Introduction
Hepatocellular carcinoma (HCC) is the the fifth most
common cancer and the third leading cause of cancer-related death
in the world (1). Its annual
incidence is estimated at 600,000 new cases, more than half of
which come from China (2). Surgical
resection is the most effective therapy for HCC. However, most of
the patients are not good candidates for surgery because of being
diagnosed in later stages. On the other hand, HCC is frequently
resistant to conventional chemotherapeutic agentsand radiation
(3). Resistance to apoptosis is one
of the reasons that HCC cells are not sensitive to chemotherapy
(4,5).
Some studies have revealed that NF-κB is
constitutively activated and plays a vital role in the regulation
of genes involved in cell apoptosis in some cancers including HCC
(6,7). Inhibition of NF-κB p65 by miRNA
resulted in a significant increased apoptosis in HCC (8). Interestingly, Rap1
(repressor/activator protein), a member of telomeres shelterin
complex, has been shown to be an essential modulator of
NF-κB-mediated pathways (9). Human
breast cancers with NF-κB hyperactivity show elevated levels of
cytoplasmic Rap1. Knockdown of Rap1 sensitizes breast cancer cells
to apoptosis (9). However, to date,
few studies have reported the biologic function of Rap1 in HCC
progression, the interactive regulation between Rap1 and NF-κB, and
the potential functional contribution of such a network to HCC
cancer progression and apoptosis have not been explored yet.
In the present study, to investigate the role of
Rap1 in HCC progression, we examined cell survival/growth and
apoptosis after downregulation of Rap1 expression by miRNA in HepG2
cells, and explored its mechanisms.
Materials and methods
Cell culture
HepG2, HuH-7, SMMC-7721, QGY-7703 and HL-7702 cell
lines were obtained from Shanghai Cell Bank. The cells were
maintained under a humidified atmosphere of 5% CO2 at
37°C in DMEM medium (HyClone), supplemented with 10% fetal bovine
serum (FBS; HyClone).
Transient transfection of HepG2 cells and
establishment of stable transfectants
Set of four Rap1-miRNA oligonucleotides were
designed and synthesized by Invitrogen for Rap1 gene knockdown
studies. The Rap1-miRNA expression vector (pcDNATM
6.2-GW/EmGPF-miR; Invitrogen; catalog no. K4936-00) was used to
construct a Rap1 miRNA plasmid by inserting the miRNA-coding
sequence. HepG2 cells were transfected with selected Rap1-miRNA
plasmid or control miRNA plasmid.
HepG2 cells were seeded on 6-well plates at a
density of 5×105 cells/well, and grown for 48 h to reach
80% confluence before transfection. Control miRNA and Rap1 miRNA
were transfected respectively into cells using Lipofectamine 2000
(Invitrogen; catalog no. 11668-027) according to the manufacturer’s
instructions. The cells were then harvested and analyzed by flow
cytometry, electron microscopy, reverse transcriptase polymerase
chain reaction (RT-PCR) and western blot analysis at 24–72 h after
the transfection. All measurements were performed in
triplicate.
The transfected cells were cultured in the medium
with Blasticidin S HCl (Invitrogen; catalog no. R210-01) at 2–10
μg/ml. After 8 weeks, surviving colonies arising from stably
transfected cells were selected and individually amplified.
MTT assay
At 48 h after transfection, cell survival was
measured by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The transfected and control cells were seeded at a density
of 5,000 cells/well in 96-well plates and incubated at 37°C in
humidified 5% CO2 for 24 h. Serially diluted 5-FU
(Sigma) was added to give the intended final concentrations. Cells
were then incubated an additional 72 h, and the MTT assay was
performed according to the manufacturer’s instructions. Absorbance
values were determined at 570 nm on a SpectraMax 250
spectrophotometer. The assays were performed three times in
triplicate. The 50% inhibitory concentration (IC50)
values were defined as the drug concentrations required to reduce
cellular proliferation to 50% of the untreated control well.
Flow cytometry for apoptosis
The HepG2 cells were harvested for evaluation of
apoptosis at 48 and 72 h after the transfection. Firstly, the cells
were washed with PBS and fixed in 4% paraformaldehyde for 30 min.
Then, the cells were re-suspended in PBS supplemented with 0.1%
Triton X-100, and incubated at 4°C for 2 min. TUNEL solution (50
μl) was added to each tube, and incubated in the dark at 37°C for
60 min. After that, the cells were washed with PBS, and
re-suspended in 250 μl PBS for flow cytometry using the FACSCalibur
system.
Electron microscopy
The cells were harvested in PBS for evaluation of
apoptosis with electron microscopy at 48 h after the transfection.
Firstly, the cells were subsequently fixed in 3.5% glutaraldehyde
and 1% OsO4, then, washed with PBS, dehydrated in
ethanol and acetone series, and embedded in Epon 618. Thin sections
were cut with a Leica Ultracut UCT microtome, stained with 2%
uranyl acetate and lead citrate. Observation followed using
JEM-1011 transmission electron microscopy.
Quantitative real-time PCR
To elucidate the mechanism by which Rap1 induces
cell growth and inhibits apoptosis in HCC, we examined the
expression of NF-κB p65 and IκB using quantitative real-time PCR at
48 h after the transfection. Total RNA was isolated from the Rap1
miRNA transfected and control cell lines with TRIzol (Invitrogen;
catalog no. 15596-026). Then, the RNA was subjected to real-time
PCR analysis with the use of a One Step SYBR PrimeScript RT-PCR kit
(Bio-Rad Laboratories) and superscript pre-amplification system
(Invitrogen Life Technologies). NF-κB p65 was amplified using
forward primer 5′-GGGAAGGAACG CTGTCAGAG-3′ and reverse primer
5′-TAGCCTCAGGG TACTCCATCA-3′. IκB was amplified using forward
primer 5′-GGATACCTGGAGGATCAGATTA-3′ and reverse primer
5′-CCACCTTAGGGAGTAGTAGATCAAT-3′. PCR amplification was performed
with 32 cycles of 45 sec denaturing at 94°C, 45 sec annealing at
55°C and 10 min extension at 72°C. A melting curve analysis was
conducted to control for the specificity of the amplification
products. The expression values were normalized by the β-actin
expression. Moreover, the amplified gene fragments were separated
in a 2% agarose gel and visualized by ethidium bromide staining and
UV light.
Western blot analysis
Western blot analysis was performed as previously
described at 24, 48 and 72 h after the transfections. The
antibodies included Rap1 monoclonal antibody (Santa Cruz
Biotechnology, 60 kDa, mouse), β-actin monoclonal antibody (Abmart;
43 kDa, mouse), goat anti-rabbit and mouse IgG-HRP (Abmart). Cells
were collected by trypsin digestion and washed twice with PBS. RIPA
lysis buffer (100 μl) (Soledad) was added. Then, protein was
extracted, and protein concentrations were determined using BCA
protein assay kit (Tiangen) based on the manufacturer’s
instructions. Protein samples (30 μl) were loaded onto a NuPAGE 8%
TA gel (Invitrogen) and run at 80 V. Next, the proteins were
transferred to PVDF membranes (Millipore) using an iBlot Gel
Transfer Device (Bio-Rad Mini Protein 3) at 200 mA for 1.5 h. The
membranes were blocked in 3% BSA blocking buffer for 2 h, exposed
to primary antibody overnight at 4°C, and secondary antibody for 2
h at room temperature. After that, the membranes were washed with
1% PBST, and rinsed with Rap1 three times (5 min each time) and
with β-actin three times (3 min each time). Excess reagent was
removed. The membranes were covered with transparent plastic wraps.
Images were acquired using darkroom development techniques.
In vivo studies
The animal protocol was approved by the the
Institutional Animal Care and Use Committee of Kunming Medical
University. Four-week-old BALB/c nude mice were obtained from Vital
River Laboratories (Beijing, China) and maintained in a specific
pathogen-free facility. These mice were randomly divided into four
groups with 10 mice each group: i) control; ii) 5-FU treated; iii)
Rap1 miRNA; and iv) Rap1 miRNA + 5-FU treated group. The mice were
implanted subcutaneously into the right flank with Rap1 miRNA
stable transfected and control HepG2 cells (1.0×107
cells in 0.2 μl PBS per mouse). When palpable tumors developed
(average volume 100 mm3), the mice in 5-FU treated and
Rap1 miRNA + 5-FU treated groups were administered
intraperitoneally with 5-FU at a dose of 30 mg/kg every 3 days.
Those in control and Rap1 miRNA groups were injected with the same
volume of 0.9% saline. Tumor size was measured at 3-day intervals
with a caliper (calculated volume = shortest diameter2 ×
longest diameter/2). Tumor growth was followed for 25 days from the
first injection.
Immunohistochemistry for Rap1 and
caspase-3
At the termination of the experiments, the tumors
were removed for histological examination. The tumor samples were
fixed in 10% formaldehyde, embedded in paraffin and then cut into
3-μm thick sections. Immunohistochemistry was conducted using
streptavidin-peroxidase method with the EnVision Kit (Maixin Bio,
Fuzhou, China). The sections were dewaxed in xylene and rehydrated
in a graded series of ethanol. The tissue sections were treated by
high temperature and pressure in 10 mmol/l sodium citrate (pH 6.0)
for 2 min for antigen retrieval. Next, the primary anti-Rap1
antibody (1:100 dilution; Santa Cruz Biotechnology; 60 kDa) was
added to the slides. The slides were incubated at 4°C overnight.
After that, the slides were stained with secondary antibody,
followed by incubation with diaminobenzidine kit for 10 min,
hematoxylin staining, vitrification with dimethylbenzene, and
coverslip with mounting medium. The Rap1 and caspase-3 expression
were assessed at the center and the invasive front of the tumor in
each section. A positive Rap1 expression was defined as nuclear
staining at both the cancer central and invasive front. A positive
caspase-3 expression was defined as cytoplasmic staining.
Statistical analysis
Data are presented as the means ± SD. Statistical
analysis was performed using the SPSS version 14. The differences
were evaluated by t-test. P-values <0.05 were considered to
indicate a statistically significant result.
Results
Expression of Rap1 in HCC cell lines
To determine the effect of Rap1 expression on the
apoptosis in HCC cells, we first determined the expression of Rap1
in four HCC cell lines and normal liver cells by quantitative
RT-PCR. Quantitative RT-PCR revealed that expression of Rap1 was
significantly higher in HCC cell lines HuH-7, SMMC-7721, QGY-7703
and HepG2 as compared with normal liver HL-7702 cells. Given the
relative high expression of Rap1 in HepG2 cells, this cell line was
selected for subsequent experiments to determine whether inhibition
of Rap1 expression by miRNA would enhance cells apoptosis.
Effects of Rap1 expression on apoptosis
in HepG2 cells
To investigate the anti-apoptotic role of Rap1 in
HCC cells, we knocked down the expression of Rap1 in HepG2 using
RNA interference (RNAi) techniques with miRNA. Firstly, Rap1
protein levels were evaluated in transfected and control HepG2
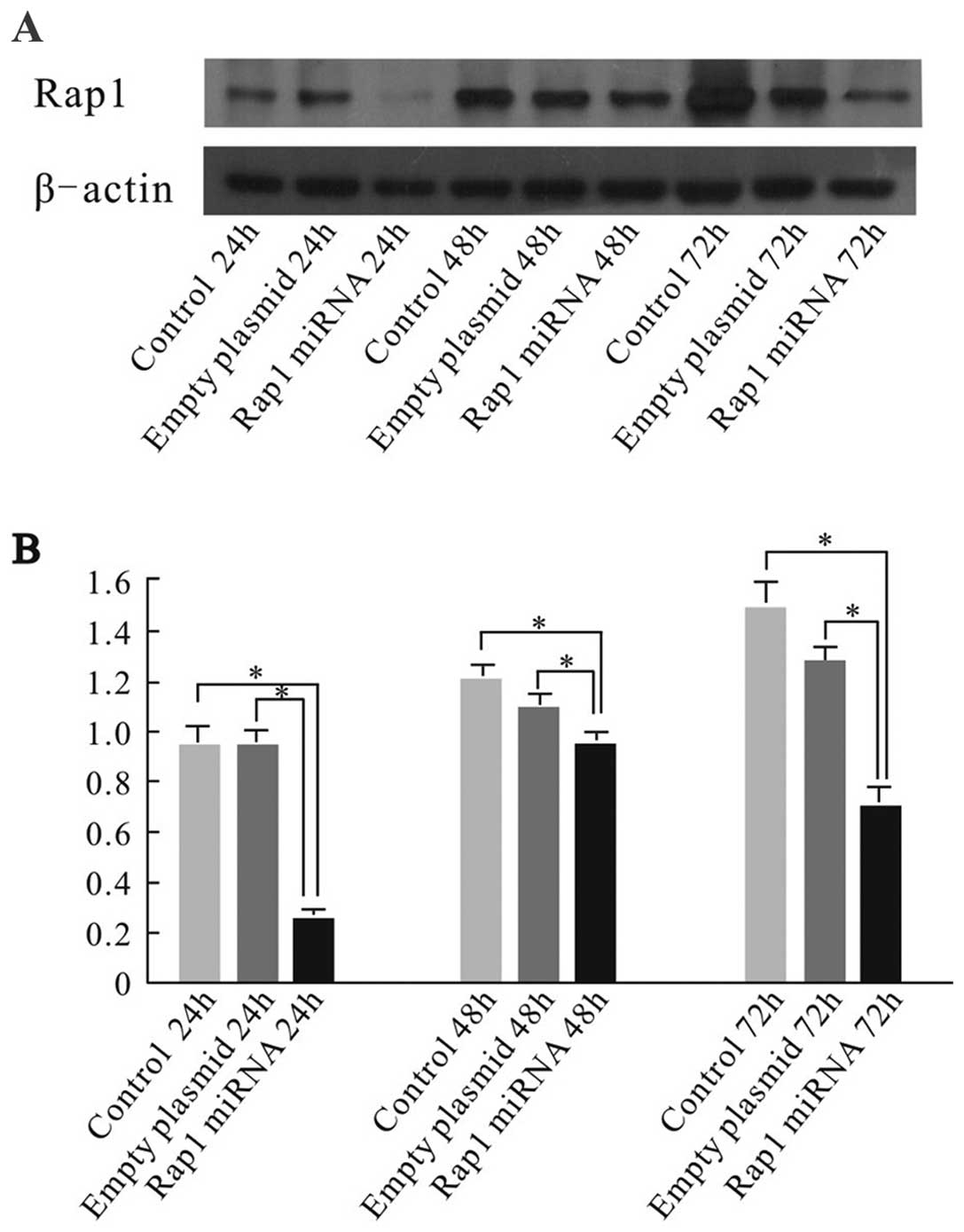
cells by western blot analysis to test RNAi efficiency. The level
of Rap1 expression in Rap1 miRNA-transfected cells was reduced
significantly compared with those in empty plasmid transfected
group, and control group at 24, 48 and 72 h after the transfections
(Fig. 1). The results suggested
that miRNA transfection led to specific suppression of Rap1 protein
expression.
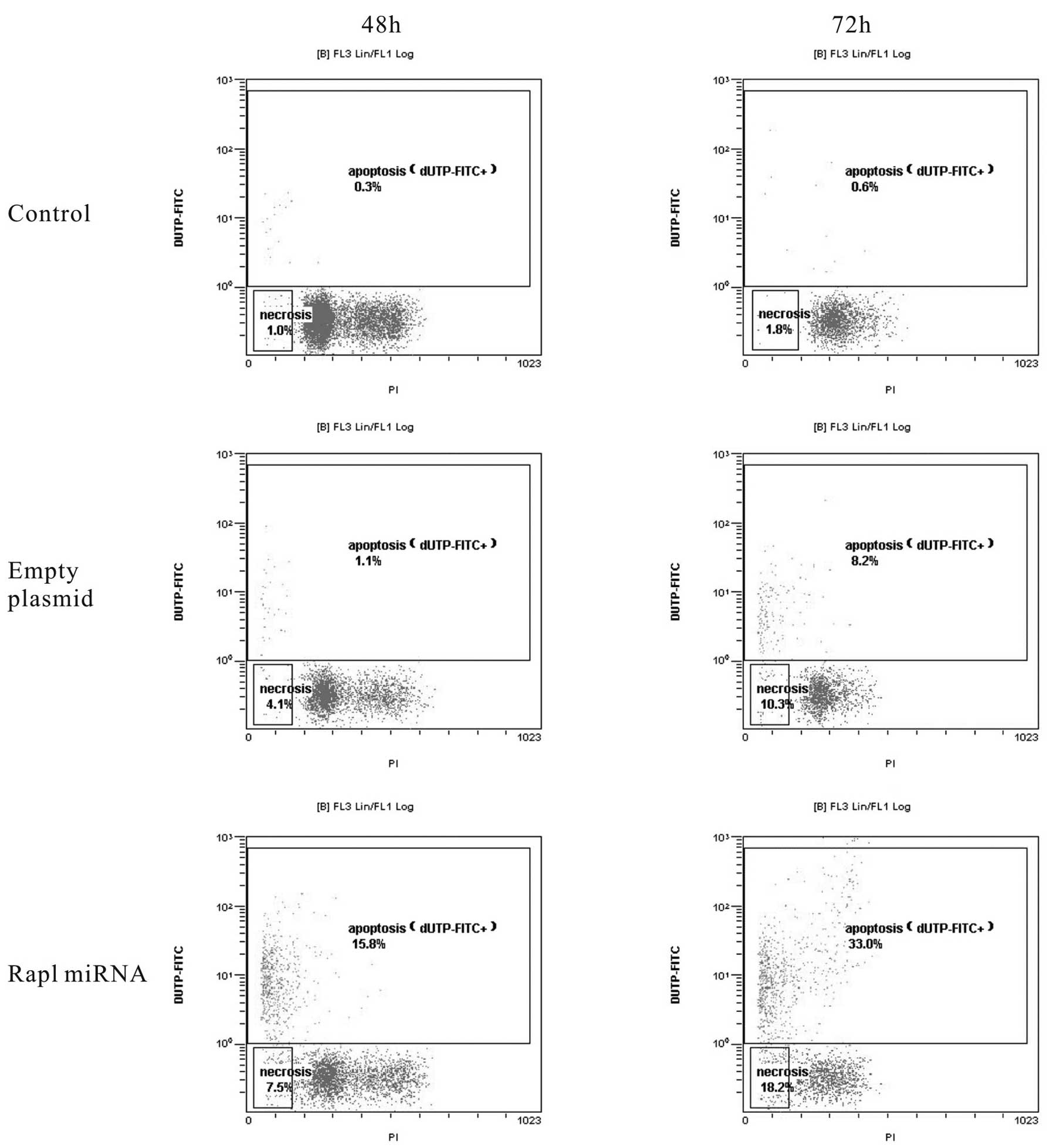
The anti-apoptotic effect of Rap1 in HepG2 cells was
subsequently confirmed by TUNEL assay and flow cytometric analysis.
The flow cytometric analysis revealed that of Rap1 knockdown cells
had a significantly higher rate of apoptosis at 48 and 72 h after
the transfection when compared with empty plasmid group and control
group (P<0.05). At 48 h, 15.8±0.5% of adherent cells were
apoptotic in Rap1 miRNA group, whereas 1.1±0.2% and 0.3±0.1% of
adherent cells were apoptotic in empty plasmid group and control
group, respectively. At 72 h, Rap1 miRNA induced apoptosis in
~33.0±2.3% of the cells, empty plasmid induced apoptosis in only
8.2±1.6% of the cells compared with 0.6±0.1% cell apoptosis in
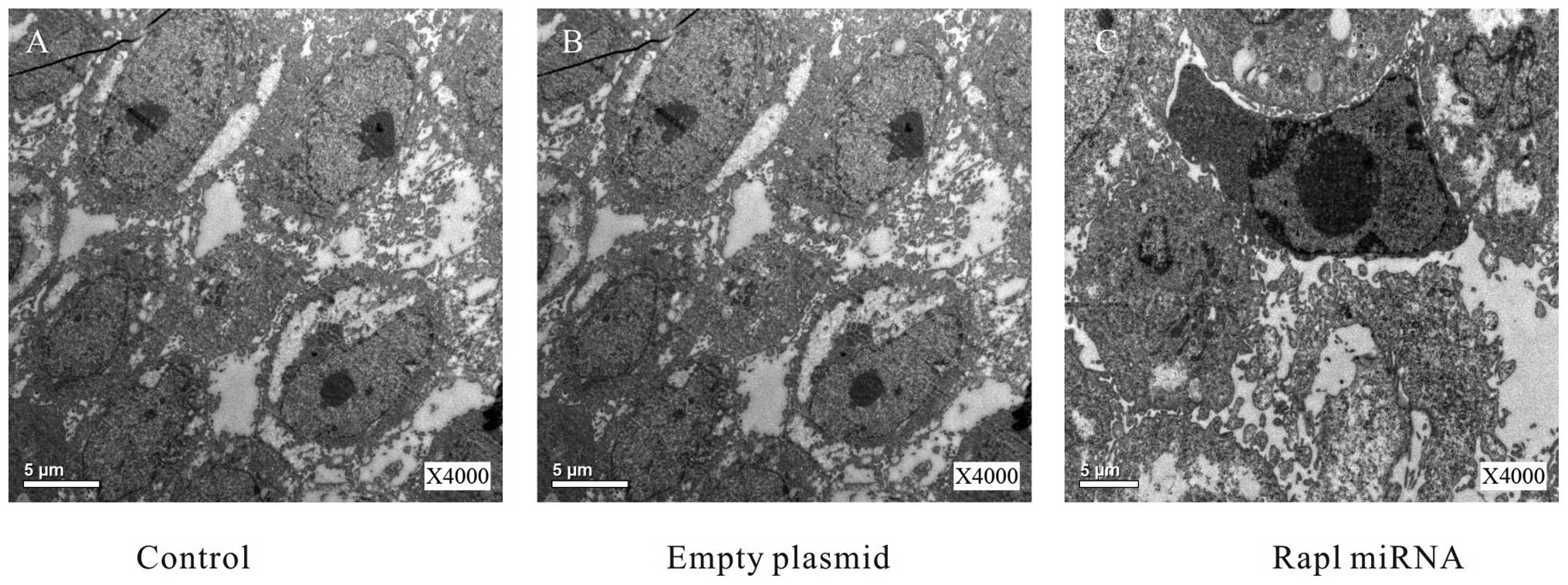
control group (Fig. 2). To gain
further information about the morphological changes, the cells from
the three groups were evaluated of apoptosis with electron
microscopy at 48 h after the transfection. Electron microscopy
revealed that some shrinking cells with a ring of condensed
chromatin at the interior surface of the nuclear envelope in Rap1
miRNA group (Fig. 3), which was
consistent with the result of flow cytometric analysis. Take
together, these results suggested Rap1 miRNA increased apoptosis in
HepG2 cells.
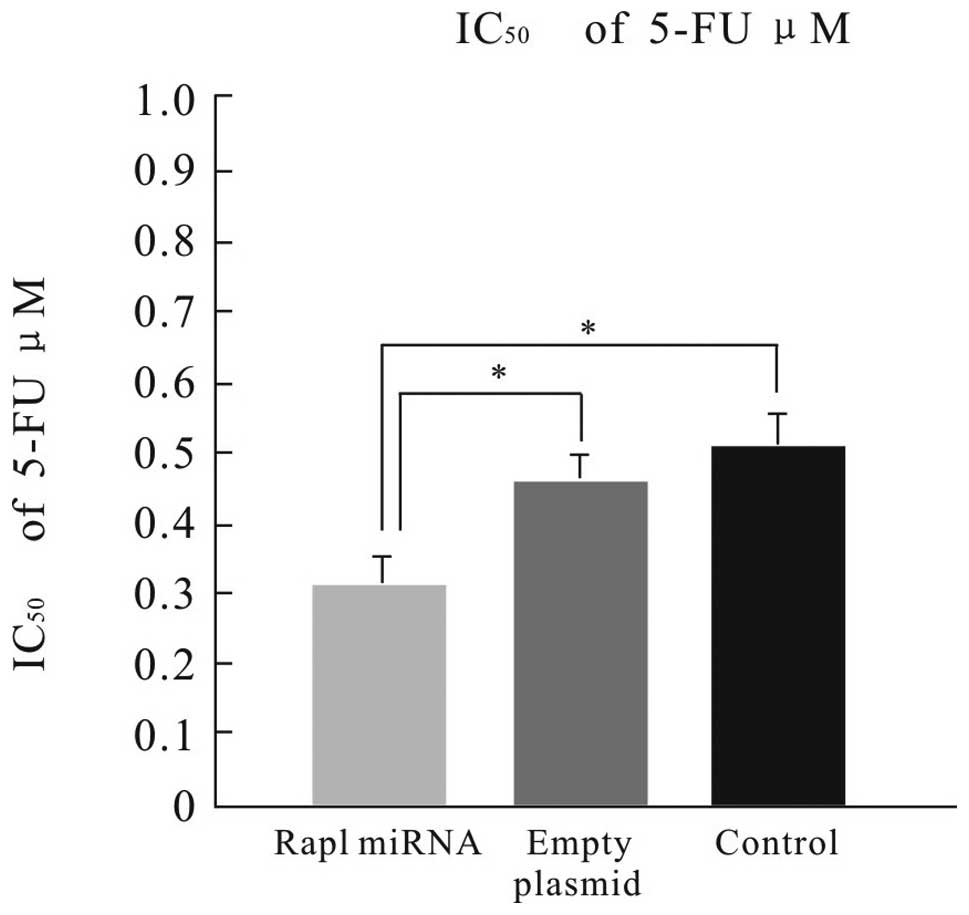
Next, we investigated whether Rap1 miRNA would
enhance chemosensitivity to 5-FU in HepG2 cells. HepG2 cells were
treated with 0, 0.1, 0.2, 0.3, 0.4, 0.5 or 0.6 μM 5-FU for 24 at 48
h after the transfection. The cell viability was evaluated using
the MTT assay. MTT assays revealed the Rap1 miRNA-transfected cells
showed increased sensitivity to 5-FU as compared with the empty
plasmid-transfected, and the control cells (Fig. 4).
Suppression of Rap1 increases cell
apoptosis through NF-κB signaling in HepG2 cells
We next investigated the mechanism responsible for
the anti-apoptotic effect of Rap1 in HepG2 cells. We examined the
expression of NF-κB p65 and IκB at mRNA level at 48 h after the
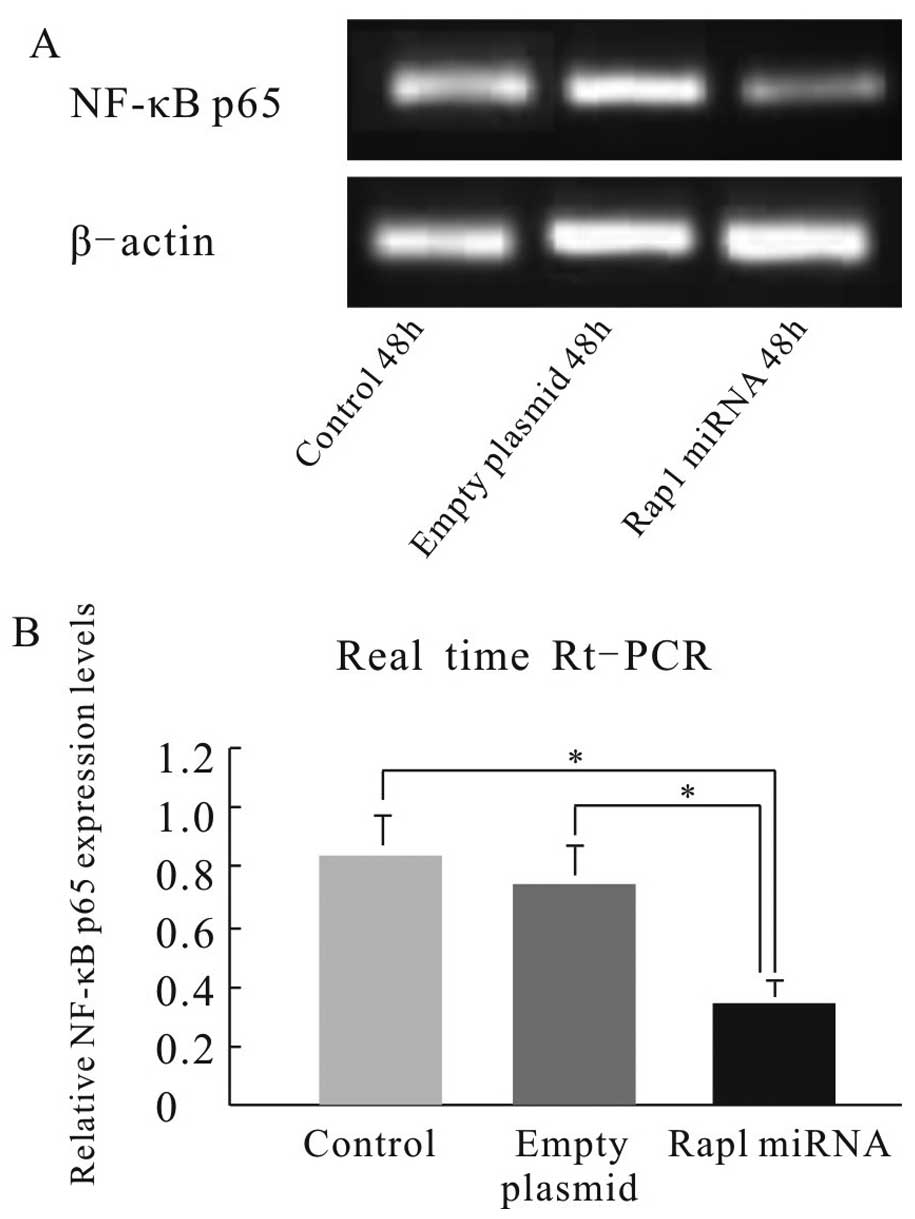
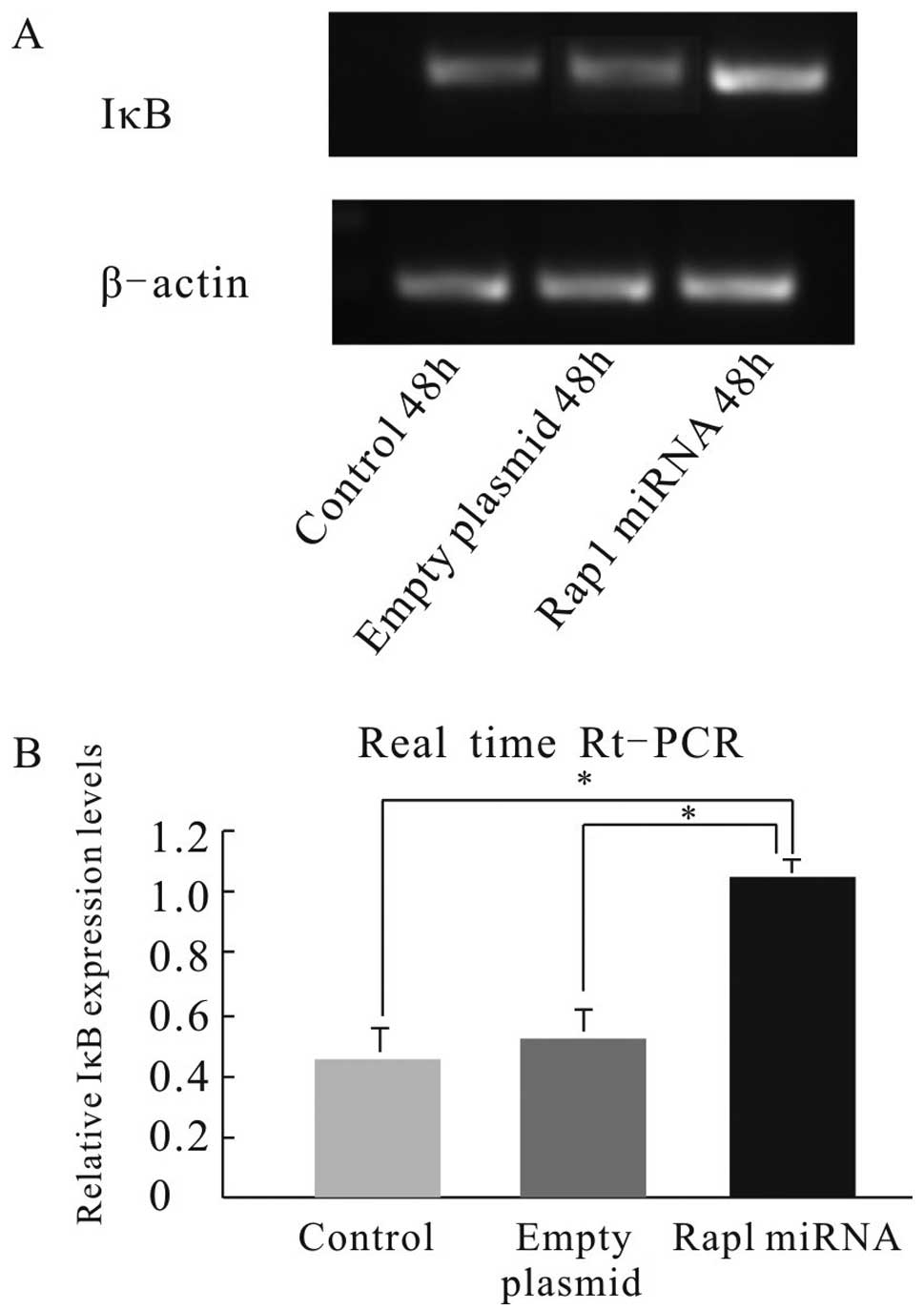
transfection. Quantitative real-time RT-PCR analysis revealed that
the NF-κB p65 mRNA was downregulated significantly, whereas the IκB
mRNA was greatly upregulated in Rap1 miRNA-transfected cells
compared with those in control cells (P<0.05) (Figs. 5 and 6), suggesting that NF-κB was suppressed in
Rap1 miRNA-transfected HepG2 cells. These results indicate that
Rap1 plays an anti-apoptotic role via the NF-κB/IκB pathway.
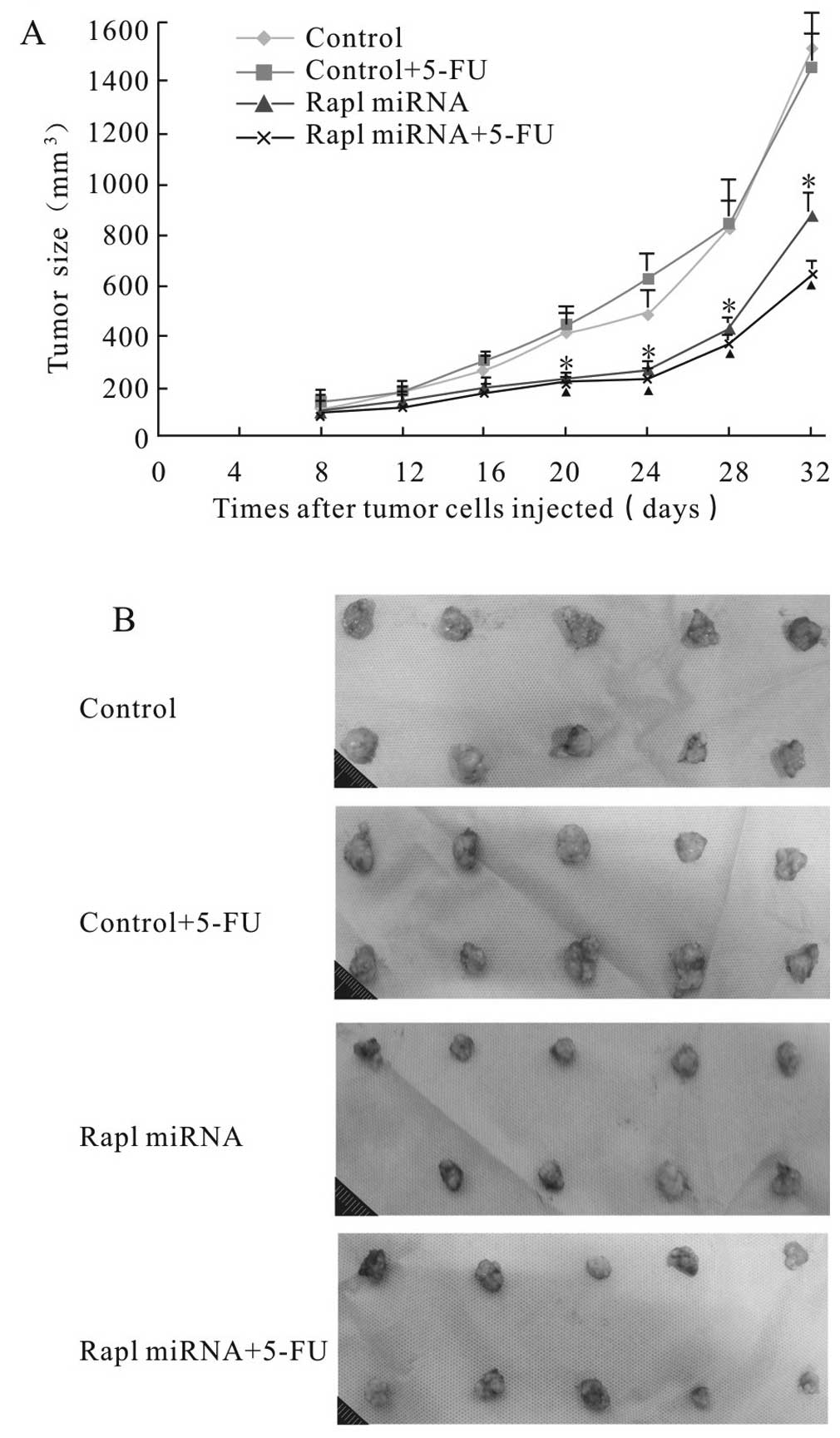
Rap1 miRNA reduces HepG2 tumor growth in
vivo
To determine the efficacy of Rap1 miRNA alone or
combined with 5-FU in vivo, we used a BALB/c murine
xenograft tumor model with Rap1-deleted and control HepG2 cells.
The size of the tumors was measured in three dimensions twice a
week for 3 weeks, and the tumor volume was calculated. Mean tumor
volume in Rap1-miRNA group was 851.2±32.6 mm3 compared
to the average tumor volume of 1494.7±126.2 mm3 in
control group at the termination of the experiment (P<0.05).
Rap1 miRNA combined with 5-FU treatment led to a reduction of tumor
growth even further as compared with 5-FU alone (624.9±45.3
mm3 vs. 1424.0±108.6 mm3; P<0.05)
(Fig. 7). The results suggested
that Rap1 miRNA inhibited liver cancer progression and enhanced
sensitivity of HepG2 cells to 5-FU in vivo.
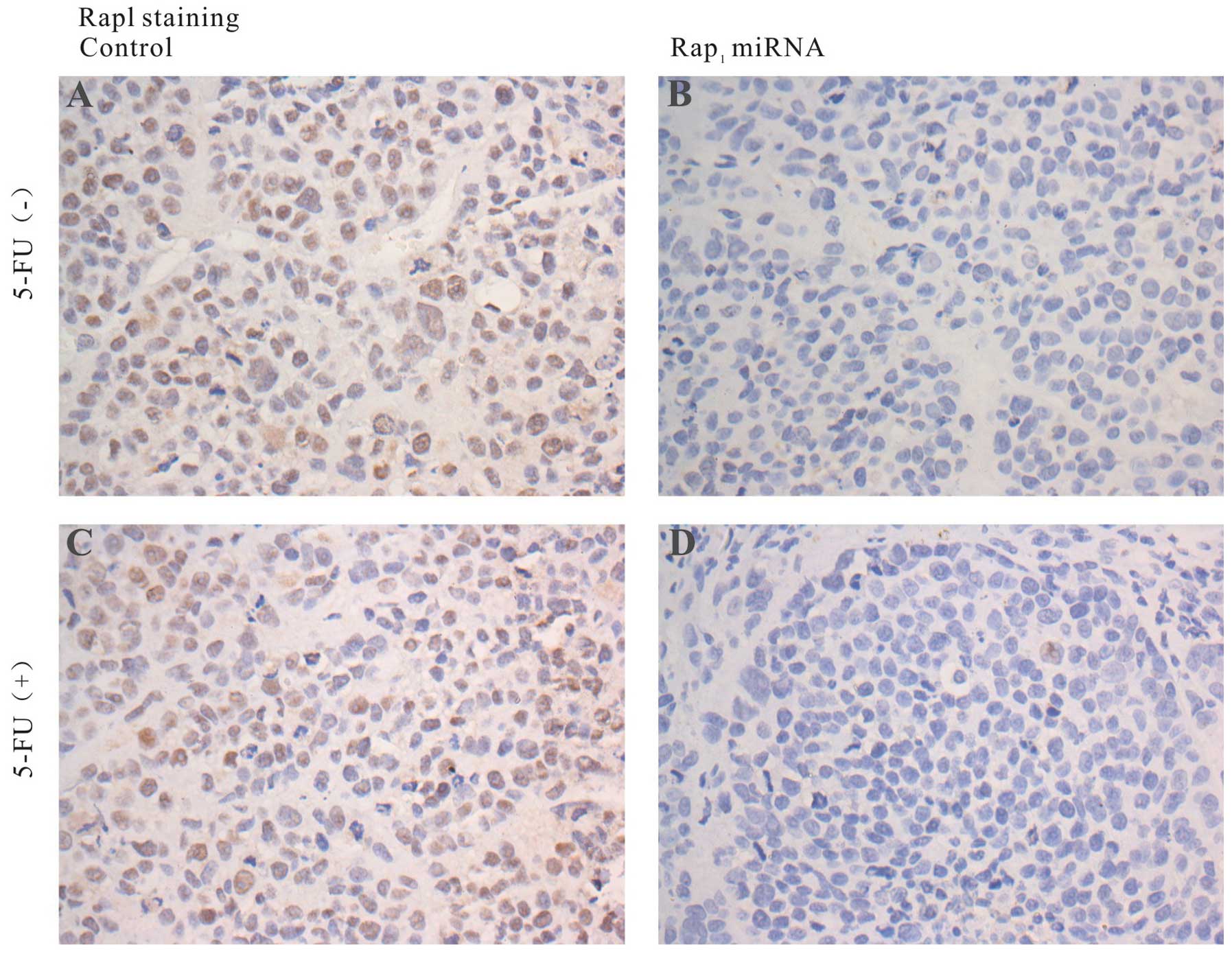
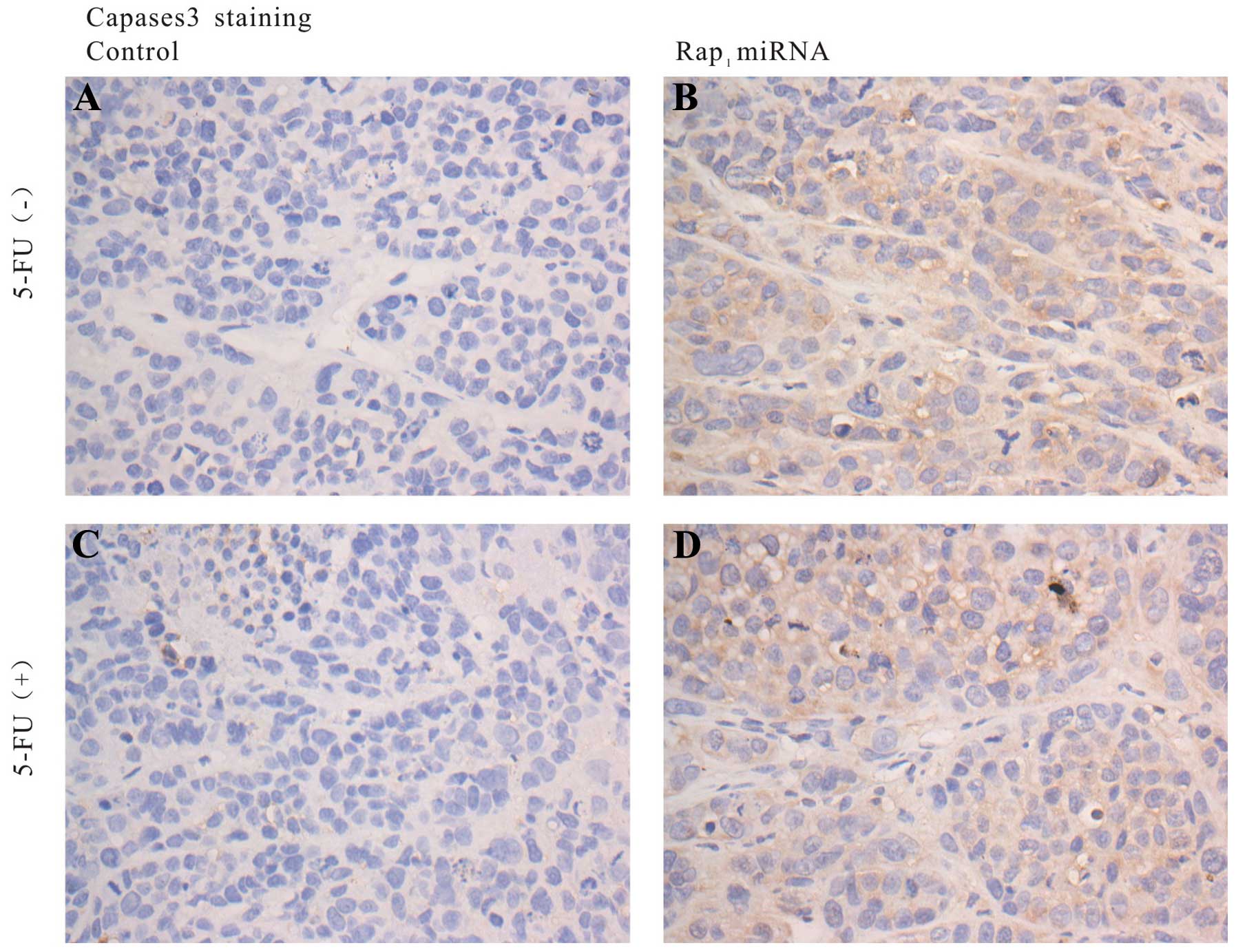
We also investigated the expression of Rap1 and
caspase-3 expression using immunohistochemical staining in
harvested tumors. Immunohistochemical staining analyses revealed
that Rap1 expression was significantly inhibited in Rap1 miRNA
tumors, whereas Rap1 overexpression was observed in control tumors
(Fig. 8). Caspase-3 expression was
positive in Rap1 miRNA treated tumors, and negative in the controls
(Fig. 9). The results demonstrated
that knockdown of Rap1 dramatically enhanced caspase-mediated
apoptosis in the mouse model of HCC.
Discussion
Previous studies have demonstrated that arterial
infusion chemotherapy improves the outcome of patients with
advanced HCC (10,11). 5-FU regimen is widely used in
arterial infusion chemotherapy for patients with advanced HCC
(10). However, HCC is highly
resistant to chemotherapy in some patients. Defect in apoptosis is
a principal mechanism of drug resistance in HCC (5). Several studies have revealed that
telomerase activity was associated with increased apoptotic and
chemotherapeutic resistance in HCC (12–14).
The expression of telomeric repeat-binding factor (TRF)1, TRF2 and
TRF1 interacting protein 2 (TIN2) was found to correlate with the
progression of hepatocarcinogenesis, and markedly upregulated from
dysplastic nodules to HCC (15,16).
As a member of telomere-binding proteins, Rap1 plays a role in
maintaining telomere homeostasis and genomic integrity (17). In a recent study, Rap1 signaling was
identified as a crucial factor in TNFα-induced apoptosis in breast
cancers (9). However, the role of
Rap1 on HCC progression and its underlying molecular mechanisms
remain largely unknown. Therefore, in the present study, we
examined the effect of knocking down Rap1 expression on apoptosis
and 5-FU chemosensitivity in HCC cells, and analyzed its
mechanisms.
Rap1 mRNA expression was initially assessed in 4 HCC
cell lines using real-time PCR analysis. Rap1 expression was
detected in all the cell lines. Given the relative high expression
of Rap1 in HepG2 cells, the cells were selected for Rap1 knockdown
assay. Western blot analyses revealed that the expression of Rap1
was significantly suppressed in Rap1 knockdown HepG2 cells compared
with those in the empty plasmid transfected, and control cells at
24, 48 and 72 h after transfection. In vivo,
immunohistochemical analyses revealed that Rap1 expression in
resected tumors was lower in the Rap1-miRNA group than that in
control group, which was consistent with the in vitro
results. The results suggested miRNA interference was effective in
knocking down the expression of Rap1 in vitro and in
vivo.
Subsequently, the effects of Rap1 miRNA on apoptosis
and 5-FU chemosensitivity were investigated in HepG2 cells. The
flow cytometric analysis revealed that the cells in Rap1 knockdown
group had a significantly higher rate of apoptosis at 48 and 72 h
after the transfection compared with those in empty plasmid group
and control group. The pro-apoptotic effect of Rap1 miRNA was
further confirmed by electron microscopy. In vivo, caspase-3
expression in resected tumors was higher in the Rap1-miRNA group
than in control group. The results demonstrated that knockdown of
Rap1 was effective in inducing apoptosis in HepG2 cells.
Rap1 knockdown also resulted in a significant
decrease in cell proliferation rate and an increase in 5-FU
sensitivity in HepG2 cells. MTT assay showed that Rap1 miRNA cells
showed increased sensitivity to 5-FU (IC50=0.31 μM) as
compared with empty plasmid-transfected cells, or control cells. At
the termination of the experiment in vivo, the mice in Rap1
miRNA transfected group had a significant reduction in tumor growth
as compared with the control group. Rap1 miRNA combined with 5-FU
treatment led to a reduction of tumor growth even further as
compared with 5-FU alone. These results are in agreement with those
reported by Teo et al (9)
who found that knockdown of Rap1 by siRNAs significantly sensitized
MCF7 cells to TNFα-induced and adriamycin-induced apoptosis.
Collectively, the findings suggest that increased Rap1 expression
plays an anti-apoptotic role in HCC cells, inhibition of Rap1 with
miRNA enhanced apoptosis and 5-FU sensitivity both in vitro
and in vivo xenografts.
We found that knockdown of Rap1 resulted in
increased NF-κB p65 and decreased IκB mRNA levels in HepG2 cell
line. Similarly, Teo et al found that there was a positive
correlation between high levels of cytoplasmic Rap1 and high levels
of nuclear NF-κB in breast cancer (9). NF-κB is a transcription factor with
five subunits: RelA (p65), RelB, c-Rel, p50/NF-κB1, and p52/NF-κB2.
The heterodimeric complexe of p50/p65 (RelA) is predominantly
detected in the cells. NF-κB dimers are complexed with the
inhibitory protein IκB and maintained in an inactive state in most
cell types (18). Previous studies
have demonstrated that 5-FU induces apoptosis, and activate the
NF-κB pathway in colon carcinoma and HCC models (19,20).
Activated NF-κB pathway is known to play a vital role in apoptosis
resistance in various malignancies including HCC through regulation
of its downstream target genes (18,21).
Inhibiting NF-κB can increase 5-FU sensitivity in HCC (20). Some studies demonstrated NF-κB
induced the expression of inhibitor of apoptosis protein-1 (c-IAP1)
and c-IAP2. NF-κB also upregulates the expression of Bcl-2 family
(such as Bcl-2 and Bcl-xL), which plays a pivotal role in the
inhibition of apoptosis (8,21). It is likely that downstream gene
targets of NF-κB are involved in Rap1-related apoptosis mechanism
in HCC cells. Rap1 promotes cell survival through an
NF-κB-dependent pathway in HCC.
In summary, the present study revealed that the
combined Rap1 inhibition and chemotherapy inhibited tumor
development and induced higher apoptosis rates than chemotherapy
alone and control group in HepG2 cells. Our data suggest that
inhibition of Rap1 is a promising target of therapeutic approaches
to render HCC cells more sensitive to apoptosis and chemotherapy.
Targeting of Rap1 via RNA interference is a potential therapeutic
strategy.
Acknowledgements
The present study was supported by grants from the
Applied Basic Research Programs of Science and Technology
Commission Foundation of Yunnan Province (no. 2011FB064 and no.
2012FB065). The authors thank Dr Eduardo Briceño for reviewing the
manuscript.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang ZY, Ye SL, Liu YK, et al: A decade’s
studies on metastasis of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 130:187–196. 2004.
|
|
3
|
Padhya KT, Marrero JA and Singal AG:
Recent advances in the treatment of hepatocellular carcinoma. Curr
Opin Gastroenterol. 29:285–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sankari SL, Masthan KM, Babu NA, et al:
Apoptosis in cancer - an update. Asian Pac J Cancer Prev.
13:4873–4878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu J, Hu D and Zhang R: Depletion of Bmi-1
enhances 5-fluorouracil-induced apoptosis and autophagy in
hepatocellular carcinoma cells. Oncol Lett. 4:723–726.
2012.PubMed/NCBI
|
|
6
|
Wang H and Cho CH: Effect of NF-κB
signaling on apoptosis in chronic inflammation-associated
carcinogenesis. Curr Cancer Drug Targets. 10:593–599. 2010.
|
|
7
|
Gocho T, Uwagawa T, Furukawa K, et al:
Combination chemotherapy of serine protease inhibitor nafamostat
mesilate with oxaliplatin targeting NF-κB activation for pancreatic
cancer. Cancer Lett. 333:89–95. 2013.PubMed/NCBI
|
|
8
|
Monks NR, Biswas DK and Pardee AB:
Blocking anti-apoptosis as a strategy for cancer chemotherapy:
NF-κB as a target. J Cell Biochem. 92:646–650. 2004.PubMed/NCBI
|
|
9
|
Teo H, Ghosh S, Luesch H, et al:
Telomere-independent Rap1 is an IKK adaptor and regulates
NF-κB-dependent gene expression. Nat Cell Biol. 12:758–767.
2010.PubMed/NCBI
|
|
10
|
Kaseb AO, Shindoh J, Patt YZ, et al:
Modified cisplatin/interferon α-2b/doxorubicin/5-fluorouracil
(PIAF)chemotherapy in patients with no hepatitis or cirrhosis is
associated with improved response rate, resectability, and survival
of initially unresectable hepatocellular carcinoma. Cancer.
119:3334–3342. 2013.
|
|
11
|
Yamashita T: Current status of
hepatocellular carcinoma treatment in Japan: hepatic arterial
infusion chemotherapy. Clin Drug Investig. 32(Suppl 2): 15–23.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spallarossa P, Altieri P, Aloi C, et al:
Doxorubicin induces senescence or apoptosis in rat neonatal
cardiomyocytes by regulating the expression levels of the telomere
binding factors 1 and 2. Am J Physiol Heart Circ Physiol.
297:H2169–H2181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato M, Nakayama M, Agata M, et al: Gene
expression levels of human shelterin complex and
shelterin-associated factors regulated by the topoisomerase II
inhibitors doxorubicin and etoposide in human cultured cells.
Tumour Biol. 34:723–733. 2013. View Article : Google Scholar
|
|
14
|
Biroccio A, Rizzo A, Elli R, et al: TRF2
inhibition triggers apoptosis and reduces tumourigenicity of human
melanoma cells. Eur J Cancer. 42:1881–1888. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh BK, Jo Chae K, Park C, et al: Telomere
shortening and telomerase reactivation in dysplastic nodules of
human hepatocarcinogenesis. J Hepatol. 39:786–792. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oh BK, Kim YJ, Park C, et al:
Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is
related to telomere shortening during human multistep
hepatocarcinogenesis. Am J Pathol. 166:73–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Connor MS, Safari A, Liu D, et al: The
human Rap1 protein complex and modulation of telomere length. J
Biol Chem. 279:28585–28591. 2004.PubMed/NCBI
|
|
18
|
Hayden MS and Ghosh S: Shared principles
in NF-κB signaling. Cell. 132:344–362. 2008.
|
|
19
|
Körber MI, Klingenbrunner S, Bartsch R, et
al: NF-κB addiction and resistance to 5-fluorouracil in a
multi-stage colon carcinoma model. Int J Clin Pharmacol Ther.
51:35–37. 2013.
|
|
20
|
Zhang H, Ozaki I, Hamajima H, et al:
Vitamin K2 augments 5-fluorouracil-induced growth inhibition of
human hepatocellular carcinoma cells by inhibiting NF-κB
activation. Oncol Rep. 25:159–166. 2011.PubMed/NCBI
|
|
21
|
Cheng Q, Lee HH, Li Y, et al: Upregulation
of Bcl-x and Bfl-1 as a potential mechanism of chemoresistance,
which can be overcome by NF-κB inhibition. Oncogene. 19:4936–4940.
2000.PubMed/NCBI
|