Introduction
Tyrosine kinase inhibitors (TKIs) have a great
impact on routine treatment in oncology. Imatinib (STI-571,
Glivec®, Gleevec®), the prototype of TKIs,
was approved in 2003 for the first-line therapy of chronic myeloid
leukemia (CML) (1). Since 2010,
various TKIs, such as nilotinib (Tasigna®), have been
developed for the first-line therapy of CML patients (2,3). Apart
from CML, TKIs such as imatinib have been approved for first-line
therapy of gastrointestinal stromal tumors (GIST), renal cell
carcinoma (RCC), melanoma or a subgroup of non-small cell lung
cancers (NSCLCs) (4–6). In CML, imatinib targets the
BCR/ABL-fusion protein known as the central oncogenic signaling
cascade resulting in a reduction of leukemic cells without
additional chemotherapy (7).
Nevertheless, some patients are or become resistant to imatinib.
Therefore, new BCR/ABL-inhibitors, such as nilotinib and dasatinib,
have been developed to overcome imatinib resistance (2,3). Apart
from its direct cell-autonomous effect, additional
immunostimulation, mediated by host cells such as dendritic cells
(DCs) and natural killer (NK) cells, was demonstrated in a murine
B16F10 melanoma model (8). NK cells
play a central role in antitumor immunity. They recognize
virus-infected or transformed tumor cells resulting in direct
cytotoxic killing of these targets and cytokine secretion
consequently inducing a general immune response (9). NK cells are defined as CD3-negative,
NKp46 and NK1.1-positive lymphocytes in C57Bl/6 mice. They can be
further subdivided into three groups by their surface expression of
CD27 and CD11b (10).
In the present study, we addressed the role of
nilotinib, as a TKI with a different selectivity profile to that of
imatinib, in combination with IL-2 in a B16 melanoma model
(11). Our results clearly
demonstrated an elevated antitumor effect of nilotinib/IL-2 when
compared to that of imatinib/IL-2. Notably, the therapeutic effect
of nilotinib/IL-2 was lost in immunodeficient mice and following
depletion of NK cells. The increase in CD27+ NK cells as
the main source of IFN-γ among NK cells as well as the abolished
antitumor efficacy of the treatment protocol in IFN-γ−/−
mice emphasize the specific importance of the subset of
IFN-γ-producing CD27+ NK cells. These new findings
provide new insights to further improve immunotherapeutic
protocols.
Materials and methods
Animals
Female C57Bl/6 mice were obtained from Elevage
Janvier (Le Genest St. Isle, France), were housed in the Franz
Penzoldt Centre (University Erlangen, Germany) and were used at 7–9
weeks of age. Rag2γc−/− mice and IFN-γ knockout mice
were kindly provided by Falk Nimmerjahn (Erlangen, Germany) and
Bernard Ryffel (Orléans, France), respectively. Animal experiments
were approved by the Regierung of Mittelfranken and Hessen,
Germany.
Flow cytometry
Single-cell suspensions were stained with FITC- or
PB-conjugated anti-CD11b (M1/70), PerCP-conjugated anti-CD3ɛ
(145-2C11), PerCP-conjugated anti-CD19 (1D3), PE-conjugated
anti-CD27 (LG.3A11), PE-Cy7-conjugated anti-NK1.1 (PK136) and APC-
and V450-conjugated anti-NKp46. Antibodies were purchased from
BioLegend (San Diego, CA, USA), BD Biosciences (Heidelberg,
Germany) and Miltenyi (Bergisch Gladbach, Germany). FACS
experiments were performed on a FACSCanto II instrument (BD
Biosciences) and analyzed by FlowJo software (Tree Star, Ashland,
OR, USA).
Functional analysis
IFN-γ production was assessed by ELISA after O/N
co-incubation with B16 cells (at an effector-target ratio of 10:1)
and 5,000 U/ml IL-2. ELISA was performed as described by the
supplier (BD Biosciences). The tumor lysis capacity of B16 melanoma
cells was investigated by crystal violet assay as previously
described (12).
Melanoma model and preparation of TKI
solutions
The B16 melanoma model was established as previously
described (13). Briefly, 500,000
B16F10 cells were injected i.v. on day 0, and mice received an oral
application of TKIs twice daily (b.i.d.) until day 11 as well as an
intraperitoneal (i.p.) injection of 100,000 U IL-2 (b.i.d.) from
day 6 until the end of the experiment. Imatinib (Novartis, Basel,
Switzerland) was used at a daily dosage of 75 or 150 mg/kg body
weight diluted in 100 μl polyethylene glycol (PEG) 300
(Sigma-Aldrich, Steinheim, Germany). Nilotinib (Novartis) was first
diluted in 1-methyl-2-pyrrolidinone solution at 0.2% and afterwards
was administered at a daily dose of 37.5 or 75 mg/kg body weight
diluted in 100 μl PEG 300. Dasatinib (Bristol-Myers Squibb, New
York, NY, USA) was used at a daily dosage of 25 mg/kg body weight
diluted in 100 μl PEG 300. Untreated controls received 100 μl PEG
300 p.o. and 100 μl PBS i.p.
Results
Nilotinib reduces the number of lung
metastases in the B16 melanoma model
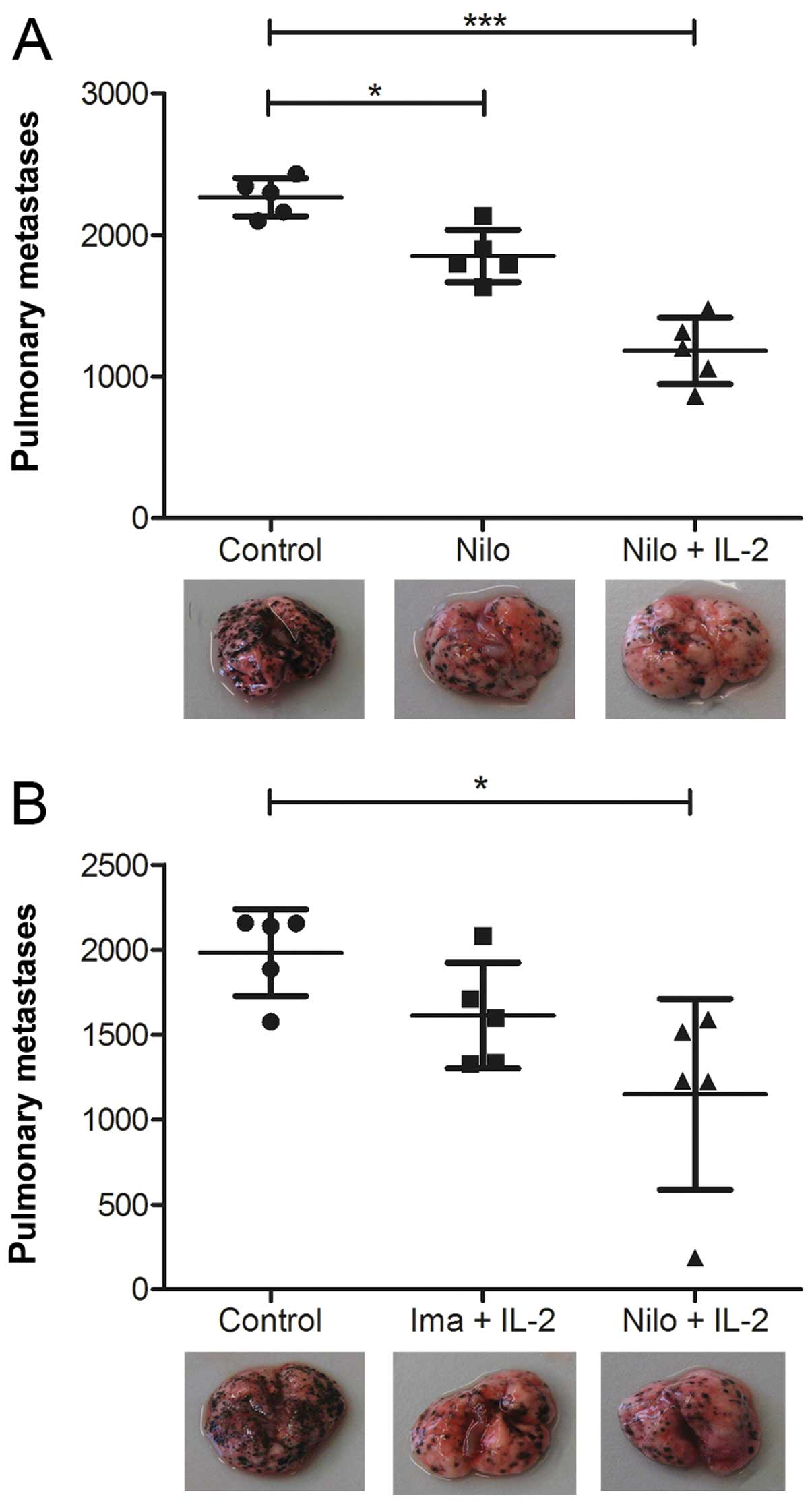
C57Bl/6 mice developing B16 melanoma metastases were
treated with either nilotinib alone (75 mg/kg per day) or in
combination with IL-2 (100,000 U), and the number of metastases
were compared to an untreated control group. Following analysis of
the number of lung metastases in the tumor-bearing mice, a
significant reduction in number was noted in the groups treated
with nilotinib alone or in combination with IL-2 (Fig. 1A). Yet, the combination of nilotinib
and IL-2 led to the most impressive antitumor effect. As it was
previously demonstrated that imatinib combined with IL-2 induces a
superior tumor response than either substance alone (8,14), we
performed experiments directly comparing imatinib/IL-2 and
nilotinib/IL-2. Our results revealed a superior effect in mice
treated with nilotinib and IL-2 (Fig.
1B). The reduced number of lung metastases is further
illustrated by images showing the lung of one representative animal
per group (Fig. 1). Notably, a dose
escalation of imatinib up to 300 mg/kg daily or nilotinib up to 150
mg/kg daily did not further improve the antitumor potency (data not
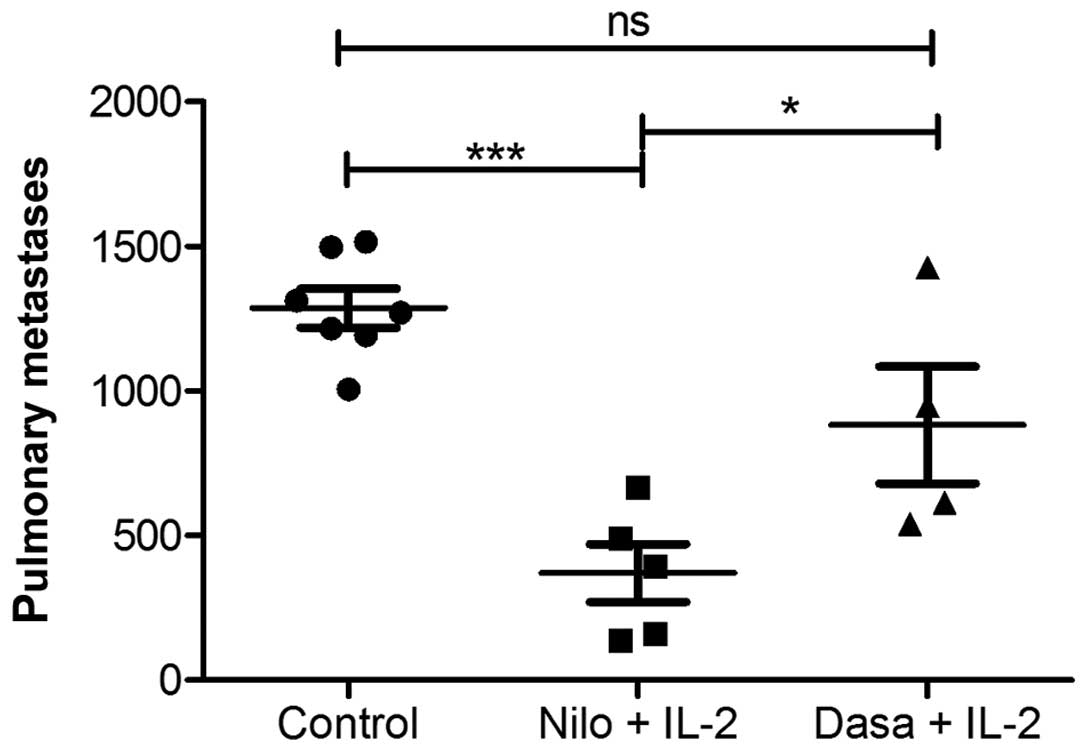
shown). In an additional experiment, we tested dasatinib as a
multi-targeted TKI also approved for first-line therapy of CML
(2,15). When directly comparing dasatinib and
nilotinib as a treatment option in the murine melanoma model, both
in combination with IL-2, dasatinib did not demonstrate an evident
reduction in lung metastases when compared to that observed
following treatment with nilotinib (Fig. 2). Furthermore, treatment with
dasatinib was less well-tolerated when compared to the other TKIs
as mice developed an effusion syndrome during dasatinib treatment.
Based on the marked antitumor potency of nilotinib/IL-2, we aimed
to further investigate the involvement of the immune system in this
therapeutic concept.
The therapeutic effect of nilotinib
combined with IL-2 is NK cell-dependent
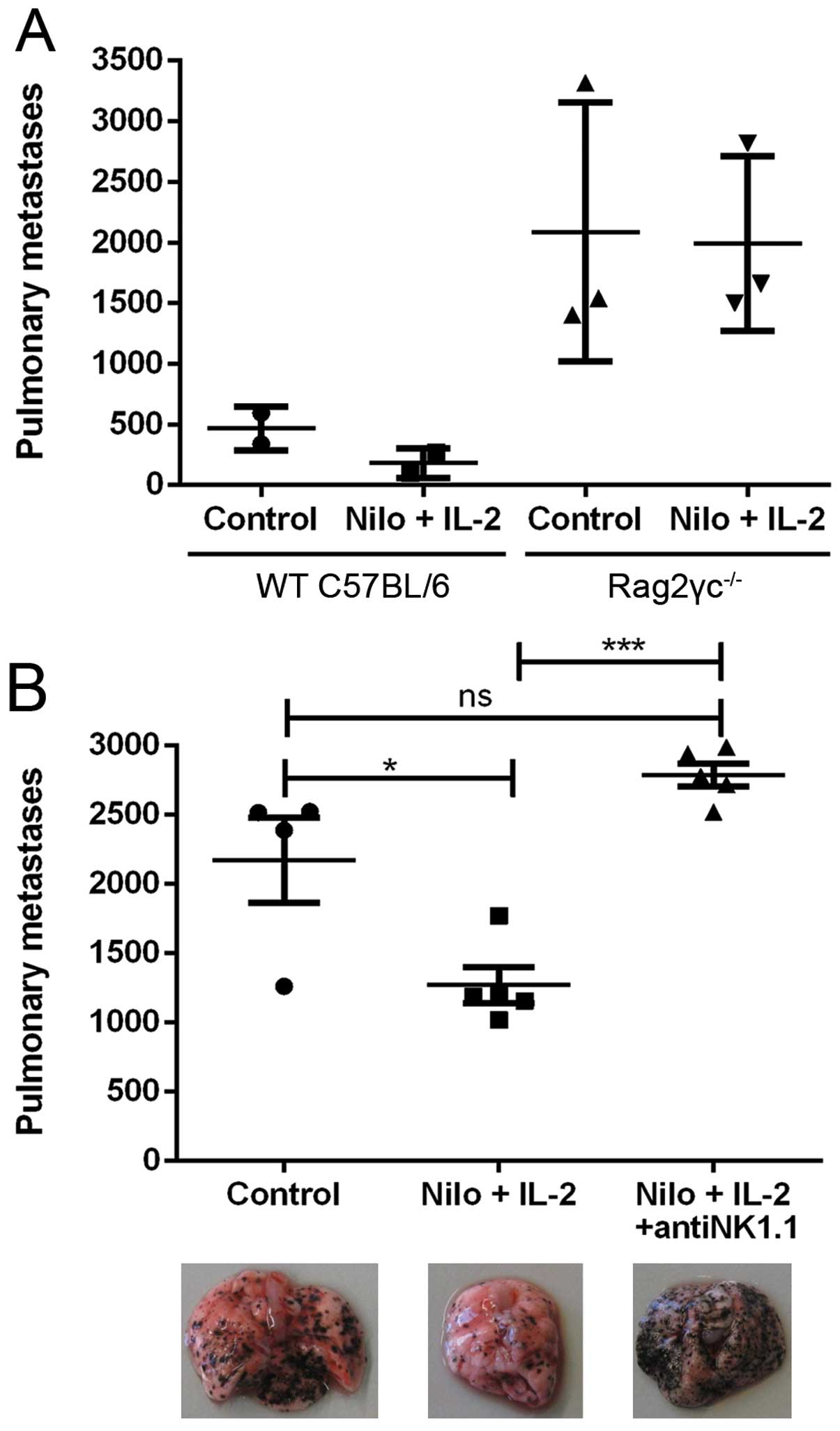
To assess the impact of the immune system on the
positive antitumor effects of nilotinib/IL-2, we injected B16
melanoma cells into Rag2γc−/− mice lacking
T-lymphocytes, B-lymphocytes and NK cells. In these immunodeficient
mice we observed at least a 2.5-fold higher number of lung
metastases when compared to this number in the C57Bl/6 wild-type
(WT) mice. Moreover, the therapeutic effect of nilotinib/IL-2 was
completely abrogated in the Rag2γc−/− mice, revealing a
possible role for T-lymphocytes, B-lymphocytes and NK cells in the
antitumor potency (Fig. 3A). Next,
we analyzed the impact of NK cells in this model. By using
anti-NK1.1-specific antibodies, we effectively depleted NK cells in
C57Bl/6 mice. In general, the mice depleted of NK cells had a
significantly higher number of lung metastases. Again, the
therapeutic effect of the nilotinib/IL-2 combination therapy was
nullified in the treated group (Fig.
3B). These data are illustrated by images of the
metastasis-bearing lungs (Fig. 3B,
below the graph).
Nilotinib/IL-2 treatment increases the
number of CD27+ IFN-γ-producing NK cells
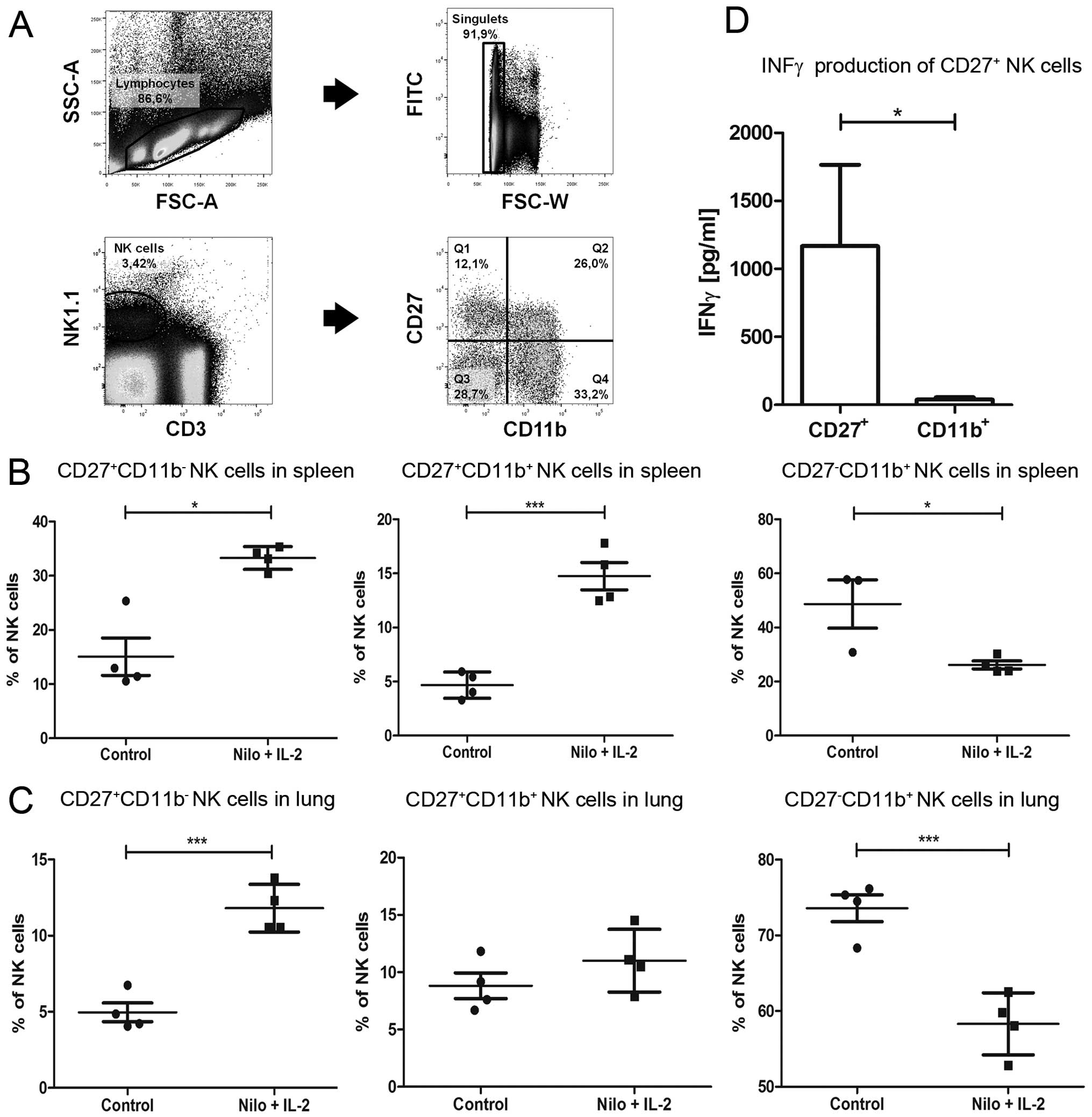
We next performed extensive immune monitoring by
flow cytometry focusing on NK cell subpopulations in different
organs of the tumor-bearing mice that were either treated or not
with nilotinib/IL-2. The gating strategy to assess NK cell subsets
distinguished by the expression of CD27 and CD11b is shown in
Fig. 4A. Regarding the distribution
of different NK cell subsets in peripheral organs, a significant
increase in CD27+CD11b− NK cells was observed
in the lung and spleen of the nilotinib/IL-2-treated mice when
compared to that in the untreated controls (Fig. 4B and C). In contrast, in the same
organs, a significant reduction in
CD27−CD11b+ NK cells was noted in the treated
mice. A significant increase in the
CD27+CD11b+ intermediate NK cell subset was
only found in the lung of the treated mice but not in the spleen.
We further addressed the functional relevance of sorted
CD27+ NK cells in comparison to CD11b+ NK
cells in vitro. Importantly, co-cultivation of purified NK
cell subpopulations with B16 melanoma cells led to a significantly
higher IFN-γ secretion of the CD27+ NK cells when
compared to that of the CD11b+ NK cell subpopulation
(Fig. 4D).
IFN-γ is suggested as a key player in the
antitumor effect mediated by nilotinib/IL-2
Based on the knowledge that the number of
IFN-γ-secreting NK cells are increased during therapy with
nilotinib/IL-2, we aimed to ascertain whether IFN-γ is relevant for
the outcome of this therapeutic regimen. To solve this issue, we
used IFN-γ knockout mice with a C57Bl/6 background injected with
B16 melanoma cells and used exactly the same therapeutic protocol
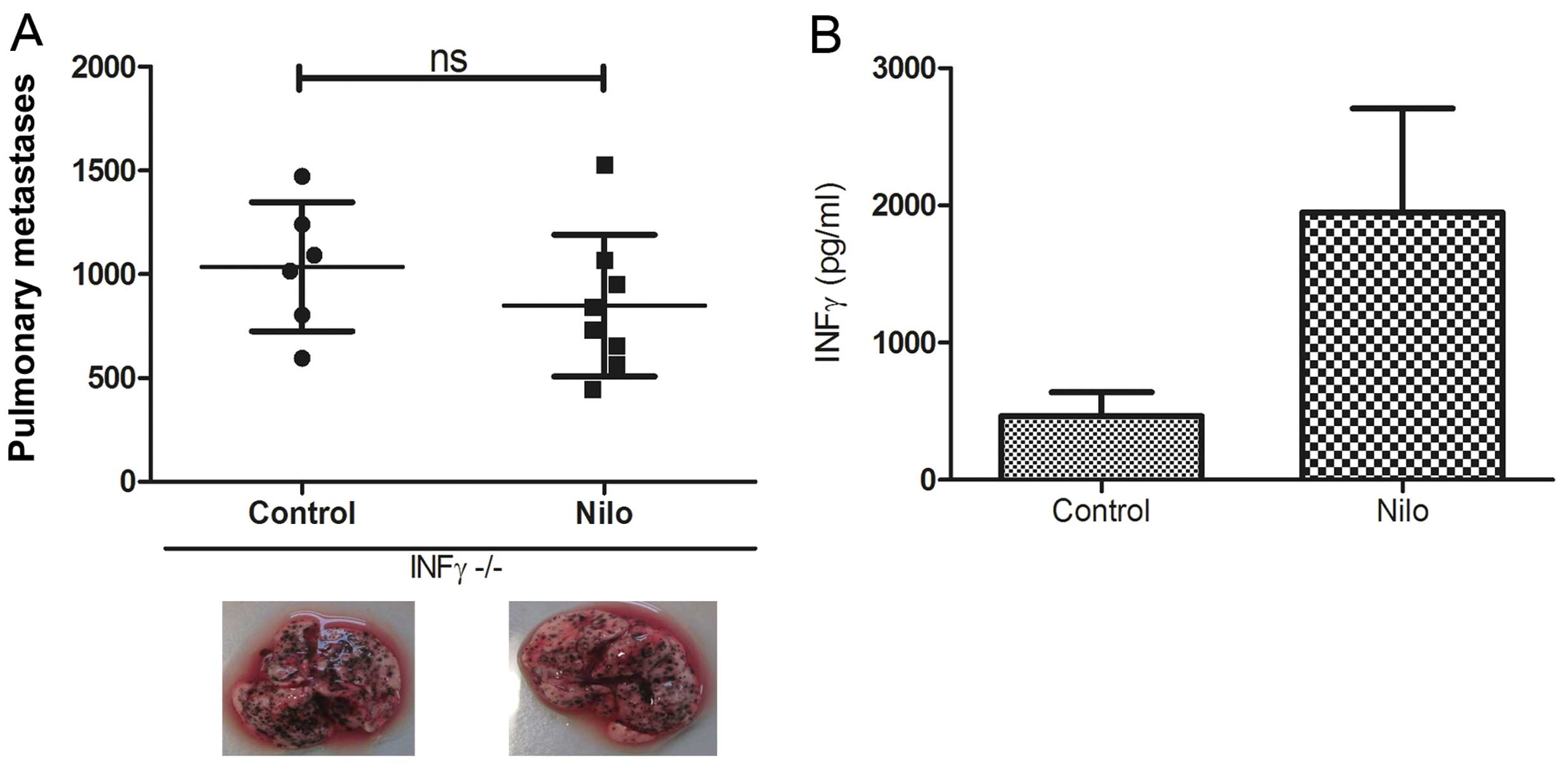
as for WT C57Bl/6 mice. Indeed, in the IFN-γ−/− mice,
nilotinib combined with IL-2 completely lost its antitumor efficacy
as shown by an equally high number of lung metastases. Images of
one representative animal per group are shown in Fig. 5A. This result is in line with our
observation of an elevated IFN-γ concentration in supernatants of
splenocytes from mice that were treated with nilotinib/IL-2 in
vivo and further stimulated with B16 tumor cells in
vitro (Fig. 5B). In summary,
these experiments emphasize the role of IFN-γ in the antitumor
immune response induced by nilotinib/IL-2 which is possibly
mediated by the subset of CD27+ NK cells.
Discussion
Since imatinib was clinically approved as the first
TKI for the treatment of CML more than 10 years ago, TKIs are
frequently used in daily clinical routine. During the last few
years, two second-generation BCR/ABL TKIs, nilotinib and dasatinib,
have been approved for the first-line therapy of CML (2,3). The
combination of imatinib and IL-2 has been reported to interfere
with the immune system, particularly with DC and NK cells (8,14).
Recently, a phase I clinical trial was carried out using a
combination of imatinib plus IL-2 and low-dose cylcophosphamid in
patients with refractory solid tumors. The level of
HLA-DR+ NK cells in the blood of the patients was
positively correlated with progression-free survival (PFS) and
overall survival (OS) (16).
In the present study, we investigated the effects on
the immune system induced by nilotinib therapy. Using a B16F10
mouse melanoma model, we observed only a moderate reduction in the
number of lung metastasis induced by nilotinib alone, possibly due
to an impaired angiogenesis as a result of PDGFR inhibition.
Interestingly, the combination of nilotinib (daily 75 mg/kg) plus
IL-2 significantly reduced the number of lung metastases when
compared to the number in the untreated controls (Fig. 1). In our murine tumor model,
escalating doses of nilotinib (150 mg/m2 daily) did not
have any additional benefit. When comparing nilotinib/IL-2 with
imatinib/IL-2, a clear advantage was observed for the therapeutic
protocol using nilotinib. Importantly, our observation in the
murine model is in line with clinical data from the international
multi-center ENESTnd trial using nilotinib as first-line therapy
for newly diagnosed Ph+ CML in the chronic phase
resulting in improved rates of major molecular response (MMR) at 12
months (43–44 vs. 22%; P<0.001) and complete cytogenetic
response rates (CCyR; 78–80 vs. 65%; P<0.001) compared to
standard treatment with imatinib (3). In addition, the DASISION trial for
first-line treatment of CML patients revealed that dasatinib
demonstrated better CCyR and MMR in comparison to imatinib
(2). In contrast, in our murine
tumor model, we did not observe a comparable positive effect by
dasatinib treatment, even in combination with IL-2 (Fig. 2). Moreover, dasatinib-treated mice
frequently developed pleural effusion and ascites as a side effect
that may be in line with reports of pleural effusion by at least
some patients included in the DASISION trial (2).
In preclinical studies, Salih et al (17) addressed the impact of imatinib,
dasatinib and nilotinib on human NK cell function in vitro.
Whereas imatinib did not have any effect on human NK cell function
(cytotoxicity and cytokine production), nilotinib impaired NK cell
cytokine production at a high dosage, and dasatinib abrogated both
NK cell cytotoxicity as well as cytokine production, possibly by
inducing apoptosis in the CD56high NK cell subset
(17).
Due to the significant reduction in tumor
progression following nilotinib/IL-2 treatment in the present
study, we further focused on the impact of this therapeutic
protocol on the immune system. It has been demonstrated that
imatinib does not exclusively act by direct cytotoxicity on B16F10
tumor cells in vitro (8,14), but
the antitumor effect is mediated by additional stimulation of the
immune system. In line with this finding, we observed a beneficial
stimulating impact of nilotinib on the immune system. By using
Rag2γc−/− mice lacking T-lymphocytes, B-lymphocytes and
NK cells, we demonstrated that the antitumor effect of nilotinib
was dependent on immune cells (Fig.
3). In particular, NK cells played an important role as the
antitumor effect observed by nilotinib/IL-2 was completely
abrogated after depletion of NK cells (Fig. 3B). Concerning the distribution of
the different NK cell subsets, we found an increase in the
cytokine-producing NK cell subset
(CD27+CD11b−) in the lung and spleen of
nilotinib/IL-2 treated mice and a decrease in mature
CD27− CD11b+ NK cells in the same
compartments (Fig. 4). We suggest
that this distribution results from a higher production of more
immature CD27+ NK cells. Importantly, the
CD27+ NK cell subpopulation was determined to be the
main producer of high amounts of IFN-γ.
Notably, in line with these observations, we
measured high amounts of IFN-γ in the supernatants of splenocytes
isolated from mice that were treated with nitolinib (Fig. 5). The fact that Salih et al
(17) demonstrated a negative
impact of nilotinib on NK cell cytokine production could be
explained by the elevated dosing when tested in vitro. In
our model, oral application may have led to a more physiologically
relevant plasma concentration of nilotinib. It is notable that
Hassold et al (18) found
that dasatinib inhibited NK cell function during functional assays
but was even able to enhance cytokine secretion and cytotoxicity
after a 24-h wash-out period of dasatinib.
We further confirmed the relevance for IFN-γ in
vivo by use of IFN-γ−/− mice as tumor bearers.
IFN-γ−/− mice exhibited no antitumor efficacy upon
treatment with nilotinib/IL-2 (Fig.
5).
In summary, we report a high antitumor potential of
the combination therapy of nilotinib/IL-2. We further suggest a
major role of IFN-γ-producing CD27+ NK cells in the
therapeutic effects observed. The immune-stimulating effect of
nilotinib/IL-2 was superior to that of imatinib/IL-2 and
dasatinib/IL-2. This positive immune modulation may be an
additional reason for the superiority of nilotinib when compared to
imatinib in first-line therapy for CML apart from the direct
inhibition of the BCR/ABL transcript.
Acknowledgements
The authors are grateful to Alexandra Abendroth,
Julia Schneider and Franziska Ganss for their technical assistance
and to PD Dr Jörg Distler and Professor Falk Nimmerjahn for
material support and helpful discussions. This study was supported
by Novartis, Bristol-Myers Squibb, and Bayer Health Care. The
Laboratory of EU was supported by the German Cancer Aid (Max Eder
Nachwuchsgruppe, Deutsche Krebshilfe), the Wilhelm Sander
Foundation and the LOEWE Center for Cell and Gene Therapy,
Frankfurt, funded by the Hessian Ministry of Higher Education,
Research and the Arts, Germany (III L 4-518/17.004).
Abbreviations:
|
CML
|
chronic myeloid leukemia
|
|
Dasa
|
dasatinib
|
|
DC
|
dendritic cell
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
IFN
|
interferon
|
|
IL
|
interleukin
|
|
IMA
|
imatinib
|
|
Nilo
|
nilotinib
|
|
NK cell
|
natural killer cell
|
|
O/N
|
over night
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
O’Brien SG, Guilhot F, Larson RA, et al:
Imatinib compared with interferon and low-dose cytarabine for newly
diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med.
348:994–1004. 2003.
|
|
2
|
Kantarjian H, Shah NP, Hochhaus A, et al:
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic
myeloid leukemia. N Engl J Med. 362:2260–2270. 2010. View Article : Google Scholar
|
|
3
|
Saglio G, Kim DW, Issaragrisil S, et al:
Nilotinib versus imatinib for newly diagnosed chronic myeloid
leukemia. N Engl J Med. 362:2251–2259. 2010. View Article : Google Scholar
|
|
4
|
Rosell R, Carcereny E, Gervais R, et al:
Erlotinib versus standard chemotherapy as first-line treatment for
European patients with advanced EGFR mutation-positive
non-small-cell lung cancer (EURTAC): a multicentre, open-label,
randomised phase 3 trial. Lancet Oncol. 13:239–246. 2012.
View Article : Google Scholar
|
|
5
|
Escudier B, Szczylik C, Hutson TE, et al:
Randomized phase II trial of first-line treatment with sorafenib
versus interferon alfa-2a in patients with metastatic renal cell
carcinoma. J Clin Oncol. 27:1280–1289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blanke CD, Rankin C, Demetri GD, et al:
Phase III randomized, intergroup trial assessing imatinib mesylate
at two dose levels in patients with unresectable or metastatic
gastrointestinal stromal tumors expressing the kit receptor
tyrosine kinase: S0033. J Clin Oncol. 26:626–632. 2008. View Article : Google Scholar
|
|
7
|
Hochhaus A, O’Brien SG, Guilhot F, et al:
Six-year follow-up of patients receiving imatinib for the
first-line treatment of chronic myeloid leukemia. Leukemia.
23:1054–1061. 2009.PubMed/NCBI
|
|
8
|
Borg C, Terme M, Taieb J, et al: Novel
mode of action of c-kit tyrosine kinase inhibitors leading to NK
cell-dependent antitumor effects. J Clin Invest. 114:379–388. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geller MA and Miller JS: Use of allogeneic
NK cells for cancer immunotherapy. Immunotherapy. 3:1445–1459.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayakawa Y and Smyth MJ: CD27 dissects
mature NK cells into two subsets with distinct responsiveness and
migratory capacity. J Immunol. 176:1517–1524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manley PW, Stiefl N, Cowan-Jacob SW,
Kaufman S, Mestan J, Wartmann M, Wiesmann M, Woodman R and
Gallagher N: Structural resemblances and comparisons of the
relative pharmacological properties of imatinib and nilotinib.
Bioorg Med Chem. 18:6977–6986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meinhardt K, Kroeger I, Abendroth A,
Müller S, Mackensen A and Ullrich E: Influence of NK cell magnetic
bead isolation methods on phenotype and function of murine NK
cells. J Immunol Methods. 378:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ullrich E, Bonmort M, Mignot G, et al:
Trans-presentation of IL-15 dictates IFN-producing killer dendritic
cells effector functions. J Immunol. 180:7887–7897. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taieb J, Chaput N, Menard C, et al: A
novel dendritic cell subset involved in tumor immunosurveillance.
Nat Med. 12:214–219. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hantschel O, Rix U and Superti-Furga G:
Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and
dasatinib. Leuk Lymphoma. 49:615–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaput N, Flament C, Locher C, et al:
Phase I clinical trial combining imatinib mesylate and IL-2:
HLA-DR+ NK cell levels correlate with disease outcome.
Oncoimmunology. 2:e230802013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salih J, Hilpert J, Placke T, et al: The
BCR/ABL-inhibitors imatinib, nilotinib and dasatinib differentially
affect NK cell reactivity. Int J Cancer. 127:2119–2128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassold N, Seystahl K, Kempf K, et al:
Enhancement of natural killer cell effector functions against
selected lymphoma and leukemia cell lines by dasatinib. Int J
Cancer. 131:E916–E927. 2012. View Article : Google Scholar : PubMed/NCBI
|