Introduction
Leukemia encompasses a group of diseases with
varying presentations, prognoses and treatments. For example, acute
lymphoblastic leukemia (ALL) is the most common type of leukemia in
young children; treatment with chemotherapy and radiotherapy
results in an 85% survival rate in this population (1). ALL also affects older adults ≥65 years
of age with 30–70% achieving remission (2). The incidence of acute myelogenous
leukemia (AML) is greatest in male adults and less common in
children. Chemotherapy results in an overall 5-year survival rate
of 40% (3). The incidence of
chronic lymphocytic leukemia (CLL) is also highest in adult males
>55 years of age. Although incurable, the 5-year survival rate
is ~79% (4). Furthermore, chronic
myelogenous leukemia (CML) occurs mainly in adults, and the 5-year
survival rate is ~93% (5).
In 2001, estimates indicate that almost 256,000
individuals worldwide developed leukemia and ~209,000 succumbed to
the disease (6). Moreover, the
incidence of leukemia in developed nations is almost double that
observed in less developed areas (7). Despite improvements in patient
survival, the exact causes of leukemia remain unknown (8). For example, exposure to ionizing
radiation, pesticides and formaldehyde has been linked to the
development of leukemia (8). In
addition, aberrant microRNA (miRNA) expression has also been
implicated in the pathogenesis of leukemia (9).
miRNAs are non-coding single-stranded RNA molecules
of 19–25 nucleotides in length that silence target gene expression
through binding of the 3′UTR, resulting in degradation of mRNA or
translation inhibition. In addition to regulating target gene
expression, some miRNAs may serve as oncogenes or tumor-suppressor
genes and may be important for genetic diagnosis, prognotic
determination, and targeted therapy of hematological tumors
(10,11). For example, miR-15a/miR-16-1
(11), miR-29 (12), miR-203 (13), and miR-181a (14) may exert antitumor effects in
hematological tumors, whereas miR-155 (15), miR-9 and let-7a (16) may confer carcinogenic effects in
lymphoma possibly through inhibition of apoptosis. Furthermore, in
acute myeloid leukemia, miR-125b-2 is highly expressed (17). In addition, miRNAs may increase the
sensitivity of tumor cells to chemotherapeutics (18); they may also regulate the tumor
microenvironment, promoting the infiltration and metastasis of
tumor cells (19).
Notably, miR-143 may also possess antitumor activity
(10), and its expression is
reduced in malignant tumors, such as prostate cancer (18), B-cell chronic lymphocytic leukemia,
Burkitt’s lymphoma (20),
nasopharyngeal cancer (21),
esophageal adenocarcinoma (22),
gastric cancer (23), lung cancer
(24), osteosarcoma (25) and colon cancer (26,27).
Reduced miR-143 expression in non-small cell lung cancer was
associated with smoking status (24). miR-143 levels were significantly
lower in esophageal adenocarcinoma as compared to Barrett’s
esophagus, suggesting a possible role in disease progression
(22).
miR-143 was also found to suppress the proliferation
of prostate (18) and gastric
(23) cancer cells and enhance
their chemosensitivity, possibly through suppression of its target
gene, KRAS (18). In
nasopharyngeal cancer, miR-143 dysregulation was inversely
correlated with genes involved in vascular endothelial growth
factor (VEGF) signaling as well as cell cycle progression (21). In addition, the antiproliferative
effects of miR-143 may be mediated through suppression of its
target genes, including ERK5 (20,23)
and Akt (23) or induction of
apoptosis by targeting Bcl-2 (25).
Moreover, miR-143 may regulate epigenetic modification via
silencing DNA methyltransferase 3A (DNMT3A) expression (26). However, the mechanism by which
miR-143 exerts its effects on hematological tumors is less
clear.
Therefore, the present study examined the hypothesis
that expression of miR-143 may impact leukemia cell growth through
altered DNMT3A expression. miR-143 expression was assessed in bone
marrow samples of leukemia patients and healthy controls, and its
correlation with DNMT3A expression was determined. The effects of
miR-143 on the growth, colony formation, cell cycle progression and
apoptosis were also determined in CML K562 cells (28). miR-143 expression was reduced in
leukemia patients and was negatively associated with DNMT3A
expression. Lentiviral-mediated miR-143 overexpression inhibited
K562 cell proliferation, transition from the G1 to S phase and
Bcl-2 expression. These data support the notion that identification
of miRNAs and their targets in cancer progression may provide novel
therapeutic strategies for the diagnosis and treatment of
leukemia.
Materials and methods
Patients and cell lines
Bone marrow cells were collected from 63 patients
with leukemia, who were hospitalized in the Affiliated Union
Hospital of Fujian Medical University from March 2009 to December
2010. The patients consisted of 37 males and 26 females with a mean
age of 36.7±17.7 years (range, 10–77 years). These patients were
diagnosed with either AML or ALL, according to the
French-American-British (FAB) classification criteria (29). Patients who underwent chemotherapy
or radiotherapy prior to the study were excluded. In addition, 15
bone marrow donors without malignant hematological diseases served
as normal controls. The present study was approved by the
Institutional Review Board of the Affiliated Union Hospital, and
informed consent was obtained from all patients and healthy
controls.
The AML (HL-60, NB4 and U937), CML (K562), acute
erythroleukemia (HEL), T lymphocytic leukemia (Jurkat and CEM),
B-cell lymphoma (CA46, Raji cells of Burkitt’s lymphoma) and
multiple myeloma (U266) cells were provided by the Institute of
Hematological Diseases in Fujian Province. All cell lines were
maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA)
containing 10% fetal bovine serum (FBS; Sijiqing Biotech, Hangzhou,
China) and 2 mM L-glutamine at 37°C in 5% CO2. Cells
were passaged every two days, and those in logarithmic growth were
used for the subsequent analyses. The peripheral blood mononuclear
cells from healthy subjects served as a negative control.
Lentiviral-mediated miR-143
expression
The PCR primers used to amplify the miR-143
gene are listed in Table I and were
synthesized by Invitrogen (Carlsbad, CA, USA). PCR was performed
with an ABI 7500 fluorescence quantitative thermal cycler (Applied
Biosystems, Foster City, CA, USA) and Platinum SYBR-Green I qPCR
SuperMix-UDG kit (Invitrogen), according to the manufacturer’s
instructions. U6 snRNA served as controls for PCR analysis. PCR
reactions were sequentially denatured at 50°C for 2 min and at 95°C
for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 30
sec. At the end of the PCR amplification, melting curve assays were
performed to ensure the purity of the amplicon. The resultant PCR
product was inserted into the pMAGic 7.1-GFP Puro lentivirus
vector, which underwent splicing by XhoI and BamHI.
After transformation into competent bacteria, the positive
recombinant plasmids were extracted, and the miR-143 gene
was confirmed by sequencing performed by Shanghai SBO Medical
Biotechnology (Shanghai, China).
 | Table IPrimer sequences used for the PCR
reactions. |
Table I
Primer sequences used for the PCR
reactions.
| Gene | Primer sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| miR-143 | F: TGT AGT TTT CGG
AGT TAG TGT CGC GC | |
| R: CCT ACG ATC GAA
AAC GAC GCG AAC G | 56 |
| U6 | F: GTT TTG TAG TTT
TTG GAG TTA GTG TTG TGT | |
| R: CTC AAC CTA CAA
TCA AAA ACA ACA CAA ACA | 96 |
| DNMT3A | F: TAT TGA TGA GCG
CAC AAG AGA GC | |
| R: GGG TGT TCC AGG
GTA ACA TTG AG | 111 |
| DNMT3B | F: GAC TTG GTG ATT
GGC GGA A | |
| R: GGC CCT GTG AGC
AGC AGA | 270 |
| DNMT1 | F: CCG AGT TGG TGA
TGG TGT GTA C | |
| R: AGG TTG ATG TCT
GCG TGG TAG C | 324 |
| Bcl-2 | F: AGA GGT CAC GGG
GGC TAA T | |
| R: CCA GGT AAC AAA
ACC CCA CA | 60 |
The recombinant plasmids were transfected into 293T
cells using Lipofectamine 2000 (Invitrogen). After 48 h, the
supernatant was collected and concentrated to determine the viral
titer by multiplicity of infection (MOI). The resultant
miR-143-expressing lentivirus was used to infect human chronic
granulocytic leukemia K562 cells, and the monoclonal cells were
screened with puromycin for one week.
Real-time PCR analysis
Bone marrow (5 ml) was collected by aspiration using
a syringe with the anticoagulant, heparin. Total RNA was extracted
from mononuclear cells using TRIzol (Invitrogen). cDNA was isolated
by reverse transcription using a kit following the manufacturer’s
instructions (Fermentas, Lafayette, CO, USA). PCR primers for
miR-143, DNMT3A, DNMT3B and DNMT1, Bcl-2, U6 snRNA are listed in
Table I. U6 snRNA served as an
internal reference. The relative mRNA expression levels of miR-143,
DNMT3A and Bcl-2 were determined using the 2−ΔΔCt
method.
Western blot analysis
Protein was extracted using lysis solution
containing protease inhibitors (all from Xiamen Lulong Biotech
Development Co., Ltd., Fujian, China). Total proteins (50 μg) were
separated by 10% SDS-PAGE (Bio-Rad Laboratories, Hercules, CA,
USA). The proteins were then transferred onto nitrocellulose
membranes, which were blocked with skim milk (Xiamen Lulong Biotech
Development, Co.), and then incubated with the following primary
antibodies at 4°C overnight: β-actin (Millipore, Billerica, MA,
USA), DNMT3B (Abcam, Cambridge, MA, USA), DNMT3A and DNMT1 (Cell
Signaling Technology, Boston, MA, USA), procaspase-3 and
procaspase-9 (Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Zhongshan, China). After washing with TBST, the membranes
were incubated with secondary antibodies (Golden Bridge
Biotechnology) and developed using ECL (Xiamen Golden
Biotechnology). Following visualization, the bands were scanned and
analyzed by a gel image analysis scanner (Gel Doe 1000; Bio-Rad
Laboratories).
Cell proliferation and colony formation
analyses
K562 cells were seeded into 96-well plates at a
density of 2×104 cells/100 μl. In each plate, there were
three groups (three wells per group): blank control group (no cells
and medium), control group (K562 transfected with lentivirus
expressing scrambled vectors), and the experimental group (K562
cells transfected with lentivirus expressing miR-143). Cell
proliferation was determined after 0, 24, 48, 72 and 96 h using the
Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Beijing, China). After 2–3 h, the optical density (OD) was measured
with a microplate reader at 450 nm using the following formula:
ODexperiment − ODblank. This experiment was
performed three times.
To assess the colony formation, K562 cells
transfected with the control scrambled lentivirus or the
miR-143-expressing lentivirus were seeded into 24-well plates (500
μl/well; 200 cells/well; three wells/group), which were pre-coated
with methyl cellulose solution (500 μl/well). The methyl cellulose
was dissolved in sterilized RPMI-1640 at a final concentration of
1.6%. After sterilization at a high pressure (10 pounds for 20
min), the methyl cellulose solution was stored at 4°C for use.
After 10–14 days, the colonies were counted under an inverted
microscope (Nikon, Tokyo, Japan). A colony was defined as an
aggregate of >40 cells.
Analysis of the cell cycle
K562 cells (1×106) were collected and
mixed with 2 ml of lysis buffer (Becton-Dickinson, Franklin Lakes,
NJ, USA). After incubation in the dark for 10 min, 1×106
cells were counted by flow cytometry followed by sequential
addition of solutions A–C (Becton-Dickinson) following the
manufacturer’s instructions.
Analysis of apoptosis
K562 cells (1×106) were collected and
independently treated with acridine orange/ethidium bromide (AO/EB)
fluorescent dye and bisbenzimide (Hoechst 33258) (both from Xiamen
Lulong Biotech), following the manufacturer’s instructions.
Apoptotic cells were observed under a fluorescence microscope
(Nikon 2000) at an excitation wavelength of 352 nm and emission
wavelength of 461 nm.
Apoptotic cells were also assessed after Annexin V
and FITC/propidium iodide (PI) staining. In brief,
1–5×105 cells were collected and resuspended in binding
buffer (Beyotime Institute of Biotechnology). A mixture of Annexin
V-FITC and PI from the apoptosis kit (Beyotime Institute of
Biotechnology) was added to the cell suspension followed by
incubation in the dark at room temperature for 5–15 min. Flow
cytometry was performed at an excitation wavelength of 488 nm and
emission wavelength of 530 nm.
Statistical analysis
Continuous variables were expressed as mean with
standard deviation. For comparisons between healthy controls and
leukemia patients, or normal control (NC) and miR-143 groups,
independent two sample t-tests were performed. For comparisons
among the various cell lines, one-way analysis of variance (ANOVA)
was used. When a significant difference between groups was
apparent, multiple comparisons were performed using the Bonferroni
procedure with type-I error adjustment. The relationship between
relative miR-143 expression and DNMT3A was evaluated using the
Pearson’s correlation coefficient test. SAS software package,
version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for the
statistical analysis. All statistical assessments were evaluated at
a two-sided α level of 0.05.
Results
Expression of miR-143 in bone marrow
cells from leukemia patients and in hematologic tumor cell
lines
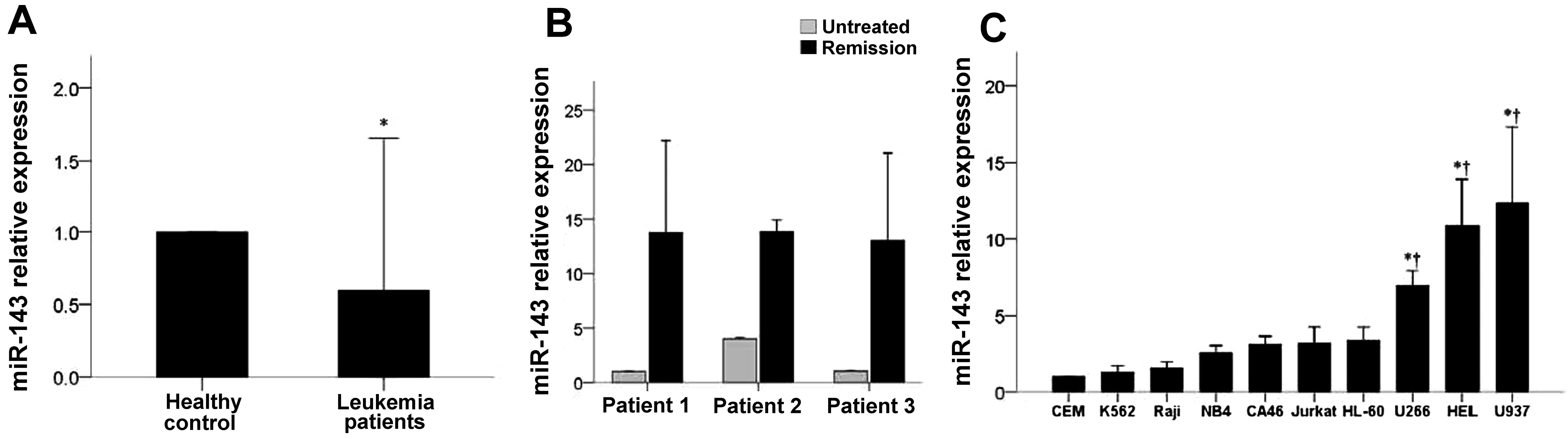
As shown in Fig. 1A,
bone marrow cells from 63 leukemia patients had significantly lower
miR-143 expression as compared to the bone marrow samples of 15
healthy controls (P=0.004). In 3 patients with disease remission,
higher relative miR-143 expression was observed as compared to the
levels prior to treatment (Fig.
1B). Furthermore, miR-143 levels in 10 different hematologic
tumor cell lines were quantified (Fig.
1C). No significant differences were observed among the CEM,
K562, Raji, NB4, CA46, Jurkat and HL-60 cell lines, while U266, HEL
and U937 expressed significantly higher levels of miR-143 when
compared to the levels in the CEM and K562 cells (all P≤0.002).
Since K562 cells expressed relatively lower levels of miR-143, they
were used for the subsequent experiments.
Effects of miR-143 on DNMT3A
expression
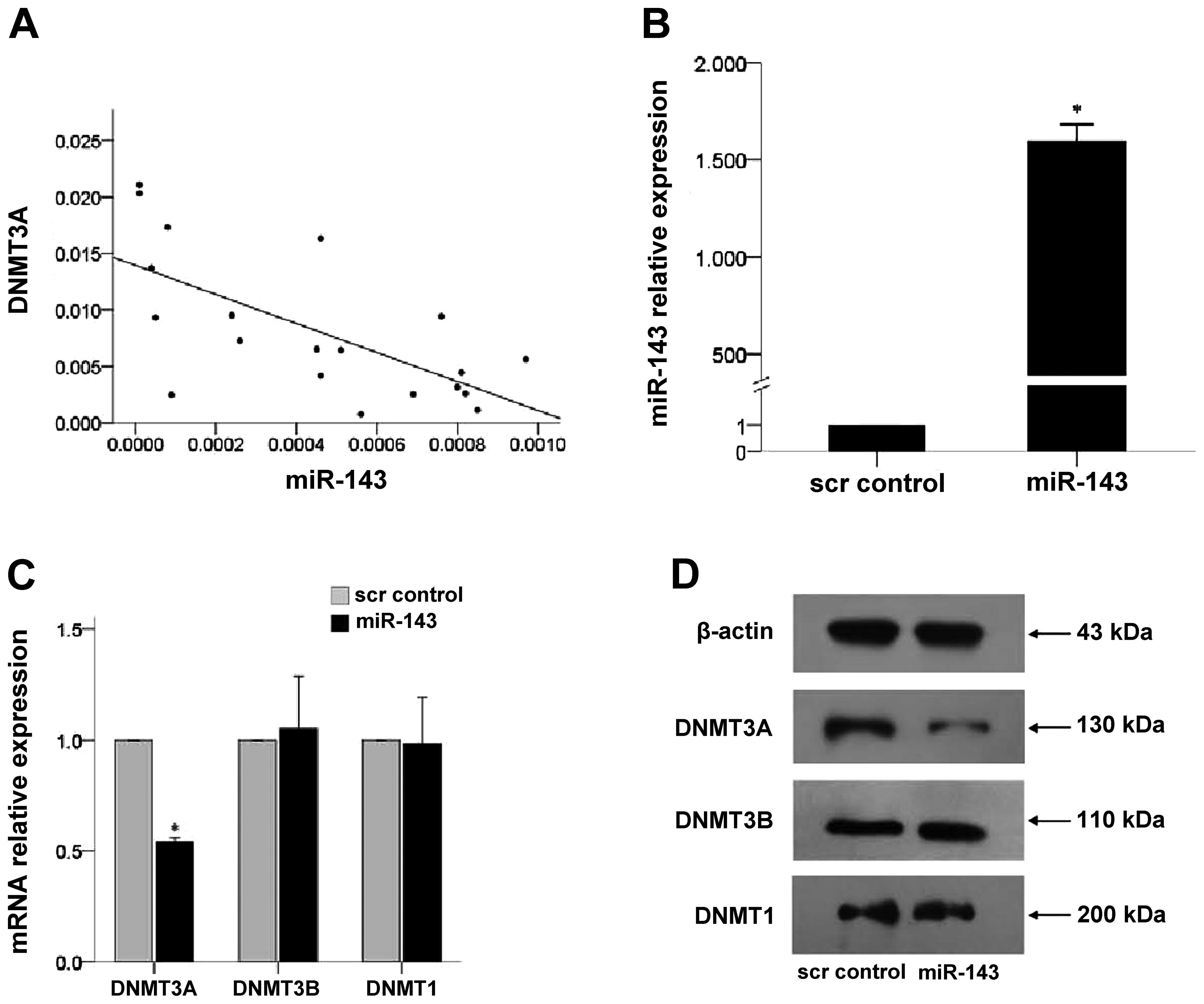
Since miR-143 may regulate epigenetic modification
via silencing its target, DNMT3A (27), the correlation between miR143 and
DNMT3A levels was assessed in 20 leukemia patient samples. As shown
in Fig. 2A, the expression levels
of miR143 and DNMTA3A were negatively correlated (r=−0.663,
P=0.001).
The effects of miR-143 expression were determined in
K562 cells after lentivirus-mediated overexpression in the cells.
As shown in Fig. 2B, the expression
of miR-143 in K562 cells infected with lenti-miR-143 was 1,594
times higher than that observed in the scrambled negative control
(scr control) group (P=0.024). Overexpression of miR-143 decreased
DNMT3A mRNA and protein expression (P<0.001; Fig. 2C and D, respectively). However, no
changes in DNMT3B and DNMT1 expression were observed.
Effects of miR-143 on K562 proliferation,
colony formation and cell cycle
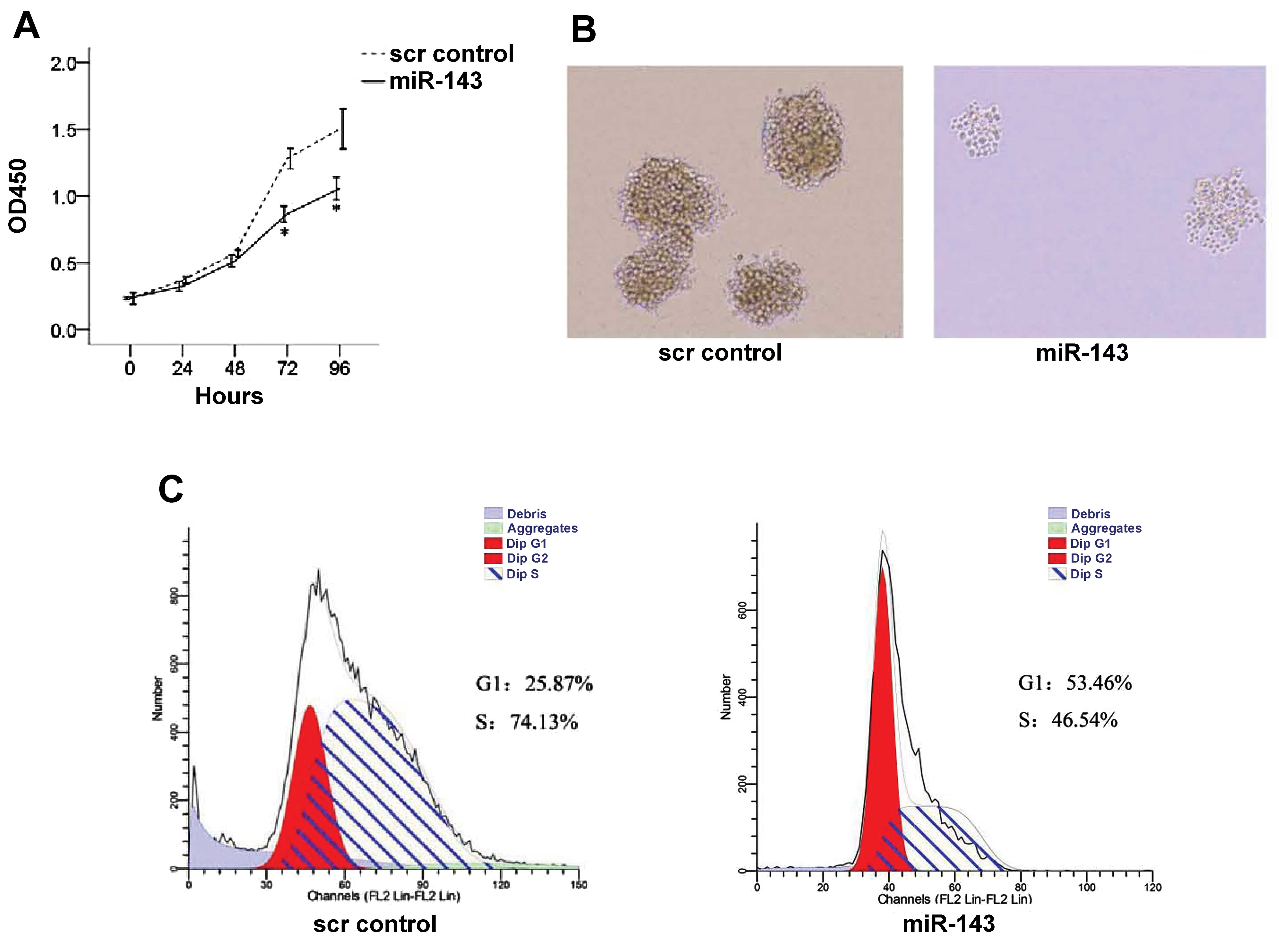
As shown in Fig. 3A,
overexpression of miR-143 significantly reduced K562 cell
proliferation at 72 and 96 h as compared to the scr control group
(both P≤0.018). In addition, reduced colony formation was observed
upon miR-143 expression (Fig. 3B).
Furthermore, cell cycle progression in scr control and
miR-143-overexpressing K562 cells was analyzed by flow cytometry. A
greater proportion of cells in the G1 phase was observed in the
miR-143-expressing K562 cells than the proportion in the scr
control group (53.46 vs. 25.87%, P=0.001; Fig. 3C); the scr control group had a
greater proportion of cells in the S phase (74.13 vs. 46.54%,
P=0.001; Fig. 3C).
Effects of miR-143 on K562 cell
apoptosis
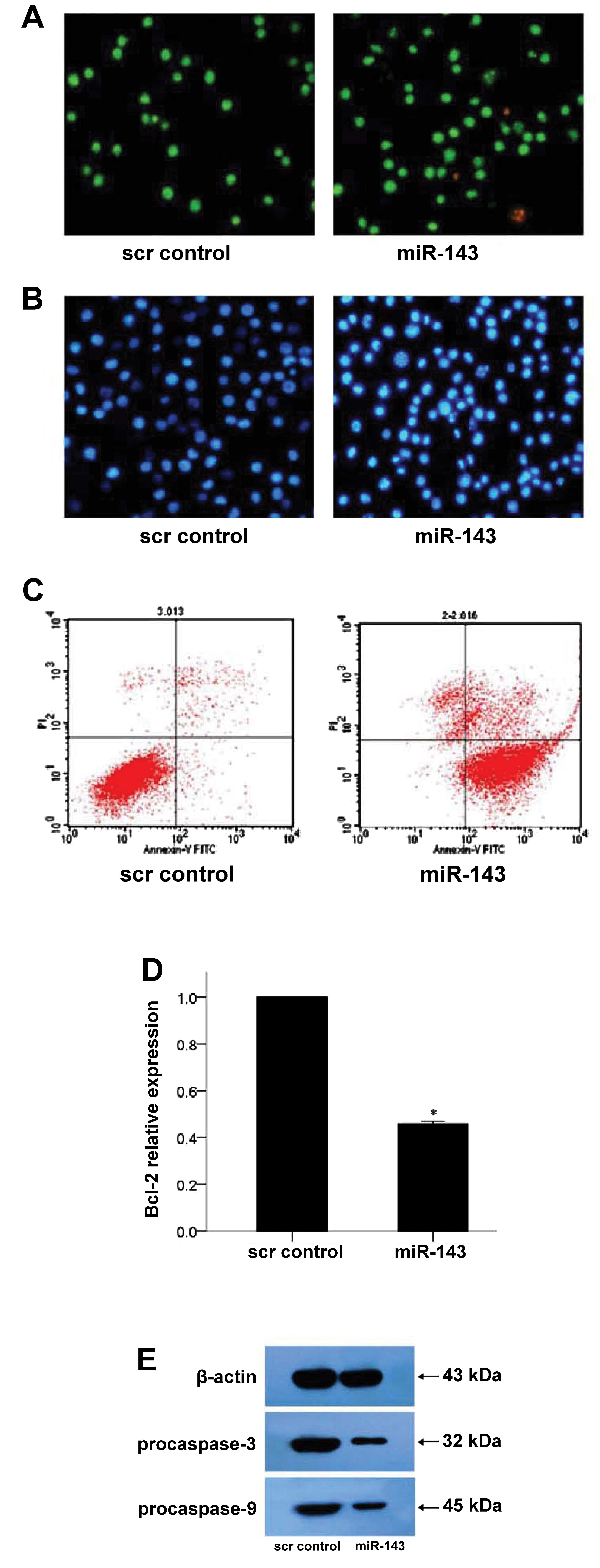
After staining with AO/EB, apoptotic K562 cells were
observed by microscopy. As shown in Fig. 4A (left panel), most cells in the scr
control group were bright green with only a few orange-red necrotic
cells. Upon miR-143 expression, the number of apoptotic K562 cells
increased (Fig. 4A, right panel).
These results were confirmed by Hoechst 33258 staining, which
revealed no apoptotic cells in the scr control group (Fig. 4B, left panel). However, in the
miR-143-transfected cells, the nuclei were dense, and apoptotic
bodies in several cells were observed (Fig. 4B, right panel). In addition, flow
cytometric analysis revealed that the early apoptosis rate was
higher in the miR-143 group as compared to the scr control group
(84.9 vs. 5.1%, P<0.001; Fig.
4C).
The effects of miR-143 on the expression of the
anti-apoptotic protein, Bcl-2, was next determined. As shown in
Fig. 4D, Bcl-2 mRNA expression was
reduced in the miR-143-expressing K562 cells (P<0.001; Fig. 4D). In addition, western blot
analysis revealed that protein expression of pro-caspase-3 and
pro-caspase-9 was reduced in the miR-143-expressing cells (Fig. 4E).
Discussion
In the present study, reduced miR-143 expression was
observed in the bone marrow cells of leukemia patients, which is
consistent with the findings of Batliner et al (30). In addition, miR-143 reduced the
expression of its target, DNMT3A, and its overexpression reduced
K562 cell proliferation, colony formation and cell cycle
progression. Furthermore, increased apoptosis was observed upon
miR-143 overexpression.
Although miR-143 expression was found to be
downregulated in colon cancer, its association with
clinicopathological features of colorectal cancer patients is
inconsistent (27,31,32).
Whereas Wang et al (32)
reported that miR-143 was not associated with clinicopathological
features, such as age, gender and TNM stage, Slaby et al
(31) observed that decreased
miR-143 was associated with tumor diameter. In addition, Calin
et al (11) observed a
microRNA signature in chronic lymphoid leukemia patients that was
correlated with patient prognosis and disease pathogenesis. In the
present study, miR-143 expression was markedly lower in the bone
marrow samples of leukemia patients than in healthy subjects, and
its expression increased with disease remission in 3 patients.
Further studies are necessary to determine whether miR-143
expression is related to disease progression, prognosis or other
clinicopathological features.
miR-143 overexpression was previously found to
inhibit the proliferation and migration of prostate cancer cells,
silencing the KRAS gene to inhibit protein kinase signaling
and increasing the chemosensitivity of cancer cells to docetaxel
(18). In addition, miR-143 levels
were negatively associated with Raji cell proliferation (20). These results are consistent with
those of the present study in which miR-143 overexpression
inhibited K562 cell proliferation, cell cycle progression and
colony formation. Although the mechanisms underlying the effects of
miR-143 on cell proliferation are unclear, we speculate that
miR-143 might act on ERK5 (33),
ErbB3 and K-ras (34) growth factor
receptor signaling to arrest the cell cycle. Further studies are
necessary to determine whether miR-143 inhibits the growth factor
receptor signaling pathway to suppress cancer cell
proliferation.
In addition to reduced cell proliferation, increased
apoptosis was observed in K562 cells exhibiting miR-143
overexpression, which is consistent with Zhang et al
(25). We postulate that miR-143
inhibits Bcl-2 expression and activates procaspase-3 and
procaspase-9 and thereby the endogenous mitochondrial pathway,
which promotes cell apoptosis. There is also evidence indicating
that Bcl-2 may be a target gene of miR-143 (25). In addition, miR-143 may target ERK5
during Fas-induced apoptosis (35).
However, the specific mechanism by which miR-143 expression induces
apoptosis requires further research.
DNA methyltransferase plays important roles in
regulating chromatin structure and gene expression via modifying
DNA methylation. DNMT3A directly influences the expression of
various oncogenes and tumor-suppressor genes (36). Although the mechanisms underlying
the effects of these DNA methyltransferases are largely unclear,
their abnormal expression has been reported in numerous types of
cancers (37,38), and their inhibition is being
explored as a potential therapy (38). In the present study, miR-143 reduced
DNMT3A expression, which is in agreement with a previous study
(26). In addition, miR-143 levels
were negatively correlated with DNMT3A levels. miR-143 may bind to
the 3′-UTRs of the DNMT3A gene to inhibit its activity.
Alternatively, an indirect inhibition of DNMT1 by miR-29b via Sp1
has also been shown (12). The
mechanism by which miR-143 inhibits DNMT3A will be assessed in
further studies. In addition, the effects of reduced DNMT3A
expression on global DNA methylation and re-expression of genes
regulated by promoter methylation, including
p15INK4b and ESR1 (12), will also be assessed in further
studies.
The small number of patients assessed in the present
study represents a study limitation. In addition, the present study
did not assess the mechanism by which miR-143 expression is
decreased in leukemia patients. Since p73 regulates miR-143
transcription during neutrophil differentiation (30), its expression and activity should be
determined in leukemia patients. Furthermore, the effects of
miR-143 overexpression and inhibition need to be analyzed in an
in vivo model of leukemia. Finally, as the study aimed to
assess the expression and function of miR-143 in leukemia cells,
specific leukemia subtypes (e.g., AML, ALL, CML and CLL) were not
individually examined. Therefore, further study is required to
assess the role of miR-143 among the various subtypes.
In conclusion, taken together, miR-143 inhibited
cell proliferation and induced apoptosis in K562 cells, which may
be mediated through silencing of DNMT3A expression in vitro.
Furthermore, miR-143 expression was significantly reduced in the
bone marrow samples of leukemia patients. Further studies are
warranted to elucidate the possible therapeutic potential and
prognostic value of miR-143 in cancer.
Acknowledgements
The present study was supported by funding from the
National and Fujian Provincial Key Clinical Specialty Discipline
Construction Program, P.R. China, the Major Research National
Natural Science Foundation of China-Young Scientist Program
(81300428), Surface Project of National Natural Science Foundation
of China (81370629), Project in Fujian Medical University
(ZD303052806), Natural Science Foundation in Fujian Province
(2011J01179), Youth Science Foundation of the Health Department in
Fujian Province (2010-01-07, 2010-01-12), Fujian Provincial Key
Laboratory on Hematology Program (2009J1004), and the Foundation
for Key Teachers of Fujian Medical University.
Abbreviations:
|
AO/EB
|
acridine orange/ethidium bromide
|
|
AML
|
acute myeloid leukemia
|
|
ANOVA
|
analysis of variance
|
|
DNMT3A
|
DNA methyltransferase 3A
|
|
FBS
|
fetal bovine serum
|
|
miRNA
|
microRNA
|
|
MOI
|
multiplicity of infection
|
|
scr control
|
scrambled negative control
|
|
SD
|
standard deviation
|
References
|
1
|
Pui CH, Mullighan CG, Evans WE and Relling
MV: Pediatric acute lymphoblastic leukemia: where are we going and
how do we get there? Blood. 120:1165–1174. 2012. View Article : Google Scholar
|
|
2
|
Marks DI: Treating the ‘older’ adult with
acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ
Program. 2010:13–20. 2010.
|
|
3
|
Colvin GA and Elfenbein GJ: The latest
treatment advances for acute myelogenous leukemia. Med Health R I.
86:243–246. 2003.PubMed/NCBI
|
|
4
|
Howlader N, Noone AM, Krapcho M, Garshell
J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,
Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ and Cronin KA: SEER
Cancer Statistics Review, 1975–2010. National Cancer Institute;
Bethesda, MD: http://seer.cancer.gov/csr/1975_2010/,
based on November 2012 SEER data submission, posted on the SEER
website, 2013.
|
|
5
|
Fausel C: Targeted chronic myeloid
leukemia therapy: seeking a cure. Am J Health Syst Pharm.
64:S9–S15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mathers CD, Boschi-Pinto C, Lopez AD and
Murray CJL: Cancer incidence, mortality and survival by site for 14
regions of the world. Global Programme on Evidence for Health
Policy Discussion Paper No. 13. World Health Organization; 2001
|
|
7
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
8
|
Polychronakis I, Dounias G, Makropoulos V,
Riza E and Linos A: Work-related leukemia: a systematic review. J
Occup Med Toxicol. 8:142013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao H, Wang D, Du W, Gu D and Yang R:
MicroRNA and leukemia: tiny molecule, great function. Crit Rev
Oncol Hematol. 74:149–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M,
Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C,
Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M and
Croce CM: A MicroRNA signature associated with prognosis and
progression in chronic lymphocytic leukemia. N Engl J Med.
353:1793–1801. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garzon R, Liu S, Fabbri M, Liu Z, Heaphy
CE, Callegari E, Schwind S, Pang J, Yu J, Muthusamy N, Havelange V,
Volinia S, Blum W, Rush LJ, Perrotti D, Andreeff M, Bloomfield CD,
Byrd JC, Chan K, Wu LC, Croce CM and Marcucci G: MicroRNA-29b
induces global DNA hypomethylation and tumor suppressor gene
reexpression in acute myeloid leukemia by targeting directly DNMT3A
and 3B and indirectly DNMT1. Blood. 113:6411–6418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bueno MJ, Pérez de Castro I, Gómez de
Cedrón M, Santos J, Calin GA, Cigudosa JC, Croce CM,
Fernández-Piqueras J and Malumbres M: Genetic and epigenetic
silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene
expression. Cancer Cell. 13:496–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pekarsky Y, Santanam U, Cimmino A,
Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG,
Rassenti L, Calin GA, Hagan JP, Kipps T and Croce CM: Tcl1
expression in chronic lymphocytic leukemia is regulated by miR-29
and miR-181. Cancer Res. 66:11590–11593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eis PS, Tam W, Sun L, Chadburn A, Li Z,
Gomez MF, Lund E and Dahlberg JE: Accumulation of miR-155 and BIC
RNA in human B cell lymphomas. Proc Natl Acad Sci USA.
102:3627–3632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie K, Gomez M, Landgraf P, Garcia JF, Liu
Y, Tan LH, Chadburn A, Tuschl T, Knowles DM and Tam W:
MicroRNA-mediated down-regulation of PRDM1/Blimp-1 in
Hodgkin/Reed-Sternberg cells: a potential pathogenetic lesion in
Hodgkin lymphomas. Am J Pathol. 173:242–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gefen N, Binder V, Zaliova M, Linka Y,
Morrow M, Novosel A, Edry L, Hertzberg L, Shomron N, Williams O,
Trka J, Borkhardt A and Izraeli S: Hsa-mir-125b-2 is highly
expressed in childhood ETV6/RUNX1 (TEL/AML1) leukemias and
confers survival advantage to growth inhibitory signals independent
of p53. Leukemia. 24:89–96. 2010. View Article : Google Scholar
|
|
18
|
Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z,
Li P, Zhang W, Wu H, Feng N, Wang Z, Hua L and Wang X: miR-143
decreases prostate cancer cells proliferation and migration and
enhances their sensitivity to docetaxel through suppression of
KRAS. Mol Cell Biochem. 350:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicoloso MS, Spizzo R, Shimizu M, Rossi S
and Calin GA: MicroRNAs - the micro steering wheel of tumour
metastases. Nat Rev Cancer. 9:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akao Y, Nakagawa Y, Kitade Y, Kinoshita T
and Naoe T: Downregulation of microRNAs-143 and -145 in B-cell
malignancies. Cancer Sci. 98:1914–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen HC, Chen GH, Chen YH, Liao WL, Liu
CY, Chang KP, Chang YS and Chen SJ: MicroRNA deregulation and
pathway alterations in nasopharyngeal carcinoma. Br J Cancer.
100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wijnhoven BP, Hussey DJ, Watson DI, Tsykin
A, Smith CM and Michael MZ: MicroRNA profiling of Barrett’s
oesophagus and oesophageal adenocarcinoma. Br J Surg. 97:853–861.
2010.
|
|
23
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
-145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao W, Yu Y, Cao H, Shen H, Li X, Pan S
and Shu Y: Deregulated expression of miR-21, miR-143 and miR-181a
in non small cell lung cancer is related to clinicopathologic
characteristics or patient prognosis. Biomed Pharmacother.
64:399–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Cai X, Wang Y, Tang H, Tong D and
Ji F: microRNA-143, down-regulated in osteosarcoma, promotes
apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol
Rep. 24:1363–1369. 2010.PubMed/NCBI
|
|
26
|
Ng EK, Tsang WP, Ng SS, Jin HC, Yu J, Li
JJ, Röcken C, Ebert MP, Kwok TT and Sung JJ: MicroRNA-143 targets
DNA methyltransferases 3A in colorectal cancer. Br J Cancer.
101:699–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh
T, Kojima K, Nakashima R, Kitade Y and Naoe T: Role of
anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer
Gene Ther. 17:398–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiyota M, Kuroda J, Yamamoto-Sugitani M,
Shimura Y, Nakayama R, Nagoshi H, Mizutani S, Chinen Y, Sasaki N,
Sakamoto N, Kobayashi T, Matsumoto Y, Horiike S and Taniwaki M:
FTY720 induces apoptosis of chronic myelogenous leukemia cells via
dual activation of BIM and BID and overcomes various types of
resistance to tyrosine kinase inhibitors. Apoptosis. 18:1437–1446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dick FR, Armitage JO and Burns CP:
Diagnostic concurrence in the subclassification of adult acute
leukemia using French-American-British criteria. Cancer.
49:916–920. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Batliner J, Buehrer E, Fey MF and Tschan
MP: Inhibition of the miR-143/145 cluster attenuated neutrophil
differentiation of APL cells. Leuk Res. 36:237–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Slaby O, Svoboda M, Fabian P, Smerdova T,
Knoflickova D, Bednarikova M, Nenutil R and Vyzula R: Altered
expression of miR-21, miR-31, miR-143 and miR-145 is related to
clinicopathologic features of colorectal cancer. Oncology.
72:397–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang CJ, Zhou ZG, Wang L, Yang L, Zhou B,
Gu J, Chen HY and Sun XF: Clinicopathological significance of
microRNA-31, -143 and -145 expression in colorectal cancer. Dis
Markers. 26:27–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clape C, Fritz V, Henriquet C, Apparailly
F, Fernandez PL, Iborra F, Avancès C, Villalba M, Culine S and
Fajas L: miR-143 interferes with ERK5 signaling, and abrogates
prostate cancer progression in mice. PLoS One. 4:e75422009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu H, Zhang SZ, Cai SR, Peng JP and Zheng
S: Effect of microRNA143 expression on cell proliferation in
colonic carcinoma. Zhonghua Zhong Liu Za Zhi. 30:498–501. 2008.(In
Chinese).
|
|
35
|
Akao Y, Nakagawa Y, Iio A and Naoe T: Role
of microRNA-143 in Fas-mediated apoptosis in human T-cell leukemia
Jurkat cells. Leuk Res. 33:1530–1538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Robertson KD and Wolffe AP: DNA
methylation in health and disease. Nat Rev Genet. 1:11–19. 2000.
View Article : Google Scholar
|
|
37
|
Shen L and Pili R: Posttranscription
regulation of prostate cancer growth. Cancer J. 14:46–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith EM, Boyd K and Davies FE: The
potential role of epigenetic therapy in multiple myeloma. Br J
Haematol. 148:702–713. 2010. View Article : Google Scholar : PubMed/NCBI
|