Introduction
Adenoid cystic carcinoma (ACC) is one of the most
common malignant neoplasms in salivary glands, which accounts for
nearly 18% of all salivary gland malignancies (1). Generally, ACC has the following
clinical and pathological characteristics: (i) progressive local
growth, contributing to the difficulties in radical local resection
and a high rate of local recurrence; (ii) aggressive histological
features. For example, perineural invasion can lead to facial
paralysis and difficulties in swallowing and pronunciation; (iii) a
high incidence of distant metastasis, especially pulmonary
originating from vascular involvement, which is the primary cause
of patient mortality (2).
Conventionally, the treatment strategies for
salivary adenoid cystic carcinoma (SACC) include local resection
combined with postoperative radiation therapy, as the response
rates of SACC to chemotherapy or molecular therapies are generally
poor (3). In general, chemotherapy
is delivered in cases of relapsed and/or metastatic disease almost
exclusively with a palliative aim. Although many chemotherapy
regimens have been attempted, to date, no randomized studies have
been conducted to identify the best therapeutic option in this
setting. Thus, combined treatments with several chemotherapy
regimens, even chemopreventive agents, are often used (3). Nonetheless, due to the cytotoxicity
and resistance of the chemotherapeutic drugs, patients always
suffer from the drug side-effects; significant improvement is
expected in local control or in overall survival.
Recently, clinicians realized that as an important
transmembrane protein belonging to the immunoglobulin super family,
extracellular matrix metalloproteinase inducer (EMMPRIN) plays a
vital role in tumor proliferation, invasiveness and patient
prognosis (4–6). Silencing of EMMPRIN expression can
both restrain the proliferation, invasion and MMP-2/MMP-9 secretion
in SACC cell lines in vitro, and suppress the tumor growth
and perineural invasion in nude mice (7). Recent immunohistochemical studies
showed that EMMPRIN expression was a significant prognosis factor
and correlated with tumor size, perineural invasion, distant
metastasis and clinical stage in patients with SACC. In addition,
patients with positive-EMMPRIN expression had much poorer prognosis
than those with negative-EMMPRIN expression (6).
As a promising physical therapy approach, nanosecond
pulsed electric field (nsPEF) exhibits many notable non-thermal
features, of which the duration of the electric pulses were
maintained in nanoseconds level. Although not widely accepted, a
few reports suggested that nsPEF may compromise the plasma membrane
barrier function by disruption of the membrane potential (8,9),
followed by multiple physiological consequences including
intracellular calcium bursts (10,11),
translocation of phosphatidylserine residues in plasma membrane
(12,13) and apoptotic cell death (10,14).
In vitro studies have confirmed that nsPEF inhibits cell
proliferation in several human malignancies including melanoma,
colon carcinoma, pancreatic cancer and squamous cell carcinoma
(15–18). Furthermore, nsPEF could create
synergistic effects in squamous cell carcinoma when combined with
low-dose of gemcitabine (19).
nsPEF was also found to suppress tumor growth in xenograft nude
mice models of melanoma and squamous cell carcinoma (15,20).
However, the mechanisms underlying the suppression of cancer growth
by nsPEF are complicated, and the effect of nsPEF on EMMPRIN
expression has not previously been discussed.
In the present study, we demonstrated the
synergistic inhibition effect of nsPEF combined with low-dose of
PYM, which is a conventional chemotherapeutic agent widely used in
China, in proliferation, apoptosis and invasion of two human SACC
cell lines (SACC-83 and SACC-LM). Furthermore, EMMPRIN expression
was investigated to clarify the synergistic effect of the nsPEF
combined with PYM.
Materials and methods
Cell culture
Human low and high metastasis cell line of ACC
(SACC-83 and SACC-LM), provided by the central laboratory of Peking
University School and Hospital of Stomatology, were cultured in
RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C in a humidified
atmosphere of 5% CO2.
Application of nsPEF
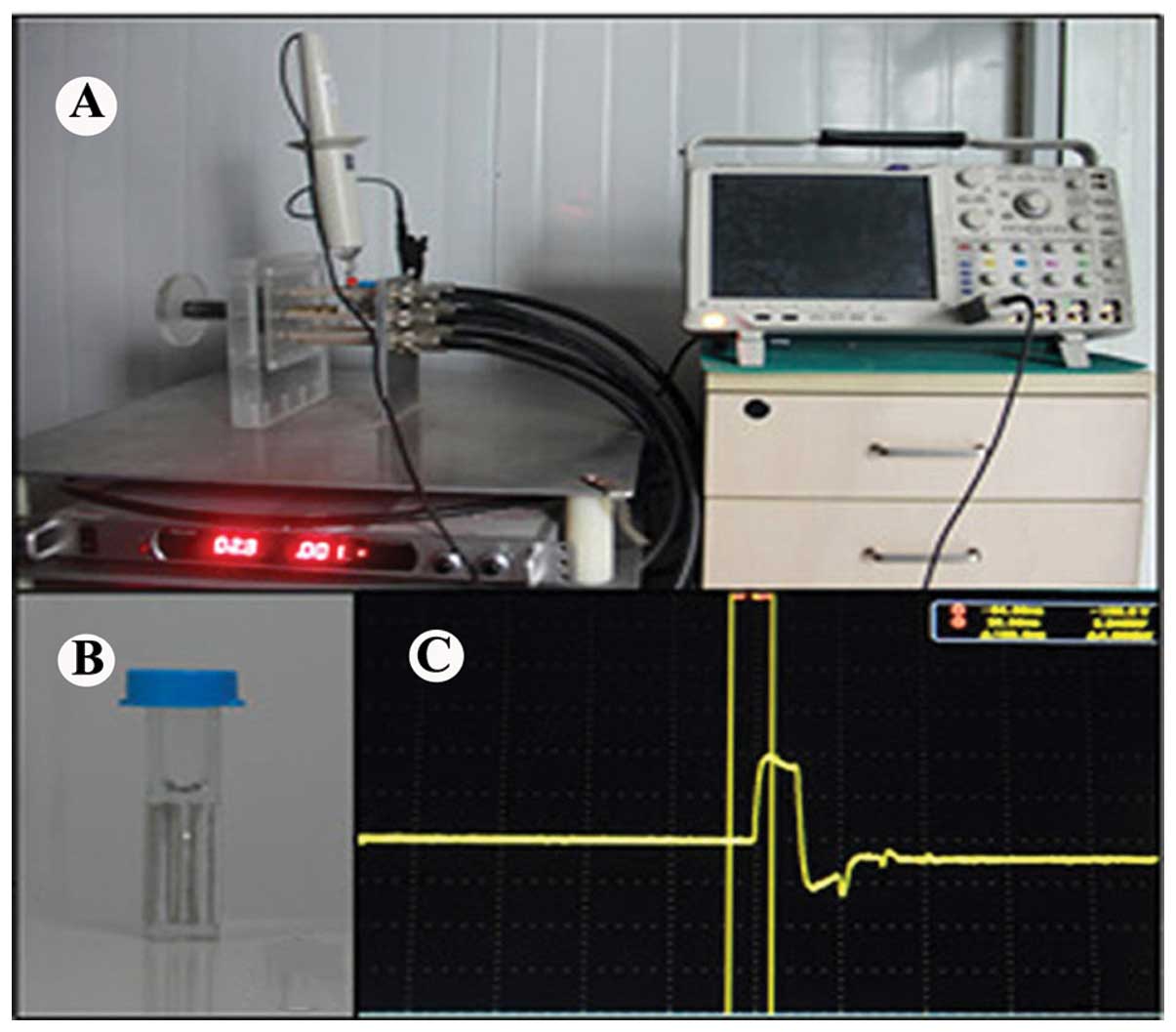
In the present study, an nsPEF generator was used
with a duration of 100 ns, and the electric fields may be adjusted
from 10 to 30 kV/cm. A digital phosphor oscilloscope (DPO4054;
Tektronix, USA) equipped with a high voltage probe (P6015A;
Tektronix) was applied to monitor the waveforms. The pulse power
device is shown in Fig. 1. The
SACC-83 and SACC-LM cells were harvested and resuspended in cell
culture media with a concentration of 2.0×106 cells/ml.
Cell suspension (1×106 cells) with 500 μl was placed in
0.2 cm gap cuvette (Fig. 1B)
(Biosmith, aluminum plate electrodes) and exposed to nsPEF. PYM
with appropriate concentrations ranging from 0.01 to 100 μg/ml was
applied to test the chemosensitivity of PYM to the SACC cells. To
explore possible synergistic effects of nsPEF combined with low
concentrations of PYM on SACC-83 and SACC-LM cells in vitro,
PYM at a concentration of 0.01 μg/ml was applied alone or in
combination after exposure to PYM. According to the different
treatment, cells were divided into four groups. Group A was the
control group in which neither nsPEF nor PYM was used. Group B was
treated only by PYM. Group C was exposed to 40 pulses nsPEF with a
field strength of 10, 20 and 30 kV/cm. Group D was treated with the
combination of nsPEF and PYM. To explore the synergistic effect of
nsPEF and PYM, the concentration of PYM in group D was as low as
0.01 μg/ml (Table I).
 | Table IBasic parameters for treatment of
SACC-83 and SACC-LM cell lines. |
Table I
Basic parameters for treatment of
SACC-83 and SACC-LM cell lines.
| Group | PYM μg/ml | Field strength
kV/cm | Durations Ns | Pulse no. |
|---|
| A | 0 | - | - | - |
| B | 0.01 | - | - | - |
| C | 0 | 10, 20, 30 | 100 | 40 |
| D | 0.01 | 10, 20, 30 | 100 | 40 |
Reagents and antibodies
Sodium pentobarbital was purchased from Sigma (St.
Louis, MO, USA). Antibodies to EMMPRIN and β-actin were purchased
from Bioworld Technology, USA. The secondary antibodies for western
blotting were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). PYM hydrochloride was purchased from Hebei
Pharmaceutical Factory (Tianjin, China). Other chemicals and
reagents were of analytical grade.
Cell proliferation test
The antiproliferative effect of PYM on SACC-83 and
SACC-LM cells was determined with the Cell Counting Kit-8 (CCK-8).
In the present study, we seeded 3×103 cells/well into
96-well flat bottom plates (Costar, Cambridge, MA, USA), treated
with PYM of the desired concentrations (0.01, 0.1, 1.0, 10, 100
μg/ml) when cells began to grow exponentially. After incubation for
24 and 48 h, 10 μl, WST-8 was added to each well, and the cells
were further incubated at 37°C for 2 h. Dye intensity was then read
on a microplate reader (Bio-Rad, Hercules, CA, USA) at 450 nm. The
inhibition rate was calculated according to the formula: Inhibition
rate (%) = (absorbency of control − absorbency of treated
cells)/absorbency of control × 100.
The inhibitory effect of PYM combined with nsPEF was
investigated in the same way. SACC-83 and SACC-LM cells were
harvested and resuspended with a concentration of
2.0×106 cells/ml. Cell suspension (1×106
cells) with 500 μl was placed in 0.2 cm gap cuvette (Biosmith) and
exposed to nsPEF. Cells in group D were then incubated with 0.01
μg/ml PYM after exposure to nsPEF. Then, we studied cell
proliferation for 24 and 48 h by CCK-8 assay.
Cell clonogenic assay
After exposure to different intensities of nsPEF of
0, 10, 20 and 30 kV/cm, SACC-83 and SACC-LM cells in control and
treatment groups were seeded into 6-well plates in triplicates in 2
ml medium containing 10% FBS at 37°C for 6 h to allow attachment to
the plastic bottom. The density of cells in group A, B, C and D
with a field strength of 10 kV/cm was 1,000 cells/well, others were
3,000 cells/well. The medium was then replaced by fresh culture
medium with 0.01 μg/ml PYM concentration and cells were allowed to
grow for another 7 days. The cell clones were fixed and stained
with 0.1% crystal violet. Colonies ≥30 cells were counted for
computing growth inhibition rate.
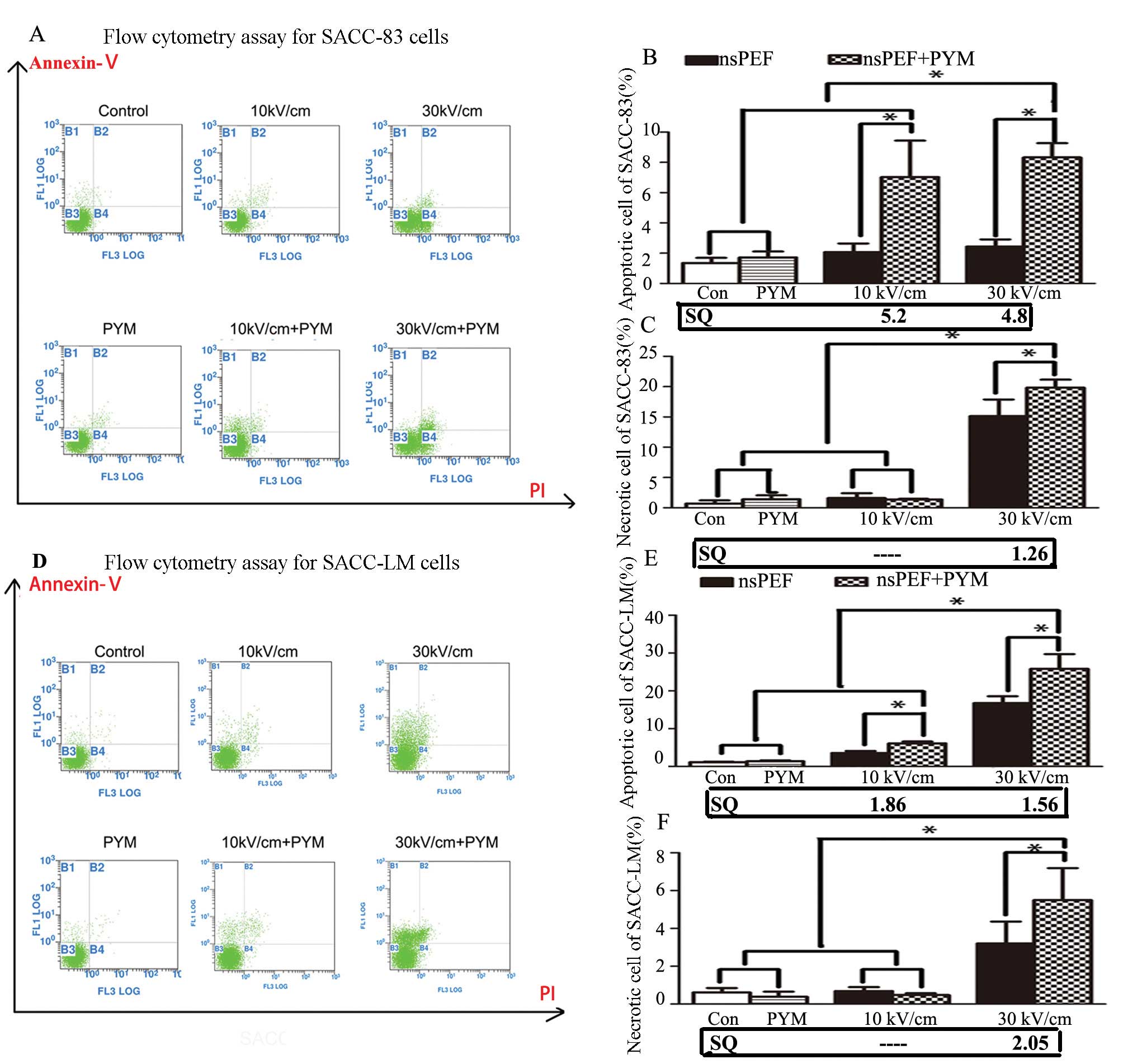
Cell apoptosis analysis by flow
cytometry
Apoptotic and necrotic cell death were analyzed by
double staining with FITC-conjugated Annexin V and PI, which was
based on the binding of Annexin V to apoptotic cells with exposed
phosphatidylserine and PI labeling of late apoptotic/necrotic cells
with membrane damage. In the present study, cells were suspended at
2×106 cells/ml in RPMI-1640 containing 10% FBS and cells
in group band group D (10 and 30 kV/cm) were incubated with 0.01
μg/ml PYM after resuspension. After exposure to nsPEF, cells were
incubated at 37°C for 2 h. All the cells were collected and stained
with FITC-conjugated Annexin V in a dark room for 3 min, and then
stained with PI on ice for 10 min. Analysis of samples was carried
out by FACSCanto flow cytometer (Becton-Dickinson, USA).
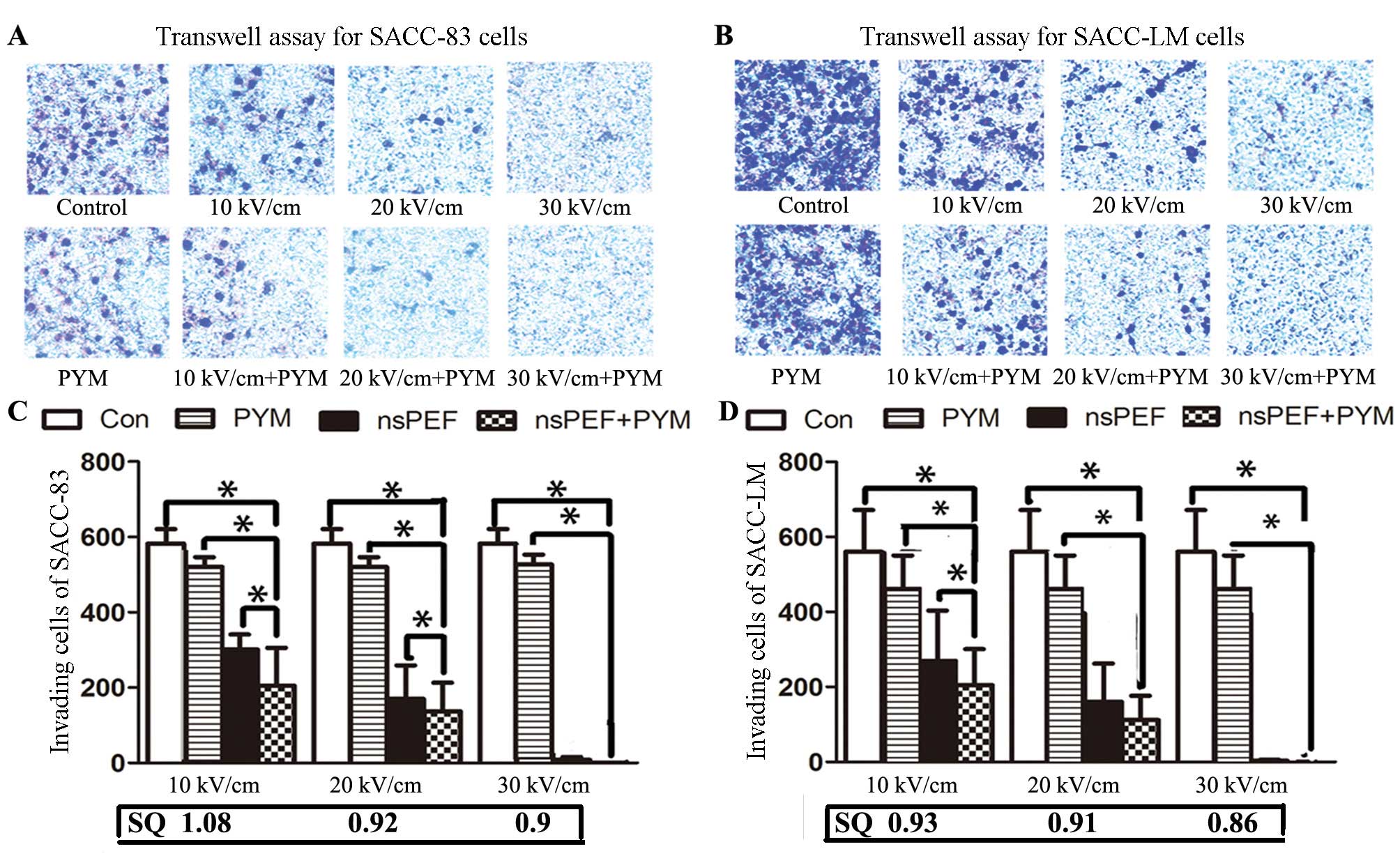
Cell invasion assay
Cell invasion was assessed by Boyden modified assay
using Transwell chambers (Costar) with 8-μm pore polycarbonate
filters that were coated with 50 μg/ml of Matrigel™ (BD
Biosciences, Bedford, MA, USA) diluted in serum-free medium.
SACC-LM and SACC-83 cells in group C and D were treated with nsPEF
(10 k, 20 and 30 kV/cm) alone or plus PYM, respectively. Then,
cells were seeded into the upper chambers with serum-free medium
(1.0×105 cells/chamber). Upper chambers were deposited
in 24-well plates filled with RPMI-1640 10% FBS and incubated for
24 h at 37°C in 5% CO2. Non-migrating cells were removed
from the upper surface of the chamber by scrubbing with a cotton
swab. Migrated cells on the lower membrane were fixed in 100%
methanol and stained with 0.1% crystal violet (Invitrogen) for 20
min. Invasion cells were counted under the microscope.
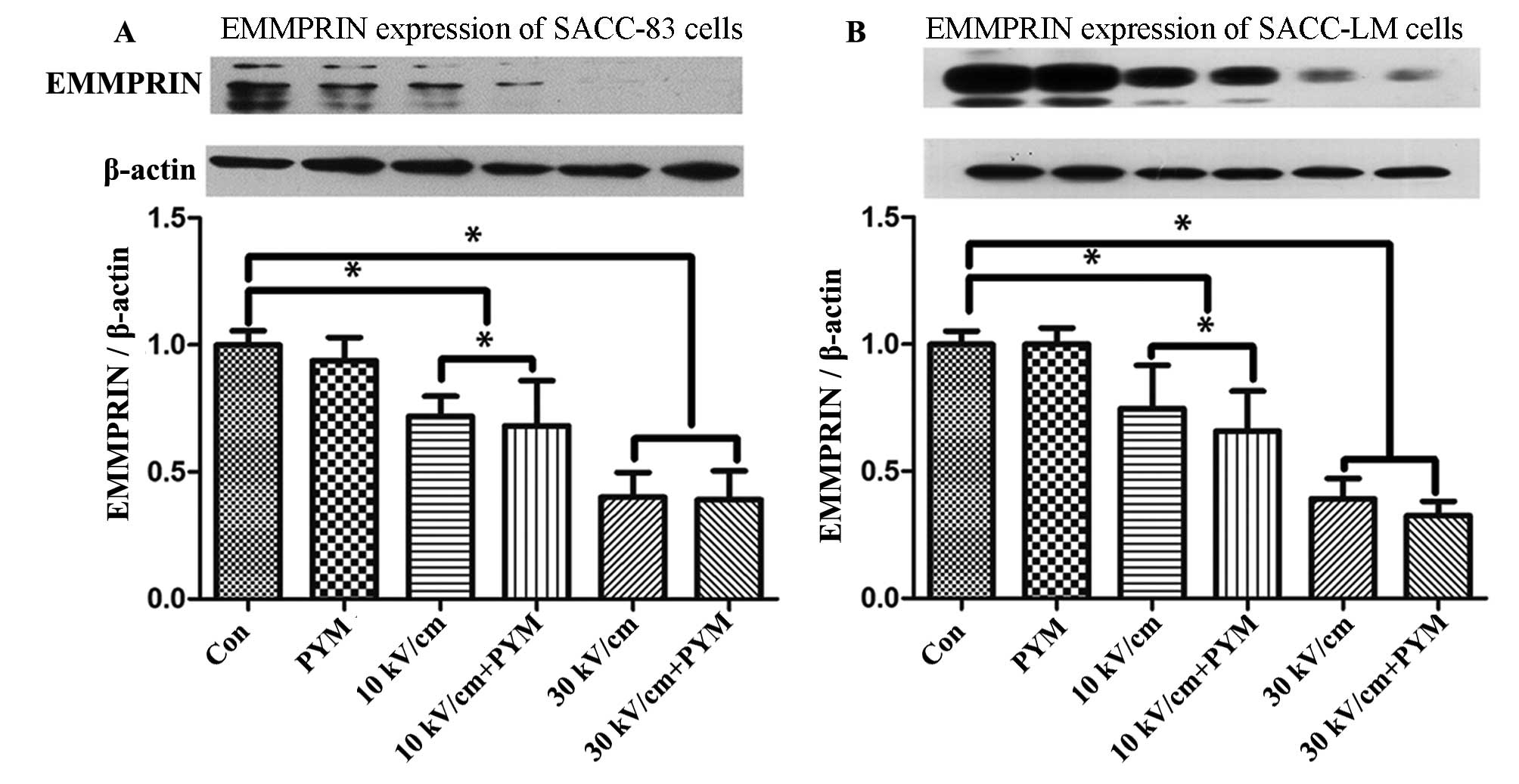
Western blot analysis
In a preliminary western blot assay, we detected the
expression of EMMPRIN of two cell lines 2 h after exposure to nsPEF
(30 kV/cm). The difference in expression of EMMPRIN to the control
group was notably detected 2 h following treatment. Therefore,
after exposure to nsPEF, the two cell lines in each group were
incubated at 37°C for 2 h. Then, cells were collected and lysed in
RIPA buffer [150 mM NaCl, 10 mM Tris-HCl, pH 8.0, 1% Nonidet P-40
(NP-40), 0.5% deoxycholic acid, 0.1% SDS, 5 mM EDTA] containing
0.7% phenylmethylsulfonyl fluoride (PMSF), 0.2% aprotinin, 0.2%
leupeptin, and sodium metavanadate. Samples (30 μg protein) were
separated on 12% (w/v) SDS-PAGE gels, and electrophoretically
transferred to PVDF membranes (Bio-Rad). Non-specific sites were
blocked with solution containing 5% non-fat milk powder in
PBS/Tween-20 (PBS/T) for 1 h at room temperature. The membrane was
probed with antibodies against β-actin and EMMPRIN in PBS/T
containing 5% bovine serum albumin (BSA) overnight at 4°C, and then
incubated with goat anti-rabbit secondary antibody at a dilution of
1:3,000.
Statistical analysis
Every group of experiments was carried out three
times repeatedly and experimental data are expressed as mean values
± standard deviation. The difference between each group was tested
for significance using one-way ANOVA, post hoc, Bonferroni and
Dunnett’s test for analyses of multiple group comparisons. The
synergism quotient (SQ) was evaluated by removing baseline values
from all treatments and then dividing the effects of combined
treatments by the sum of individual treatments. Synergism quotient
>1.0 indicates that a synergism exists for a given measured
response.
Results
nsPEF with PYM enhances the suppression
effect of proliferation in the two cell lines
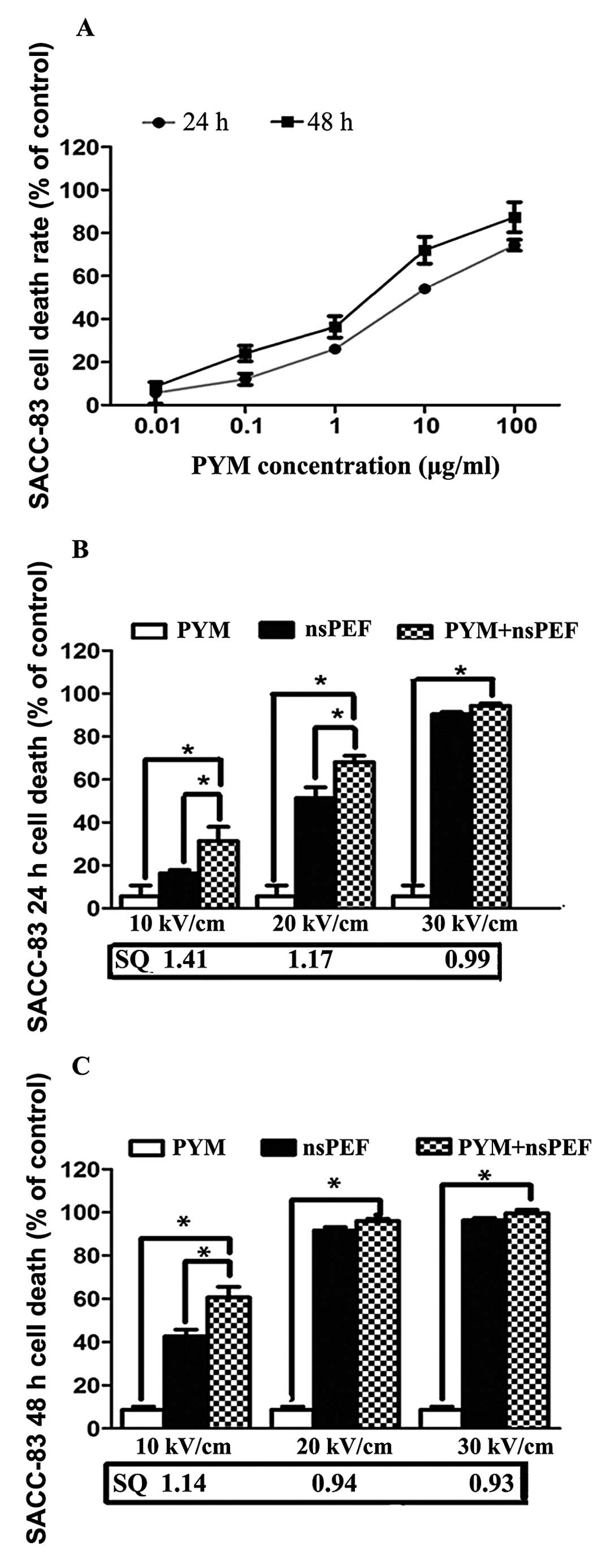
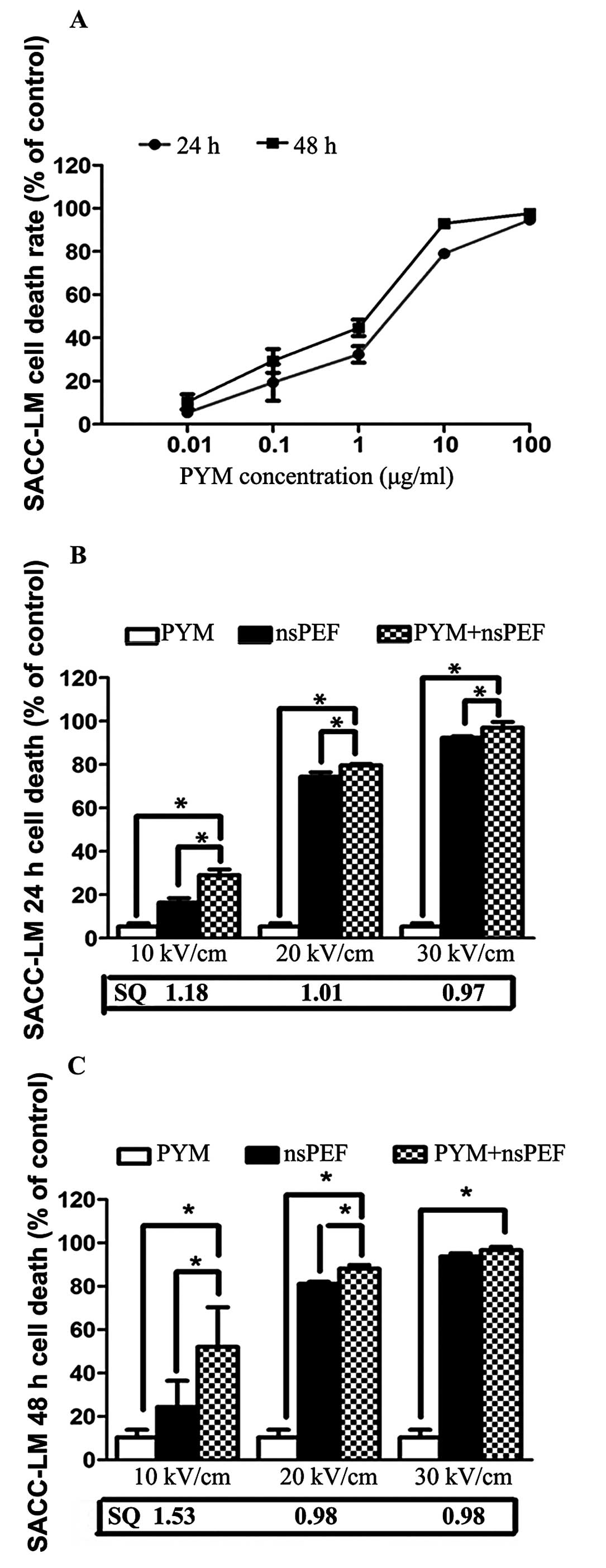
The inhibitory effect of PYM on proliferation of
SACC-83 and SACC-LM was determined by CCK-8 assay. As shown in
Figs. 2A and 3A, PYM significantly inhibited
proliferation in both cell lines and the suppression effect is
time- and dose-related. Based on these results, we set 0.01 μg/ml
as the extremely low concentration for the combination treatment,
which is much lower than the IC50 of PYM to SACC cells.
Fig. 2B and C and Fig. 3B and C demonstrate the effects of
nsPEF (10, 20 and 30 kV/cm) and PYM alone and in combination in a
CCK-8 cell death assay 24 and 48 h following treatment. When used
alone, there was a time- and electric field-dependent increase in
nsPEF-induced cell death. The combination group shows an obviously
synergistic effect of inhibition with the lower field strength 24
and 48 h after exposure, and the IC50 in group D is much
lower than that of PYM. However, as the field strength of nsPEF
increased, no marked synergistic but an enhancement effect was
detected.
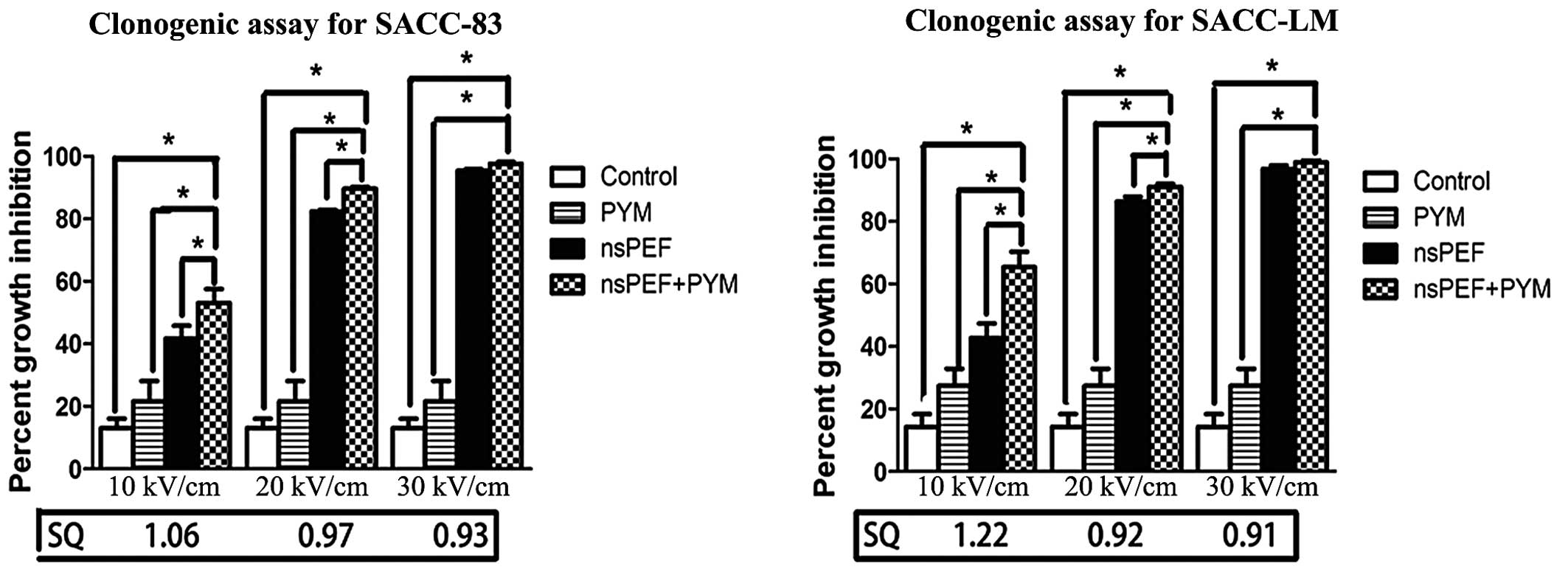
nsPEF with PYM enhances the inhibition
effect of colony formation
As shown in Fig. 4,
the results from the clonogenic assay are consistent with those of
CCK-8 assay. Again, there is a field strength-dependent increase in
inhibition of colony formation with either nsPEF treatment alone or
in combination with PYM. Furthermore, nsPEF combined with low
concentrations of PYM significantly inhibited cell growth in both
cell lines.
nsPEF with PYM induces apoptosis and
necrosis of both cell lines
The numbers of apoptotic and necrotic cells were
determined by Annexin V-FITC and PI double staining. With a lower
electric field, nsPEF played a mild role in inducing cell apoptosis
in the two cell lines. When the voltage was increased to 30 kV/cm,
a great number of SACC-83 cells underwent necrosis whereas numerous
SACC-LM cells underwent apoptosis (Fig.
5). When combined with PYM, a synergistic effect on apoptosis
and necrosis was detected in both cell lines although no
significant effect on promoting apoptosis or necrosis was detected
when PYM was used alone.
nsPEF with PYM inhibits the invasion of
SACC-83 and SACC-LM cells
As shown in Fig. 6,
SACC-LM cells exhibited more aggressive behavior than SACC-83
cells, which is in consistent with the characteristics of the two
cell lines. Both nsPEF and PYM inhibit the invasion potential of
SACC cells. In addition, the inhibition of nsPEF alone or in
combination with PYM for migration in both cell lines was
electrical field strength-related, and when the field strength
increased to 30 kV/cm, the cells that transmit the polycarbonate
filters were hardly detected. As is shown in Fig. 6, PYM statistically enhanced the
effect of nsPEF although only a weak synergism was detected in
SACC-83 cells with a lower electric field (10 kV/cm).
nsPEF and PYM inhibit the expression of
EMMPRIN in SACC-83 and SACC-LM cells
The difference in EMMPRIN expression of SACC-83 and
SACC-LM was investigated 2 h after exposure to nsPEF, as shown in
Fig. 7. The results suggested that
EMMPRIN expression suppression of nsPEF on SACC-83 and SACC-LM
cells is electric field-correlated. In addition, it was found that
PYM does not affect EMMPRIN expression when used alone with a low
concentration (0.01 μg/ml), even under the administration of
nsPEF.
Discussion
Adenoid cystic carcinoma (ACC) is characterized by
extensive local invasion and a tendency for distant metastasis
(21–23). The mainstay of treatment is
comprehensive therapy which includes local resection combined with
postoperative radiation. However, both may lead to aesthetic or
functional complications, especially for young people. The present
study found that nsPEF combined with low-dose PYM may act
synergistically and develop an effective inhibitory effect in the
proliferation, apoptosis and invasion of SACC cells in
vitro. As the side-effects of nsPEF and chemotherapy in a low
dose are minimal, it provides a potential strategy for SACC
treatment.
nsPEF has emerged as an anticancer strategy by
inducing cell death in many tumors (16,19,24,25).
nsPEF may interact with plasma membranes and intracellular
organelles, resulting in the DNA damage, cellular calcium bursts,
apoptosis or other forms of cell death (10,26).
It has been confirmed that nsPEF may inhibit proliferation of
several types of neoplasm in vitro and in vivo, and
the death induced by nsPEF is depended on cell type (17). Our results showed that nsPEF induced
apoptosis of the two cell lines in a relatively low field strength,
whereas when the field strength increased to 30 kV/cm, the
proportion of SACC-83 cells that underwent necrosis was more than
that of apoptosis. Moreover, we observed that nsPEF inhibited
proliferation of SACC-83 and SACC-LM cells significantly and the
suppression was electric field-dependent. Although ignorable when
applied with 10 kV/cm alone to SACC-LM cells, the inhibitory effect
of the applied nsPEF may be improved significantly when combined
with low-dose of PYM.
PYM has been applied in clinical chemotherapy in
China since 1979 (27,28), and it has been fairly extensively
used in chemotherapy for treatment of squamous cell carcinoma,
malignant lymphoma, Hodgkin’s disease, breast cancer and ACC
(3,29–31).
Principally, PYM exerts its chemotherapeutic effects through damage
to cellular DNA (32). Tai et
al found that the cytotoxic effect of PYM on KB cells was
concentration-dependent (33).
Morphological changes in KB cells treated with a lower dose of PYM
showed features of necrosis 24 h after exposure, whereas the number
of apoptotic cells increased significantly when the concentration
was increased 100-fold. Tai et al proposed that the
mechanism of cell death resulted from PYM depended on the number of
PYM molecules present inside the cell and PYM may cross the cell by
passive diffusion. However, PYM overdose often leads to several
complications involving multiple organs and systems, especially
pulmonary fibrosis (34–36). Thus, the comprehensive treatment was
widely considered to minimize the side-effects and improve the
therapeutic efficacy.
The synergism of nsPEF with very low doses of PYM
found in our study demonstrates a possibility for further clinical
use. First, the results showed that PYM inhibited proliferation of
SACC cells in a dose- and time-dependent manner. Meanwhile, we
found the significant synergistic activity with combination
treatment of nsPEF and low dose of PYM to inhibit proliferation,
induce apoptosis and necrosis in the two cell lines as detected by
flow cytometry. However, not synergism but an enhancement effect
was exhibited in the high field strength group in CCK-8 assay,
clonogenic assay and Transwell assay, which contributed to the high
efficiency of nsPEF. The PYM crosses the cell by passive diffusion
and the nano-pores caused by nsPEF were small and short lived
(37); thus, we speculated that the
sites or pathways that nsPEF and PYM acted on were different,
rather than enhancement of PYM passive diffusion.
EMMPRIN is widely expressed in various cancer cell
lines and is associated with an invasive phenotype of various types
of tumors, including SACC (4,38,39).
In the present study, we observed that the suppression effect of
nsPEF on EMMPRIN expression was field strength-dependent. When
combined with a low dose of PYM, EMMPRIN expression was not
decreased significantly, which suggests that EMMPRIN was not a
common target for the two treatments. Grass et al proved
that EMMPRIN may induce breast epithelial cell invasiveness by
promoting the EGFR-Ras-ERK signaling pathway (5). Furthermore, PYM could inhibit
esophageal squamous cell carcinoma proliferation by downregulating
EGFR expression (30). It has been
proved that EGFR is highly expressed in ACC (40,41)
and is involved in cell proliferation, motility, adhesion,
invasion, angiogenesis and survival (42,43).
In other words, PYM may not inhibit SACC cell proliferation and
invasion through EMMPRIN directly, but may suppress EGFR expression
to play a synergistic effect combined with nsPEF.
Multi-drug resistance (MDR) of tumor cells towards
chemotherapeutic drugs is the main reason for failure of
chemotherapy. EMMPRIN played a vital role in MDR (44), and mediated chemoresistance of
various types of cancer in several pathways, such as increasing the
expression of P-glycoprotein (MDR1/ABCB1), stimulating
phosphoinositide 3-kinase/AKT cell survival signaling pathway (an
anti-apoptosis pathway), and upregulating MMP expression (45,46).
However, the relationship between PYM resistance and the pathways
referred above remain unknown. Zheng et al (47) found that carbonic anhydrase 9 (CA9)
served as a contributor to PYM resistance in human tongue cancer
and silencing CA9 may improve PYM chemosensitivity. Recent studies
have shown that EMMPRIN is associated with CA9 expression and
indicates an adverse prognosis in breast cancer (48). Hence, we proposed that nsPEF
enhanced the chemosensitivity of SACC cell lines to PYM indirectly
through suppression of EMMPRIN expression and led to the
synergism.
It was of vital clinical significance that
regardless the pathways or sites that PYM impacted, nsPEF was able
to inhibit the EMMPRIN expression that played a central role in
MDR. In other words, nsPEF may improve the chemosensitivity of
other chemotherapeutic drugs which shared the mechanism of
chemoresistance referred. Furthermore, as shown in the present
study, nsPEF with appropriate electric field may also serve as an
effective treatment for SACC. The proposed approach which combines
nsPEF with PYM may be a promising way to inactivate SACC tumor
cells, and it may also markedly reduce the side-effects due to the
extremely low dose of chemotherapy used.
In conclusion, the present study demonstrated that
there is a synergistic effect in SACC cell lines when nsPEF is
combined with a low dose of PYM, a commonly used chemotherapy
agent, and it may provide a valuable tool to treat SACC. Western
blot results suggested that the mechanism for this synergism is
probably due to the suppression of EMMPRIN expression induced by
nsPEF. Further studies will be carried out in EMMPRIN-targeted
cancer therapy induced by nsPEF.
Acknowledgements
The authors thank Shenglin Li, Xin Cong and Lingfei
Jia for their excellent technical assistance.
References
|
1
|
Li LJ, Li Y, Wen YM, Liu H and Zhao HW:
Clinical analysis of salivary gland tumor cases in West China in
past 50 years. Oral Oncol. 44:187–192. 2008.PubMed/NCBI
|
|
2
|
Rapidis AD, Givalos N, Gakiopoulou H, et
al: Adenoid cystic carcinoma of the head and neck.
Clinicopathological analysis of 23 patients and review of the
literature. Oral Oncol. 41:328–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dodd RL and Slevin NJ: Salivary gland
adenoid cystic carcinoma: a review of chemotherapy and molecular
therapies. Oral Oncol. 42:759–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fink K and Boratyński J: The role of
metalloproteinases in modification of extracellular matrix in
invasive tumor growth, metastasis and angiogenesis. Postepy Hig Med
Dosw (Online). 66:609–628. 2012.(In Polish).
|
|
5
|
Grass GD, Tolliver LB, Bratoeva M and
Toole BP: CD147, CD44, and the epidermal growth factor receptor
(EGFR) signaling pathway cooperate to regulate breast epithelial
cell invasiveness. J Biol Chem. 288:26089–26104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Dai J, Li T, et al: Expression of
EMMPRIN in adenoid cystic carcinoma of salivary glands: correlation
with tumor progression and patients’ prognosis. Oral Oncol.
46:755–760. 2010.PubMed/NCBI
|
|
7
|
Yang X, Zhang P, Ma Q, et al: EMMPRIN
silencing inhibits proliferation and perineural invasion of human
salivary adenoid cystic carcinoma cells in vitro and in vivo.
Cancer Biol Ther. 13:85–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kotnik T and Miklavcic D: Theoretical
evaluation of voltage inducement on internal membranes of
biological cells exposed to electric fields. Biophys J. 90:480–491.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gowrishankar TR and Weaver JC: Electrical
behavior and pore accumulation in a multicellular model for
conventional and supra-electroporation. Biochem Biophys Res Commun.
349:643–653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beebe SJ, Fox PM, Rec LJ, Willis EL and
Schoenbach KH: Nanosecond, high-intensity pulsed electric fields
induce apoptosis in human cells. FASEB J. 17:1493–1495.
2003.PubMed/NCBI
|
|
11
|
Vernier PT, Sun Y, Marcu L, Salemi S,
Craft CM and Gundersen MA: Calcium bursts induced by nanosecond
electric pulses. Biochem Biophys Res Commun. 310:286–295. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vernier PT, Sun Y, Marcu L, Craft CM and
Gundersen MA: Nanoelectropulse-induced phosphatidylserine
translocation. Biophys J. 86:4040–4048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vernier PT, Ziegler MJ, Sun Y, Gundersen
MA and Tieleman DP: Nanopore-facilitated, voltage-driven
phosphatidylserine translocation in lipid bilayers - in cells and
in silico. Phys Biol. 3:233–247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beebe SJ, Blackmore PF, White J, Joshi RP
and Schoenbach KH: Nanosecond pulsed electric fields modulate cell
function through intracellular signal transduction mechanisms.
Physiol Meas. 25:1077–1093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nuccitelli R, Pliquett U, Chen X, et al:
Nanosecond pulsed electric fields cause melanomas to self-destruct.
Biochem Biophys Res Commun. 343:351–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hall EH, Schoenbach KH and Beebe SJ:
Nanosecond pulsed electric fields induce apoptosis in p53-wildtype
and p53-null HCT116 colon carcinoma cells. Apoptosis. 12:1721–1731.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren W and Beebe SJ: An apoptosis targeted
stimulus with nanosecond pulsed electric fields (nsPEFs) in E4
squamous cell carcinoma. Apoptosis. 16:382–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nuccitelli R, Lui K, Kreis M, Athos B and
Nuccitelli P: Nanosecond pulsed electric field stimulation of
reactive oxygen species in human pancreatic cancer cells is
Ca2+-dependent. Biochem Biophys Res Commun. 435:580–585.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Guo J, Wu S, et al: Synergistic
effects of nanosecond pulsed electric fields combined with low
concentration of gemcitabine on human oral squamous cell carcinoma
in vitro. PLoS One. 7:e432132012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Kolb JF, Swanson RJ, Schoenbach KH
and Beebe SJ: Apoptosis initiation and angiogenesis inhibition:
melanoma targets for nanosecond pulsed electric fields. Pigment
Cell Melanoma Res. 23:554–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szanto PA, Luna MA, Tortoledo ME and White
RA: Histologic grading of adenoid cystic carcinoma of the salivary
glands. Cancer. 54:1062–1069. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van der Wal JE, Becking AG, Snow GB and
van der Waal I: Distant metastases of adenoid cystic carcinoma of
the salivary glands and the value of diagnostic examinations during
follow-up. Head Neck. 24:779–783. 2002.PubMed/NCBI
|
|
23
|
Gao M, Hao Y, Huang MX, et al:
Clinicopathological study of distant metastases of salivary adenoid
cystic carcinoma. Int J Oral Maxillofac Surg. 42:923–928. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren W, Sain NM and Beebe SJ: Nanosecond
pulsed electric fields (nsPEFs) activate intrinsic
caspase-dependent and caspase-independent cell death in Jurkat
cells. Biochem Biophys Res Commun. 421:808–812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nuccitelli R, Tran K, Sheikh S, Athos B,
Kreis M and Nuccitelli P: Optimized nanosecond pulsed electric
field therapy can cause murine malignant melanomas to self-destruct
with a single treatment. Int J Cancer. 127:1727–1736. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stacey M, Stickley J, Fox P, et al:
Differential effects in cells exposed to ultra-short, high
intensity electric fields: cell survival, DNA damage, and cell
cycle analysis. Mutat Res. 542:65–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He NG, Zhang HQ, Wang RH, Yang XL and Xue
SB: Enhancement of antitumor activity of bleomycin A5 in mouse
sarcoma 180 cells in vitro and in vivo by verapamil. Zhongguo Yao
Li Xue Bao. 11:381–384. 1990.(In Chinese).
|
|
28
|
He NG, Zhang HQ, Song PG, Liu ZM and Xue
SB: Mechanism of enhancement of bleomycin A5 antitumor activity by
verapamil. Yao Xue Xue Bao. 26:15–19. 1991.(In Chinese).
|
|
29
|
Yue H, Qian J, Elner VM, et al: Treatment
of orbital vascular malformations with intralesional injection of
pingyangmycin. Br J Ophthalmol. 97:739–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong JH, Liu XJ, Li Y and Zhen YS:
Pingyangmycin downregulates the expression of EGFR and enhances the
effects of cetuximab on esophageal cancer cells and the xenograft
in athymic mice. Cancer Chemother Pharmacol. 69:1323–1332. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong L, Lou JY, Wang P, Zhang JW, Liu H
and Peng ZL: Clinical evaluation of neoadjuvant chemotherapy
followed by radical surgery in the management of stage IB2-IIB
cervical cancer. Int J Gynaecol Obstet. 117:23–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tai KW, Chang YC, Chou LS and Chou MY:
Cytotoxic effect of pingyangmycin on cultured KB cells. Oral Oncol.
34:219–223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tai KW, Chou MY, Hu CC, Yang JJ and Chang
YC: Induction of apoptosis in KB cells by pingyangmycin. Oral
Oncol. 36:242–247. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji Y, Wang T, Wei ZF, et al: Paeoniflorin,
the main active constituent of Paeonia lactiflora roots,
attenuates bleomycin-induced pulmonary fibrosis in mice by
suppressing the synthesis of type I collagen. J Ethnopharmacol.
149:825–832. 2013.PubMed/NCBI
|
|
35
|
Trujillo G, Hartigan AJ and Hogaboam CM: T
regulatory cells and attenuated bleomycin-induced fibrosis in lungs
of CCR7−/− mice. Fibrogenesis Tissue Repair. 3:182010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou C, Han W, Zhang P, Cai M, Wei D and
Zhang C: Lycopene from tomatoes partially alleviates the
bleomycin-induced experimental pulmonary fibrosis in rats. Nutr
Res. 28:122–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pakhomov AG, Shevin R, White JA, et al:
Membrane permeabilization and cell damage by ultrashort electric
field shocks. Arch Biochem Biophys. 465:109–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang ZQ, Chen WL, Li HG, Li JS, Xu ZY and
Lin ZY: Extracellular matrix metalloproteinase inducer expression
in salivary gland tumors: a correlation with microvessel density. J
Craniofac Surg. 21:1855–1860. 2010. View Article : Google Scholar
|
|
39
|
Kim H, Zhai G, Liu Z, et al: Extracelluar
matrix metalloproteinase as a novel target for pancreatic cancer
therapy. Anticancer Drugs. 22:864–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hitre E, Budai B, Takácsi-Nagy Z, et al:
Cetuximab and platinum-based chemoradio- or chemotherapy of
patients with epidermal growth factor receptor expressing adenoid
cystic carcinoma: a phase II trial. Br J Cancer. 109:1117–1122.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang Y, Yu T, Fu X, et al: EGFR
inhibition prevents in vitro tumor growth of salivary adenoid
cystic carcinoma. BMC Cell Biol. 14:132013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang SM and Harari PM: Epidermal growth
factor receptor inhibition in cancer therapy: biology, rationale
and preliminary clinical results. Invest New Drugs. 17:259–269.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Woodburn JR: The epidermal growth factor
receptor and its inhibition in cancer therapy. Pharmacol Ther.
82:241–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen H, Wang L, Beretov J, Hao J, Xiao W
and Li Y: Co-expression of CD147/EMMPRIN with monocarboxylate
transporters and multiple drug resistance proteins is associated
with epithelial ovarian cancer progression. Clin Exp Metastasis.
27:557–569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kuang YH, Chen X, Su J, et al: RNA
interference targeting the CD147 induces apoptosis of multi-drug
resistant cancer cells related to XIAP depletion. Cancer Lett.
276:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang JM, Xu Z, Wu H, Zhu H, Wu X and Hait
WN: Overexpression of extracellular matrix metalloproteinase
inducer in multidrug resistant cancer cells. Mol Cancer Res.
1:420–427. 2003.PubMed/NCBI
|
|
47
|
Zheng G, Zhou M, Ou X, et al:
Identification of carbonic anhydrase 9 as a contributor to
pingyangmycin-induced drug resistance in human tongue cancer cells.
FEBS J. 277:4506–4518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pinheiro C, Sousa B, Albergaria A, et al:
GLUT1 and CAIX expression profiles in breast cancer correlate with
adverse prognostic factors and MCT1 overexpression. Histol
Histopathol. 26:1279–1286. 2011.PubMed/NCBI
|