Introduction
Human NTERA-2 D1 cells (NT2 cells) belong to
embryonal carcinoma cells that have cancer and stem cell
characteristics (1–4). Therefore, NT2 cells are often used as
cell models for cancer therapy and neuron differentiation studies
(5–7). Currently, cisplatin, fisetin and
nucleoside drugs are applied to NT2 cell treatment. These drugs can
activate the MAPK and caspase-dependent pathway resulting in NT2
cell death (8,9). On the other hand, NT2 cells can be
induced to differentiate into neuron cells by treatment with
differentiation agents including retinoic acid, AraC, DAC, valproic
acid and berberine (7,8,10,11).
Among these differentiation agents, retinoic acid is generally used
for neuron differentiation studies (3,6,12).
Previous studies have demonstrated that retinoic acid can induce
NT2 cells to give rise to cell aggregation, neuron-like morphology
and to express neuronal markers (13–15).
Although the mechanisms of retinoic acid-induced neuron
differentiation in NT2 cells remain to be elucidated, it has been
reported that retinoic acid induced NT2 cells to differentiate into
neural cells via Wnt, Nitric oxide and cGMP signal pathways
(16,17).
Many ribonucleases have been demonstrated to have
anticancer activities (18–21). RC-RNase and onconase are frog
ribonucleases purified from Rana catesbeiana and Rana
pipiens, respectively. Both belong to the RNase A superfamily
(22–25). RC-RNase and onconase exert cytotoxic
effects on various cancer cells such as hepatoma, cervical cancer,
breast cancer, leukemia, mesothelioma, lung cancer, lymphoma,
myeloma and prostate carcinoma (21,26–31).
Although the mechanisms of frog ribonuclease-exerted cytotoxicity
remain to be elucidated, it is noteworthy that onconase has been
used as an anticancer drug in clinical trails (32–35).
Previous studies indicated that RC-RNase and onconase exert
different cytotoxic effects in different cancer cell types
(28,30,31,36–41).
In addition, several studies suggested that RC-RNase- and
onconase-induced cell cytotoxicity may be related to the caspase
cascade and MAPK signal pathway (36,38,42–44).
Similar to RC-RNase, RC-6 (Rana catesbeiana ribonuclease-6)
is also a frog ribonuclease derived from Rana catesbeiana
(29,45). Previous studies demonstrated that
onconase and RC-RNase can induce cell death in various cancer cells
(21,26–31).
However, only few studies showed that RC-6 inhibited cell growth in
cervical cancer and hepatoma cells (29,45).
Therefore, whether RC-6 can exert anticancer activities in various
cancer cell types and the mechanisms of RC-6-induced cytotoxic
effects on cancer cells remain unclear. In addition, to date, there
are no studies to demonstrate whether frog ribonucleases (RC-RNase,
onconase, RC-6) can inhibit cell growth in embryonal carcinoma
cells.
Here, RC-6-induced anticancer effects on NT2 cells
were investigated. The present study demonstrated that RC-6 can
inhibit cell growth and induce caspase-9/-3 cascade activation in
NT2 cells. On the other hand, some NT2 cells remained alive after
RC-6 treatment. Of note, these remaining live cells displayed cell
aggregation and neuron-like morphology similar to retinoic
acid-differentiated NT2 cells. However, when we compared
RC-6-treated NT2 cells with retinoic acid-treated NT2 cells, neuron
marker was found in retinoic acid-treated NT2 cells but not in
RC-6-treated NT2 cells. In addition, senescence characteristics
were observed in a small fraction of NT2 cells after RC-6
treatment. Taken together, our study is the first to indicate that
RC-6 can induce caspase-9/-3 activities and senescence
characteristics in NT2 cells and it can also induce cell
aggregation, neuron-like morphology in the remaining live
cells.
Materials and methods
Materials
RC-6 was kindly provided by Dr Jaang-Jiun Wang
(Division of Pediatric Infectious Diseases, Emory University School
of Medicine, Atlanta, GA, USA). p16 and Tau antibodies were
purchased from BD Biosciences (San Jose, CA, USA). p21 and p27
antibodies were bought from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). Horseradish peroxidase-conjugated secondary antibodies
were from Sigma-Aldrich (St. Louis, MO, USA). FITC-conjugated
secondary antibody was obtained from Jackson ImmunoResearch (West
Grove, PA, USA). Ac-LEHD-pNA
(acetyl-Leu-Glu-His-Asp-p-nitroanilide; caspase-9 substrate),
Ac-DEVD-pNA (Acetyl-Asp-Glu-Val-Asp-p-nitroanilide; caspase-3-like
substrate), and Ac-IETD-pNA (acetyl-Ile-Glu-Thr-Asp-p-nitroanilide;
caspase-8 substrate) were purchased from AnaSpec (San Jose, CA,
USA). Fetal bovine serum, DMEM, non-essential amino acid,
L-glutamine and penicillin/streptomycin were purchased from
Gibco-BRL.
Cell lines and cell cultures
The NT2 cell line was obtained from Bioresources
Collection and Research Center (BCRC, Hsinchu, Taiwan). NT2 cells
were cultured in DMEM supplemented with 10% fetal bovine serum, 2
mM L-glutamine, 100 IU/ml penicillin/streptomycin, and 0.1 mM
non-essential amino acids and maintained at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability assay
Cell viability assay was performed as previously
described (31,44). In brief, cells were grown on 6-well
cell culture plates overnight. After 24 h, cells were treated with
50 μg/ml RC-6 (experimental group) or without RC-6 (control group).
Every 24 h, cells were collected and stained with trypan blue and
counted on a hemocytometer.
Caspase substrate cleavage assay
Caspase activities were determined using caspase
substrate cleavage assay (31,44).
Briefly, cells were lysed with lysis buffer (50 mM Tris-HCl, 120 mM
NaCl, 1 mM EDTA, 1% NP-40, pH 7.5) and were then treated with
protease inhibitors. Subsequently, cell pellets were cleaned
through centrifugation (15,000 × g, at 4°C, for 30 min). Caspase-3,
−8 and −9 activities were determined. The working solutions were
prepared involving experimental sample (80 μg total protein), 158
μl reaction buffer (20% glycerol, 0.5 mM EDTA, 5 mM dithiothreitol,
100 mM HEPES, pH 7.5), and 2 μl fluorogenic substrate (Ac-LEHD-pNA,
Ac-DEVD-pNA or Ac-IETD-pNA). Then, the working solutions were
incubated for 8 h at 37°C. The fluorogenic substrate was determined
at 405 nm in an ultra-microplate reader (Bio-Tek Instruments). Fold
increase in caspase activity was calculated using the following
formula: (A405sample−
A405control)/A405control.
Senescence-associated β-galactosidase
(SA-β-Gal) assay
SA-β-gal activity was assessed as described in a
previous study (46). In brief,
cells were fixed with 0.5% glutaraldehyde solution for 15 min.
Next, cells were treated with 0.02% NP-40 and 0.1% sodium
deoxycholate for 15 min. Then, cells were incubated with 1 mg/ml
X-gal substrate solution
(5-bromo-4-chloro-3-indolyl-bd-galactopyranoside) containing 5 mM
potassium ferricyanide and 2 mM magnesium chloride at an acidic pH
6.0 for 16 h under CO2-free and dark conditions.
Western blot assay
Cells were treated with lysis buffer (50 mM
Tris-HCl, 120 mM NaCl, 1 mM EDTA, 1% NP-40 and pH 7.5). After
centrifugation, (16,000 × g) at 4°C for 10 min, the suspension
fraction containing protein was collected. The protein
concentration of the cell lysates was measured with a Bio-Rad
protein assay kit (Bio-Rad Laboratories) according to the
manufacturer’s instructions. Next, proteins were separated by 13.3%
SDS-PAGE and transferred to polyvinylidene difluoride membranes
(Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skim milk for 4 h at room temperature then probed with primary
antibodies overnight at 4°C. Membranes were washed three times with
0.1% Tween-20 (15 min/every time), then incubated with
HRP-conjugated secondary antibody (1:1,000 dilution) for 2 h at
room temperature. All proteins were observed using Western
Lightning Chemiluminescence reagent plus (Perkin-Elmer, Waltham,
MA, USA).
Immunofluorescent assay
Cells were fixed with 4% paraformaldehyde for 15 min
then treated with 0.03% Triton X-100 and blocked with 3% BSA. The
cells were incubated with Tau antibody overnight at 4°C. After
washing three times with PBS (15 min/time), cells were incubated
with a secondary goat anti-rabbit FITC antibody at room temperature
for 1 h. After washing three times with PBS (15 min/time), the
cells were observed under fluorescent microscopy.
Statistical analysis
Data were obtained from four independent triplicate
experiments and are presented as the mean values of the chosen
triplicate groups. The data are shown as means ± standard
deviations (SD).
Results
Cell growth inhibition and caspase-9/-3
cascade are induced in NT2 cells with RC-6 treatment
In the present study, whether RC-6 had antitumor
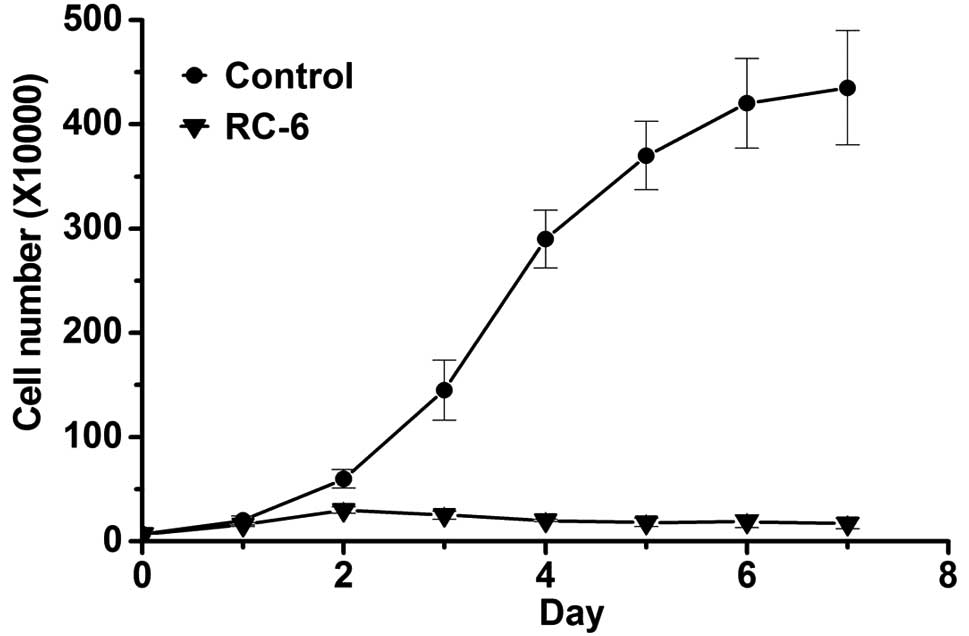
effects on NT2 cells was determined by cell number observation.
Following RC-6 treatment, viable cells were counted by using cell
viability assay with trypan blue stain under a hemocytometer
(31,44). When comparing RC-6-treated cells
with control cells, the cell number continuously increased in
control cells while cell growth was clearly inhibited in
RC-6-treated cells (Fig. 1). In
addition, as shown in Fig. 1, there
were few remaining live cells after RC-6 treatment on day 7. Next,
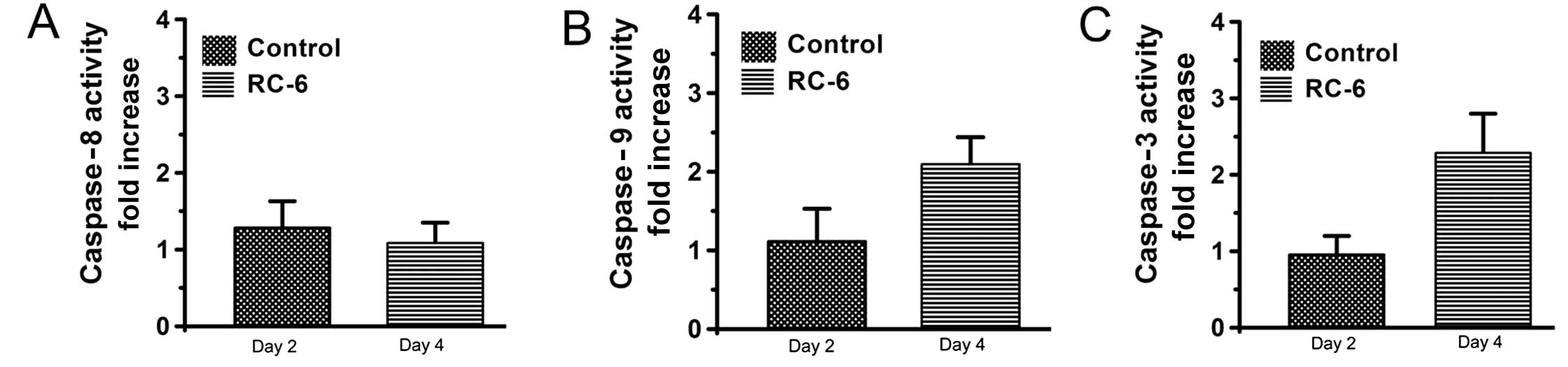
caspase activation was determined by using substrate cleavage assay
(30,36). Our results showed that caspase-9, −8
and −3 were not activated in NT2 cells with RC-6 treatment at day
2, whereas caspase-9 and −3 activities were observed on day 4
(Fig. 2B and C). However, caspase-8
activity was not clearly found in RC-6-treated cells on day 2 and
day 4 (Fig. 2A). These results
suggested that RC-6 exerts an antitumor effect in NT2 cells and
induces cell cytotoxicity related to the caspase-9/-3 cascade
pathway.
Senescence characteristics in NT2 cells
after RC-6 treatment
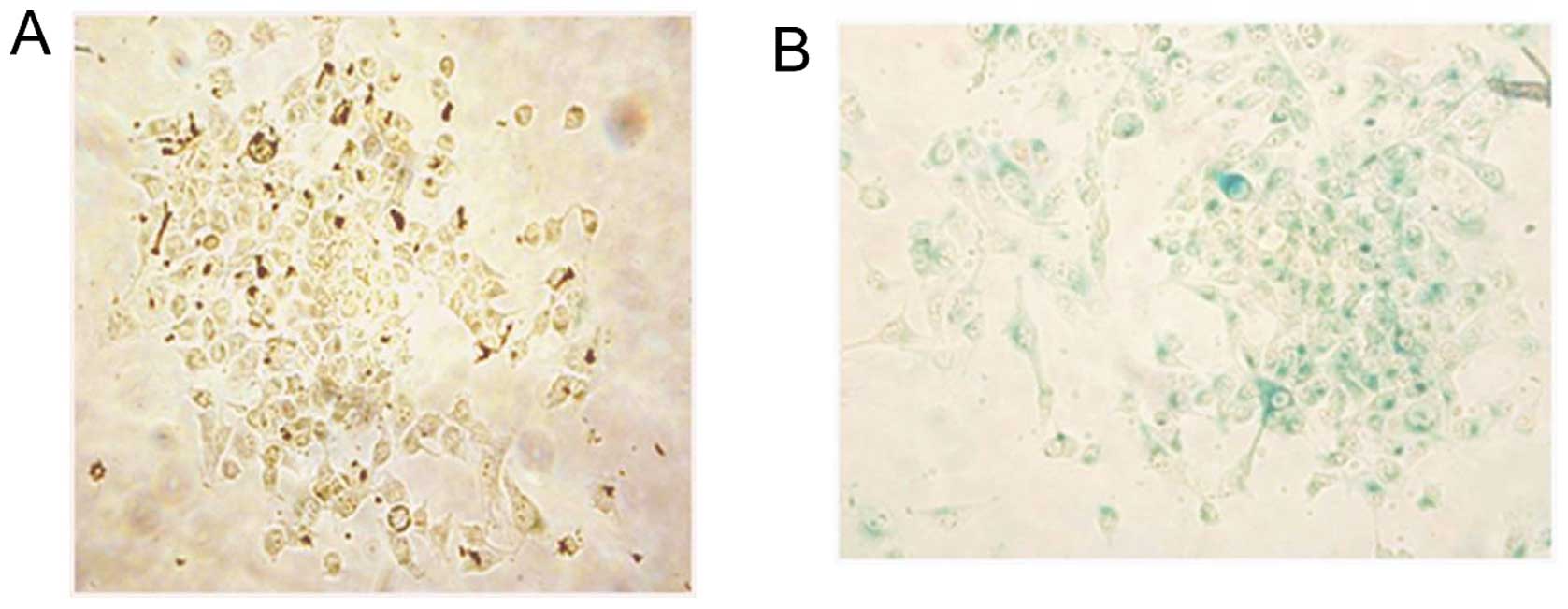
Our observations of cell morphology and cell growth
of RC-6-treated cells under a microscope showed that a small
fraction of NT2 cells had flat phenotype and these cells were not
able to proliferate similar to cellular senescence as in previous
studies (46–49). In order to investigate whether RC-6
induced cellular senescence, SA-β-Gal assay was performed. Our
results showed that some flat, enlarged RC-6-treated cells had
SA-β-Gal activity which appeared as blue color inside the cells
(Fig. 3B). However, blue color was
not found in control cells (Fig.
3A). Thus, RC-6 induced some cells to give rise to cellular
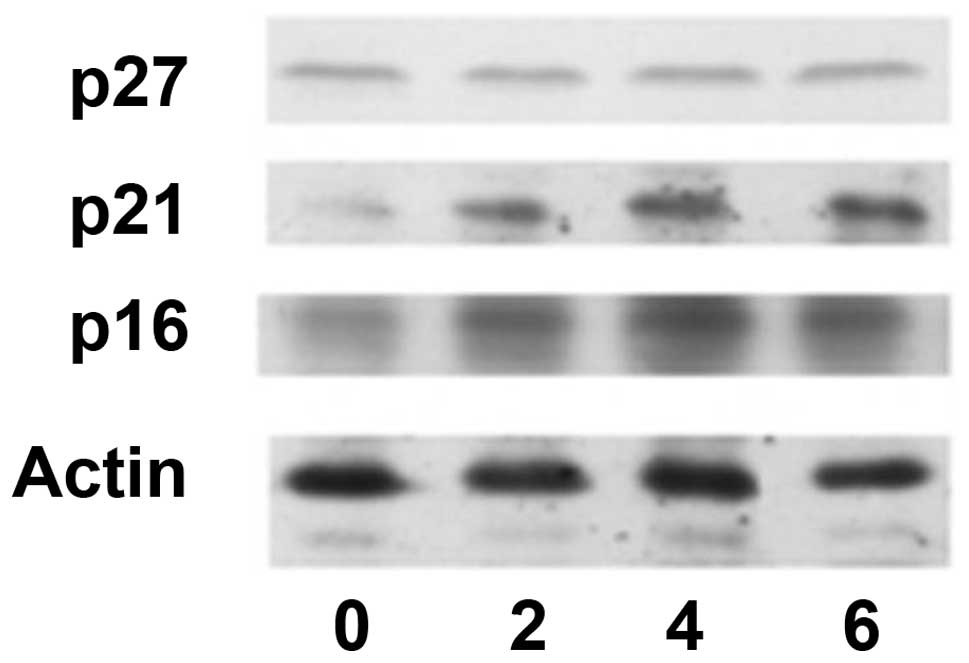
senescence. Previous studies indicated that p16, p21 and p27 are
related to cellular senescence in senescence cells (49–53).
Therefore, these proteins were determined in the present study.
Western blotting assay (Fig. 4)
demonstrated that p16 and p21 levels were increased in RC-6-treated
cells whereas p27 level was not increased in RC-6-treated cells.
Hence, RC-6 was able to induce p16 and p21 protein increase and
cause cellular senescence in a small fraction of NT2 cells.
Neuron-like morphology in the remaining
live cells following RC-6 treatment
After RC-6 treatment for 7 days, most cells died,
while few cells survived. These remaining live cells were collected
and re-cultured with fresh media under RC-6-free conditions.
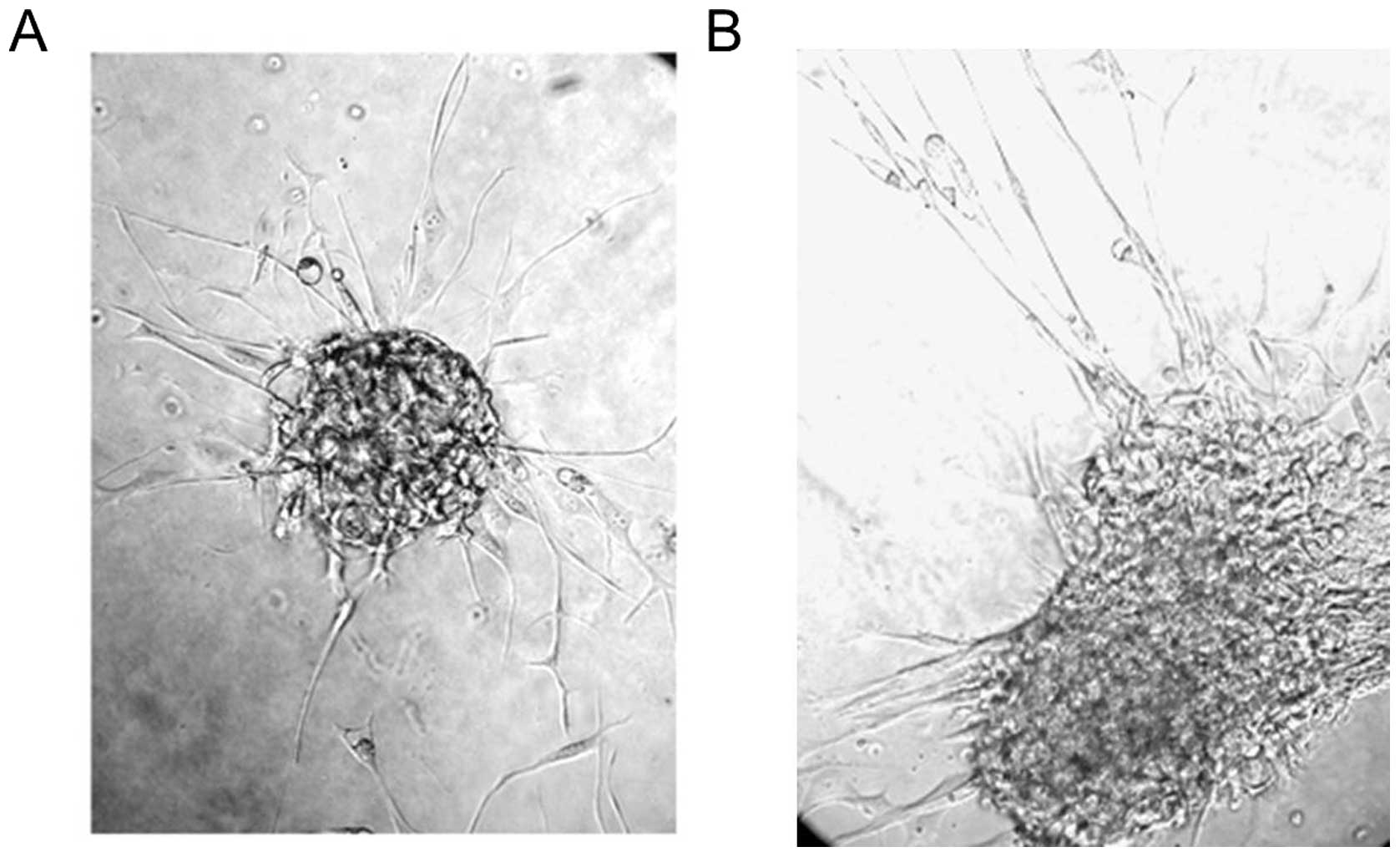
Notably, the remaining live cells aggregated and presented a neural
sphere-like phenotype similar to retinoic acid-differentiated NT2
cells (Fig. 5). Subsequently, the
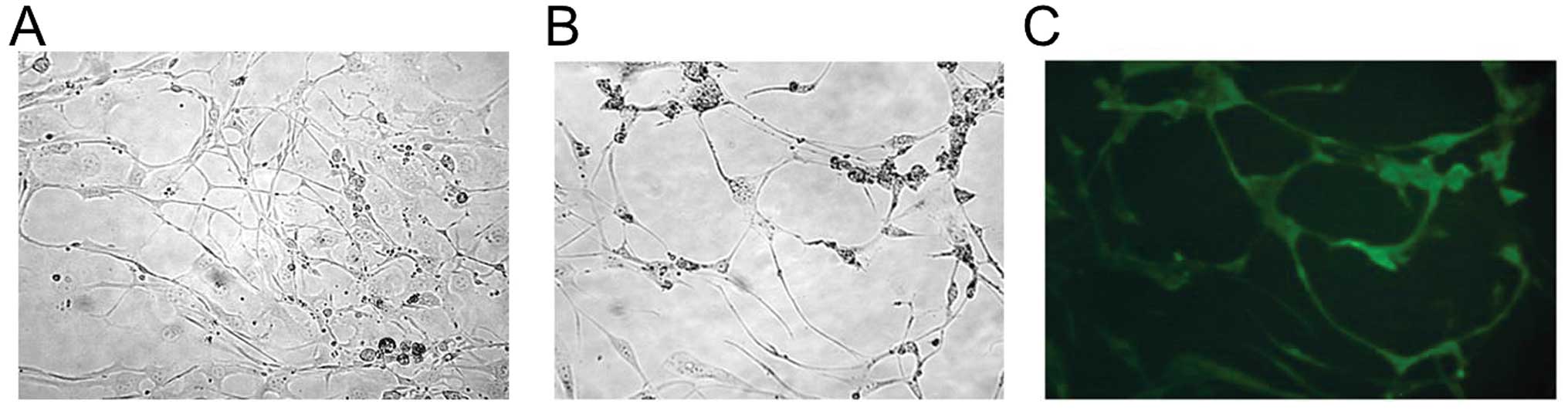
remaining live cells presented a neuron-like morphology (Fig. 6A) similar to retinoic
acid-differentiated NT2 cells (Fig.
6B). When comparing the remaining live cells with retinoic
acid-differentiated cells, neuron marker Tau only appeared in
retinoic acid-differentiated cells (Fig. 6C); however, Tau was not found in the
remaining live cells after RC-6 treatment (data not shown). The
present study suggests that RC-6 induced NT2 cells to form
neuron-like cells whereas these cells did not have neuron
function.
Discussion
RC-6 has been reported to exert anticancer effects
on human cervical cancer (HeLa) and hepatoma (HepG2) cells
(29,45). In the present study, we further
demonstrated that RC-6 also exerted anticancer effects on human
embryonal carcinoma cells (NT2 cells). The results suggested that
RC-6 may exert anticancer effects on various types of cancer, such
as the RC-RNase- and onconase-exerted anticancer effects (21,26–31).
Although the mechanisms of RC-6-induced cell cytotoxicity on
cervical cancer cells has not been studied (29), RC-6-induced cell cytotoxicity in
HepG2 cells was related to caspase-9/-3 cascade (45). Here, caspase-9 and −3 activities
were found in RC-6-treated NT2 cells similar to RC-6-treated-HepG2
cells. Thus, we considered that the caspase-9/-3 cascade is an
important signal pathway in RC-6-induced cell death. RC-6, RC-RNase
and onconase are all frog ribonucleases (22–25).
RC-RNase and onconase can induce caspase-9 and −3 activities in
cancer cells; however, they cannot induce caspase-8 activity in
cancer cells (31,36,38,54).
In the present study, RC-6 also induced caspase-9 and −3 activities
but did not induce caspase-8 activity. Based on these findings, we
suggested that frog ribonuclease-induced cell death was related to
the caspase-9/-3 cascade but not the caspase-8/-3 cascade.
Retinoic acid induces NT2 cells to differentiate
into neuron-like cells, as has previously been reported (2,6,55–57).
Numerous studies have demonstrated that cell aggregation and
neuron-like morphology appear in retinoic acid-differentiated NT2
cells (13–15). Moreover, caspase activities were
observed in NT2 cells during retinoic acid treatment (8,56,58).
Here, cell aggregation, neuron-like morphology was also observed in
the remaining live NT2 cells after RC-6 treatment. In addition,
caspase activities were activated in NT2 cells during RC-6
treatment. Our results indicated that RC-6-treated NT2 cells were
similar to retinoic acid-treated NT2 cells. Therefore, we consider
that RC-6 and retinoic acid may induce certain similar signal
pathways in NT2 cells, whereas these signal pathways are still
unknown and remain to be studied in the future. On the other hand,
retinoic acid induced neuron marker expression in NT2 cells in the
present study as well as in previous studies (4,13).
However, neuron markers were not clearly found in RC-6-treated NT2
cells in the present study. A previous study showed that RC-6
having ribonuclease activity could cleave RNAs (29). There is no evidence to show that
retinoic acid can induce RNA degradation similar to ribonucleases.
Based on these findings, we speculated that neuron marker RNAs may
be degraded in NT2 cells during RC-6 treatment; therefore, neuron
markers cannot be found in RC-6-treated NT2 cells unlike retinoic
acid-differentiated NT2 cells.
Recent studies demonstrated that senescence can be
induced in many cell types under various conditions and treatments
such as hypoxia, uremia, UVB, radiation, H2O2
and oxidized low-density lipoprotein (59–64).
Previous studies indicated that β-galactosidase activity is a major
characteristic found in senescence cells and is generally applied
to senescence detection (65–67).
In addition, many studies have reported that p16 and p21 protein
levels are increased in senescence cells (50–53).
At present, a small fraction of RC-6-treated NT2 cells can express
β-galactosidase activity. Moreover, p16 and p21 protein levels are
increased in RC-6-treated NT2 cells. Thus, our results indicated
that RC-6 can induce senescence characteristics in NT2 cells.
Previous studies demonstrated that p27 protein increase was also
found in senescence cells (49,68,69).
However, p27 protein levels were not increased significantly in
RC-6-treated NT2 cells. Our results indicated that p16 and p21 play
more important roles than p27 in RC-6-induced senescence.
In summary, the present study is the first to show
that RC-6 can induce embryonal carcinoma cells to give rise to
caspase-9/-3 activation, senescence characteristics and neuron-like
morphology.
Acknowledgements
The present study was supported by the following
grants: NSC99-2320-B-039-030-MY3, NSC99-2632-B-039-001-MY3,
NSC101-2321-B-039-004 and NHRI-EX102-10245BI.
References
|
1
|
Biswal BK, Beyrouthy MJ, Hever-Jardine MP,
et al: Acute hypersensitivity of pluripotent testicular
cancer-derived embryonal carcinoma to low-dose 5-aza deoxycytidine
is associated with global DNA damage-associated p53 activation,
anti-pluripotency and DNA demethylation. PLoS One. 7:e530032012.
View Article : Google Scholar
|
|
2
|
Coyle DE, Li J and Baccei M: Regional
differentiation of retinoic acid-induced human pluripotent
embryonic carcinoma stem cell neurons. PLoS One. 6:e161742011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Favaedi R, Shahhoseini M and Akhoond MR:
Comparative epigenetic analysis of Oct4 regulatory region in
RA-induced differentiated NT2 cells under adherent and non-adherent
culture conditions. Mol Cell Biochem. 363:129–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tegenge MA, Roloff F and Bicker G: Rapid
differentiation of human embryonal carcinoma stem cells (NT2) into
neurons for neurite outgrowth analysis. Cell Mol Neurobiol.
31:635–643. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drakulic D, Krstic A and Stevanovic M:
Establishment and initial characterization of SOX2-overexpressing
NT2/D1 cell clones. Genet Mol Res. 11:1385–1400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jezierski A, Deb-Rinker P, Sodja C, et al:
Involvement of NOS3 in RA-Induced neural differentiation of human
NT2/D1 cells. J Neurosci Res. 90:2362–2377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oz S, Maercker C and Breiling A: Embryonic
carcinoma cells show specific dielectric resistance profiles during
induced differentiation. PLoS One. 8:e598952013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Musch T, Oz Y, Lyko F and Breiling A:
Nucleoside drugs induce cellular differentiation by
caspase-dependent degradation of stem cell factors. PLoS One.
5:e107262010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tripathi R, Samadder T, Gupta S, Surolia A
and Shaha C: Anticancer activity of a combination of cisplatin and
fisetin in embryonal carcinoma cells and xenograft tumors. Mol
Cancer Ther. 10:255–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang KS: Down-regulation of c-Ki-ras2
gene expression associated with morphologic differentiation in
human embryonal carcinoma cells treated with berberine. J Formos
Med Assoc. 90:10–14. 1991.
|
|
11
|
Skladchikova G, Berezin V and Bock E:
Valproic acid, but not its non-teratogenic analogue
2-isopropylpentanoic acid, affects proliferation, viability and
neuronal differentiation of the human teratocarcinoma cell line
NTera-2. Neurotoxicology. 19:357–370. 1998.PubMed/NCBI
|
|
12
|
Kakhki SA, Shahhoseini M and Salekdeh GH:
Comparative SRY incorporation on the regulatory regions of
pluripotency/differentiation genes in human embryonic carcinoma
cells after retinoic acid induction. Mol Cell Biochem. 376:145–150.
2013. View Article : Google Scholar
|
|
13
|
Megiorni F, Mora B, Indovina P and
Mazzilli MC: Expression of neuronal markers during NTera2/cloneD1
differentiation by cell aggregation method. Neurosci Lett.
373:105–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jain P, Cerone MA, Leblanc AC and Autexier
C: Telomerase and neuronal marker status of differentiated NT2 and
SK-N-SH human neuronal cells and primary human neurons. J Neurosci
Res. 85:83–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheung WM, Fu WY, Hui WS and Ip NY:
Production of human CNS neurons from embryonal carcinoma cells
using a cell aggregation method. Biotechniques. 26:946–954.
1999.PubMed/NCBI
|
|
16
|
Snow GE, Kasper AC, Busch AM, et al: Wnt
pathway reprogramming during human embryonal carcinoma
differentiation and potential for therapeutic targeting. BMC
Cancer. 9:3832009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tegenge MA and Bicker G: Nitric oxide and
cGMP signal transduction positively regulates the motility of human
neuronal precursor (NT2) cells. J Neurochem. 110:1828–1841. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ardelt W, Ardelt B and Darzynkiewicz Z:
Ribonucleases as potential modalities in anticancer therapy. Eur J
Pharmacol. 625:181–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang CH, Gupta P, Michel R, et al:
Ranpirnase (frog RNase) targeted with a humanized, internalizing,
anti-Trop-2 antibody has potent cytotoxicity against diverse
epithelial cancer cells. Mol Cancer Ther. 9:2276–2286. 2010.
View Article : Google Scholar
|
|
20
|
Fang EF and Ng TB: Ribonucleases of
different origins with a wide spectrum of medicinal applications.
Biochim Biophys Acta. 1815:65–74. 2011.PubMed/NCBI
|
|
21
|
Zwolinska M and Smolewski P: Onconase: a
ribonuclease with antitumor activity. Postepy Hig Med Dosw
(Online). 64:58–66. 2010.(In Polish).
|
|
22
|
Chang CF, Chen C, Chen YC, Hom K, Huang RF
and Huang TH: The solution structure of a cytotoxic ribonuclease
from the oocytes of Rana catesbeiana (bullfrog). J Mol Biol.
283:231–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gahl RF, Narayan M, Xu G and Scheraga HA:
Dissimilarity in the oxidative folding of onconase and ribonuclease
A, two structural homologues. Protein Eng Des Sel. 21:223–231.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gahl RF and Scheraga HA: Oxidative folding
pathway of onconase, a ribonuclease homologue: insight into
oxidative folding mechanisms from a study of two homologues.
Biochemistry. 48:2740–2751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosenberg HF, Zhang J, Liao YD and Dyer
KD: Rapid diversification of RNase A superfamily ribonucleases from
the bullfrog, Rana catesbeiana. J Mol Evol. 53:31–38.
2001.PubMed/NCBI
|
|
26
|
Ita M, Halicka HD, Tanaka T, et al:
Remarkable enhancement of cytotoxicity of onconase and
cepharanthine when used in combination on various tumor cell lines.
Cancer Biol Ther. 7:1104–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee I: Ranpirnase (Onconase), a cytotoxic
amphibian ribonuclease, manipulates tumour physiological parameters
as a selective killer and a potential enhancer for chemotherapy and
radiation in cancer therapy. Expert Opin Biol Ther. 8:813–827.
2008. View Article : Google Scholar
|
|
28
|
Hu CC, Lee YH, Tang CH, Cheng JT and Wang
JJ: Synergistic cytotoxicity of Rana catesbeiana
ribonuclease and IFN-γ on hepatoma cells. Biochem Biophys Res
Commun. 280:1229–1236. 2001.PubMed/NCBI
|
|
29
|
Liao YD, Huang HC, Leu YJ, Wei CW, Tang PC
and Wang SC: Purification and cloning of cytotoxic ribonucleases
from Rana catesbeiana (bullfrog). Nucleic Acids Res.
28:4097–4104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yiang GT, Yu YL, Chou PL, et al: The
cytotoxic protein can induce autophagocytosis in addition to
apoptosis in MCF-7 human breast cancer cells. In Vivo. 26:403–409.
2012.PubMed/NCBI
|
|
31
|
Wei CW, Hu CC, Tang CH, Lee MC and Wang
JJ: Induction of differentiation rescues HL-60 cells from Rana
catesbeiana ribonuclease-induced cell death. FEBS Lett.
531:421–426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Costanzi J, Sidransky D, Navon A and
Goldsweig H: Ribonucleases as a novel pro-apoptotic anticancer
strategy: review of the preclinical and clinical data for
ranpirnase. Cancer Invest. 23:643–650. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mikulski S, Grossman A, Carter P, Shogen K
and Costanzi J: Phase I human clinical trial of
ONCONASE®* (P-30 protein) administered intravenously on
a weekly schedule in cancer patients with solid tumors. Int J
Oncol. 3:57–64. 1993.PubMed/NCBI
|
|
34
|
Mikulski SM, Costanzi JJ, Vogelzang NJ, et
al: Phase II trial of a single weekly intravenous dose of
ranpirnase in patients with unresectable malignant mesothelioma. J
Clin Oncol. 20:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vogelzang NJ, Aklilu M, Stadler WM, Dumas
MC and Mikulski SM: A phase II trial of weekly intravenous
ranpirnase (Onconase), a novel ribonuclease in patients with
metastatic kidney cancer. Invest New Drugs. 19:255–260. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang CH, Hu CC, Wei CW and Wang JJ:
Synergism of Rana catesbeiana ribonuclease and IFN-γ
triggers distinct death machineries in different human cancer
cells. FEBS Lett. 579:265–270. 2005.PubMed/NCBI
|
|
37
|
Tseng HH, Yu YL, Chen YL, et al:
RC-RNase-induced cell death in estrogen receptor positive breast
tumors through downregulation of Bcl-2 and estrogen receptor. Oncol
Rep. 25:849–853. 2011.PubMed/NCBI
|
|
38
|
Grabarek J, Ardelt B, Du L and
Darzynkiewicz Z: Activation of caspases and serine proteases during
apoptosis induced by onconase (Ranpirnase). Exp Cell Res.
278:61–71. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Halicka HD, Ardelt B, Shogen K and
Darzynkiewicz Z: Mild hyperthermia predisposes tumor cells to
undergo apoptosis upon treatment with onconase. Int J Oncol.
30:841–847. 2007.PubMed/NCBI
|
|
40
|
Michaelis M, Cinatl J, Anand P, et al:
Onconase induces caspase-independent cell death in chemoresistant
neuroblastoma cells. Cancer Lett. 250:107–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ramos-Nino ME, Vianale G, Sabo-Attwood T,
et al: Human mesothelioma cells exhibit tumor cell-specific
differences in phosphatidylinositol 3-kinase/AKT activity that
predict the efficacy of Onconase. Mol Cancer Ther. 4:835–842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iordanov MS, Wong J, Newton DL, et al:
Differential requirement for the stress-activated protein
kinase/c-Jun NH(2)-terminal kinase in RNAdamage-induced apoptosis
in primary and in immortalized fibroblasts. Mol Cell Biol Res
Commun. 4:122–128. 2000. View Article : Google Scholar
|
|
43
|
Altomare DA, Rybak SM, Pei J, et al:
Onconase responsive genes in human mesothelioma cells: implications
for an RNA damaging therapeutic agent. BMC Cancer. 10:342010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yiang GT, Yu YL, Hu SC, Chen MH, Wang JJ
and Wei CW: PKC and MEK pathways inhibit caspase-9/-3-mediated
cytotoxicity in differentiated cells. FEBS Lett. 582:881–885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei CW, Chou PL, Hung YT and Yiang GT:
Synergistic cytotoxicity of 1,3-bis(2-chloroethyl)-1-nitrosourea
and Rana catesbeiana ribonuclease-6 in hepatoma cells. Tzu
Chi Med J. 23:9–15. 2011. View Article : Google Scholar
|
|
46
|
Hu Z, Gu Y, Han B, et al: Knockdown of
AGR2 induces cellular senescence in prostate cancer cells.
Carcinogenesis. 33:1178–1186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Giovannini C, Gramantieri L, Minguzzi M,
et al: CDKN1C/P57 is regulated by the Notch target gene Hes1
and induces senescence in human hepatocellular carcinoma. Am J
Pathol. 181:413–422. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leontieva OV, Natarajan V, Demidenko ZN,
Burdelya LG, Gudkov AV and Blagosklonny MV: Hypoxia suppresses
conversion from proliferative arrest to cellular senescence. Proc
Natl Acad Sci USA. 109:13314–13318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ewald JA, Desotelle JA, Church DR, et al:
Androgen deprivation induces senescence characteristics in prostate
cancer cells in vitro and in vivo. Prostate. 73:337–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Burns TF, Dobromilskaya I, Murphy SC, et
al: Inhibition of TWIST1 leads to activation of oncogene-induced
senescence in oncogene-driven non-small cell lung cancer. Mol
Cancer Res. 11:329–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tu Z, Zhuang X, Yao YG and Zhang R: BRG1
is required for formation of senescence-associated heterochromatin
foci induced by oncogenic RAS or BRCA1 loss. Mol Cell Biol.
33:1819–1829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Capparelli C, Chiavarina B,
Whitaker-Menezes D, et al: CDK inhibitors (p16/p19/p21) induce
senescence and autophagy in cancer-associated fibroblasts,
‘fueling’ tumor growth via paracrine interactions, without an
increase in neo-angiogenesis. Cell Cycle. 11:3599–3610.
2012.PubMed/NCBI
|
|
53
|
Wu X, Jia S, Zhang X, Si X, Tang W and Luo
Y: Two mechanisms underlying the loss of p16Ink4a
function are associated with distinct tumorigenic consequences for
WS MEFs escaping from senescence. Mech Ageing Dev. 133:549–555.
2012.PubMed/NCBI
|
|
54
|
Iordanov MS, Ryabinina OP, Wong J, et al:
Molecular determinants of apoptosis induced by the cytotoxic
ribonuclease onconase: evidence for cytotoxic mechanisms different
from inhibition of protein synthesis. Cancer Res. 60:1983–1994.
2000.
|
|
55
|
Kvissel AK, Orstavik S, Oistad P, Rootwelt
T, Jahnsen T and Skalhegg BS: Induction of Cβ splice variants and
formation of novel forms of protein kinase A type II holoenzymes
during retinoic acid-induced differentiation of human NT2 cells.
Cell Signal. 16:577–587. 2004.
|
|
56
|
Patel NA, Song SS and Cooper DR: PKCδ
alternatively spliced isoforms modulate cellular apoptosis in
retinoic acid-induced differentiation of human NT2 cells and mouse
embryonic stem cells. Gene Expr. 13:73–84. 2006.
|
|
57
|
Misiuta IE, Saporta S, Sanberg PR, Zigova
T and Willing AE: Influence of retinoic acid and lithium on
proliferation and dopaminergic potential of human NT2 cells. J
Neurosci Res. 83:668–679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pistritto G, Papaleo V, Sanchez P, Ceci C
and Barbaccia ML: Divergent modulation of neuronal differentiation
by caspase-2 and −9. PLoS One. 7:e360022012.PubMed/NCBI
|
|
59
|
Zhang XP, Zhang GH, Wang YY, et al:
Oxidized low-density lipoprotein induces hematopoietic stem cell
senescence. Cell Biol Int. 37:940–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carracedo J, Buendia P, Merino A, et al:
Cellular senescence determines endothelial cell damage induced by
uremia. Exp Gerontol. 48:766–773. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim HD, Yu SJ, Kim HS, et al: IL-4 induces
senescence in human renal carcinoma cell lines through STAT6 and
p38 MAPK. J Biol Chem. 288:28743–28754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Luo H, Yount C, Lang H, et al: Activation
of p53 with Nutlin-3a radiosensitizes lung cancer cells via
enhancing radiation-induced premature senescence. Lung Cancer.
81:167–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Suo R, Zhao ZZ, Tang ZH, et al: Hydrogen
sulfide prevents H2O2-induced senescence in
human umbilical vein endothelial cells through SIRT1 activation.
Mol Med Rep. 7:1865–1870. 2013.PubMed/NCBI
|
|
64
|
Mo J, Sun B, Zhao X, et al:
Hypoxia-induced senescence contributes to the regulation of
microenvironment in melanomas. Pathol Res Pract. 209:640–647. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bassaneze V, Miyakawa AA and Krieger JE:
Chemiluminescent detection of senescence-associated β
galactosidase. Methods Mol Biol. 965:157–163. 2013.PubMed/NCBI
|
|
66
|
Pospelova TV, Chitikova ZV and Pospelov
VA: An integrated approach for monitoring cell senescence. Methods
Mol Biol. 965:383–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mortuza R, Chen S, Feng B, Sen S and
Chakrabarti S: High glucose induced alteration of SIRTs in
endothelial cells causes rapid aging in a p300 and FOXO regulated
pathway. PLoS One. 8:e545142013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Diep CH, Charles NJ, Gilks CB, Kalloger
SE, Argenta PA and Lange CA: Progesterone receptors induce
FOXO1-dependent senescence in ovarian cancer cells. Cell Cycle.
12:1433–1449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang H, Xu Y, Fang Z, Chen S, Balk SP and
Yuan X: Doxycycline regulated induction of AKT in murine prostate
drives proliferation independently of p27 cyclin dependent kinase
inhibitor downregulation. PLoS One. 7:e413302012. View Article : Google Scholar
|