Introduction
Ovarian cancer, one of the leading causes of
mortality involving gynecologic malignancies, is a highly
metastatic disease characterized by widespread peritoneal
dissemination and ascites (1). The
development of new treatment protocols depends on the improved
knowledge of the molecular mechanisms controlling metastasis as the
treatment of patients in advanced stages yields low survival rates
(2). Studies have shown that a
morphological conversion known as epithelial-to-mesenchymal
transition (EMT) is associated with the acquisition of malignant
characteristics in ovarian cancer cells (3–9).
EMT was initially characterized as an important
program during normal embryonic development (10,11);
however, studies have further suggested that carcinoma cells can
reactivate the EMT program during tumor progression (12). Similar to cells that undergo EMT
during normal development, carcinoma cells that undergo EMT lose
cell-to-cell contacts, undergo major changes in their cytoskeleton,
and acquire a mesenchymal-like morphology that enhances invasive
and migratory abilities (13).
Several signaling molecules present in tumor microenvironments can
initiate EMT and metastasis; many of these same factors are
aberrantly activated and/or overexpressed in human cancer (14–16).
Studies have revealed that EMT is governed by
various regulatory networks. EMT is triggered by extracellular
stimuli, such as TGF-β1, fibroblast growth factor, hepatocyte
growth factor and endothelin-1 (6,7,17).
These factors activate specific signaling pathways, thereby
inducing changes in cytoskeletal organization and disrupting
cell-to-cell junctions. In addition to these signaling pathways,
transcription factors, such as Snail, Slug, Twist, Zeb1 and SIP1,
are important in the promotion of EMT (18). Another example is forkhead box
protein C2 (FOXC2), an EMT-inducing factor that has been detected
in breast cancer (19). FOXC2
potently acts on mesenchymal tissues, suggesting that FOXC2 enables
cancer cells to thrive in environments favorable for invasion and
metastasis (20). Therefore, FOXC2
may act as an oncogene. However, whether or not FOXC2 is involved
in ovarian cancer development and metastasis remains unknown.
This study showed that FOXC2 expression was
increased in ovarian cancer tissues and cell lines. In human
ovarian cancer cells, migration and invasion were significantly
enhanced when FOXC2 was overexpressed. By contrast, these
properties were inhibited when FOXC2 was knocked down. These
results indicated that FOXC2 is required for the maintenance of a
mesenchymal phenotype after TGF-β1 induced EMT in human ovarian
cancer cells.
Materials and methods
Samples, cells and antibodies
Human normal ovarian and ovarian cancer tissue
samples were provided by Shandong Provincial Hospital affiliated to
Shandong University of China. All experiments were approved by the
Ethics Committee of the Shandong Provincial Hospital affiliated to
Shandong University and informed consent was obtained from all
patients prior to specimen collection. ES-2 and SKOV3 cell lines
were obtained from American Type Culture Collection (Manassas, VA,
USA). NOS4, K2 and TAOV were established in the Department of
Obstetrics and Gynecology, Nagoya University Graduate School of
Medicine (21). OVSAHO was obtained
from JCRB Cell Bank (Japanese Collection of Research Bioresources
Cell Bank). All ovarian cancer cells were cultured in RPMI
supplemented with 10% fetal bovine serum (FBS) and antibiotics.
Mouse monoclonal FOXC2, E-cadherin and vimentin antibodies were
purchased from Abcam (Cambridge, MA, USA). Mouse monoclonal β-actin
antibody was purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA).
FOXC2-specific siRNA inhibition
To knock down FOXC2 expression in ovarian cancer
cells, the following two siRNAs against FOXC2 were purchased from
GenePharma (Shanghai, China): siFOXC2-no.1 (5′-GCAGTCTTATC
TAACTATGATGCAA-3′), and siFOXC2-no.2 (5′-TCGCA AAGGGCATGAACTA-3′).
Cells were grown in dishes until they reached 75% confluence and
were then transfected for 24 h with siRNA specific to FOXC2 using
the Lipofectamine 2000 transfection reagent, according to the
manufacturer’s instructions. FOXC2 expression was then confirmed by
RT-PCR and western blot analyses.
Plasmid construct and generation of
stable cell lines
Human cDNA of FOXC2 was cloned as previously
reported (22). Full-length cDNAs
were subcloned into multiple cloning sites of the pBabe plasmid,
forming the pBabeFOXC2 expression plasmids. TAOV cells were
transfected with the pBabe or pBabe-FOXC2 plasmid respectively,
using the Lipofectamine 2000 according to the manufacturer’s
instructions (Invitrogen, Grand Island, NY, USA). Stable
transfectants were obtained after selection with puromycin (10
μg/ml; Invitrogen) for 2 weeks. RT-PCR and western blot analyses
were carried out to determine the expression of FOXC2 mRNA and
protein in stable cell lines, respectively.
RT-PCR
Total RNA was extracted from tissues and cell lines
using the TRIzol reagent (Invitrogen). Reverse transcription was
performed using the Thermo script RT System (Invitrogen). Hot start
PCR conditions were: 45 sec at 94°C, 30 sec at 55°C, and 1 min at
72°C for 28 to 30 cycles or 26 cycles. This study used the
following primers: FOXC2 (sense, 5′-CCTACC TGAGCGAGCAGAAT-3′ and
antisense, 5′-ACCTTGACGA AGCACTCGTT-3′); E-cadherin (sense,
5′-GGCACTTTTGAA GATCATTTTCTC-3′ and antisense, 5′-CTGTGTTGAGGGC
AATGAG-3′); vimentin (sense, 5′-GGCACTTTTGAAGATC ATTTTCTC-3′ and
antisense, 5′-CTGTGTTGAGGGCAAT GAG-3′); and GAPDH (sense,
5′-TGCCTCCTGCACCACCA ACT-3′ and antisense,
5′-CCCGTTCAGCTCAGGGATGA-3′).
Western blotting
Samples and cells were solubilized in
radioimmunoprecipitation assay lysis buffer [50 mmol/l Tris-HCl (pH
7.4), 1% NP40, 0.25% Na-deoxycholate, 150 mmol/l NaCl, 1 mmol/l
EDTA, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mg/ml each of
aprotinin, leupeptin and pepstatin, 1 mmol/l
Na3VO4, 1 mmol/l NaF]. The supernatants,
which contained the whole-cell protein extracts, were obtained
after centrifugation of the cell lysates at 10,000 × g for 10 min
at 4°C. A total of 20 μg of protein samples were loaded on a sodium
dodecyl sulfate-PAGE gel (5% stacking gel and 12% separating gel).
These proteins were then transferred to polyvinylidene difluoride
membranes (Millipore, Bedford, MA, USA). The membranes were
initially probed with a primary antibody and then with a secondary
antibody. The bound antibody was detected by enhanced
chemiluminescence detection reagents (Amersham Biosciences,
Piscataway, NJ, USA) according to the manufacturer’s instructions.
The band intensity was quantitated using ImageQuant software
(Molecular Dynamics, Sunnyvale, CA, USA).
Confocal immunofluorescence
microscopy
Cell lines were plated on culture slides (Costar,
Manassas, VA, USA). After 24 h, the cells were rinsed with PBS and
fixed with 4% paraformaldehyde, and cell membrane was permeabilized
using 0.5% Triton X-100. These cells were then blocked for 30 min
in 10% BSA and then incubated with primary antibodies overnight at
4°C. After three washes in PBS, the slides were incubated for 1 h
in the dark with FITC-conjugated secondary antibodies (Invitrogen).
After three further washes, the slides were stained with DAPI for 5
min to visualize the nuclei, and examined using a Carl Zeiss
confocal imaging system (LSM 780; Carl Zeiss, Jena, Germany).
Wound healing assay
Equal numbers of different cells were seeded in
six-well tissue culture plates. When 90% confluence was reached, a
single wound was created by gently removing the attached cells
using a sterile plastic pipette tip. Debris was removed by washing
the cells with serum-free medium. Cell migration of the cells into
the wounded area was observed and noted at different time points.
Various migrations and extended protrusion of cells from the border
of the wound were visualized and photo-documented using an inverted
microscope.
Cell invasion and motility assay
Cell invasion was measured in Matrigel-coated (BD,
Franklin Lakes, NJ, USA) Transwell inserts (6.5 mm; Costar,
Manassas, VA, USA) containing polycarbonate filters with 8 μm pores
as previously described in detail (23). The inserts were coated with 50 μl of
1 mg/ml Matrigel matrix according to the manufacturer’s
recommendations. Cells (2×105) in 200 μl of serum-free
medium were plated in the upper chamber, whereas 600 μl of medium
with 10% FBS were added to the lower well. After 24 h incubation,
the top cells were removed and the bottom cells were counted. The
cells that migrated to the lower surface of the membrane were fixed
in 4% paraformaldehyde and stained with 0.5% crystal violet. For
each membrane, five random fields were counted at ×10
magnification. The mean was calculated, and data are presented as
means ± SD from three independent experiments performed in
triplicate.
Motility assays were performed using Transwell
membrane inserts containing polycarbonate filters with 8 μm pores.
The methods used in the cell migration assay were similar to those
in the Matrigel invasion assay, except that the Transwell insert
was not coated with Matrigel.
Immunohistochemistry
Surgically excised specimens were fixed with 10%
neutral formalin, embedded in paraffin, and 4 μm specimen sections
were prepared. Immunostaining was performed using the
avidin-biotin-peroxidase complex method (UltrasensitiveTM; MaiXin
Bio Co., Fuzhou, China). The sections were deparaffinized in
xylene, rehydrated with graded alcohol, and boiled in 0.01 M
citrate buffer (pH 6.0)for 2 min with an autoclave. Hydrogen
peroxide (0.3%) was applied to block endogenous peroxide activity,
and the sections were incubated with normal goat serum to reduce
non-specific binding. Tissue sections were incubated with different
monoclonal antibodies. Mouse immunoglobulin (with the same
concentration of the antigen-specific antibody) was used as a
negative control. Staining for both antibodies was performed at
room temperature for 2 h. Biotinylated goat anti-mouse serum IgG
was used as a secondary antibody. The sections were washed and
incubated with streptavidin-biotin conjugated with horseradish
peroxidase. The peroxidase reaction was developed with
3,30-diaminobenzidine tetrahydrochloride. Two independent blinded
investigators randomly examined all tumor slides. Five views were
examined per slide, and 100 cells were observed per view at ×400
magnification.
Statistical analysis
Experimental data are shown as the means ± SD. A
two-tailed Student’s t-test was used to compare the results from
the different treatment groups. Differences with P<0.05 were
considered statistically significant; SPSS/Win11.0 software (SPSS,
Inc., Chicago, IL, USA) was used to analyze the data.
Results
FOXC2 is overexpressed in metastatic
ovarian cancer cell lines and invasive ovarian cancer tissues
To identify whether or not FOXC2 can initiate
ovarian cancer invasion and metastasis, we initially evaluated the
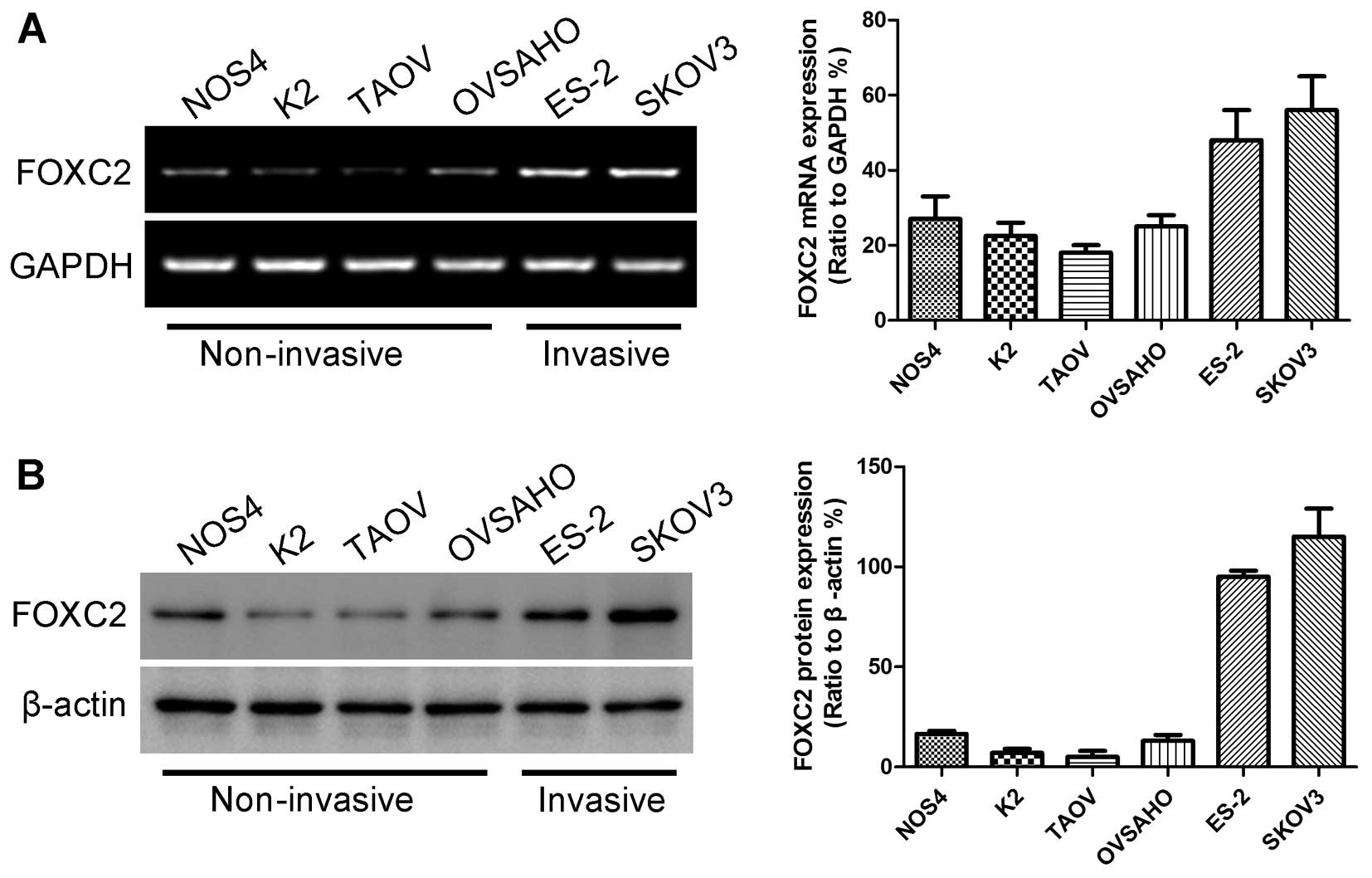
mRNA expression levels of FOXC2 in six ovarian cancer cell lines
(NOS4, K2, TAOV, OVSAHO, ES-2 and SKOV3) by conducting RT-PCR
analysis. The highest FOXC2 expression was observed in ES-2 and
SKOV3 cells (Fig. 1A). The mRNA
expression level of FOXC2 was related to the malignant
characteristics of ovarian cancer cells. ES-2 and SKOV3 cells were
more invasive than other ovarian cancer cell lines and could grow
under anchorage-independent conditions (24). Western blotting results also showed
the protein expression levels of FOXC2 in the six ovarian cancer
cell lines (Fig. 1B).
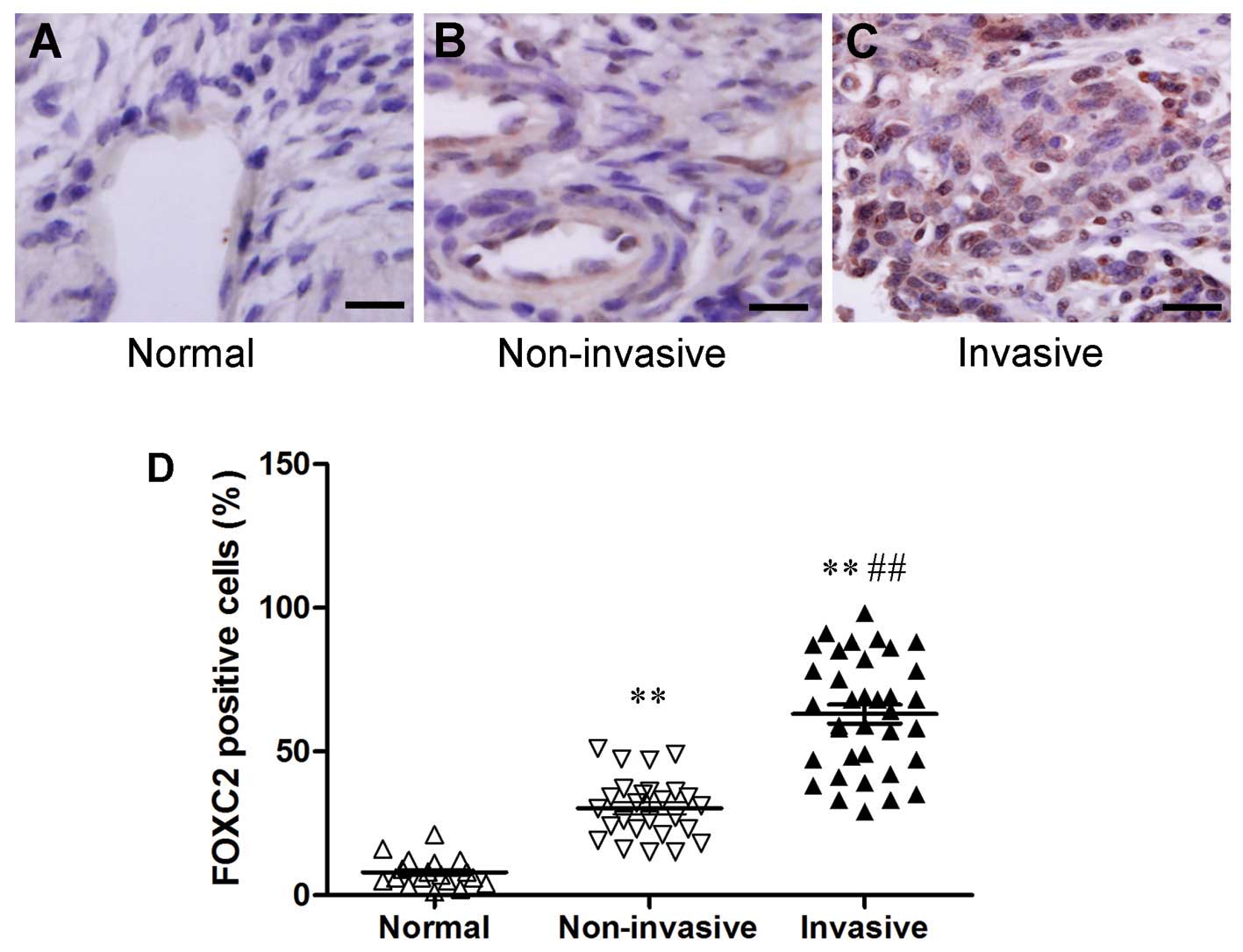
FOXC2 expression was also analyzed in normal ovarian
tissues and cancer tissues by immunohistochemistry (normal ovarian
tissues, 18; non-invasive ovarian cancer tissues, 26; invasive
ovarian cancer tissues, 36). Human normal ovarian tissues did not
show immunostaining properties (Fig.
2A). FOXC2 protein, which was localized in the nuclei of tumor
cells, was expressed in all the human ovarian cancer samples
(Fig. 2B and C). We also found a
strong expression of FOXC2 protein in some cancer tissues, and this
expression was correlated with metastatic property (Fig. 2D). These associations suggested that
FOXC2 expression was induced during tumor progression. FOXC2 may
also exhibit a causal function, enabling metastatic
dissemination.
FOXC2 depletion induces the reversion of
EMT
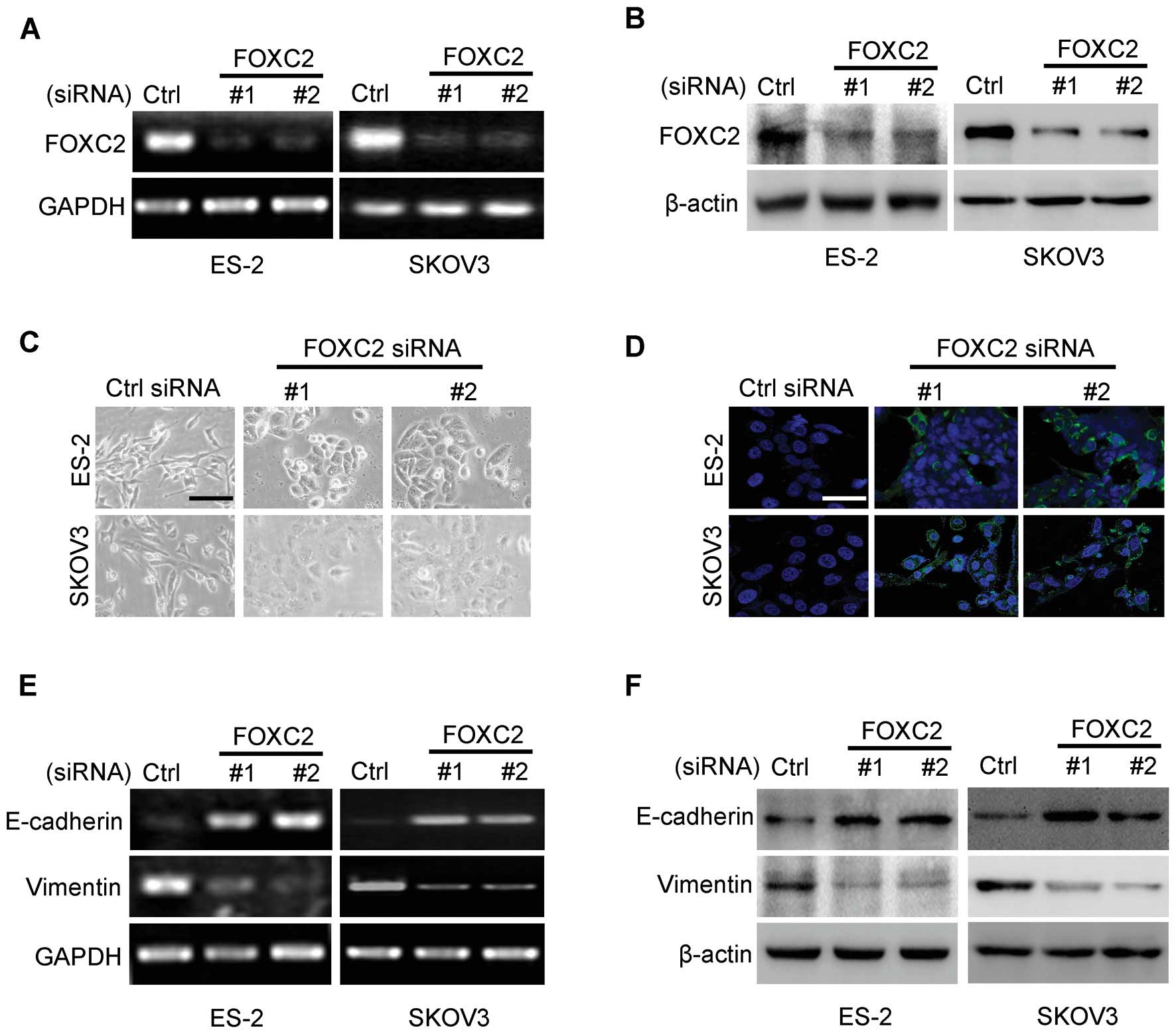
We determined whether or not the expression of FOXC2
contributes to the EMT of ovarian cancer cells. We suppressed FOXC2
expression in the cells with high FOXC2 expression (ES-2 and SKOV3)
by constructing two siRNA oligonucleotides targeting the mRNA of
human FOXC2. The two siRNAs of FOXC2 reduced the level of FOXC2
expression at mRNA and protein levels by >70% (Fig. 3A and B).
We then examined the effects of FOXC2 depletion in
the cell lines with high FOXC2 expression. FOXC2 depletion induced
the recovery of cell-to-cell adhesions; the cellular morphologies
of these cell lines were similar to those of epithelial cells
(Fig. 3C). The results of
immunostaining analysis revealed that a clear recovery of
E-cadherin localization was induced in ES-2 and SKOV3 cells by
knocking down FOXC2 expression (Fig.
3D). This result suggested that the cells underwent a reversion
of EMT when FOXC2 level was reduced in ES-2 and SKOV3 cells. To
test this hypothesis, we examined the expression levels of
epithelial and mesenchymal markers. The upregulation of E-cadherin
and the downregulation of vimentin were detected at mRNA and
protein levels in ES-2 and SKOV3 cells (Fig. 3E and F). These results indicated
that FOXC2 is important for SKOV3 and ES-2 cells to maintain
mesenchymal characteristics.
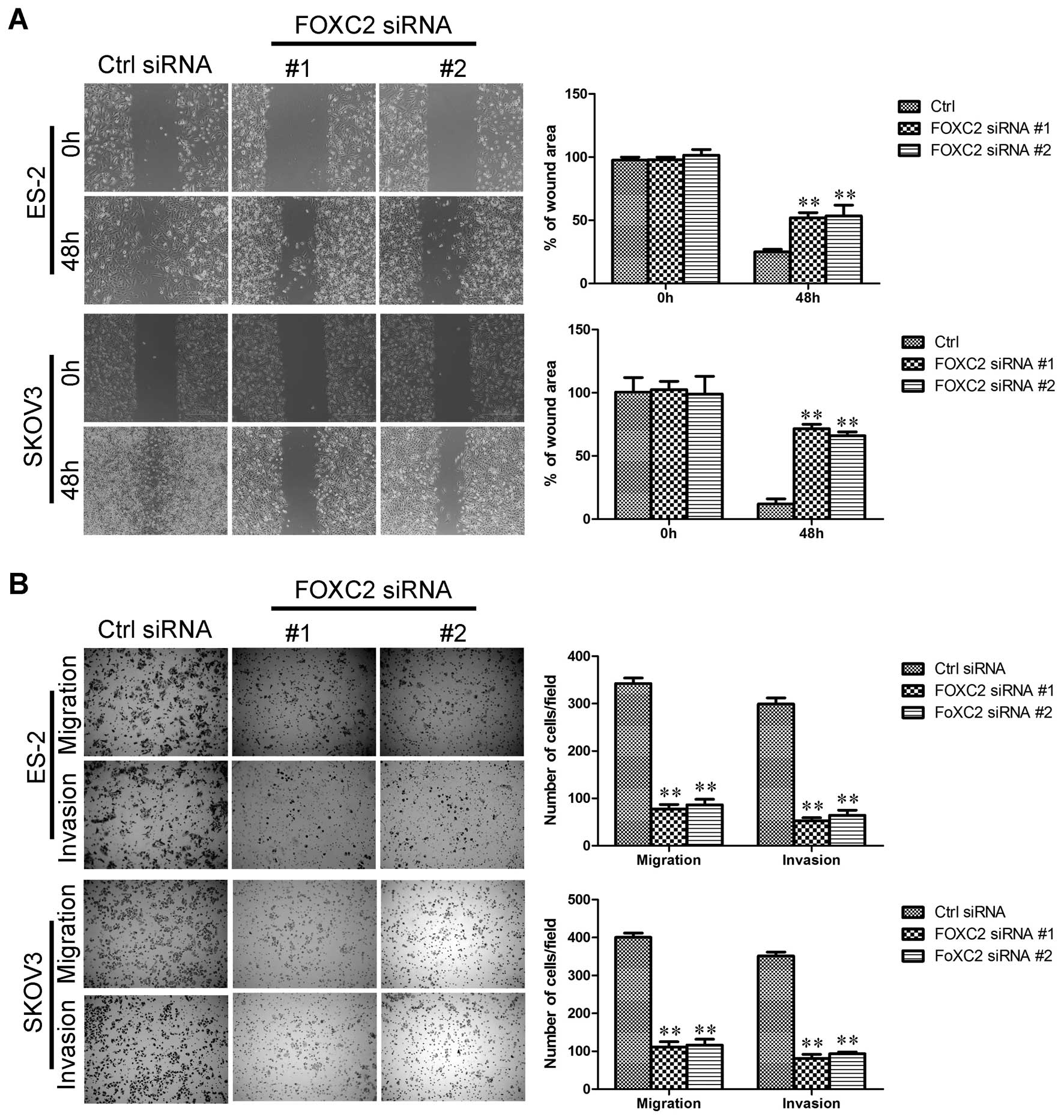
FOXC2 depletion inhibits ovarian cancer
cell migration and invasion
EMT is associated with malignant properties, such as
migration and invasion (25,26).
We investigated whether or not FOXC2 is required for these
properties in ovarian cancer cells. The effect of FOXC2 on cell
migration was initially assessed by wound healing assay. ES-2
siFOXC2 and SKOV3 siFOXC2 cells exhibited a significantly slower
closure of the wound area than the control cells (Fig. 4A). This result was confirmed by
Boyden’s chamber assay (Fig. 4B).
Moreover, the invasion of SKOV3 and ES-2 cells through the Matrigel
was significantly suppressed by FOXC2 knockdown (Fig. 4B).
Ectopic FOXC2 expression induces EMT and
promotes the migration and invasion of ovarian cancer cells in
vitro
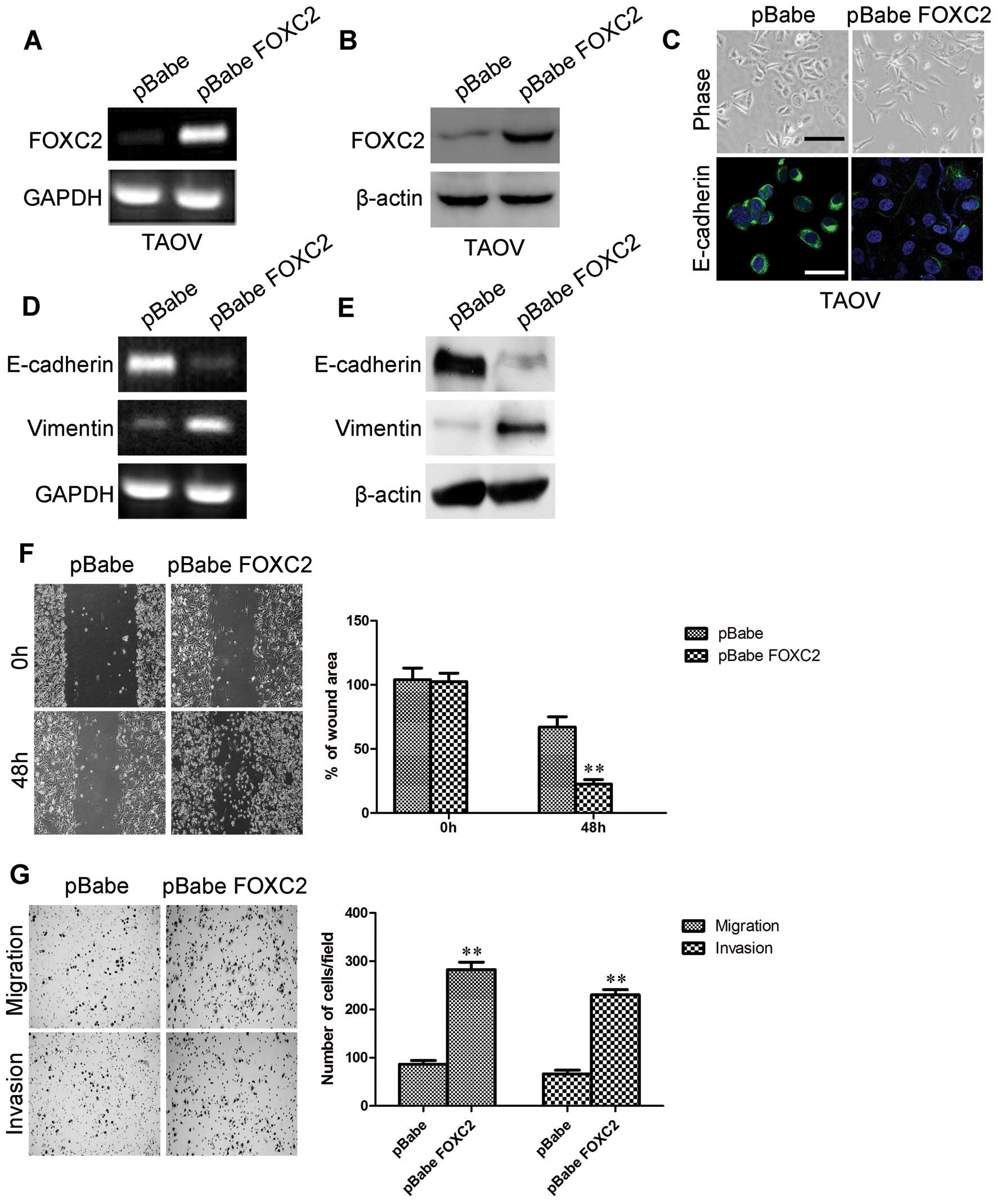
To evaluate the oncogenic activity of FOXC2 in
ovarian cancer, we retrovirally established the stable
overexpression of FOXC2 in TAOV cells (designated as TAOV-pBabe
FOXC2). The FOXC2 expression in the resultant cell line was
verified by RT-PCR (Fig. 5A) and
western blotting (Fig. 5B). We
initially observed the morphological changes and found that
TAOV-pBabe FOXC2 cells exhibited a fibroblastic morphology
(Fig. 5C). This observation was
further confirmed by analyzing the expression levels of epithelial
and mesenchymal markers. We showed that FOXC2 overexpression
decreased the levels of epithelial markers (E-cadherin; Fig. 5C and E) and increased the levels of
mesenchymal markers (vimentin; Fig.
5E) in TAOV-pBabe FOXC2 cells. The mRNA expression levels were
correlated with the corresponding protein levels (Fig. 5D), suggesting that FOXC2 affected
the expression of epithelial and mesenchymal markers at the
transcript level.
The effect of FOXC2 on cell migration was initially
assessed by wound healing assay. TAOV-pBabe FOXC2 cells exhibited a
significantly faster closure of the wound area than the control
cells (Fig. 5F). This result was
confirmed by Boyden’s chamber assay; TAOV-pBabe FOXC2 cells
migrated by more than two-fold in the Transwell membranes than the
control cells after 48 h of incubation (Fig. 6F). TAOV-pBabe FOXC2 cells also
exhibited a higher degree of invasion in the Matrigel (Fig. 6F). These results indicated that
FOXC2 promoted the migratory and invasive behaviors of ovarian
cancer cells.
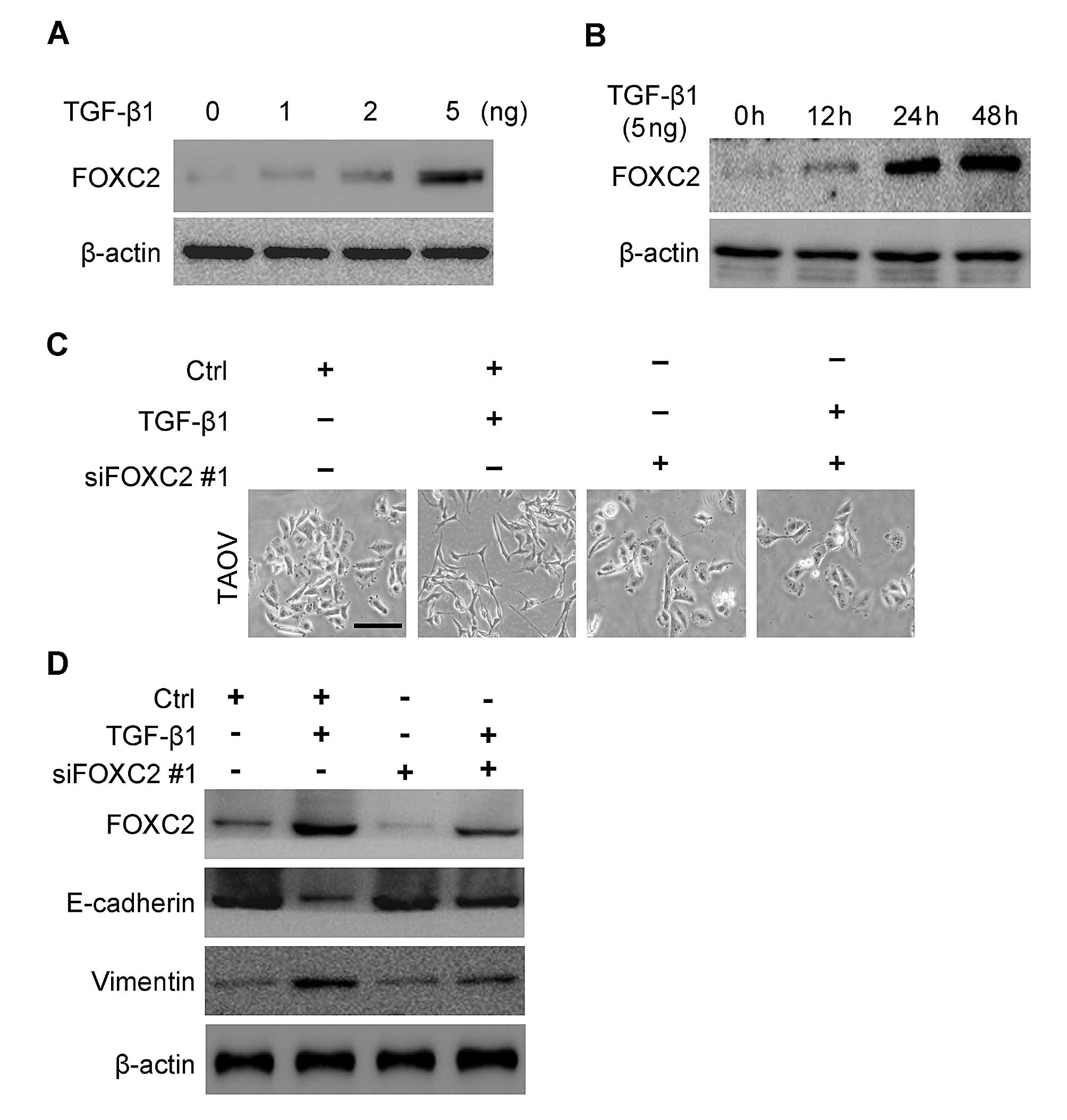
TGF-β1 stimulation induces FOXC2
upregulation
Although various growth factors and cytokines
orchestrate EMT, TGF-β1 exhibits a major function in this process
(27,28). Considering that FOXC2 is associated
with EMT, we then investigated whether or not the expression of
FOXC2 is upregulated by TGF-β1 in ovarian cancer cells (TAOV).
TGF-β1 increased FOXC2 expression at 12 h after treatment and
reached the highest level at 24 h (Fig.
6A) compared with the untreated cells. We also found that the
increasing FOXC2 expression stimulated by TGF-β1 was
concentration-dependent. Hence, FOXC2 level increased gradually as
TGF-β1 level increased (Fig.
6B).
FOXC2 suppression attenuates
TGF-β1-induced EMT in TAOV cells
Considering that TGF-β1 stimulation can induce EMT
and upregulate FOXC2 expression in TAOV cells, we proposed that
FOXC2 may be involved in TGF-β1-induced EMT of TAOV cells. To
understand the specific biological functions that FOXC2 performs
during TGF-β1-induced EMT, we knocked down FOXC2 in TAOV cells
transfected with a FOXC2-targeting siRNA. We initially observed the
morphological changes in TGF-β1-induced TAOV cells after FOXC2
silencing was performed. siRNA-transfected cells showed epithelial
morphology (Fig. 6C) compared with
the control-transfected cells. FOXC2 expression was then observed
by western blotting after 48 h of TGF-β1 treatment. In Fig. 6D, FOXC2 expression in FOXC2
siRNA-transfected cells was downregulated significantly compared
with that in the control-transfected cells. These results showed
that FOXC2 levels were decreased in FOXC2 siRNA-transfected cells
and the silencing of FOXC2 expression reversed TGF-β1-induced
morphological transition (Fig. 6C).
We subsequently determined the underlying molecular mechanism. In
Fig. 6D, FOXC2 expression was
detected after TGF-β1 was stimulated for 48 h in the cells
transfected with FOXC2 siRNA or with the control cells. The FOXC2
expression in FOXC2 siRNA-transfected cells significantly decreased
compared with that of the control cells. These results showed that
siRNA against FOXC2 could efficiently reduce FOXC2 expression. At
the same time, the downregulation of FOXC2 resulted in a decrease
in the mesenchymal marker vimentin and an increase in the
epithelial marker E-cadherin (Fig.
6D).
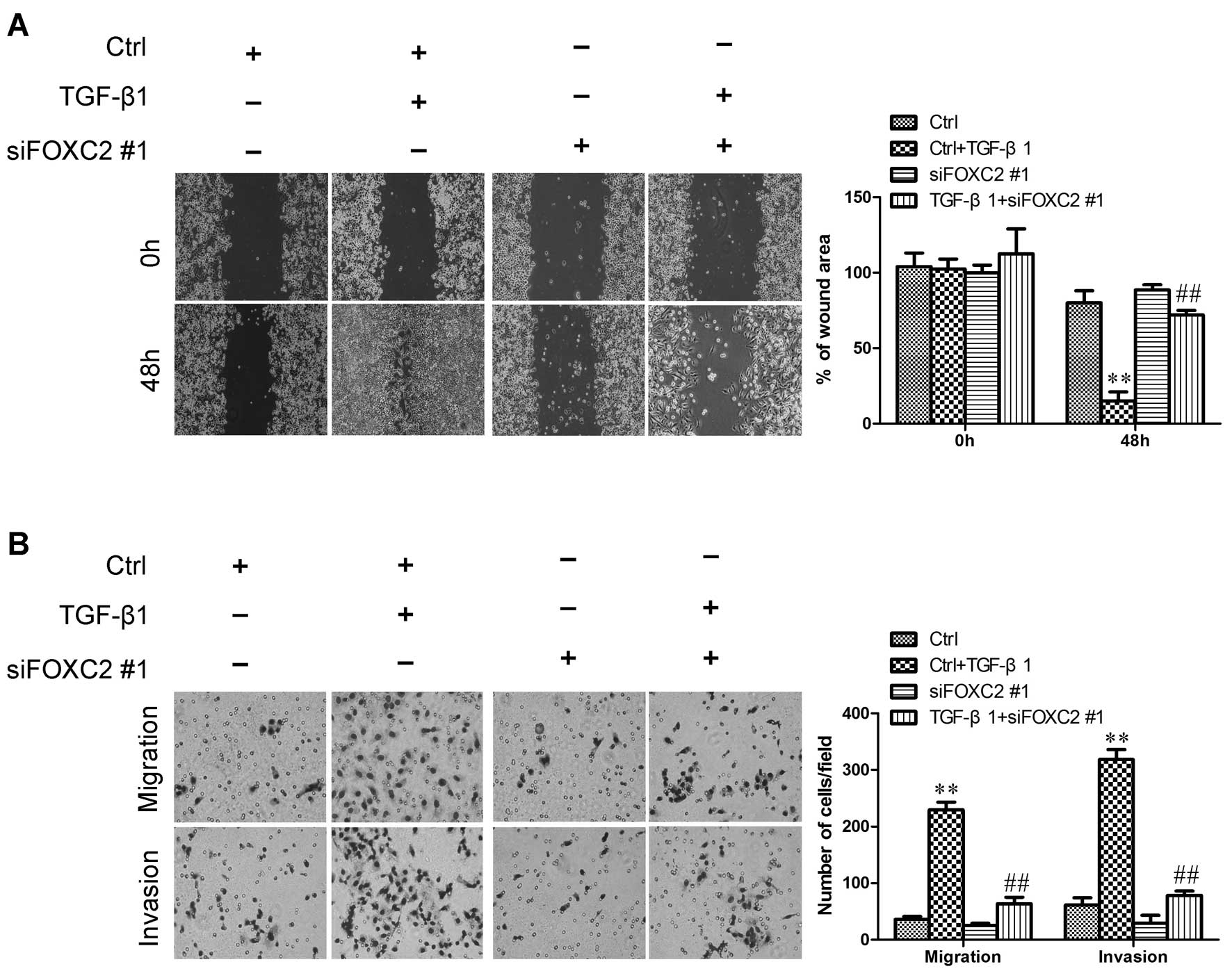
FOXC2 suppression effectively suppresses
the TGF-β1-induced migration and invasion of TAOV cells
Considering that EMT can increase cell motility, we
then examined whether or not FOXC2 can modulate the migratory and
invasive abilities of TGF-β1-induced ovarian cancer cells. To
determine the functional changes in the behavior of TAOV cells
after FOXC2 is suppressed, we initially assessed the effect of
FOXC2 on TGF-β1-induced cell migration by conducting a wound
healing assay. The cells were treated with TGF-β1; as a result, the
cells were stimulated and migrated, thereby closing the wound. By
comparison, TGF-β1-mediated migration was reversed when the cells
were co-incubated with siFOXC2. Mock treatment alone did not
markedly affect cell migration (Fig.
7A). This result was further evaluated by Boyden’s chamber
assay, in which the cells migrated through the Transwell membranes
to a lesser extent than their TGF-β1-induced cells after 48 h of
incubation (Fig. 7B). Moreover,
FOXC2 suppression markedly reduced the invasion ability through the
Matrigel (Fig. 7B). These results
indicated that FOXC2 suppression effectively inhibited the
TGF-β1-induced migration of TAOV cells.
Discussion
To the best of our knowledge, the present study is
the first to show that FOXC2 is overexpressed in ovarian cancer
cells and is involved in ovarian cancer cell migration and
invasion. FOXC2 overexpression in ovarian cancer cells induced EMT,
migration, and invasion in vitro. By contrast, silencing
FOXC2 aggressively reversed these events and invaded the ovarian
cancer cells. We also showed that TGF-β1 stimulation induced the
upregulation of FOXC2. Furthermore, FOXC2 exhibited an important
function in the TGF-β1-induced EMT of ovarian cancer cells. On the
basis of these results, we proposed a model for FOXC2 regulation of
EMT and metastasis of ovarian cancer.
Ovarian cancer is a major cause of cancer-related
mortality among women as this disease is typically not diagnosed
until the disease is at advanced stages when cancer is highly
metastatic (1). Although current
methodologies (surgery, radiation and chemotherapy) are considered
relatively effective for the treatment of primary ovarian tumors,
many patients treated at advanced stages eventually suffer
recurrence at metastatic sites (29). Considering that cancer metastasis is
possibly initiated by EMT, researchers focused on the
identification of possible therapeutic agents that may retard or
reverse EMT (30).
A high incidence of FOXC2 expression has been
observed in some types of cancer, and such expression has been
significantly associated with metastatic disease; however, limited
information is available on the function of FOXC2 expression in
ovarian cancer (20,31–33).
Previous studies showed that FOXC2 is important for vascular
formation during development (34).
Other studies have shown that FOXC2 is expressed in the endothelium
of tumors in humans and mice (22,35).
The present study is the first to define the functional roles of
human FOXC2 in ovarian cancer.
Motility and invasion are major events in cancer
metastasis; such events are also associated with poor prognosis in
patients with cancer (36).
However, the mechanisms associated with cell invasiveness remain
poorly understood. Metastasis is a complex phenomenon regulated by
many components that facilitate the detachment of tumor cells from
primary tumors to secondary sites (36). EMT promotes the distribution of a
single carcinoma cell from primary tumor sites to distant organs
(metastasis); EMT also exhibits a major function in ovarian cancer
progression (37). Another study
showed that FOXC2 is a transcription factor that can induce EMT
(19). Our study further
demonstrated that FOXC2 promotes the migratory and invasive
potential of ovarian cancer cells. The ectopic FOXC2 expression in
ovarian cancer cells increased the migratory and invasive behaviors
in vitro. This observation is consistent with that in a
previous study, in which FOXC2 was involved in the EMT of breast
cancer cells (19). The findings of
our study revealed an important function of FOXC2 in the
tumorigenesis and metastasis of ovarian cancer cells.
TGF-β1 is a pleiotropic factor that exhibits a
physiological function in regulating proliferation,
differentiation, development, wound healing and angiogenesis
(27). In addition, TGF-β1-induced
EMT has been well established as an important mechanism of ovarian
cancer progression (38). However,
TGF-β1-induced EMT is a very complicated process, and the exact
function of TGF-β1 in the stimulation of EMT is poorly understood.
In the current study, ovarian cancer cells underwent EMT changes
after these cells were exposed to TGF-β1. Our data also showed an
increased ability of TGF-β1-induced TAOV cells to undergo cell
migration and invasion compared with that of the untreated cells.
These results are consistent with those in previous studies. We
also found that TGF-β1-treated TAOV cells showed a high FOXC2
expression compared with the untreated cells. Our novel finding is
noteworthy as it connects two very important molecules, namely,
TGF-β1 and TAOV, of the developmental pathway to ovarian tumor
aggressiveness. Our result is also consistent with that in
previously published reports, in which the function of EMT in tumor
aggressiveness and metastasis was demonstrated.
Our results also suggested that the maintenance of
EMT phenotype in TGF-β1-treated cells may be related to the
sustained activation of FOXC2. The inhibition of TGF-β1-induced
FOXC2 by siRNA decreased the ability of TGF-β1-treated TAOV cells
to migrate and invade.
We further showed the importance of FOXC2 in EMT, in
which the siRNA-inhibited FOXC2 signaling could downregulate
mesenchymal markers, such as vimentin. This result is consistent
with the upregulation of epithelial markers, such as E-cadherin.
These results also suggested that the attenuation of FOXC2
signaling could reverse the EMT phenotype to
mesenchymal-to-epithelial transition, resulting in decreased cell
migration, invasion and tumorigenic potential. Our data showed for
the first time that TGF-β1-induced EMT is mediated by upregulating
FOXC2 as the knockdown of FOXC2 by FOXC2-specific siRNA
significantly attenuated EMT induction by TGF-β1 treatment.
In conclusion, this study is the first to show that
FOXC2 promotes ovarian cancer cell proliferation and invasion
properties. FOXC2 also exhibited an important function in the
enhancement of malignancy by TGF-β1 in ovarian cancer cells. The
results of this study could be used as guidelines to target novel
proteins located downstream of FOXC2 and disrupt the signaling
pathways involved in the proliferation, motility and invasion of
ovarian cancer.
Acknowledgements
This project was supported by the Natural Science
Foundation of China (81172180).
References
|
1
|
Naora H and Montell DJ: Ovarian cancer
metastasis: integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmitt J and Matei D: Targeting
angiogenesis in ovarian cancer. Cancer Treat Rev. 38:272–283. 2012.
View Article : Google Scholar
|
|
3
|
Davidson B, Tropé CG and Reich R:
Epithelial-mesenchymal transition in ovarian carcinoma. Front
Oncol. 2:332012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Comamala M, Pinard M, Theriault C, et al:
Downregulation of cell surface CA125/MUC16 induces
epithelial-to-mesenchymal transition and restores EGFR signalling
in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer. 104:989–999.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng JC, Auersperg N and Leung PC:
EGF-induced EMT and invasiveness in serous borderline ovarian tumor
cells: a possible step in the transition to low-grade serous
carcinoma cells? PLoS One. 7:e340712012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng JC, Auersperg N and Leung PC:
TGF-beta induces serous borderline ovarian tumor cell invasion by
activating EMT but triggers apoptosis in low-grade serous ovarian
carcinoma cells. PLoS One. 7:e424362012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bagnato A and Rosanò L:
Epithelial-mesenchymal transition in ovarian cancer progression: a
crucial role for the endothelin axis. Cells Tissues Organs.
185:85–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahmed N, Thompson EW and Quinn MA:
Epithelial-mesenchymal interconversions in normal ovarian surface
epithelium and ovarian carcinomas: an exception to the norm. J Cell
Physiol. 213:581–588. 2007. View Article : Google Scholar
|
|
9
|
Elloul S, Vaksman O, Stavnes HT, Tropé CG,
Davidson B and Reich R: Mesenchymal-to-epithelial transition
determinants as characteristics of ovarian carcinoma effusions.
Clin Exp Metastasis. 27:161–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Wen M, Kwon Y, et al: CUL4A
induces epithelial-mesenchymal transition and promotes cancer
metastasis by regulating ZEB1 expression. Cancer Res. 74:520–532.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang RY, Chung VY and Thiery JP:
Targeting pathways contributing to epithelial-mesenchymal
transition (EMT) in epithelial ovarian cancer. Curr Drug Targets.
13:1649–1653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Liu Y, Lu J, et al: Rapamycin
inhibits FBXW7 loss-induced epithelial-mesenchymal transition and
cancer stem cell-like characteristics in colorectal cancer cells.
Biochem Biophys Res Commun. 434:352–356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu J, Wen M, Huang Y, et al: C2ORF40
suppresses breast cancer cell proliferation and invasion through
modulating expression of M phase cell cycle genes. Epigenetics.
8:571–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Ma G, Wang Q, et al: Involvement
of CUL4A in regulation of multidrug resistance to P-gp substrate
drugs in breast cancer cells. Molecules. 19:159–176. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosano L, Spinella F, Di Castro V, et al:
Endothelin-1 promotes epithelial-to-mesenchymal transition in human
ovarian cancer cells. Cancer Res. 65:11649–11657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: an alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollier BG, Tinnirello AA, Werden SJ, et
al: FOXC2 expression links epithelial-mesenchymal transition and
stem cell properties in breast cancer. Cancer Res. 73:1981–1992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishida N, Mimori K, Yokobori T, et al:
FOXC2 is a novel prognostic factor in human esophageal squamous
cell carcinoma. Ann Surg Oncol. 18:535–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kajiyama H, Kikkawa F, Suzuki T, Shibata
K, Ino K and Mizutani S: Prolonged survival and decreased invasive
activity attributable to dipeptidyl peptidase IV overexpression in
ovarian carcinoma. Cancer Res. 62:2753–2757. 2002.PubMed/NCBI
|
|
22
|
Mortazavi F, An J, Dubinett S and Rettig
M: p120-catenin is transcriptionally downregulated by FOXC2 in
non-small cell lung cancer cells. Mol Cancer Res. 8:762–774. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu J, Lv X, Lin H, et al: Ubiquitin ligase
cullin 7 induces epithelial-mesenchymal transition in human
choriocarcinoma cells. J Biol Chem. 285:10870–10879. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan H, Kajiyama H, Ito S, et al: ALX1
induces snail expression to promote epithelial-to-mesenchymal
transition and invasion of ovarian cancer cells. Cancer Res.
73:1581–1590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, betacatenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsuno Y, Lamouille S and Derynck R:
TGF-beta signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nougaret S, Addley HC, Colombo PE, et al:
Ovarian carcinomatosis: how the radiologist can help plan the
surgical approach. Radiographics. 32:1775–1803. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu JL, Song YX, Wang ZN, et al: The
clinical significance of mesenchyme forkhead 1 (FoxC2) in gastric
carcinoma. Histopathology. 62:1038–1048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe T, Kobunai T, Yamamoto Y, et al:
Gene expression of mesenchyme forkhead 1 (FOXC2) significantly
correlates with the degree of lymph node metastasis in colorectal
cancer. Int Surg. 96:207–216. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mani SA, Yang J, Brooks M, et al:
Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is
associated with aggressive basal-like breast cancers. Proc Natl
Acad Sci USA. 104:10069–10074. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seo S, Fujita H, Nakano A, Kang M, Duarte
A and Kume T: The forkhead transcription factors, Foxc1 and Foxc2,
are required for arterial specification and lymphatic sprouting
during vascular development. Dev Biol. 294:458–470. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cederberg A, Grände M, Rhedin M, Peng XR
and Enerbäck S: In vitro differentiated adipocytes from a Foxc2
reporter knock-in mouse as screening tool. Transgenic Res.
18:889–897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chou JL, Chen LY, Lai HC and Chan MW:
TGF-β: friend or foe? The role of TGF-β/SMAD signaling in
epigenetic silencing of ovarian cancer and its implication in
epigenetic therapy. Expert Opin Ther Targets. 14:1213–1223.
2010.
|