyr204) rabbit mAb; phospho-STAT3
(Tyr705) rabbit mAb; EGFR rabbit mAb; ATK rabbit mAb; ERK1/2 rabbit
mAb; STAT3 rabbit mAb; Cell Signaling Technology, Inc.] and then
with the appropriate HRP-conjugated secondary antibodies. Proteins
were detected using the enhanced chemiluminescence detection kit
(Thermal Science, Rockford, IL, USA). For loading control, the
membrane was probed with a monoclonal antibody for GAPDH (Kangchen
Biotechnology, Shanghai).
RNA isolation
Total RNA was isolated from the cultured cells with
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions and quantified by a spectrophotometer. To assess the
purity of RNA, optical density (OD) was measured at 260 and 280 nm
for determination of OD260/OD280 ratio. The
RNAs with >1.8 OD ratios were used in this study. Relative
abundance and integrity of 18s and 28s ribosomal bands were
assessed with formaldehyde denaturing agarose gel. Those RNAs that
exhibited intact 18s and 28s ribosomal bands with 1:1.5 relative
abundance ratios were used in the present study.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR) analysis
Expression of mature miRNAs was examined by qRT-PCR
analysis using a Real-time PCR Universal Reagent kit according to
the manufacturer’s instructions (GenePharma, Co., Ltd., Shanghai).
In brief, two-step qRT-PCR procedure was performed. Reverse
transcription was performed in 20 μl volume, starting with 10 ng of
total RNA. The reaction mixture was initially heated to 25°C for 30
min, 42°C for 45 min, 85°C for 10 min and finally to 4°C for 5 min.
In the PCR step, PCR products were amplified from cDNA samples
using specific miRNA primers and all assays were performed in
triplicate on the MX3000P Real-time PCR Instrument (Stratagene,
USA). The primers used were: miR-145, 5′-ATC GTC CAG TTT TCC CAG
G-3′ (forward), and 5′-CGC CTC CAC ACA CTC ACC-3′ (reverse); U6
snRNA, 5′-ATT GGA ACG ATA CAG AGA AGA TT-3′ (forward), and 5′-GGA
ACG CTT CAC GAA TTT G-3′ (reverse). The assay tubes were initially
heated to 95°C for 3 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 60 sec. The expression levels of candidate miRNAs were
evaluated by the comparative CT method and were normalized using U6
snRNA as the endogenous control. Relative quantitative expression
levels of miRNAs were determined by the 2−ΔΔCT
method.
Transient transfection of small
interfering RNA (siRNA)
The human cell lines were seeded in a 6-well plate
and cultured to 70% confluence. siRNA oligonucleotides targeting
STAT3, AKT, ERK1/2 and negative control were purchased from
GenePharma. The siRNA sequences were: STAT3 siRNA sense, 5′-GCA GCA
GCU GAA CAA CAU GTT-3′ and antisense, 5′-CAU GUU GUU CAG CUG CUG
CTT-3′; AKT siRNA sense, 5′-UGC CCU UCU ACA ACC AGG ATT-3′ and
antisense, 5′-UCC UGG UUG UAG AAG GGC ATT-3′; ERK1 siRNA sense,
5′-GAC CGG AUG UUA ACC UUU ATT-3′ and antisense, 5′-UAA AGG UUA ACA
UCC GGU CTT-3′; ERK2 siRNA sense, 5′-CAC CAA CCA UCG AGC AAA UGT
T-3′ and antisense, 5′-CAU UUG CUC GAU GGU UGG UGT T-3′ and
negative control sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and
antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. Cells were
transfected with siRNA (100 nM) by using Lipofectamine 2000
(Invitrogen) following the manufacturer’s protocol. The selective
silencing of STAT3, AKT, ERK1/2 was confirmed by western blot
analysis.
Statistical analysis
All experiments were repeated a minimum of three
times. Error bars indicate standard errors of mean. Microsoft Excel
or Instat software (GraphPad Prism4; San Diego, CA, USA) was used
to analyze the data. A Student’s t-test or one-way analysis of
variance (ANOVA) was used for parametric data. Correlation analysis
was made by using Pearson’s correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
The EGFR signaling pathway is associated
with the downregulation of miR-145 in lung cancer cells
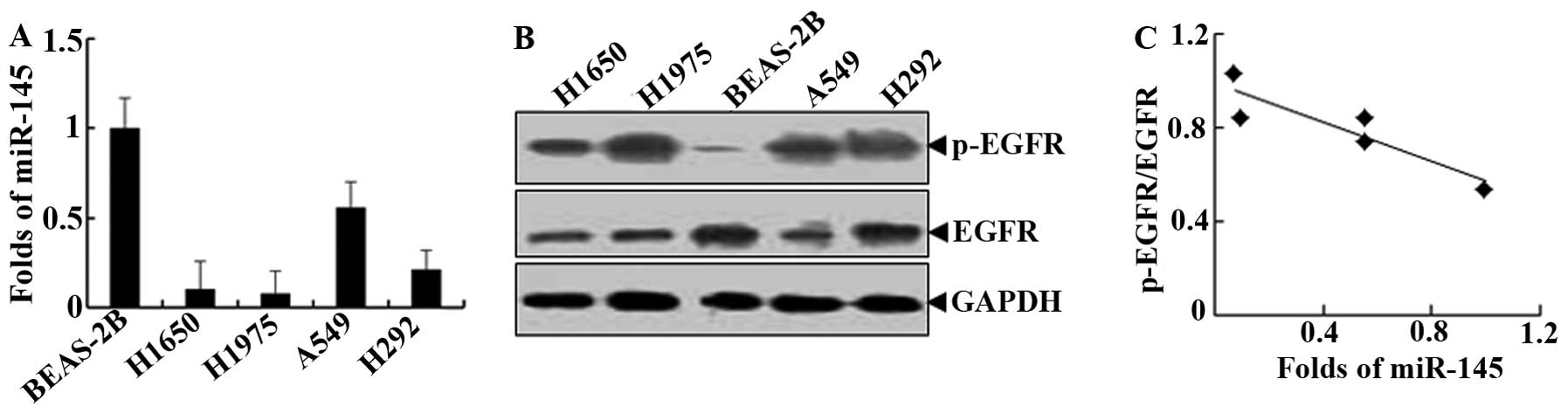
The levels of miR-145 in human normal lung bronchial
epithelial cells (BEAS-2B) and lung cancer cells (H1975, H1650,
A549 and H292) were determined by qRT-PCR. The results showed that
the levels of miR-145 in four lung cancer cell lines were
significantly lower than in normal lung epithelial cells (P=0.025;
Fig. 1A). Although there were no
significant changes in EGFR protein between human normal lung
bronchial epithelial cells and lung cancer cells, EGFR activity in
the form of pi-EGFR expression in lung cancer cells was higher than
in normal lung epithelial cells (P=0.032; Fig. 1B). The quantitative comparison of
miR-145 and p-EGFR levels showed a significant negative correlation
between these two factors (Pearson’s correlation, r=−0.926,
P<0.05). These results suggest that the activated EGFR signaling
pathway may be functionally associated with miR-145
downregulation.
Activation of the EGFR signaling pathway
downregulates the expression of miR-145 and inhibition of the EGFR
signaling pathway restores the expression of miR-145 in lung cancer
cells
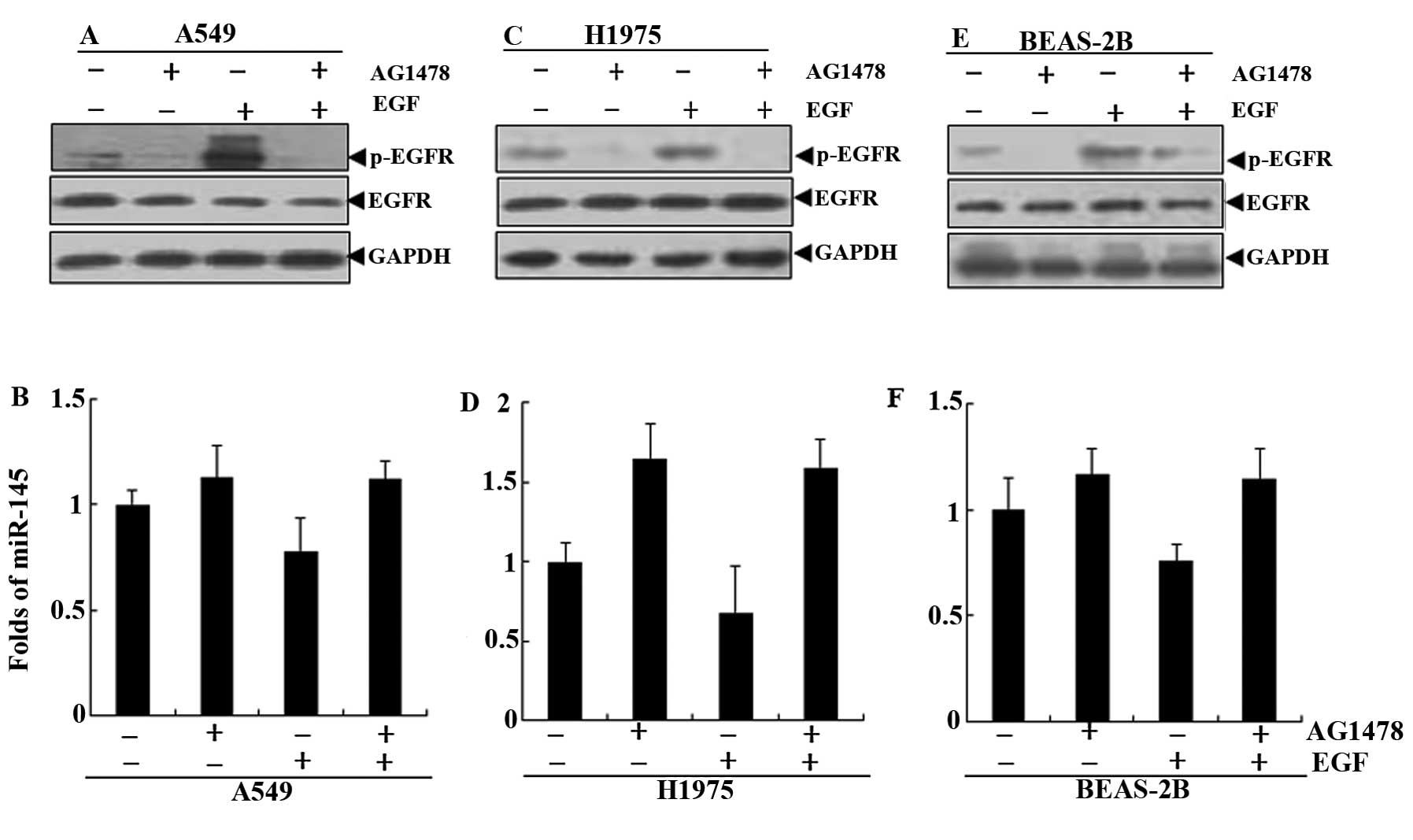
To further investigate the effect of EGFR status on
miR-145 expression, EGFR-mutant H1975 cells, EGFR wild-type A549
cells and human normal lung BEAS-2B cells were first starved for 24
h with serum free culture and then treated with AG1478 (a specific
inhibitor of EGFR) in the presence or absence of EGF (12,13).
The results showed that there was no change in the total EGFR
protein. In the three cells, the levels of p-EGFR protein were
significantly increased in the cells treated with EGF, compared
with the respective control group. The AG1478 completely blocked
the activation of EGF to EGFR (Fig. 2A,
C and E). The levels of miR-145 were significantly decreased in
the treatment of EGF. The AG1478 may restore the downregulation of
EGF to miR-145 in A549, H1975 and BEAS-2B cells (Fig. 2B, D and F).
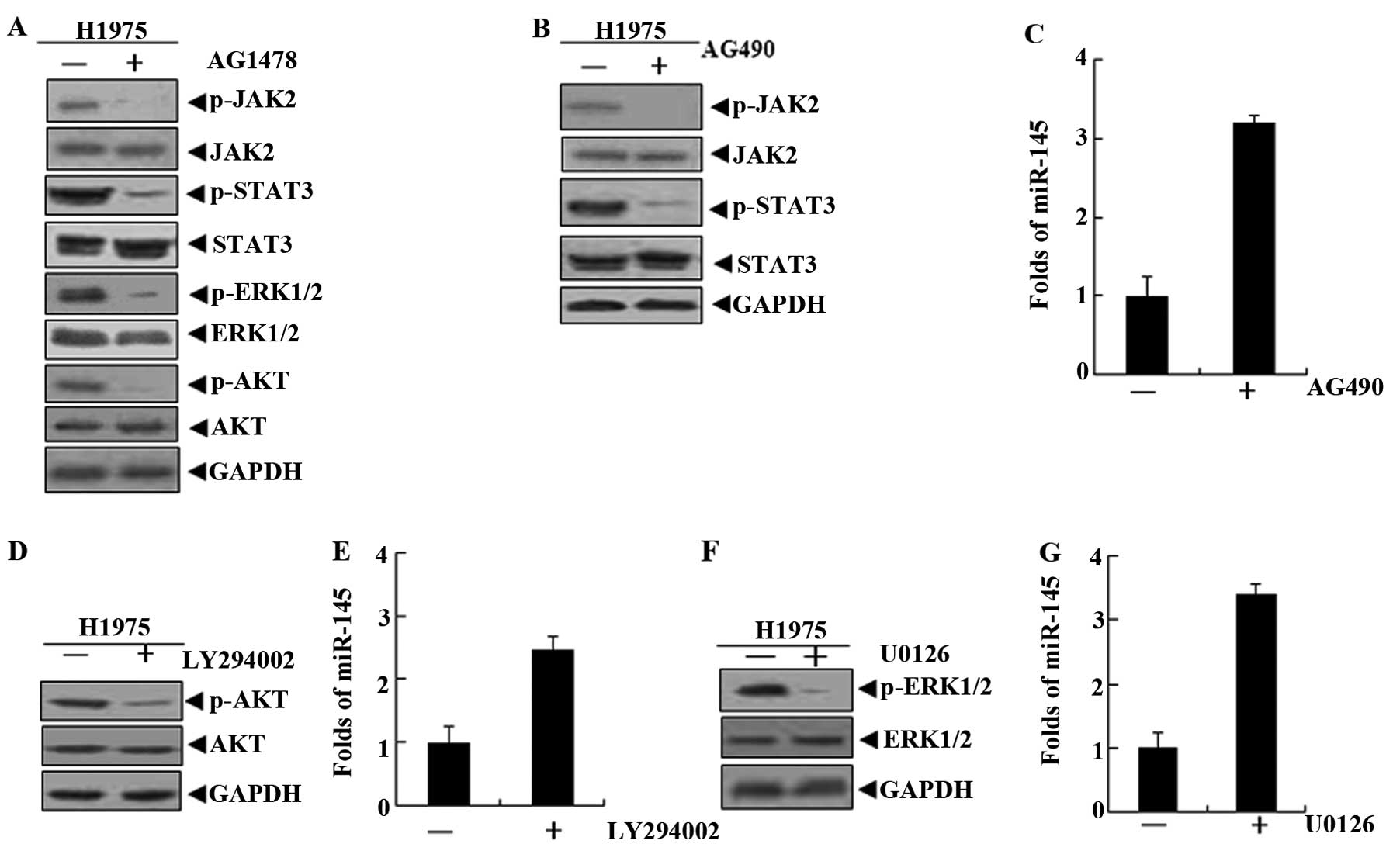
AG490, LY294002 and U0126 upregulate the
levels of miR-145 in lung cancer cells
Due to the mutation of EGFR in H1975 cells, the EGFR
signaling pathway is highly activated (14). This was confirmed by detecting the
EGFR downstream signaling molecules STAT3, ERK1/2 and AKT. STAT3,
ERK1/2 and AKT activity was inhibited when AG1478 blocked EGFR
phosphorylation in H1975 cells (Fig.
3A). However, it was not clear whether these signaling
molecules were involved in the process of miR-145 downregulation by
EGF-EGFR. Therefore, H1975 cells were treated with the three
signaling molecule inhibitors AG490, LY294002 and U0126 (15–17).
The data obtained indicated that the levels of miR-145 were
upregulated after the inhibitors blocked the corresponding signal
molecules (Fig. 3B–G).
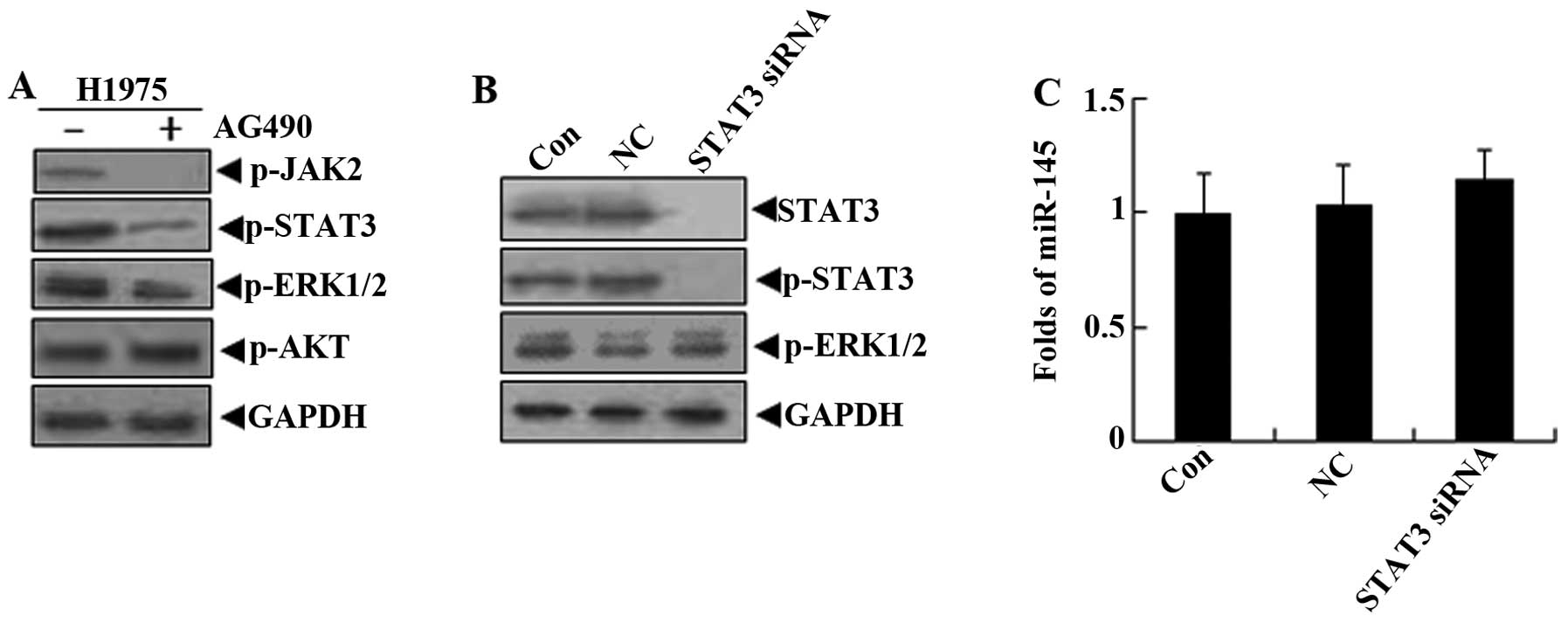
STAT3 signaling molecules are not
involved in the regulation of miR-145 downregulated by EGFR in
H1975 cells
Although the AG490, a relatively specific chemical
inhibitor for STAT3, may not inhibit the phosphorylation of AKT, it
reduces the levels of p-ERK1/2 (18). Thus, we hypothesized that the levels
of miR-145 in lung cancer cells may be restored by the non-specific
inhibition of AG490. In order to determine whether STAT3 signaling
molecules are involved in the regulation of miR-145 expression, the
siRNA against STAT3 was used to treat H1975 cells. The results
showed that the levels of miR-145 presented almost no change while
STAT3 expression and its phosphorylation levels were suppressed;
there was also no effect on p-ERK1/2 (Fig. 4). These observations suggest that
STAT3 signaling molecules were not involved in the downregulation
of miR-145 by the activation of EGFR in lung cancer cells.
AKT signaling molecules are involved in
the regulation of miR-145 expression through the activation of
ERK1/2
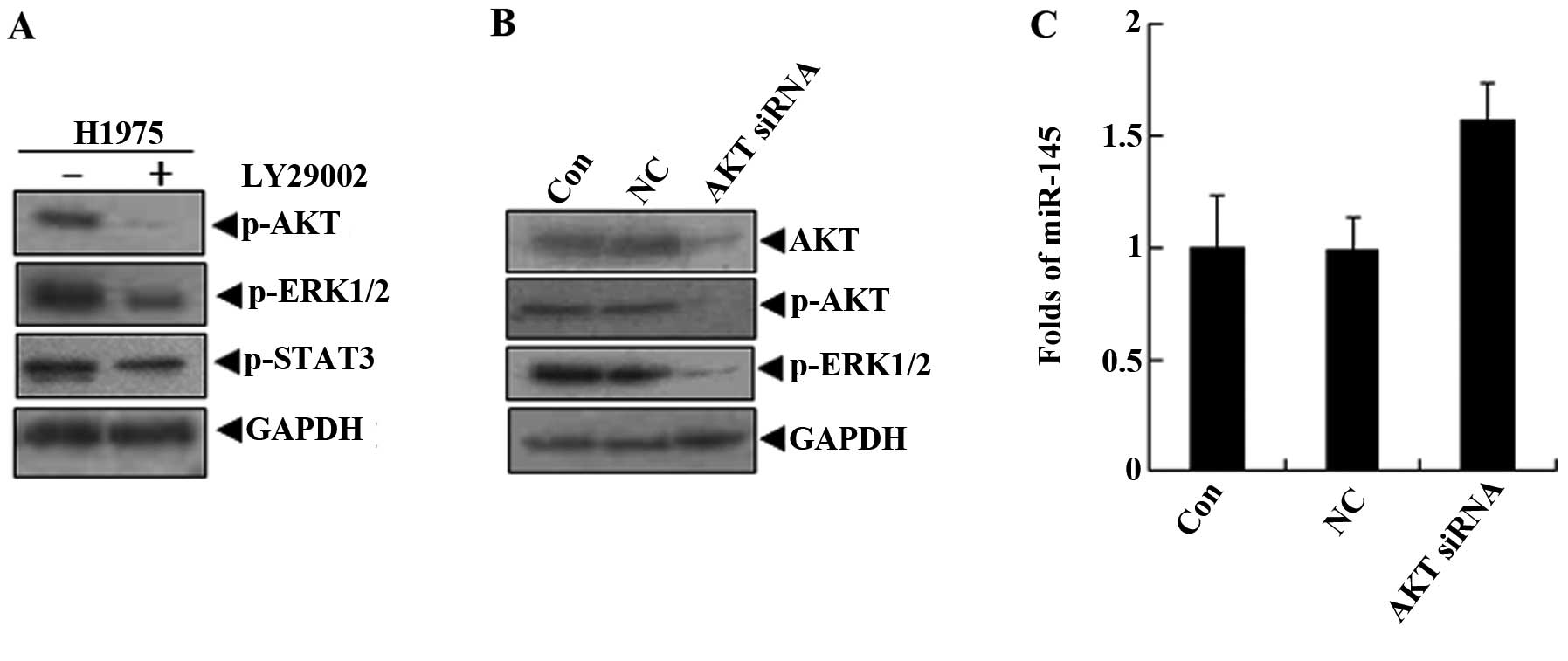
LY294002 is a potent inhibitor of phosphoinositide
3-kinases (PI3Ks), an upstream molecule of AKT (19). In H1975 cells treated with LY294002,
the activation of AKT and ERK1/2 was effectively suppressed, but
LY294002 did not inhibit the STAT3 activity (Fig. 5A). At the same time, the miR-145
levels were increased by LY294002 in H1975 cells. Therefore, we
hypothesized that the levels of miR-145 in lung cancer cells may be
restored by the pi-ERK1/2 inhibition of LY294002. To explore the
role of AKT in the regulation of miR-145, the siRNA against AKT was
used to treat H1975 cells. The data demonstrated that ERK1/2
phosphorylation levels were blocked after the specific inhibition
of the activation of AKT signaling molecules (Fig. 5B). We detected that miR-145
expression was upregulated in the AKT siRNA group (Fig. 5C). This indicated that the miR-145
levels were regulated by AKT via the activation of ERK1/2.
ERK1/2 is involved in the downregulation
of miR-145 expression by EGFR
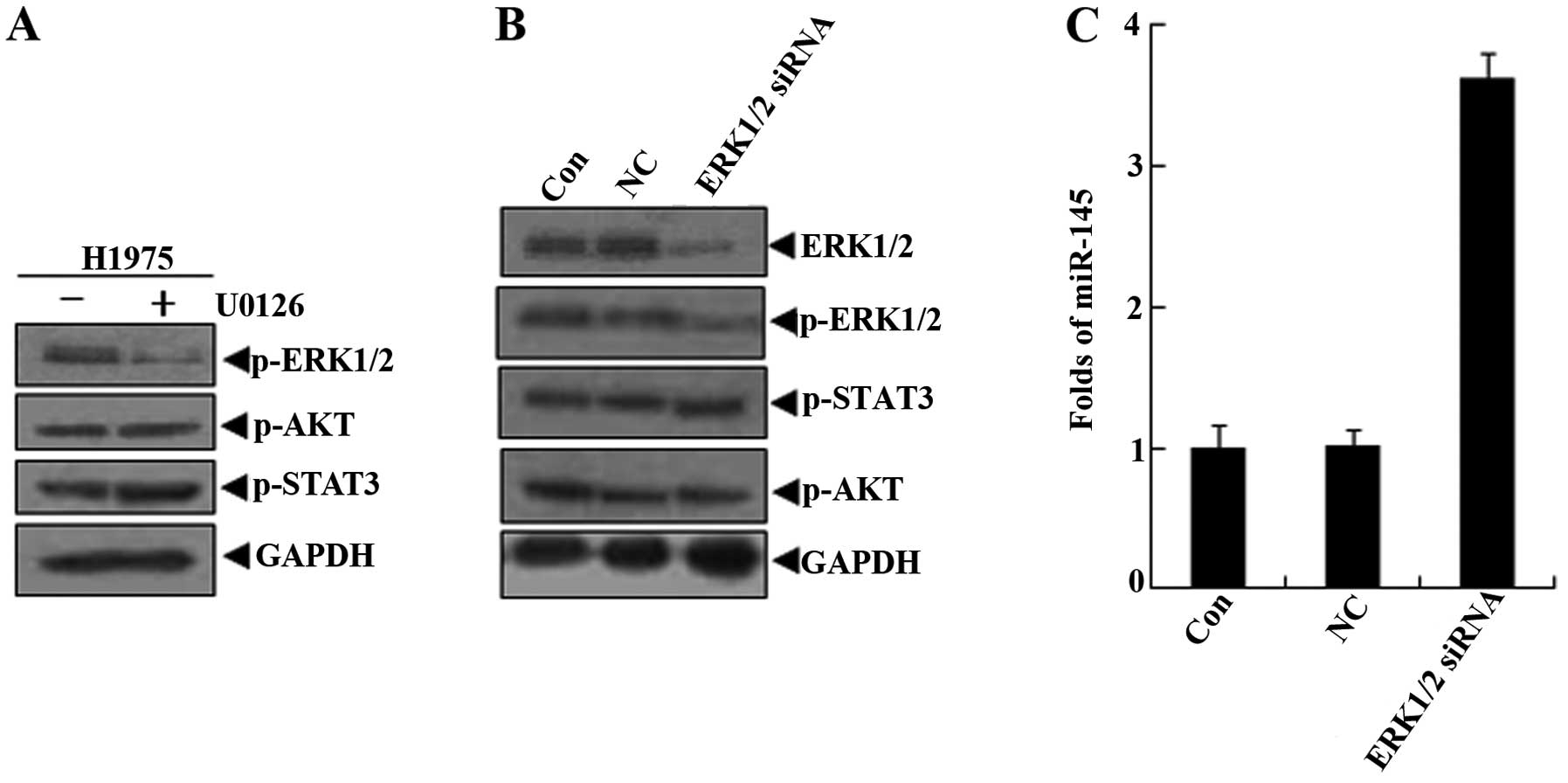
U0126, is a highly selective inhibitor of both MEK1
and MEK2, a type of MAPK/ERK kinase (20,21).
In H1975 cells treated with U0126 for 24 h, western blot results
confirmed that U0126 only inhibited the activation of ERK1/2, and
there were no inhibitory effects on the AKT and STAT3 signaling
molecules (Fig. 6A). Similarly, in
applications for ERK1/2 siRNA in H1975 cells, no effect on the
activation of AKT and STAT3 was observed, while the ERK1/2 activity
was specifically silenced (Fig.
6B). The miR-145 levels were upregulated, compared with the
control group and negative control group (Fig. 6C). These findings suggested that
ERK1/2 mediated the downregulation of miR-145 in the EGF-EGFR
signaling pathway in lung cancer cells.
Discussion
It has been confirmed that miR-145 has a tumor
suppressor function (22,23). miR-145 had a lower level of
expression in lung, breast, prostate and colorectal cancer cells
(24–26). However, it is unclear why miR-145 is
downregulated in these tumor cells. The EGFR signaling pathway is
activated and treated as the target molecule in lung cancer cells.
Therefore, we chose the lung cancer cells to explore how the EGFR
signaling pathway may be involved in the molecular mechanism of
miR-145 downregulation. The results showed that the EGFR signaling
pathway mediated the downregulation of miR-145 through ERK1/2
signaling molecules in lung cancer cells.
There are generally two forms of the activation of
EGFR in lung cancer cells. First, it is the excessive activation of
wild-type EGFR; second, it is more important that the mutation of
EGFR protein in ATP binding sites causes its conformational change,
which is easier to bind ATP (9,27).
Therefore, we chose the wild-type EGFR (A549 and H292), the mutant
EGFR (H1650 and H1975) and normal lung bronchial epithelial cells
(BEAS-2B) to investigate the link between the activation of EGFR
and the downregulation of miR-145. The results indicated that the
wild-type and mutant EGFR in lung cancer cells were activated
compared to normal EGFR in lung bronchial epithelial cells under
conventional culture conditions, which was consistent with the
expected results. The levels of miR-145 in lung cancer cells were
not the same, but they were less than in normal lung bronchial
epithelial cells. To determine the relationship between the
activation of the EGFR and the levels of miR-145 expression in lung
cancer cells, the correlation of the both was analyzed. Finally,
statistical results indicated that the activation of EGFR was
negatively correlated with the levels of miR-145 in lung cancer
cells.
In order to verify the existence of negative contact
between them, EGF, a physiological ligand of EGFR, stimulated the
A549, H1975 and BEAS-2B cells. The results showed that EGF may
significantly reduce the levels of miR-145 in lung cancer cells or
in normal cells, while it activated the EGFR in corresponding
cells. In addition, AG1478, a potent and selective inhibitor of
EGFR, may block EGF downregulation of the levels of miR-145 in
these cells. We observed that the activation of EGFR and the
expression of miR-145 had indeed a negative correlation through the
activation and inhibition of EGFR, indicating that the activation
of EGFR may reduce the levels of miR-145 in lung cancer cells.
EGFR is a membrane protein. It is obvious that it
may downregulate the expression of miR-145 by the associated
signaling molecules. STAT3, AKT and ERK1/2 are the downstream
signaling molecules of EGFR (28–30).
AG1478 may inhibit the activation of STAT3, AKT and ERK1/2 in H1975
cells. Next, we applied STAT3, AKT and ERK1/2 signaling molecule
inhibitors (AG490, LY294002 and U0126) to explore their role in the
downregulation of miR-145. Notably, the results showed that three
inhibitors may restore the expression of miR-145, indicating that
these signaling molecules were involved in the regulation of
miR-145 expression. We first considered whether there was
interaction among them. Western blot results showed that AG490 or
LY294002 not only inhibited the activation of STAT3 or AKT, but
also blocked ERK1/2 phosphorylation. U0126 only inhibited the
activation of ERK1/2 and had no effect on the phosphorylation
levels of STAT3 and AKT. These data suggest that the signal
molecule inhibitors may suppress the activation of ERK1/2. Thus, we
assume that EGFR mediates the downregulation of miR-145 through the
activation of ERK1/2 in lung cancer cells.
To confirm this conjecture, siRNAs for STAT3, AKT
and ERK1/2 were used. siRNAs against STAT3 did not increase the
levels of miR-145 and there was no effect on ERK1/2
phosphorylation. This showed that the activation of STAT3 was not
involved in the process of the downregulation of miR-145 and it
also suggested that AG490 may be a non-specific inhibitor for
ERK1/2. siRNAs for AKT significantly increased the levels of
miR-145 and inhibited the activation of ERK1/2. This indicated that
AKT may be an upstream molecule of ERK1/2. It is possible that
LY294002 and siRNAs for AKT blocked the activity of ERK1/2. siRNAs
against ERK1/2 only significantly elevated the miR-145 and had no
effect on the activation of STAT3 and AKT. This strongly supported
that EGFR mediated the expression of miR-145 through the activation
of ERK1/2 in lung cancer cells. Regarding the downregulation of
miR-145 by ERK1/2, this requires further in-depth study.
Collectively, we found that the EGFR activation was
negatively correlated with the downregulation of miR-145 in lung
cancer cells. The further signal molecules studied revealed that
EGFR mediated the downregulation of miR-145 through ERK1/2
activation. These data provide a molecular mechanism which
elaborated the downregulation of miR-145 in lung cancer cells.
Moreover, it also provides a possible direction for the
intervention of miR-145 expression in lung cancer cells.
Acknowledgements
This study was supported in part by grants from the
National Natural Science Foundation of China (81172322), the
Science and Technology Commission of Shanghai Municipality
(10JC1409200 and 11ZR1421000), and the Science and Technology Fund
of Shanghai Jiaotong University School of Medicine (11XJ22014 and
YZ1027).
References
|
1
|
Pogribny IP: MicroRNA dysregulation during
chemical carcinogenesis. Epigenomics. 1:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takai D: Recent proceedings in epigenetics
research of lung cancer. Nihon Rinsho. 67:2387–2396. 2009.(In
Japanese).
|
|
3
|
Wang Q, Wang S, Wang H, Li P and Ma Z:
MicroRNAs: novel biomarkers for lung cancer diagnosis, prediction
and treatment. Exp Biol Med. 237:227–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang J, Lee SY, Lee SY, et al:
microRNA-99b acts as a tumor suppressor in non-small cell lung
cancer by directly targeting fibroblast growth factor receptor 3.
Exp Ther Med. 3:149–153. 2012.PubMed/NCBI
|
|
5
|
Darido C, Georgy SR, Wilanowski T, et al:
Targeting of the tumor suppressor GRHL3 by a miR-21-dependent
proto-oncogenic network results in PTEN loss and tumorigenesis.
Cancer Cell. 20:635–648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu Y, Govindan R, Wang L, et al: MicroRNA
profiling and prediction of recurrence/relapse-free survival in
stage I lung cancer. Carcinogenesis. 33:1046–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Z, Zeng H, Guo Y, et al: miRNA-145
inhibits non-small cell lung cancer cell proliferation by targeting
c-Myc. J Exp Clin Cancer Res. 29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gregersen LH, Jacobsen AB, Frankel LB, Wen
J, Krogh A and Lund AH: MicroRNA-145 targets YES and
STAT1 in colon cancer cells. PLoS One. 5:e88362010.
View Article : Google Scholar
|
|
9
|
Dhomen NS, Mariadason J, Tebbutt N and
Scott AM: Therapeutic targeting of the epidermal growth factor
receptor in human cancer. Crit Rev Oncog. 17:31–50. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ayoola A, Barochia A, Belani K and Belani
CP: Primary and acquired resistance to epidermal growth factor
receptor tyrosine kinase inhibitors in non-small cell lung cancer:
an update. Cancer Invest. 30:433–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Wang X, Zhang J, et al: MicroRNAs
involved in the EGFR/PTEN/AKT pathway in gliomas. J Neurooncol.
106:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmidt-Ullrich RK, Mikkelsen RB, Dent P,
et al: Radiation-induced proliferation of the human A431 squamous
carcinoma cells is dependent on EGFR tyrosine phosphorylation.
Oncogene. 15:1191–1197. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellis AG, Doherty MM, Walker F, et al:
Preclinical analysis of the analinoquinazoline AG1478, a specific
small molecule inhibitor of EGF receptor tyrosine kinase. Biochem
Pharmacol. 71:1422–1434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pao W, Miller VA, Politi KA, et al:
Acquired resistance of lung adenocarcinomas to gefitinib or
erlotinib is associated with a second mutation in the EGFR kinase
domain. PLoS Med. 2:e732005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burke WM, Jin X, Lin HJ, et al: Inhibition
of constitutively active Stat3 suppresses growth of human ovarian
and breast cancer cells. Oncogene. 20:7925–7934. 2001. View Article : Google Scholar
|
|
16
|
Pene F, Claessens YE, Muller O, et al:
Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase
pathways in the proliferation and apoptosis in multiple myeloma.
Oncogene. 21:6587–6597. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh T, Nakatsuka D, Watanabe Y, Nagata
I, Kikuchi H and Namura S: Neuroprotection by MAPK/ERK kinase
inhibition with U0126 against oxidative stress in a mouse neuronal
cell line and rat primary cultured cortical neurons. Neurosci Lett.
288:163–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seo IA, Lee HK, Shin YK, et al: Janus
kinase 2 inhibitor AG490 inhibits the STAT3 signaling pathway by
suppressing protein translation of gp130. Korean J Physiol
Pharmacol. 13:131–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walker EH, Pacold ME, Perisic O, et al:
Structural determinants of phosphoinositide 3-kinase inhibition by
wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol
Cell. 6:909–919. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Favata MF, Horiuchi KY, Manos EJ, et al:
Identification of a novel inhibitor of mitogen-activated protein
kinase kinase. J Biol Chem. 273:18623–18632. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sachdeva M, Zhu S, Wu F, et al: p53
represses c-Myc through induction of the tumor suppressor
miR-145. Proc Natl Acad Sci USA. 106:3207–3212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takaoka Y, Shimizu Y, Hasegawa H, et al:
Forced expression of miR-143 represses ERK5/c-Myc and p68/p72
signaling in concert with miR-145 in gut tumors of
ApcMin Mice. PLoS One. 7:e421372012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou CH, Yang SF and Li PQ: Human lung
cancer cell line SPC-A1 contains cells with characteristics of
cancer stem cells. Neoplasma. 59:685–692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z, Zhang X, Yang Z, et al: MiR-145
regulates PAK4 via the MAPK pathway and exhibits an antitumor
effect in human colon cells. Biochem Biophys Res Commun.
427:444–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gotte M, Mohr C, Koo CY, et al:
miR-145-dependent targeting of junctional adhesion molecule A and
modulation of fascin expression are associated with reduced breast
cancer cell motility and invasiveness. Oncogene. 29:6569–6580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eck MJ and Yun CH: Structural and
mechanistic underpinnings of the differential drug sensitivity of
EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta.
1804:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukohara T, Kudoh S, Yamauchi S, et al:
Expression of epidermal growth factor receptor (EGFR) and
downstream-activated peptides in surgically excised non-small-cell
lung cancer (NSCLC). Lung Cancer. 41:123–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koivunen JP, Mermel C, Zejnullahu K, et
al: EML4-ALK fusion gene and efficacy of an ALK kinase
inhibitor in lung cancer. Clin Cancer Res. 14:4275–4283. 2008.
View Article : Google Scholar
|
|
30
|
Chen H, Kovar J, Sissons S, et al: A
cell-based immunocytochemical assay for monitoring kinase signaling
pathways and drug efficacy. Anal Biochem. 338:136–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|