Introduction
Even after decades of research investments, ovarian
cancer remains the most lethal gynecological malignancy. The 5-year
survival rate is only 44% in the USA (1). Due to histologic and molecular
heterogeneity, epithelial ovarian cancer (EOC) is not usually
considered a single entity. EOC can be divided into four major
subtypes: serous, mucinous, endometrioid and clear cell
adenocarcinomas. Serous adenocarcinoma is the most common among the
subtypes and consists of low-grade and high-grade carcinomas. Most
ovarian cancer deaths are associated with high-grade serous ovarian
carcinoma (HG-SOC) (2), which
originates from the fallopian tube fimbriae according to recent
studies (3–7). Metastasis is the major cause for the
high mortality rate. Therefore, it is crucial to clarify the
molecular mechanisms that influence the metastasis of HG-SOC.
Epithelial-mesenchymal transition (EMT) is known as
a key regulatory mechanism of migration and invasion in many types
of cancers including ovarian cancer (8,9). EMT
is a morphological change where cells switch from a polarized
epithelial phenotype to a highly motile mesenchymal phenotype
(10). During the process of EMT,
cells lose epithelial adhesion molecules (such as E-cadherin) and
acquire mesenchymal markers (such as N-cadherin), with increased
migration and invasion. Several transcriptional factors such as
Twist, ZEB and Snail have been reported as inducers of EMT
(11). Among these factors, the
zinc-finger E-box binding homeobox 1 (ZEB1) is known to be
overexpressed in ovarian cancer and may directly repress the
epithelial marker E-cadherin to induce EMT (12).
MicroRNAs (miRNAs) are a class of small (~22
nucleotides) non-coding RNAs that negatively modulate gene
expression in a sequence-specific manner, and they are conserved in
evolution (13). miRNAs play
important roles in numerous biological processes, such as cell
cycle, differentiation, proliferation, apoptosis and angiogenesis
(14–16). The relationship between miRNAs and
cancer was first discovered in chronic lymphocytic leukemia
(17). Since then, numerous miRNAs
have been found abnormally expressed in many types of cancers
including ovarian cancer (18). EMT
was also found to be modified by many miRNAs, such as the miR-200
family, miR-23b, miR-29b and miR-150 (19–23).
In the present study, we examined the expression of miR-1236-3p in
HG-SOC and normal fallopian tube tissues. We found that miR-1236-3p
was downregulated in HG-SOC. We then investigated the effect of
miR-1236-3p on tumor metastasis and showed that miR-1236-3p
overexpression suppressed the migration and invasion of ovarian
cancer cells by targeting EMT-inducer ZEB1.
Materials and methods
Patients and tissue samples
The present study consisted of 20 samples of HG-SOC
and 12 normal fallopian tube (FT) tissues. All samples were
collected between March and June 2013 at Qilu Hospital during
surgery and were immediately stored at −80°C. For the use of these
samples, signed informed consent was obtained. All the diagnoses
were confirmed by at least two pathologists.
Cell lines and culture conditions
Human ovarian cancer cell lines A2780 and SKOV3 were
originally obtained from the American Type Culture Collection
(ATCC, Rockville, MD, USA). Roswell Park Memorial Institute
(RPMI)-1640 medium was purchased from Gibco-BRL (Rockville, MD,
USA). Fetal bovine serum (FBS) was supplied by Haoyang Biological
Manufacture Co. Ltd. (Tianjin, China). The human ovarian cancer
cell lines A2780 and SKOV3 were cultured in RPMI-1640 medium
supplemented with 10% FBS and 100 U penicillin-streptomycin at 37°C
in a humidified atmosphere containing 5% CO2.
Synthesis and transfection of miRNA
mimics and miRNA inhibitors
miRNA mimics and miRNA inhibitors (2′-O-methyl
modified) for in vitro transfection and its negative control
(miR-nc) were designed and synthesized by RiboBio (Guangzhou,
China). A2780 (3.5×105) and SKOV3 (3.0×105)
cells were seeded into 6-well plates and incubated overnight.
Transfection was performed using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
After 6 h, the medium was replaced with fresh medium. During
transfection, the medium was antibiotic-free.
Dual-luciferase reporter assay
The 3′-untranslated region (3′UTR) of ZEB1 mRNA
containing the putative miR-1236-3p binding site was cloned into
the pGL3 vector according to the manufacturer’s instructions. The
forward primer (5′-CGAGCT CGACAGCACAGAGCAGGAA-3′) and the reverse
primer (5′-CCCTCGAGTAGTTAGCACGGGTTGGA-3′) were used to amplify the
3′UTR of ZEB1. The forward primer contained a Sac1
restriction site and the reverse primer contained an Xho1
site. The binding site of miR-1236-3p in the ZEB1 3′UTR was mutated
by using primers: F, 5′-CAGGCGCTTAAAGG AAGCTGATTAAT-3′ and R,
5′-AGCGCCTGTATTGTTGC TCTCTGAGT-3′. SKOV3 cells were plated at
1.5×104/well in a 96-well plate one day before
transfection. Cells were co-transfected with 50 ng of wild-type or
mutant ZEB1 3′UTR, and 5 pmol of miR-1236-3p mimics or negative
control. After 48 h, luciferase activity was measured using the
Dual-Luciferase Reporter assay system (Promega).
RNA extraction and quantitative real-time
PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen) according to the manufacturer’s protocol. Then
cDNA was synthesized using the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). Quantitative real-time PCR
(qRT-PCR) was performed with the SYBR Premix Ex Taq (Takara) by
using the Applied Biosystems StepOnePlus™ Real-Time PCR System
according to the manufacturer’s protocol.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
endogenous control.
Western blot analysis
The cells were washed 3 times with PBS chilled to
4°C and lysed on ice in RIPA buffer (Shenneng Bocai, Shanghai,
China) containing protease inhibitors (1 mM). The density of the
protein was measured using the BCA protein assay kit (Merck,
Darmstadt, Germany). The same amount of protein was separated by
5–10% SDS-PAGE and then transferred to a PVDF membrane (Millipore)
using a semi-dry blotting apparatus (Bio-Rad Laboratories,
Hercules, CA, USA). Membranes were blocked with 5% (w/v) non-fat
milk in Tris-buffered saline with Tween-20 (TBST) (100 mM NaCl, 50
mM Tris and 0.1% Tween-20) at room temperature for 1 h. After the
blocking step, the membranes were incubated overnight with primary
antibodies (Cell Signaling Technology, Beverly, MA, USA) at 4°C.
The membranes were then washed 3 times with TBST and incubated with
horseradish peroxidase-labeled secondary antibodies for 2 h.
Signals were detected with an ECL system (Merck) according to the
manufacturer’s instructions. GAPDH was used as the loading
control.
Invasion and migration assays
Invasion assays were performed in a Transwell system
(24-wells, 8-μm pore size) coated with 2 mg/ml of Matrigel (both
from BD Biosciences, Bedford, MA, USA). First, the cells were
transfected with miR-1236-3p. After 48 h, the transfected cells
were digested with trypsin, and 1.0×105 cells were
suspended in serum-free RPMI-1640 and seeded into the upper chamber
of each well. The lower chamber was filled with RPMI-1640
supplemented with 20% FBS. Then the cells were fixed with
formaldehyde, permeabilized with 100% methanol and stained with
0.5% crystal violet. The number of cells that had attached to the
lower surface of the membrane were counted in 6 random fields under
a light microscope and analyzed statistically. The cell migration
assays were performed in a similar manner, except that the upper
chambers were Matrigel-free.
Statistical analysis
All data are expressed as means ± standard deviation
(SD). Student’s t-test (paired, 2-tail) was employed to analyze the
significance of two groups. P-value <0.05 was considered to
indicate a statistically significant result. All of the experiments
were repeated at least 3 times.
Results
Expression of miR-1236-3p is
downregulated in HG-SOC
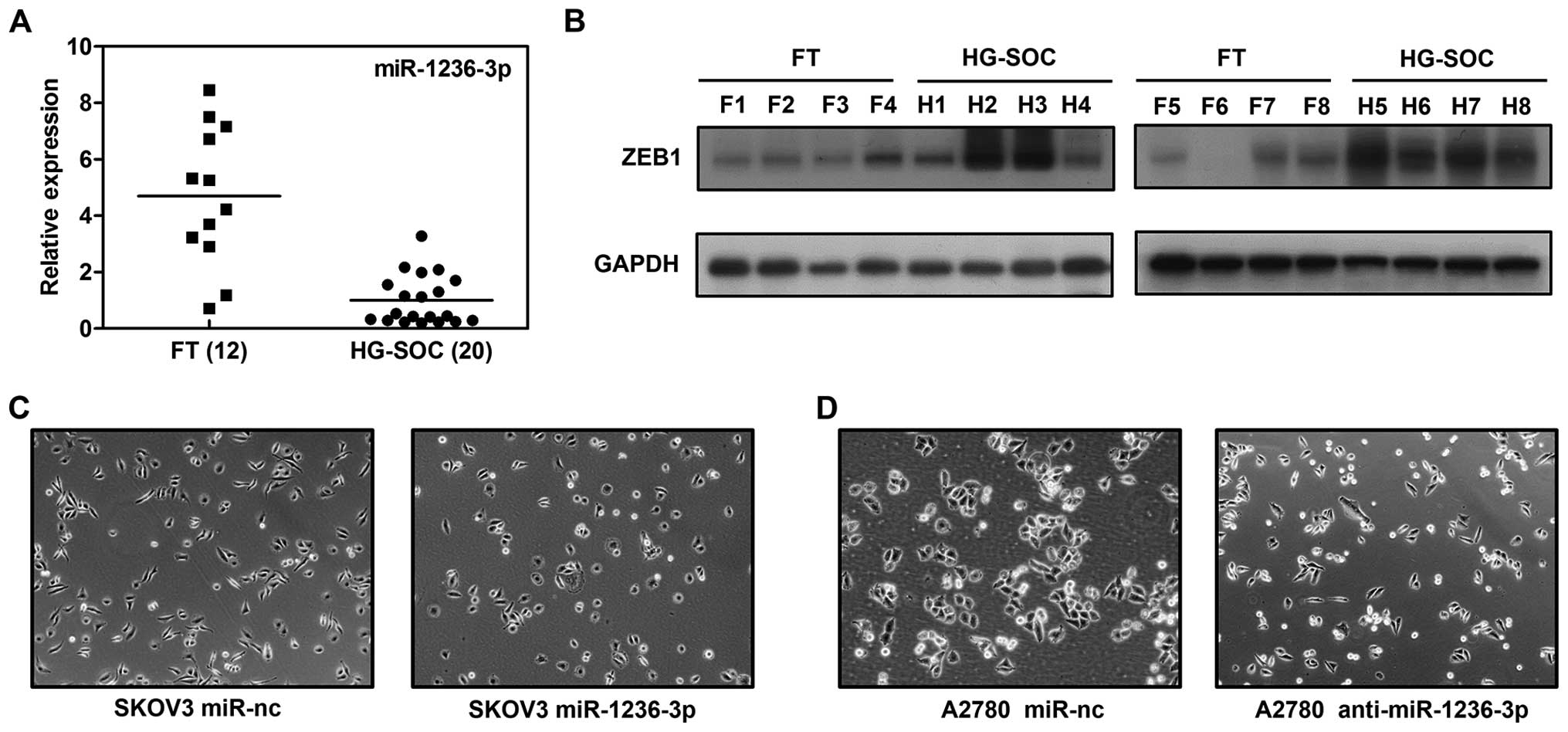
To confirm the expression of miR-1236-3p in HG-SOC
tissue samples, we employed qRT-PCR to quantify and compare the
miR-1236-3p expression levels in the HG-SOC (n=20) and FT (n=12)
tissue samples. As shown in Fig.
1A, the expression of miR-1236-3p was generally decreased in
the HG-SOC when compared to the FT samples (P<0.001). This
suggests that miR–1236-3p acts as a tumor suppressor in HG-SOC.
Manipulation of miR-1236-3p-induced
morphological change in SKOV3 and A2780 cells
To explore the influence of miR-1236-3p on ovarian
cancer cells, we transfected SKOV3 and A2780 cells with miR-1236-3p
mimics or inhibitors. As shown in Fig.
1C, SKOV3 cells treated with miR-1236-3p mimics presented a
cobblestone-like appearance, while cells treated with the negative
control exhibited a spindle-like morphology. We then inhibited the
expression of miR-1236-3p in A2780 cells. A2780 cells transfected
with the miR-1236-3p inhibitors were narrower than cells
transfected with the negative control (Fig. 1D). As known, the classic
morphological change correlated with EMT is the conversion from a
rounded, epithelial-like form to a spindle-shaped, mesenchymal form
(8,9). These changes indicated that the
manipulation of miR-1236-3p inhibited or stimulated the process of
EMT.
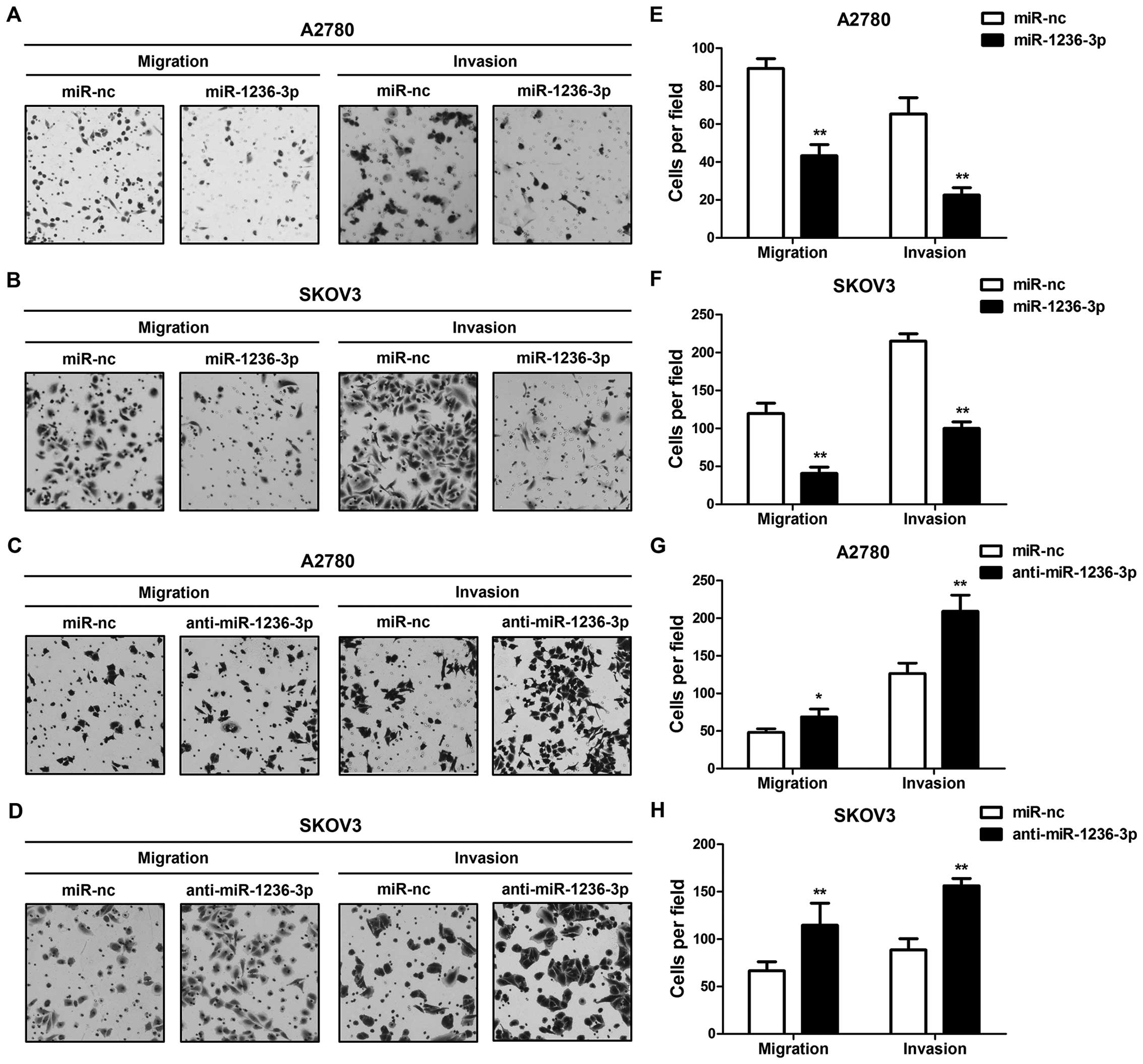
Migratory and invasive abilities of
ovarian cancer cells are regulated by miR-1236-3p
To further confirm the role of miR-1236-3p in
ovarian cancer cells, we used a two-chamber assay to evaluate the
migratory and invasive capacities of SKOV3 and A2780 cells treated
with miR-1236-3p mimics or inhibitors. As shown in Fig. 2, increased miR-1236-3p significantly
suppressed migration and invasion of the ovarian cancer cells. In
contrast, decreased miR-1236-3p promoted these abilities. Our
results showed that miR-1236-3p greatly influenced migration and
invasion of both ovarian cancer cell lines.
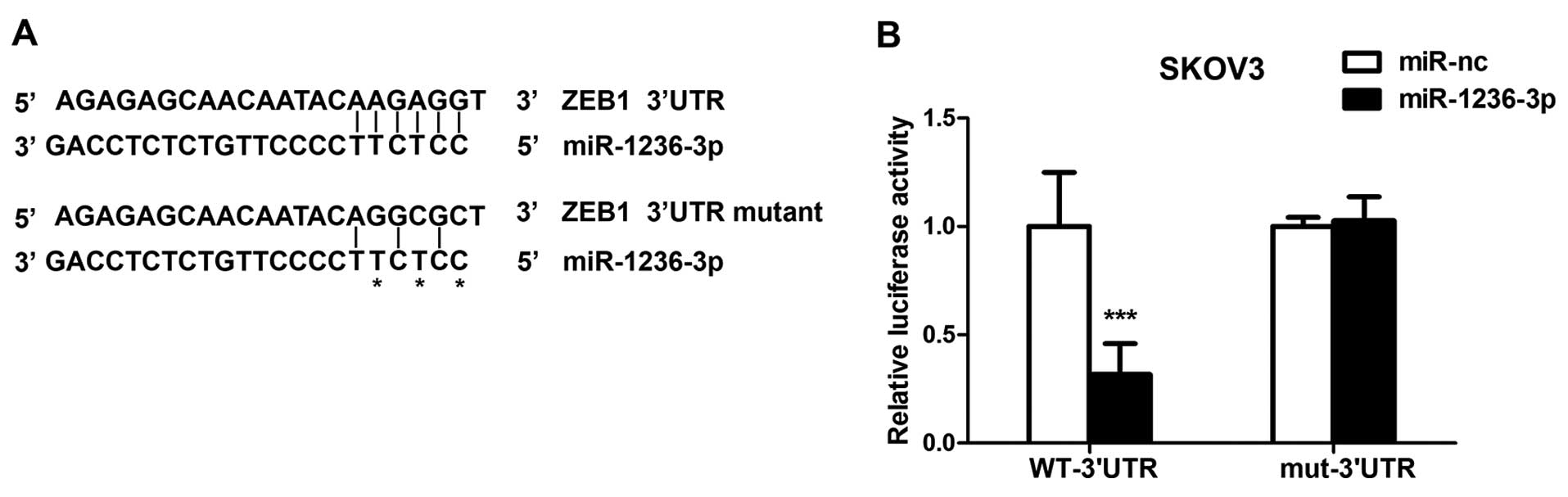
ZEB1 is targeted by miR-1236-3p
Based on the above findings, we hypothesized that
miR-1236-3p regulates genes associated with EMT. Using online miRNA
target prediction tools, such as microRNA.org, we found that the
3′UTR of ZEB1 mRNA contained a putative binding site for
miR-1236-3p. To investigate the ability of miR-1236-3p to bind and
regulate the 3′UTR of ZEB1, we performed the luciferase reporter
assay. Wild-type or the mutant ZEB1 3′UTR sequence was cloned into
the pGL3 vector (Fig. 3A). SKOV3
cells were co-transfected with miR-1236-3p (mimics or negative
control) and the pGL3 vector (wild-type or mutant). After 48 h,
cells were lysed to measure the luciferase activity using the
Dual-Luciferase Reporter assay system. Overexpression of
miR-1236-3p significantly reduced the luciferase activity in the
wild-type (P<0.001) but not the mutant ZEB1 3′UTR (Fig. 3B). This demonstrated that ZEB1 was
directly targeted by miR-1236-3p.
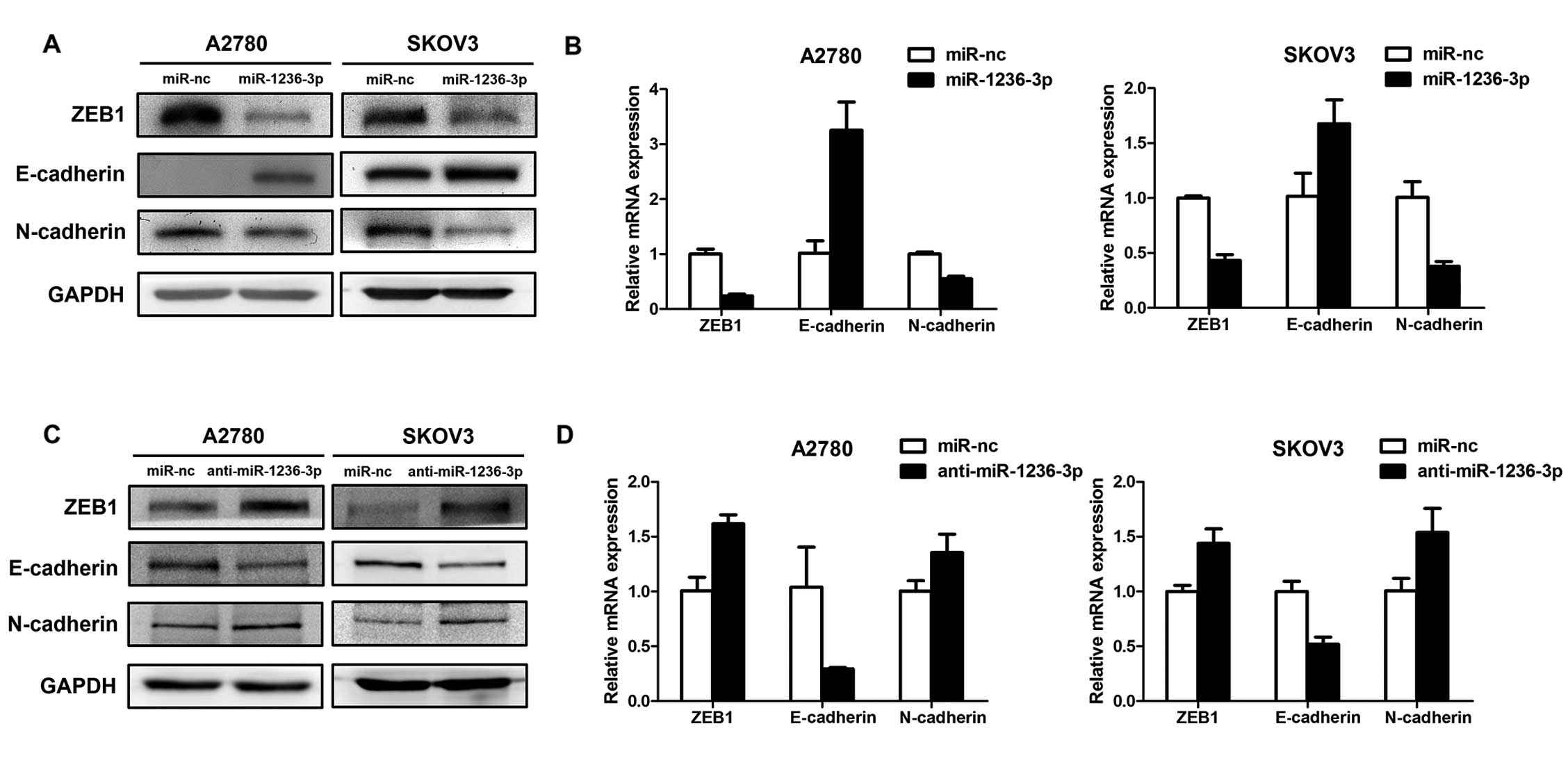
miR-1236-3p regulates the expression of
ZEB1 and EMT-related genes
We then tested whether miR-1236-3p modulates the
expression of ZEB1 and EMT-related genes in ovarian cancer. First,
the SKOV3 and A2780 cell lines were transfected with miR-1236-3p
mimics or miR-nc. The transfected cells were analyzed by qRT-PCR
and western blotting. As shown in Fig.
4A and B, ZEB1 was downregulated by miR-1236-3p mimics at both
the protein and mRNA levels. We also detected the expression of
EMT-related markers E-cadherin and N-cadherin. The results showed
that the expression of E-cadherin was upregulated and the
N-cadherin expression was downregulated. Second, we downregulated
the expression of miR-1236-3p in the two cell lines by using
miR-1236-3p inhibitors. As expected, the expression of ZEB1 and
EMT-related genes showed an opposite pattern at the protein level
(Fig. 4C) and mRNA level (Fig. 4D) compared to the previous assay. As
known, a switch from epithelial marker E-cadherin to mesenchymal
marker N-cadherin is a classical molecular change during EMT
(8,9). Thus, our results indicated that
miR-1236-3p may regulate the process of EMT.
Expression of ZEB1 protein is decreased
in HG-SOC
Finally, we tested whether miR-1236-3p-induced ZEB1
suppression confirmed in our research was clinically relevant. As
shown in Fig. 1B, we found that the
expression of ZEB1 protein was generally upregulated in HG-SOC
(n=8) when compared to that in the FT (n=8) samples, and its
expression was inversely correlated with miR-1236-3p. Taken
together, our findings revealed that miR-1236-3p downregulation
induced overexpression of ZEB1, and consequently influenced
migration and invasion of HG-SOC cells.
Discussion
Early-stage ovarian cancer has few visible symptoms;
therefore, most patients are diagnosed at advanced stages of
disease when cancer cells have already spread (24). Current treatments (surgery,
radiation and chemotherapy) are relatively ineffective for advanced
ovarian cancer. Most of these patients will eventually relapse at
metastatic sites. Thus, it is vital to understand the molecular
mechanisms of metastasis. The present study showed that miR-1236-3p
expression was decreased in HG-SOC tissue samples. Functional
studies demonstrated that decreased miR-1236-3p enhanced ovarian
cancer cell migration and invasion in vitro. These findings
suggest that miR-1236-3p may play a potential inhibitory role in
HG-SOC, and loss of miR-1236-3p may be critical for HG-SOC
metastasis.
According to previous studies, miRNAs are highly
tissue specific and function as tumor suppressors or oncogenes
(25). The diagnostic, therapeutic
and prognostic potential of miRNAs in cancer is promising (26). The miR-200 family plays an important
role in the regulation of EMT by targeting the mRNA of ZEB1 and
ZEB2. E-cadherin expression was also found to be correlated with
miR-200 family expression in tissue samples from ovarian cancer
patients (19,20). miR-1236-3p is reported to be
involved in the regulation of VEGFR-3 and TLR4 (27,28).
Meanwhile, TLR4 and VEGFR-3 are both associated with metastasis and
the poor survival of ovarian cancer patients (29–32).
Therefore miR-1236-3p may be relevant to the prognosis of ovarian
cancer.
EMT facilitates the ability of cancer cells to
detach themselves from primary lesions, migrate to distant organs
or to invade adjacent tissue, and eventually form tumor metastases.
EMT is considered to be the most important step in the progression
of cancer from primary tumors to other organs. Thus, blocking of
EMT is an efficient approach to inhibit the spread of cancer. ZEB1
has been previously reported to be involved in cancer progression,
and is known as an important transcriptional repressors of
E-cadherin. In the present study, we found that miR-1236-3p may
influence the process of EMT in vitro. We demonstrated that
overexpression of miR-1236-3p suppressed ZEB1 expression and the
motility of ovarian cancer cells, while downregulation of
miR-1236-3p promoted them. Based on our findings, further studies
may be required to verify the function of miR-1236-3p in
vivo and whether the expression of miR-1236-3p is associated
with the overall survival of HG-SOC patients.
In conclusion, the present study first demonstrated
that miR-1236-3p directly targets the EMT-inducer ZEB1 and is
downregulated in HG-SOC. Manipulation of miR-1236-3p regulated the
invasion and migration of ovarian cancer cells. In addition, the
present study indicates that miR-1236-3p may be a potential target
for the prognosis and treatment of HG-SOC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China to B.K. (no. 81272857), and the
National High Technology Research and Development Program of China
(863 project, no. 2012AA02A507).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Integrated genomic analyses of ovarian
carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar
|
|
3
|
Levanon K, Crum C and Drapkin R: New
insights into the pathogenesis of serous ovarian cancer and its
clinical impact. J Clin Oncol. 26:5284–5293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Fadare O, Xiang L, Kong B and Zheng
W: Ovarian serous carcinoma: recent concepts on its origin and
carcinogenesis. J Hematol Oncol. 5:82012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Liu J, Segura MF, et al:
MiR-182 overexpression in tumourigenesis of high-grade
serous ovarian carcinoma. J Pathol. 228:204–215. 2012. View Article : Google Scholar
|
|
6
|
Kim J, Coffey DM, Creighton CJ, Yu Z,
Hawkins SM and Matzuk MM: High-grade serous ovarian cancer arises
from fallopian tube in a mouse model. Proc Natl Acad Sci USA.
109:3921–3926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Z, Gersbach E, Zhang X, et al:
miR-106a represses the RB tumor suppressor p130 to regulate
cellular proliferation and differentiation in high-grade serous
ovarian carcinoma. Mol Cancer Res. 11:1314–1325. 2013. View Article : Google Scholar
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vergara D, Merlot B, Lucot JP, et al:
Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett.
291:59–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berx G, Raspé E, Christofori G, Thiery JP
and Sleeman JP: Pre-EMTing metastasis? Recapitulation of
morphogenetic processes in cancer. Clin Exp Metastasis. 24:587–597.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang G, Yuan J and Li K: EMT transcription
factors: implication in osteosarcoma. Med Oncol. 30:6972013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spaderna S, Schmalhofer O, Wahlbuhl M, et
al: The transcriptional repressor ZEB1 promotes metastasis and loss
of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das E, Jana NR and Bhattacharyya NP:
MicroRNA-124 targets CCNA2 and regulates cell cycle in
STHdhQ111/HdhQ111
cells. Biochem Biophys Res Commun. 437:217–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakano H, Yamada Y, Miyazawa T and Yoshida
T: Gain-of-function microRNA screens identify miR-193a regulating
proliferation and apoptosis in epithelial ovarian cancer cells. Int
J Oncol. 42:1875–1882. 2013.PubMed/NCBI
|
|
16
|
Icli B, Wara AK, Moslehi J, et al:
MicroRNA-26a regulates pathological and physiological angiogenesis
by targeting BMP/SMAD1 signaling. Circ Res. 113:1231–1241. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calin GA, Dumitru CD, Shimizu M, et al:
Frequent deletions and down-regulation of micro- RNA genes
miR15 and miR16 at 13q14 in chronic lymphocytic
leukemia. Proc Natl Acad Sci USA. 99:15524–15529. 2002.PubMed/NCBI
|
|
18
|
Dahiya N and Morin PJ: MicroRNAs in
ovarian carcinomas. Endocr Relat Cancer. 17:F77–F89. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bendoraite A, Knouf EC, Garg KS, et al:
Regulation of miR-200 family microRNAs and ZEB transcription
factors in ovarian cancer: evidence supporting a
mesothelial-to-epithelial transition. Gynecol Oncol. 116:117–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Wang L, Matyunina LV, Hill CG and
McDonald JF: Overexpression of miR-429 induces
mesenchymal-to-epithelial transition (MET) in metastatic ovarian
cancer cells. Gynecol Oncol. 121:200–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao M, Seike M, Soeno C, et al: MiR-23a
regulates TGF-β-induced epithelial-mesenchymal transition by
targeting E-cadherin in lung cancer cells. Int J Oncol. 41:869–875.
2012.
|
|
22
|
Ru P, Steele R, Newhall P, Phillips NJ,
Toth K and Ray RB: miRNA-29b suppresses prostate cancer metastasis
by regulating epithelial-mesenchymal transition signaling. Mol
Cancer Ther. 11:1166–1173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yokobori T, Suzuki S, Tanaka N, et al:
MiR-150 is associated with poor prognosis in esophageal
squamous cell carcinoma via targeting the EMT inducer ZEB1.
Cancer Sci. 104:48–54. 2013. View Article : Google Scholar
|
|
24
|
Buys SS, Partridge E, Black A, et al:
Effect of screening on ovarian cancer mortality: the Prostate,
Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized
Controlled Trial. JAMA. 305:2295–2303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sempere LF, Christensen M, Silahtaroglu A,
et al: Altered microRNA expression confined to specific epithelial
cell subpopulations in breast cancer. Cancer Res. 67:11612–11620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schaefer A, Jung M, Mollenkopf HJ, et al:
Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
27
|
Sato K, Yoshimura A, Kaneko T, et al: A
single nucleotide polymorphism in 3′-untranslated region
contributes to the regulation of Toll-like receptor 4 translation.
J Biol Chem. 287:25163–25172. 2012.
|
|
28
|
Jones D, Li Y, He Y, Xu Z, Chen H and Min
W: Mirtron microRNA-1236 inhibits VEGFR-3 signaling during
inflammatory lymphangiogenesis. Arterioscler Thromb Vasc Biol.
32:633–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yokoyama Y, Charnock-Jones DS, Licence D,
et al: Vascular endothelial growth factor-D is an independent
prognostic factor in epithelial ovarian carcinoma. Br J Cancer.
88:237–244. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim KH, Jo MS, Suh DS, et al: Expression
and significance of the TLR4/MyD88 signaling pathway in ovarian
epithelial cancers. World J Surg Oncol. 10:1932012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Y, Huang JM, Zhang GN, Zha X and Deng
BF: Prognostic significance of MyD88 expression by human epithelial
ovarian carcinoma cells. J Transl Med. 10:772012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klasa-Mazurkiewicz D, Jarząb M, Milczek T,
Lipińska B and Emerich J: Clinical significance of VEGFR-2 and
VEGFR-3 expression in ovarian cancer patients. Pol J Pathol.
62:31–40. 2011.PubMed/NCBI
|