Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy
particularly prevalent in the southern Chinese population of
Guangdong, in the Inuit population of Alaska and in native
Greenlanders (1,2). The 5-year survival rates of stage I
and II NPC range from 72 to 90%; however, the 5-year survival rates
of stage III and IV drop to 55 and 30% (3). NPC detection rate and early diagnosis
are major factors adversely affecting the effect of treatment
(4). Finding a biomarker detecting
early stage of NPC remains the most promising to facilitate the
early NPC diagnosis and therapy, probably improving the long-term
survival of patients.
Phosphatase and tensin homolog (PTEN) has been
identified and mapped to chromosome 10q23 (5). Considerable attention has been paid to
the tumor suppressor gene PTEN, since it may suppress tumor cell
growth by antagonizing protein tyrosine kinases and regulate the
first step of tumor cell invasion and metastasis through its
interaction with focal adhesions (5,6). The
loss of function of PTEN leads to increases in cellular
proliferation, survival and growth in many types of cancer
(7). Recombinant PTEN is able to
dephosphorylate phosphatidylinositol 3,4,5-trisphosphate and
phosphatidylinositol 3,4-bisphosphate as well as antagonize the
phosphoinositide 3-kinase pathway (8). Notably, CpG island hypermethylation
has been identified as an alternative mechanism of PTEN
inactivation in cancers including lung cancer, endometrial
carcinoma, prostate cancer, brain tumors, EBV-associated gastric
carcinoma, cervical neoplasm, malignant melanoma and hematologic
malignancies (9–17). However, to date, this mechanism of
PTEN inactivation has not been reported in NPC.
In the present study, we evaluated the PTEN
expression in NPC specimens and control samples by quantitative
RT-PCR (qPCR) and examined the CpG island methylation status of
PTEN using methylation-specific polymerase chain reaction (MSP) and
sequencing. 5-aza-dC treatment can lead to DNA demethylation via
inhibition of DNA methyltransferase activity (18). We also treated NPC cell lines
(HONE1, CNE1 and 6–10B) with 5-aza-dC and then examined PTEN
expression. Thus, we preliminarily demonstrated the role of PTEN
methylation in relation to the mechanism of PTEN inactivation in
NPC.
Materials and methods
Methylation-specific PCR
The methylation status of the PTEN promoter region
was determined by MSP using bisulfite-modified DNA. The targets of
the promoter regions were one site. The sequences of the methylated
and unmethylated primer pairs are listed in Table I with the other primer sequences
used in the study. DNA was modified by the bisulfite reaction using
an EpiTect Bisulfite kit (Qiagen). Methylated and unmethylated
genomic regions can be distinguished by PCR using each
sequence-specific pair of primers. MSP experiments were performed
at least in duplicate.
 | Table ISummary of the primers used in the
present study. |
Table I
Summary of the primers used in the
present study.
| Primer | Sequence | Sequence product size
(bp) | Annealing temperature
(°C) |
|---|
| qRT-PCR |
| GAPDH-forward |
5′-CATGGGTGTGAACCATGAGA-3′ | 165 | 60 |
| GAPDH-reverse |
5′-GTCTTCTGGGTGGCAGTGAT-3′ | | |
| PTEN-forward |
5′-TGCAGAAGAAGCCCCGCCA-3′ | 208 | 60 |
| PTEN-reverse |
5′-ACGCCTTCAAGTCTTTCTGCAGG-3′ | | |
| Methylated specific
PCR assay |
| Methylated |
| PTEN-forward |
5′-TTCGTTCGTCGTCGTCGTATTT-3′ | 207 | 58 |
| PTEN-reverse |
5′-GCCGCTTAACTCTAAACCGCAA-3′ | | |
| Unmethylated |
| PTEN-forward |
5′-GTGTTGGTGGAGGTAGTTGTTT-3′ | 163 | 58 |
| PTEN-reverse |
5′-ACCACTTAACTCTAAACCACAACCA-3′ | | |
| PCR primers for 6
PTEN exons |
| Exon 1-forward |
5′-TTCTGCCATCTCTCTCCTCC-3′ | 194 | 60 |
| Exon 1-reverse |
5′-ATCCGTCTACTCCCACGTTC-3′ | | |
| Exon 2-forward |
5′-GTTTGATTGCTGCATATTTCA-3′ | 201 | 50 |
| Exon 2-reverse |
5′-TCTAAATGAAAACACAACATGAA-3′ | | |
| Exon 3-forward |
5′-AGCTCATTTTTGTTAATGGTGG-3′ | 178 | 60 |
| Exon 3-reverse |
5′-CCTCACTCTAACAAGCAGATAACTTTC-3′ | | |
| Exon 4-forward |
5′-AAAGATTCAGGCAATGTTTGTTAG-3′ | 200 | 60 |
| Exon 4-reverse |
5′-TGACAGTAAGATACAGTCTATCGGG-3′ | | |
| Exon
5-1-forward |
5′-TTTTTTCTTATTCTGAGGTTATC-3′ | 184 | 50 |
| Exon
5-1-reverse |
5′-TCATTACACCAGTTCGTCC-3′ | | |
| Exon
5-2-forward |
5′-TCATGTTGCAGCAATTCAC-3′ | 176 | 50 |
| Exon
5-2-reverse |
5′-GAAGAGGAAAGGAAAAACATC-3′ | | |
| Exon
6-forward |
5′-ATGGCTACGACCCAGTTACC-3′ | 284 | 60 |
| Exon
6-reverse |
5′-AAGAAAACTGTTCCAATACATGG-3′ | | |
Patients and tissue samples
All NPC and NP samples were collected from the
Department of Otorhinolaryngology, Head and Neck Surgery, Nanfang
Hospital, Affiliated Hospital of Southern Medical University,
Guangzhou, China. For the use of these clinical materials for
research purposes, prior written informed consent and ethics
approval were obtained from all participants and the Ethics
Committees of the Nanfang Hospital, respectively.
Cells and culture conditions
NPC cell lines (5–8F, CNE1, CNE2, 6–10B, SUNE1 and
HONE1) were available from the Cancer Institute of Southern Medical
University (Guangzhou, China). All cell lines used in the present
study were maintained in DMEM medium (Invitrogen) with 10% FBS
(HyClone), 100 U/ml penicillin, 100 mg/ml streptomycin
(Invitrogen), and incubated at 5% CO2 at 37°C.
Quantitative RT-PCR (qRT-PCR)
Total RNA of tissues and cells was reverse
transcribed using PrimeScript® RT reagent kit (Takara).
Quantitative real-time PCR was performed using SYBR®
Premix Ex Taq™ II (Takara) on a StrataGene Mx3005P System. The
sequences of the qPCR primer pairs are listed in Table I.
Cell viability assay
Cell viability was analyzed using an MTT assay
(Sigma, St. Louis, MO, USA). Briefly, 1×103 cells were
seeded into a 96-well plate with quadruplicate repeat for each
condition. After 24 h of incubation, MTT reagent was added to each
well and incubated for 4 h. The formazan crystals formed by viable
cells were then solubilized in DMSO and measured at 490 nm for the
absorbance values. Each experiment was performed in triplicate. The
5-aza-dC concentration required to inhibit cell growth by 50%
(IC50) was calculated from survival curves. Survival
percent was calculated using the following formula: Survival
percent (%) = (mean experimental absorbance/mean control
absorbance) × 100%.
Statistical analysis
The data are presented as mean ± SEM, unless
otherwise indicated, of at least three independent experiments.
Statistical analysis was performed using an SPSS 13.0 package
system. Statistical significance was assessed by the Student’s
t-test, the Pearson’s Chi-square test, Fisher’s exact test or
one-way ANOVA analysis (*P<0.05;
**P<0.01; ***P<0.001).
Results
PTEN is downregulated in clinical NPC
specimens and human NPC cell lines
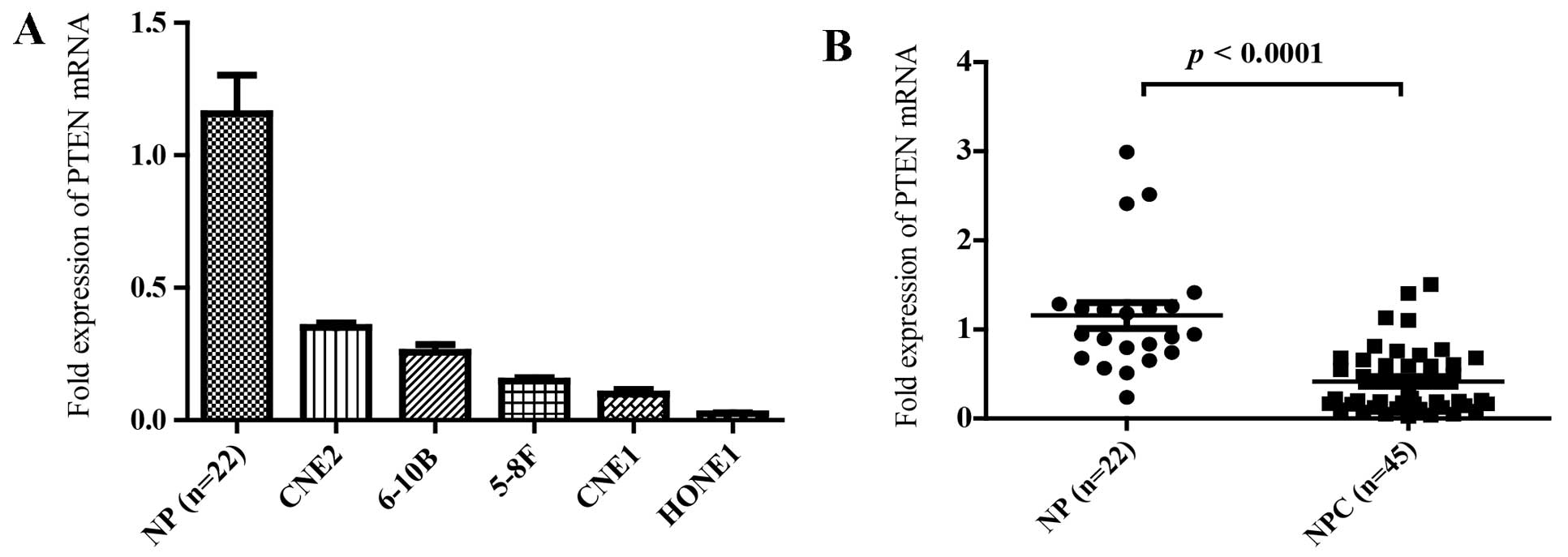
We first examined the expression level of PTEN in 45
NPC specimens and 22 non-tumor nasopharyngeal epithelial (NP)
tissues. The average expression level of PTEN was significantly
lower in NPC specimens compared with non-tumor NP tissues (Fig. 1B; P<0.0001). A panel of human NPC
cell lines was also analyzed for the expression level of PTEN.
Similarly, the expression level of PTEN was observed to be
decreased in all 5 NPC cell lines compared with the non-tumor NP
tissues (Fig. 1A). These data
supported that PTEN was downregulated in NPC.
Hypermethylation of PTEN in clinical NPC
specimens and human NPC cell lines
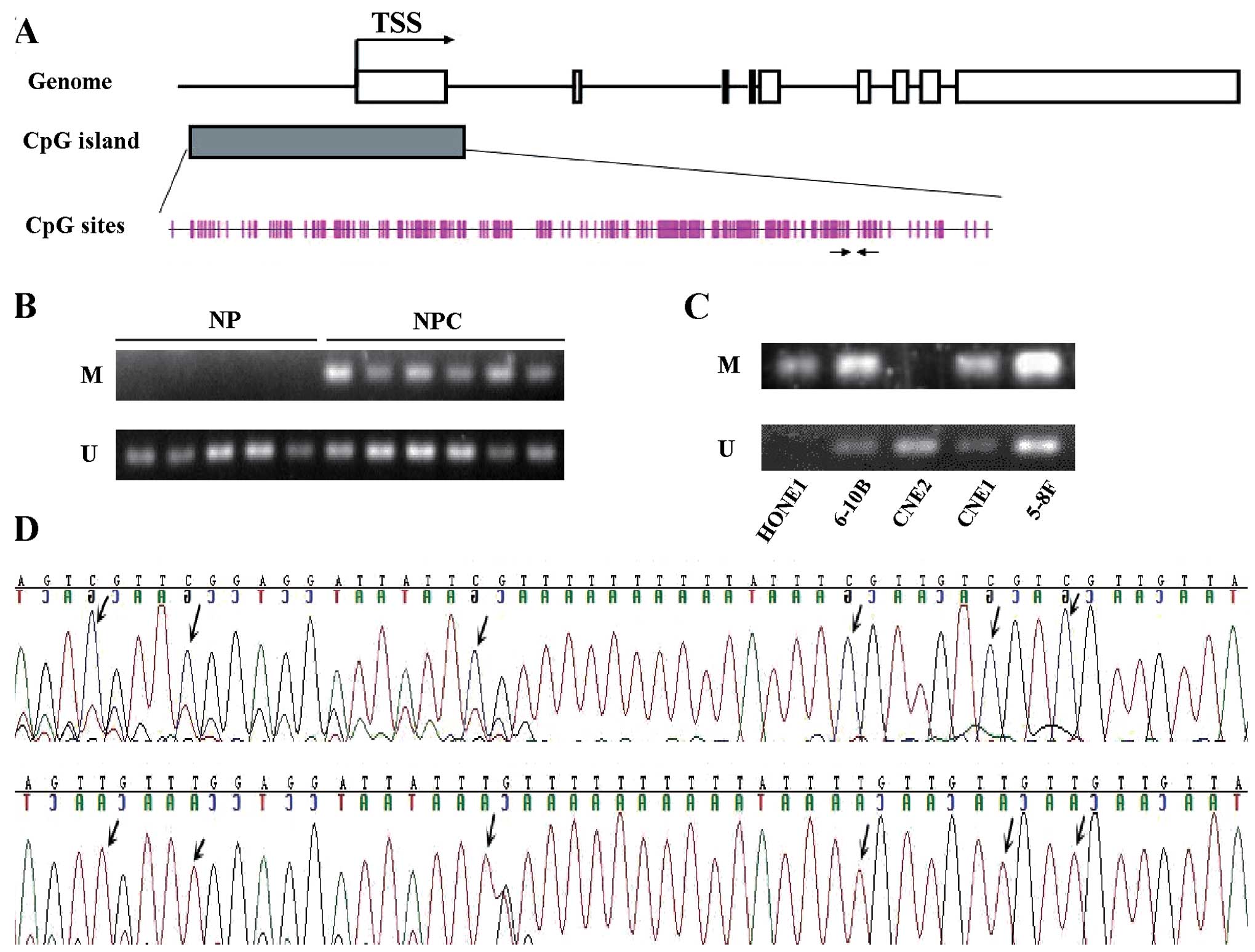
In order to explore the potential role of CpG island
methylation in the transcriptional silencing of the PTEN gene, we
investigated the methylation status of PTEN in clinical specimens
and NPC cell lines. As the 5′ region of PTEN contained many CpG
islands spanning ~3 kb, we focused only on its promoter region
(Fig. 2A) in the present study.
Our MSP analysis showed that CpG islands in the PTEN
promoter region were methylated in 82.2% (37/45) of NPC tissues,
whereas the methylated PTEN appeared in only 5.3% (1/19) of the
non-tumor NP tissues (Fig. 2B) and
in 80% (4/5) of NPC cell lines (Fig.
2C). The difference in the hypermethylation level between NPC
tissues and non-tumor NP tissues was statistically significant
(P<0.0001).
To validate MSP results, we sequenced M-MSP and
U-MSP products amplified from two NPC tissues using either M or U
primers of PTEN. The sequencing results showed that all the
cytosine residues in the M-MSP product were converted to thymines,
except for those in CpG dinucleotides, indicating the presence of
methylated cytosines in these CpG dinucleotides. The representative
sequencing results of M- and U-MSP products of PTEN are shown in
Fig. 2D.
Restoration of PTEN in NPC cell lines
after 5-aza-dC treatment
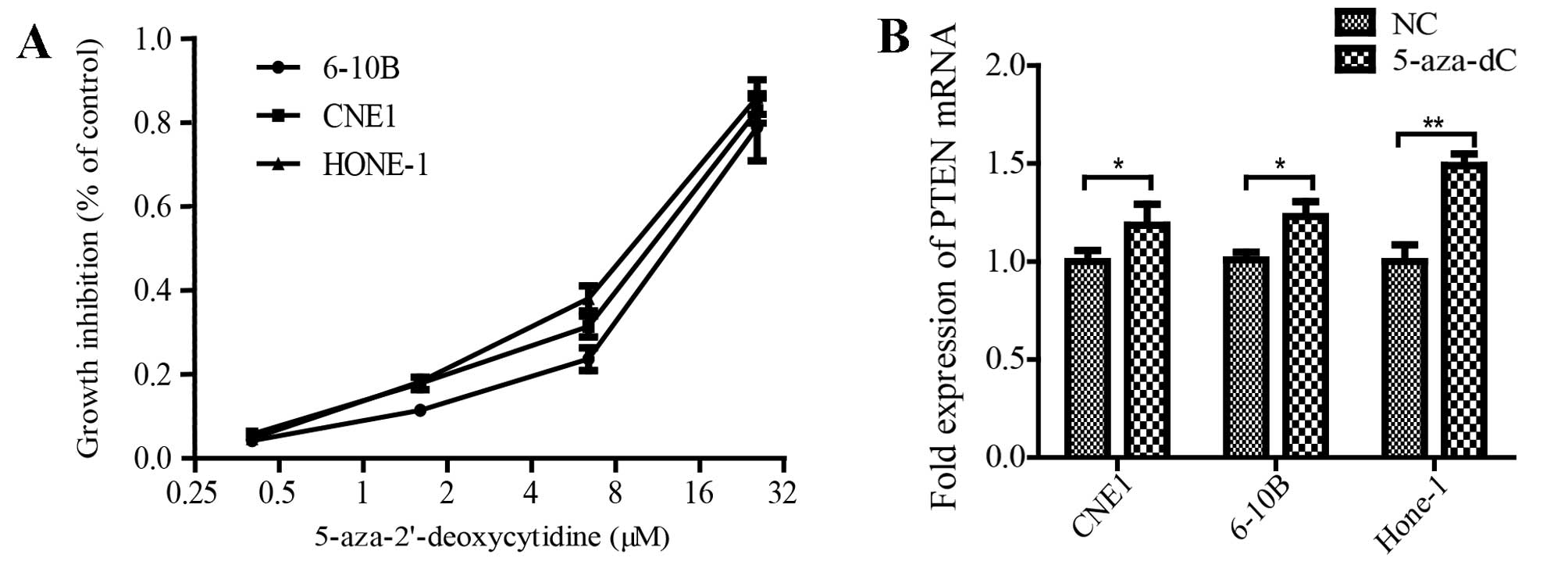
To directly test the effect of promoter methylation
on PTEN inactivation, we treated 3 NPC cell lines (HONE-1, CNE1 and
6–10B) with 5-aza-dC for 3 days (Fig.
3A), and then examined PTEN mRNA expression changes. We
observed that PTEN mRNA expression was clearly upregulated in all 3
NPC cell lines after 5-aza-dC treatment (Fig. 3B). These results suggested that
methylation of promoter region plays a regulatory role in silencing
the expression of PTEN in NPC cells.
PTEN mutation in NPC cell lines
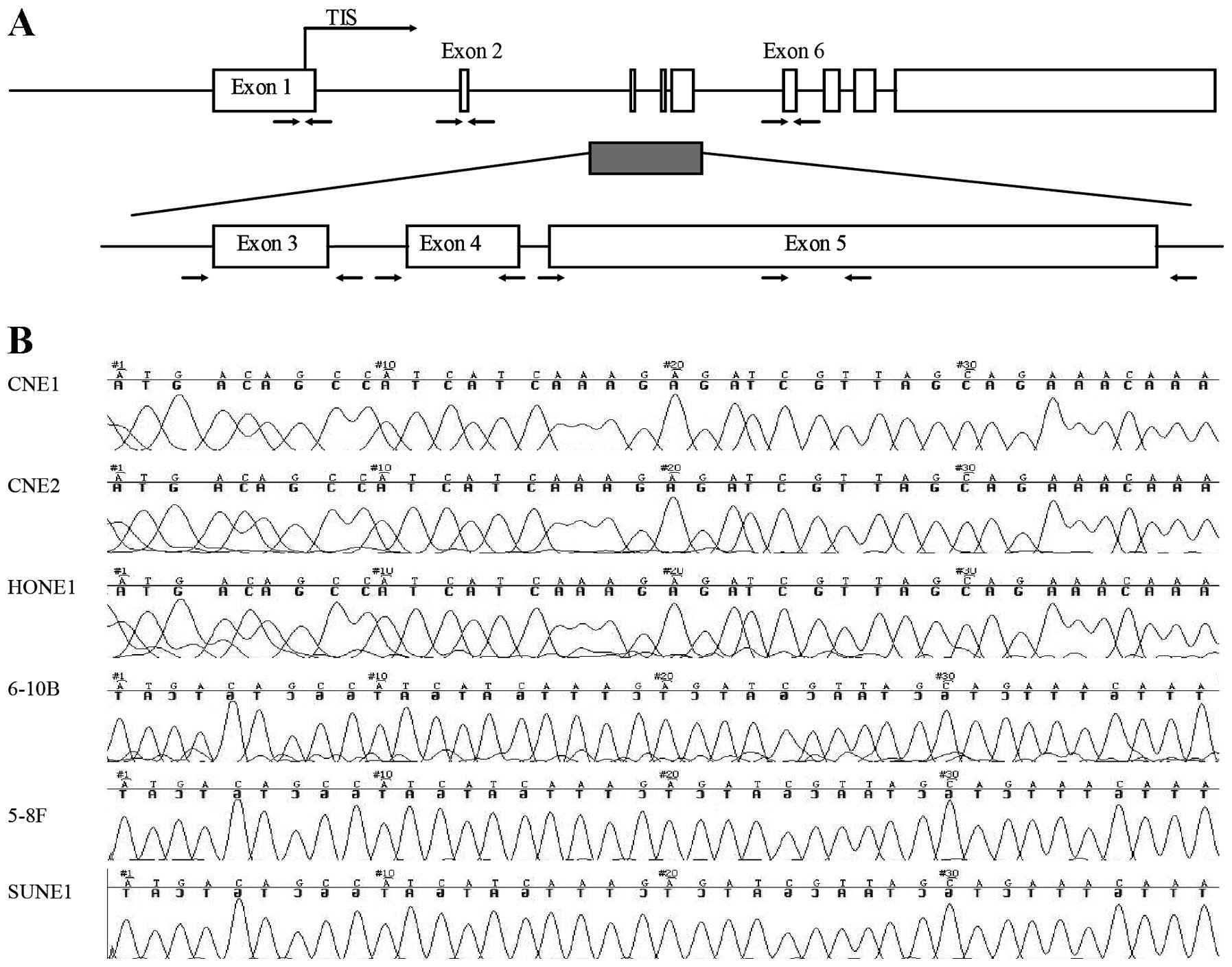
To detect the mutation of tumor suppressor PTEN in
NPC, DNA sequencing was used to detect the mutation from exon 1 to
exon 6 (Fig. 4A) of the PTEN gene
in 6 NPC cell lines (5–8F, CNE1, CNE2, 6–10B, SUNE1 and HONE1).
None of the NPC cell lines showed mutation in exon 1 to exon 6 of
the PTEN gene (Fig. 4B).
Discussion
NPC is one of the most common types of cancer in
southern China. In contrast to other head and neck malignancies,
NPC is highly sensitive to radiation and chemotherapy. High
survival rates are reported for early stage (stages I and II) of
diseases, but the prognosis for advanced stage (stages III and IV)
remains poor; in patients with advanced NPC at the local site or
with distant metastases, the disease will subsequently recur in
30–40% (19). Unfortunately, the
majority of NPCs are diagnosed at an advanced stage due to the
difficulty of a thorough nasopharyngeal exam, as well as
non-specific presenting symptoms (cervical nodal enlargement,
headache, nasal and aural dysfunction). In light of these, the
molecular targets for early diagnosis of NPC need to be
clarified.
In NPC, gene silencing by deletion, insertion and
mutation of tumor suppressor genes (TSGs), such as p53 (20), are uncommon events in
carcinogenesis. The present study demonstrated that no mutation was
found in functional DNA regions of PTEN by PCR sequencing, which is
in accordance with the results by PCR-SSCP in NPC. Downregulation
of TSG expression by aberrant methylation is increasingly emerging
as an important mechanism of nasopharyngeal tumorigenesis (21–24).
Phosphatase and tensin homolog (PTEN) deleted on
chromosome 10 was originally cloned as a tumor suppressor. It has
been confirmed that PTEN is deleted or inactivated in many tumor
types, including renal (25),
melanoma (26), endometrial
(27), breast (5), prostate (5), lung (9), bladder (28), thyroid (29) and NPC (30), identifying PTEN as an important
tumor suppressor. On the other hand, promoter methylation is widely
considered to be an important epigenetic mechanism in the
carcinogenesis of NPC, and has been proven promising for early
diagnosis of multiple types of tumors (31–33).
To the best of our knowledge, this is the first
study to demonstrate an essential role of PTEN methylation in the
carcinogenesis of NPC. We found that 82.2% (37/45) of NPC specimens
and 80% (4/5) of NPC cell lines present PTEN CpG island DNA
methylation, compared with 5.3% (1/19) of non-tumor NP tissues;
PTEN methylation was not related to age, gender, lymphatic node
metastasis and tumor local grade (Table II). However, our present study did
not demonstrate a significant difference between methylation
frequency in early and late stage NPC cases. Considering PTEN
expression in tumor specimens and cell lines (Table III), PTEN methylation may be an
early event in the carcinogenesis of NPC. As to whether the
epigenetic change of PTEN is one of the potential markers for early
diagnosis, a profound study is currently in progress in the local
high risk population and preclinical disease.
 | Table IIAssociation between PTEN methylation
and clinicopathological parameters of NPC. |
Table II
Association between PTEN methylation
and clinicopathological parameters of NPC.
| PTEN
methylation |
|---|
|
|
|---|
| Total no. | Positive n/total
(%) | Negative n/total
(%) | P-valuea |
|---|
| Gender |
| Male | 36 | 30/36 (83.3) | 6/36 (16.7) | 0.65 |
| Female | 9 | 7/9 (77.8) | 2/9 (22.2) | |
| Age (years) |
| <60 | 35 | 28/35 (80) | 7/35 (20) | 0.66 |
| ≥60 | 10 | 9/10 (90) | 1/10 (10) | |
| T gradeb |
| 1, 2 | 31 | 27/31 (87.1) | 4/31 (12.9) | 0.23 |
| 3, 4 | 14 | 10/14 (71.4) | 4/14 (28.6) | |
| N stageb |
| 0 | 7 | 6/7 (85.7) | 1/7 (14.3) | 1 |
| 1, 2, 3, 4 | 38 | 31/38 (81.6) | 7/38 (18.4) | |
| Stageb |
| I, II | 20 | 18/20 (90) | 2/20 (10) | 0.27 |
| III, IV | 25 | 19/25 (76) | 6/25 (24) | |
| Histology |
| Keratinizing
squamous cell carcinoma | 45 | 37/45 (82.2) | 8/45 (17.8) | |
| Non-keratinizing
carcinoma | 0 | | | |
 | Table IIISummary of the PTEN methylation in
human tumor/normal tissues and cell lines. |
Table III
Summary of the PTEN methylation in
human tumor/normal tissues and cell lines.
| Tumor type
(Ref.) | Testing method | Histology | Methylation
rate | Tumor cell
lines | Methylation
rate |
|---|
| Lung cancer
(9) | MSP | PTEN-negative
NSCLC | 7/20 (35) | NSCLC cell
lines | 11/16 (69) |
| MSP | PTEN-positive
NSCLC | 0/10 (0) | | |
| Endometrial
carcinoma (10) | MSP | Endometrial
carcinoma | 26/138 (19) | | |
| Prostate cancer
(11) | MSP | Primary prostate
tumor | 0/6 (0) | | |
| Brain tumor
(12) | MSP | Non-tumor
brain | 0/13 (0) | | |
| | Gliomas | 44/90 (49) | | |
| EBV-associated
gastric carcinoma (13) | MSP | EBV-negative
gastric cancer | 26/87 (30) | | |
| | EBV-associated
gastric cancer | 18/28 (64) | | |
| Malignant
melanomaa (15) | MSP | Malignant
melanoma | | Melanoma cell
lines | 3/13 (23) |
| Gastric cancer
(14) | MSP | Gastric cancer | 26/66 (39) | | |
| | PTEN-negative
gastric cancer | 19/26 (73) | | |
| Cervical neoplasm
(17) | MSP | CIN-Hb | 4/10 (40) | | |
| | Squamous cell
carcinoma | 36/62 (58) | | |
| Nasopharyngeal
carcinoma (present study) | MSP | Nasopharyngeal
epithelial | 1/19 (5.3) | NPC cell lines | 4/5 (80) |
| | Nasopharyngeal
carcinoma | 37/45 (82.2) | | |
In brief, our more recent studies indicated that
PTEN methylation and loss of PTEN expression are early events in
the development of NPC and may serve as a biomarker for early
diagnosis.
Acknowledgements
This study was sponsored by NSFC of China (no.
81172585) and Guangdong SFC (nos. S2011010003828 and
S2013010016388).
References
|
1
|
Parkin DM and Muir CS: Cancer incidence in
five continents. Comparability and quality of data. IARC Sci Publ;
pp. 45–173. 1992
|
|
2
|
Nielsen NH, Mikkelsen F and Hansen JP:
Nasopharyngeal cancer in Greenland. The incidence in an Arctic
Eskimo population. Acta Pathol Microbiol Scand A. 85:850–858.
1977.PubMed/NCBI
|
|
3
|
DeNittis AS, Liu L, Rosenthal DI and
Machtay M: Nasopharyngeal carcinoma treated with external
radiotherapy, brachytherapy, and concurrent/adjuvant chemotherapy.
Am J Clin Oncol. 25:93–95. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanguineti G, Geara FB, Garden AS, et al:
Carcinoma of the nasopharynx treated by radiotherapy alone:
determinants of local and regional control. Int J Radiat Oncol Biol
Phys. 37:985–996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Yen C, Liaw D, et al: PTEN, a
putative protein tyrosine phosphatase gene mutated in human brain,
breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar
|
|
6
|
Liotta LA: Tumor invasion and metastases -
role of the extracellular matrix: Rhoads Memorial Award lecture.
Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
7
|
Leslie NR and Downes CP: PTEN function:
how normal cells control it and tumour cells lose it. Biochem J.
382:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Senechal K, Neshat MS, Whang YE and
Sawyers CL: The PTEN/MMAC1 tumor suppressor phosphatase functions
as a negative regulator of the phosphoinositide 3-kinase/Akt
pathway. Proc Natl Acad Sci USA. 95:15587–15591. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soria JC, Lee HY, Lee JI, et al: Lack of
PTEN expression in non-small cell lung cancer could be related to
promoter methylation. Clin Cancer Res. 8:1178–1184. 2002.PubMed/NCBI
|
|
10
|
Salvesen HB, MacDonald N, Ryan A, et al:
PTEN methylation is associated with advanced stage and
microsatellite instability in endometrial carcinoma. Int J Cancer.
91:22–26. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cairns P, Okami K, Halachmi S, et al:
Frequent inactivation of PTEN/MMAC1 in primary prostate
cancer. Cancer Res. 57:4997–5000. 1997.
|
|
12
|
Wiencke JK, Zheng S, Jelluma N, et al:
Methylation of the PTEN promoter defines low-grade gliomas
and secondary glioblastoma. Neuro Oncol. 9:271–279. 2007.
|
|
13
|
Hino R, Uozaki H, Murakami N, et al:
Activation of DNA methyltransferase 1 by EBV latent membrane
protein 2A leads to promoter hypermethylation of PTEN gene
in gastric carcinoma. Cancer Res. 69:2766–2774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YH, Lee HS and Kim WH: Promoter
methylation and silencing of PTEN in gastric carcinoma. Lab Invest.
82:285–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Furuta J, Umebayashi Y, Miyamoto K, et al:
Promoter methylation profiling of 30 genes in human malignant
melanoma. Cancer Sci. 95:962–968. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Montiel-Duarte C, Cordeu L, Agirre X, et
al: Resistance to Imatinib Mesylate-induced apoptosis in acute
lymphoblastic leukemia is associated with PTEN down-regulation due
to promoter hypermethylation. Leuk Res. 32:709–716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheung TH, Lo KW, Yim SF, et al:
Epigenetic and genetic alternation of PTEN in cervical
neoplasm. Gynecol Oncol. 93:621–627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jones PA and Taylor SM: Cellular
differentiation, cytidine analogs and DNA methylation. Cell.
20:85–93. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teo P, Yu P, Lee WY, et al: Significant
prognosticators after primary radiotherapy in 903 nondisseminated
nasopharyngeal carcinoma evaluated by computer tomography. Int J
Radiat Oncol Biol Phys. 36:291–304. 1996. View Article : Google Scholar
|
|
20
|
Sun Y, Hegamyer G, Cheng YJ, et al: An
infrequent point mutation of the p53 gene in human nasopharyngeal
carcinoma. Proc Natl Acad Sci USA. 89:6516–6520. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Sun D, Van Do N, Tang A, Hu L and
Huang G: Inactivation of RASSF2A by promoter methylation
correlates with lymph node metastasis in nasopharyngeal carcinoma.
Int J Cancer. 120:32–38. 2007.
|
|
22
|
Mo Y, Midorikawa K, Zhang Z, et al:
Promoter hypermethylation of Ras-related GTPase gene
RRAD inactivates a tumor suppressor function in
nasopharyngeal carcinoma. Cancer Lett. 323:147–154. 2012.
|
|
23
|
Shu XS, Li L, Ji M, et al: FEZF2, a
novel 3p14 tumor suppressor gene, represses oncogene EZH2
and MDM2 expression and is frequently methylated in
nasopharyngeal carcinoma. Carcinogenesis. 34:1984–1993. 2013.
View Article : Google Scholar
|
|
24
|
Li L, Tao Q, Jin H, et al: The tumor
suppressor UCHL1 forms a complex with p53/MDM2/ARF to promote p53
signaling and is frequently silenced in nasopharyngeal carcinoma.
Clin Cancer Res. 16:2949–2958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brenner W, Farber G, Herget T, Lehr HA,
Hengstler JG and Thuroff JW: Loss of tumor suppressor protein PTEN
during renal carcinogenesis. Int J Cancer. 99:53–57. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guldberg P, Thor SP, Birck A, Ahrenkiel V,
Kirkin AF and Zeuthen J: Disruption of the MMAC1/PTEN gene
by deletion or mutation is a frequent event in malignant melanoma.
Cancer Res. 57:3660–3663. 1997.PubMed/NCBI
|
|
27
|
Oda K, Stokoe D, Taketani Y and McCormick
F: High frequency of coexistent mutations of PIK3CA and
PTEN genes in endometrial carcinoma. Cancer Res.
65:10669–10673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cairns P, Evron E, Okami K, et al: Point
mutation and homozygous deletion of PTEN/MMAC1 in primary
bladder cancers. Oncogene. 16:3215–3218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liaw D, Marsh DJ, Li J, et al: Germline
mutations of the PTEN gene in Cowden disease, an inherited
breast and thyroid cancer syndrome. Nat Genet. 16:64–67. 1997.
|
|
30
|
Qu C, Liang Z, Huang J, et al: MiR-205
determines the radioresistance of human nasopharyngeal carcinoma by
directly targeting PTEN. Cell Cycle. 11:785–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mutter GL, Lin MC, Fitzgerald JT, et al:
Altered PTEN expression as a diagnostic marker for the earliest
endometrial precancers. J Natl Cancer Inst. 92:924–930. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heyn H, Carmona FJ, Gomez A, et al: DNA
methylation profiling in breast cancer discordant identical twins
identifies DOK7 as novel epigenetic biomarker.
Carcinogenesis. 34:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun D, Zhang Z, Van Do N, Huang G, Ernberg
I and Hu L: Aberrant methylation of CDH13 gene in nasopharyngeal
carcinoma could serve as a potential diagnostic biomarker. Oral
Oncol. 43:82–87. 2007. View Article : Google Scholar : PubMed/NCBI
|