Introduction
Intrahepatic cholangiocarcinoma (ICC) is a
life-threatening and treatment-refractory disease with a dismal
outcome. The lethality of the disease is due to both rapid tumor
growth and the tendency to invade adjacent organs and metastasize
(1). Although ICC is relatively
rare, its incidence in the Western world is showing an alarming
rise (2). ICC accounts for 13% of
all annual cancer-related deaths worldwide and for 3% of deaths in
Western countries (3). To date,
little is known about the mechanism of tumor initiation,
progression, metastasis formation and drug resistance of ICC. Due
to advances in surgical techniques and peri-operative management,
the best curative treatment for this malignancy is complete
resection with histologically negative tumor margins (4). However, only 13–27% of patients are
eligible for surgical treatment due to extensive perineural
invasion, lymphatic metastasis and vascular encasement (5). For the majority of patients presenting
with advanced disease, systemic chemotherapy has been
disappointing, as ICC has low sensitivity to most chemotherapeutic
drugs, including 5-fluorouracil (5-FU) (6,7).
Notch is an evolutionarily-conserved single-pass
transmembrane receptor involved in numerous cell fate decisions
during development, stem cell renewal and differentiation in
postnatal tissues. Accumulating evidence suggests that Notch
signaling plays a critical role in the development of several types
of cancer, functioning as a tumor promoter or a tumor suppressor
(8–15).
In the present study, we reported common
upregulation of Notch1 in ICC tissues (35/44, 79.5%). In functional
analysis, we showed knockdown of Notch1 could reduce cell
proliferation and invasiveness of ICC cells. Also, depletion of
Notch1 sensitized ICC cells to the chemotherapeutic drug 5-FU. The
identification and functional characterization of Notch1 in ICC
provides new insights into the possible use of Notch1 as a
therapeutic target.
Materials and methods
Patients and tumor tissue specimens
Forty-four surgically-resected liver tissue
specimens of ICC were examined. None of the patients had received
chemotherapy or radiation therapy prior to the radical tumor
resection. The project was approved by the Ethics Committee of the
Hospital and was in accordance with the Helsinki Declaration of
1975. Written informed consent was obtained from either the
patients or their guardians.
Immunohistochemical staining
Immunohistochemical staining was carried out
according to the protocol defined in the PV Two-Step kit
instructions (Zhongshan Goldenbridge Biotechnology, Beijing,
China). Briefly, sections of a paraffin-embedded tissue block were
deparaffinized twice in xylene for 15 min and rehydrated through
graded ethanol solutions. Sections were subsequently heated in a
microwave oven twice for antigen retrieval for 8 min. Citrate
buffer (10 mmol/l, pH 6.0) was used as the antigen retrieval
buffer. Endogenous peroxidases were inactivated by immersing the
sections in 0.3% hydrogen peroxide for 15 min. Then, the slides
were incubated at 4°C overnight in a humidified chamber with Notch1
monoclonal antibody (Cell Signaling Technology Inc., Boston, MA,
USA) in a final dilution of 1:100. The sections were further
incubated with goat anti-rabbit immunoglobulin G-horseradish
peroxidase conjugate for 30 min at room temperature. Finally, the
sections were developed with DAB color solution (50 μl/section) for
2 min at room temperature. Hematoxylin (Boster Biotechnology,
Wuhan, China) was then used as a chromogen (50 μl/section) and the
slides were consecutively counter-stained for 30 sec. With the
exception of the omission of primary antibodies, negative controls
were processed in the same manner as above. All sections were
washed three times in phosphate-buffered saline (PBS; pH 7.4) for 5
min after each step.
Semi-quantitative method for the
immunohistochemical expression of Notch1
The semi-quantitative method was applied for the
immunohistochemical expression of Notch1. The percentages of
positively stained cells were determined by examination under a
microscope of 5 randomly selected foci, composed of >100 cells
each. Scoring was based on distribution and intensity according to
a previous report (16).
Cell culture
Two human ICC cell lines, RBE and HCCC-9810, were
purchased from the Chinese Academy of Sciences Shanghai Branch Cell
Bank and cultured in RPMI-1640 (Gibco BRL, Gaithersburg, MD, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Biological Industries, Kibbutz Beit Haemek, Israel) as recommended
by the supplier. All cultures were maintained in a humidified
atmosphere containing 5% CO2 at 37°C.
siRNA interference
For the RNAi analyses, human Notch1 small
interfering RNA (siRNA) with the nucleotide sequence
5′-UACAGUACUGACCUGUCCACUCUGG-3′ (sense) and
5′-CCAGAGUGGACAGGUCAGUACUGUA-3′ (antisense), corresponding to part
of the Notch1 mRNA were designed and purchased from Shanghai
GenePharma (Shanghai, China). As a negative control, we used a
control scrambled siRNA oligonucleotide (NC siRNA; sense,
5′-UUCUCCGAACGUGUCACG UTT-3′, antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) targeting a sequence not sharing
homology with the human genome (GenePharma). Briefly, RBE and
HCCC-9810 cells were cultured in RPMI-1640 medium containing 10%
FBS, incubated for 18–22 h at 37°C, 5% CO2 and grown to
50% confluence in well plates. Cells were transfected using the
Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s protocol. Notch1 siRNA or NC siRNA
(final 100 nM) solutions were prepared and the siRNA-Lipofectamine
transfection complexes were incubated for 20 min at room
temperature. The culture medium was replaced with OPTI-MEM
(Invitrogen) and suspension was added drop-wise onto the cells.
After 6 h of transfection, the culture medium was recovered to a
normal medium (RPMI-1640 medium containing 10% FBS).
RNA isolation and real-time qRT-PCR
analysis
Total RNA was isolated using RNAiso Plus reagent
according to the manufacturer’s protocol (Takara Biotechnology,
Tokyo, Japan). cDNA was synthesized using the PrimeScript RT
Reagent (Takara). Portions of double-stranded cDNA were subjected
to PCR with a SYBR-Green Premix Ex Taq (Takara). Primer sets
(design and synthesis by Takara) used for qRT-PCR are shown in
Table I. As a control, the levels
of glyceraldehyde phosphate dehydrogenase (GAPDH) expression were
also analyzed. The amplification protocol comprised incubations at
95°C for 30 sec, 95°C for 5 sec and 65°C for 20 sec. Incorporation
of the SYBR-Green dye into PCR products was monitored in real-time
with LightCycler real-time PCR detection system (Roche Applied
Science, Indianapolis, IN, USA), thereby allowing for the
determination of the threshold cycle (Ct) at which exponential
amplification of products begins. Fold change was determined by
2−ΔΔCt.
 | Table IPrimers for Notch1, MRP-1, ABCB-1,
ABCG-2 and reference genes. |
Table I
Primers for Notch1, MRP-1, ABCB-1,
ABCG-2 and reference genes.
| Gene | Primer Sequence |
|---|
| Notch1 | F:
5′-GAGGCGTGGCAGACTATGC-3′
R: 5′-CTTGTACTCCGTCAGCGTGA-3 |
| MRP-1 | F:
5′-GAGGAACCATATTACAGGTCCGT-3′
R: 5′-AGGGGATCATCGAAGAGGTAAAT-3′ |
| ABCB-1 | F:
5′-GGGAGCTTAACACCCGACTTA-3′
R: 5′-GCAAAATCACAAGGGTTAGCTT-3′ |
| ABCG-2 | F:
5′-CAGGTGGAGGCAAATCTTCGT-3′
R: 5′-ACCCTGTTAATCCGTTCGTTTT-3′ |
| GAPDH | F:
5′-ACAACTTTGGTATCGTGGAAG-3′
R: 5′-ACAACTTTGGTATCGTGGAAG-3′ |
Protein purification and western blot
analysis
Cells were rinsed three times with cold PBS and
lysed on ice with a lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl,
1% NP-40, 0.1% SDS], 1% protease inhibitor phenylmethane-sulfonyl
fluoride (PMSF; Bocai Bio, Shanghai, China) for 30 min and
centrifuged down to collect the supernatant. Protein concentrations
in the supernatant were determined using a BCA protein assay kit
(Bocai Bio). Proteins were separated on 10% SDS-PAGE gels and
transferred to a PVDF membrane followed by western blot analysis.
In brief, 5% milk in TBS containing 0.1% Tween-20 was used to block
the non-specific binding. The blot was subsequently incubated with
primary antibodies for Notch1 (1:1,000; Cell Signaling Technology),
MRP-1 (1:50, Chemicon Inc., Temecula, CA, USA), ABCB-1 (1:1,000;
Epitomics Inc., Burlingame, CA, USA), ABCG-2 (1:1,000; Epitomics)
and with the goat anti-rabbit secondary antibody (1:2,000; Cell
Signaling Technology). β-tubulin (1:2,000; Cell Signaling
Technology) was used as an internal control. After each antibody
incubation, blots were extensively washed in TBS containing 0.1%
Tween-20. For detection, we used the ECL Plus Western Blotting
Detection System (Amersham Biosciences, Piscataway, NJ, USA). The
intensity of western bands was measured by the Quantity One
software (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Cell proliferation assay by CCK-8
One day before transfection, the cells
(5×103/well) were cultured in 96-well tissue culture
plates and then transfected with Notch1 siRNA or NC siRNA. After
transfection (24, 48 and 72 h), viability of the cells was
determined using the Cell Counting Kit-8 (CCK-8) that was purchased
from the Dojindo Molecular Technologies (Dojindo China Co.,
Shanghai, China). Briefly, after washing in PBS, 100 μl medium
containing 10% CCK-8 solution were added to each well and incubated
for 2 h at 37°C. Samples were read directly in the wells using an
absorbance of the 450 nm wavelength by an enzyme linked
immunosorbent assay (ELISA) plate reader. In order to adjust the
background, the blank control absorbance (100 μl medium containing
10% CCK-8 alone) was subtracted from the experimental
absorbance.
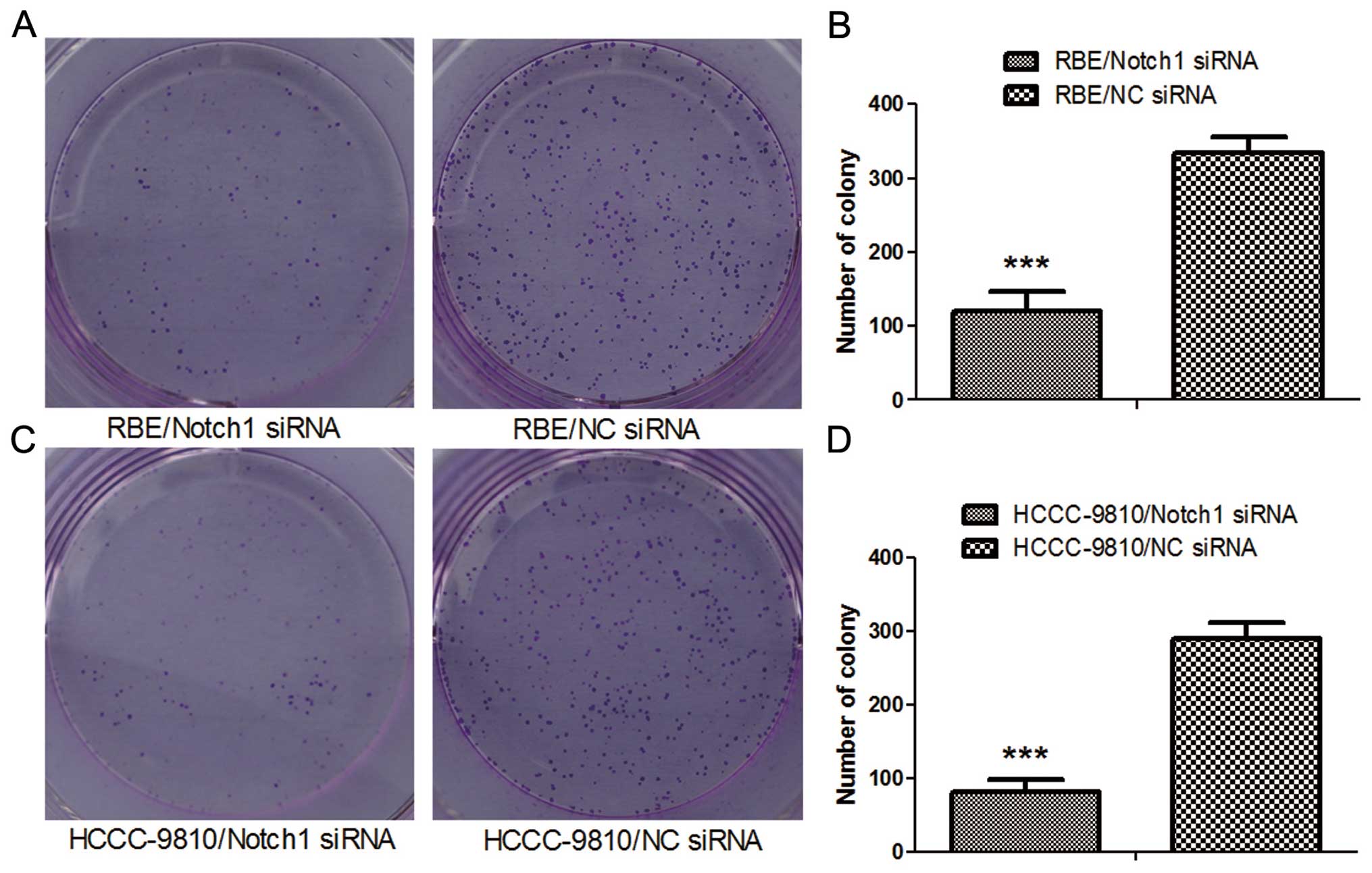
Colony formation assay
Twenty-four hours after transfection, the
transfected cells were seeded in 6-well tissue culture plates (500
cells/well). After an incubation period of 10 days, the medium was
decanted and each well was washed twice with PBS. The cells were
stained with 1% crystal violet (in 100% methanol) for 15 min,
followed by detaining. Colonies (>20 cells/colony) were
counted.
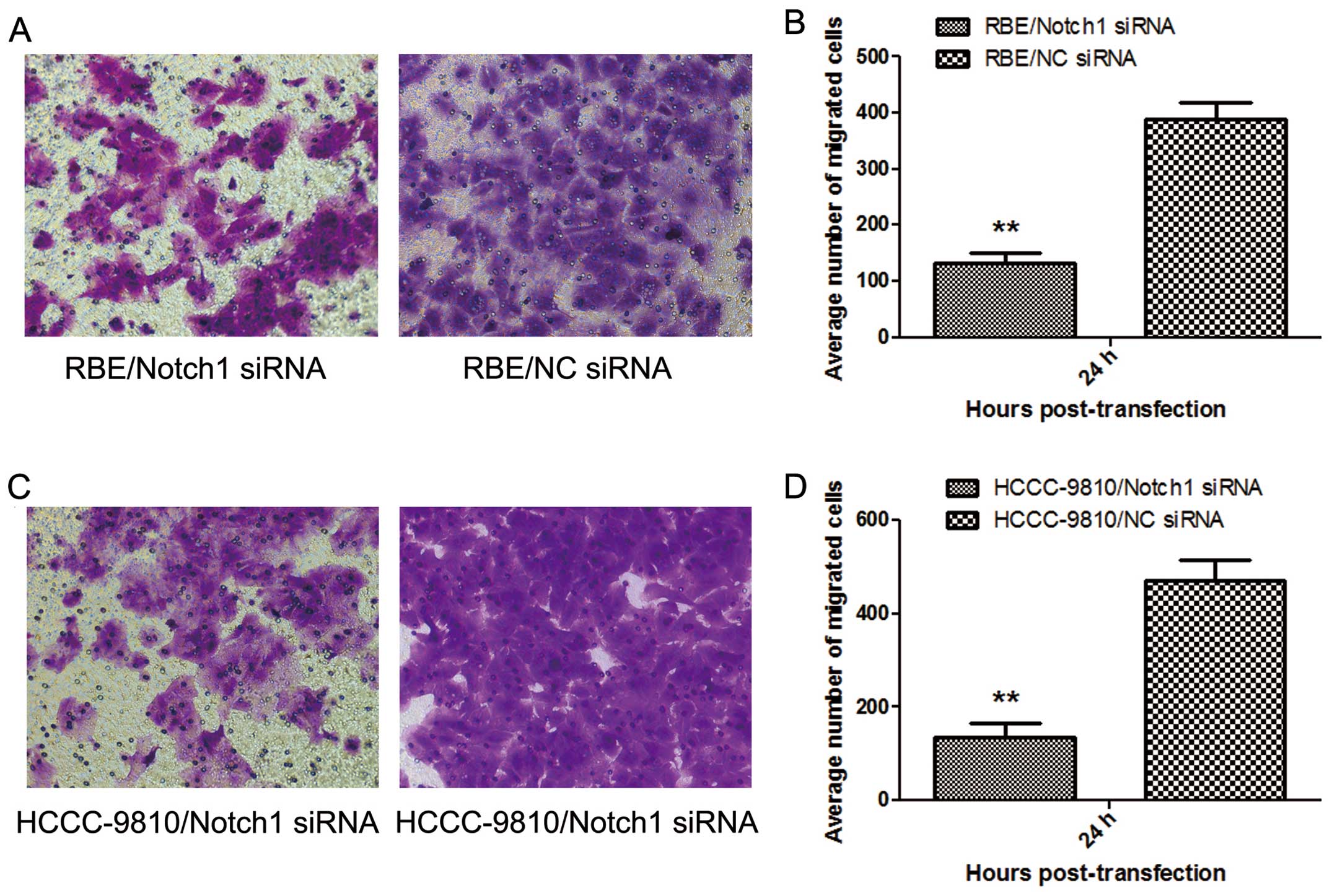
Transwell invasive assay
Twenty-four hours after transfection,
1×105 cells in 0.1 ml serum-free medium were placed into
the upper chamber (24-well plate) of the insert (Corning Inc.,
Corning, NY, USA) with Matrigel (BD Biosciences, San Jose, CA,
USA), whereas the lower chamber was 0.6 ml RPMI-1640 medium
containing 20% FBS. After 24 h of incubation, the cells that were
still on the upper side of the filters were mechanically removed
with cotton swabs. Cells that had migrated to the lower side were
fixed with 4% paraformaldehyde and were counterstained with 0.1%
crystal violet. The cells that had migrated into the lower chamber
were observed and counted under a light microscope. Finally, the
number of the migrating cells was calculated.
Measurement of cytotoxicity by CCK-8
Twenty-four hours after transfection, the cells were
treated with various concentrations (50, 100, 200 and 400 μg/ml,
respectively) of 5-FU (Baiyunshan Pharmaceutical Co., Guangzhou,
China) for 48 h. Then, the cell viability was determined by CCK-8
assay. The rate of cell growth inhibition (IR) was calculated
according to the following equation: IR
=[1-A450(drug)/A450(control)] ×100%, where A450 (drug) is the
absorbance of the cells exposed to 5-FU and A450 (control) is the
absorbance of the cells without 5-FU treatment.
DAPI staining
4,6-Diamidino-2-phenylindole (DAPI; Invitrogen)
staining was performed according to the manufacturer’s protocol. In
brief, cells were fixed with 4% paraformaldehyde for 30 min, washed
three times with cold PBS, then exposed to 1 μg/ml DAPI solution
for 15 min in the dark at room temperature. Stained cells were
observed under a laser scanning microscope (Nikon, Tokyo,
Japan).
Flow cytometry
Twenty-four hours after transfection, cells were
treated with 5-FU (50 and 100 μg/ml) for 48 h. To detect the
apoptosis of ICC cells, the cells were doubly stained with Annexin
V-FITC (BD Bioscience) and propidium iodide (Sigma Chemical Co.,
St. Louis, MO, USA) followed by flow cytometry analysis. Apoptotic
ratio was determined on the basis of Annexin
V+PI+ and Annexin V+PI−
fractions.
Statistical analysis
Each assay was performed in triplicate. Statistical
analysis was conducted with the SPSS software package (version
13.0; SPSS, Inc., Chicago, IL, USA). Data are presented as the
means ± standard deviation (SD). The relation between the
expression of Notch1 in ICC samples and the clinico-pathological
data was statistically analyzed by χ2 test, and the
other data was compared using paired Student’s t-test. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
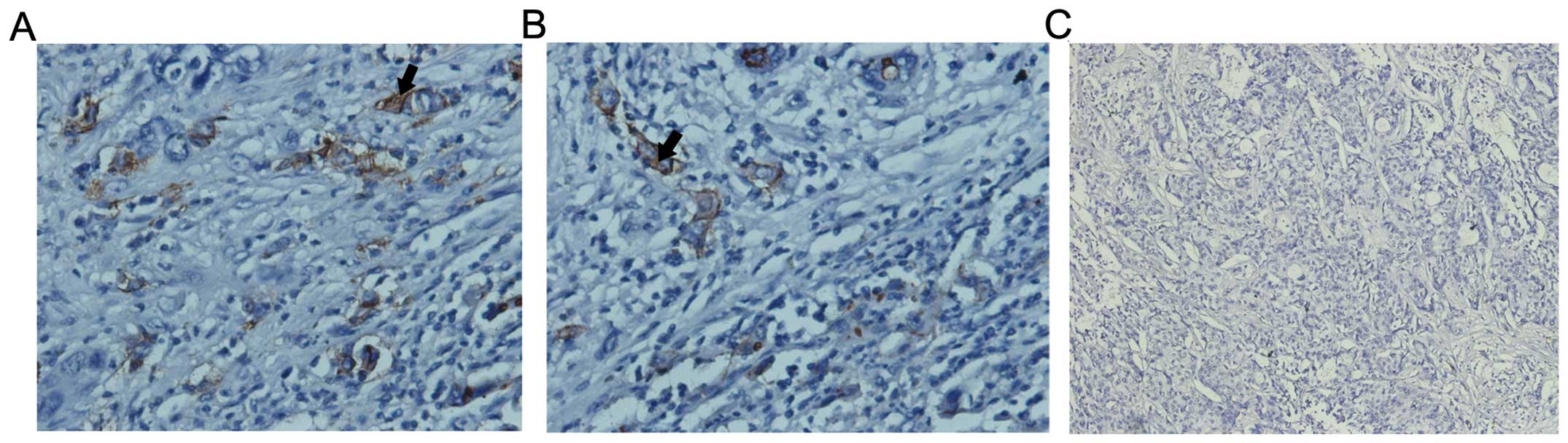
Notch1 is overexpressed in ICC
Notch1 was overexpressed in cell membranes and
cytoplasm of human ICC compared with the adjacent liver tissue
(35/44, 79.5%; Fig. 1). Among them,
Notch1 was more common in cases with tumor size >5 cm (95.5%,
21/22) than in cases with tumor size ≤5 cm (63.6%, 14/22, p=0.021).
In addition, Notch1 was more expressed in ICC patients with HBs-Ag
positive (100%, 15/15) than in patients with HBs-Ag negative
(69.0%, 20/29, p=0.018). There was no statistically significant
difference in age, gender, liver cirrhosis, capsular invasion,
portal vein tumor thrombi, bile duct tumor thrombi, lymphatic or
organ metastasis, tumor number, tumor stage, histological grade,
serum α fetoprotein (AFP) level, serum CA199 level and serum CA125
level between Notch1 positive and Notch1 negative patients
(Table II).
 | Table IIClinicopathological data of 44
intrahepatic cholangiocarcinoma patients. |
Table II
Clinicopathological data of 44
intrahepatic cholangiocarcinoma patients.
| | Group | |
|---|
| |
| |
|---|
| Characteristics | N | Notch1+ | Notch1− | P-value |
|---|
| Age (years) | | | | 0.659 |
| ≤50 | 8 | 6 | 2 | |
| >50 | 36 | 29 | 7 | |
| Gender | | | | 0.227 |
| Male | 32 | 27 | 5 | |
| Female | 12 | 8 | 4 | |
| Cirrhosis | | | | 0.566 |
| Yes | 4 | 4 | 0 | |
| No | 40 | 31 | 9 | |
| Capsular
invasion | | | | 0.124 |
| Yes | 16 | 15 | 1 | |
| No | 28 | 20 | 8 | |
| Portal vein tumor
thrombi | | | | 1.000 |
| Yes | 12 | 10 | 2 | |
| No | 32 | 25 | 7 | |
| Bile duct tumor
thrombi | | | | 1.000 |
| Yes | 3 | 3 | 0 | |
| No | 41 | 32 | 9 | |
| Lymphatic
metastasis | | | 0.477 | |
| Yes | 24 | 18 | 6 | |
| No | 20 | 17 | 3 | |
| Organ invasion | | | | 0.716 |
| Yes | 17 | 13 | 4 | |
| No | 27 | 22 | 5 | |
| Tumor number | | | | 0.319 |
| Single | 38 | 29 | 9 | |
| Multiple | 6 | 6 | 0 | |
| Tumor size | | | | 0.021 |
| ≤5 cm | 22 | 14 | 8 | |
| >5 cm | 22 | 21 | 1 | |
| Tumor stage (UICC,
2010) | | | | 0.411 |
| I + II | 11 | 10 | 1 | |
| III + IV | 33 | 25 | 8 | |
| Histological
grade | | | | 0.235 |
| G1 + G2 | 29 | 25 | 4 | |
| G3 + G4 | 15 | 10 | 5 | |
| HBs-Ag | | | | 0.018 |
| Positive | 15 | 15 | 0 | |
| Negative | 29 | 20 | 9 | |
| Serum AFP | | | | 0.566 |
| ≤25 ng/ml | 40 | 31 | 9 | |
| >25 ng/ml | 4 | 4 | 0 | |
| CA199 | | | | 0.092 |
| >35 μ/ml | 34 | 25 | 9 | |
| ≤35 μ/ml | 10 | 10 | 0 | |
| CA125 | | | | 0.262 |
| >35 μ/ml | 20 | 18 | 2 | |
| ≤35 μ/ml | 26 | 19 | 7 | |
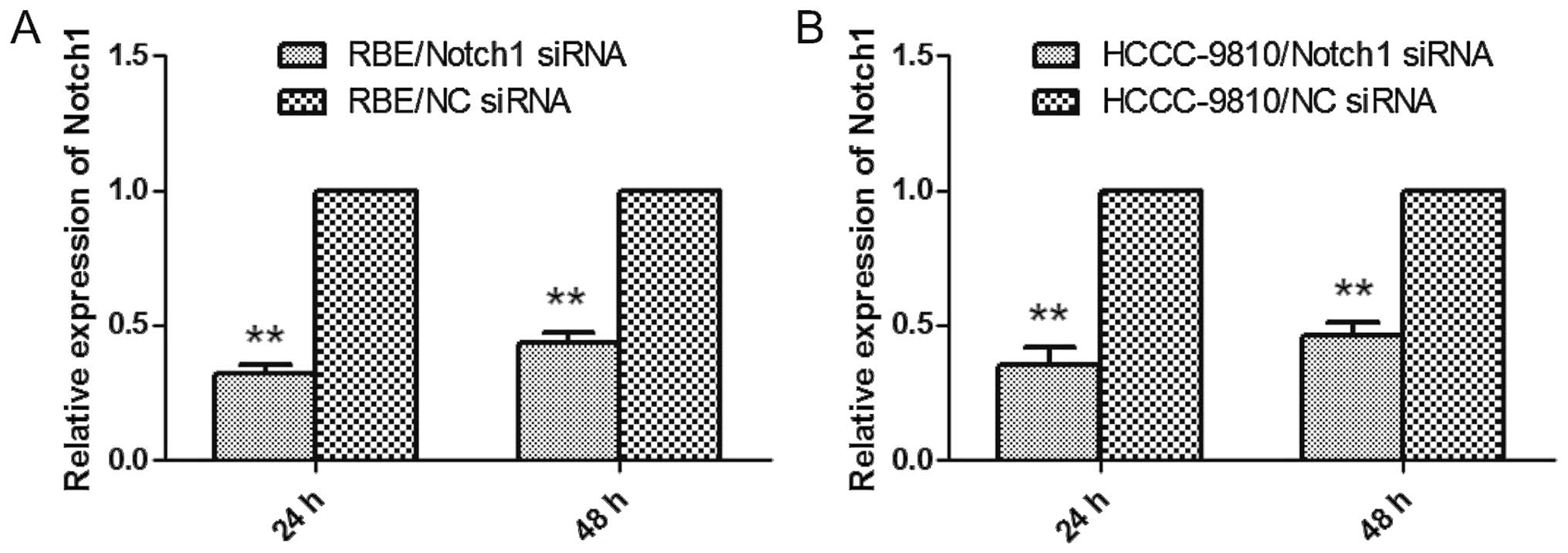
siRNAs targeting Notch1 gene downregulate
Notch expression in ICC cells
To address the functional importance of the Notch1
gene, we employed RNAi to deplete its expression in RBE and
HCCC-9810 cells, both of which were treated with NC-siRNA or siRNA
targeting Notch1. After 24 h, the cells were examined by qRT-PCR
and western blot analysis. As shown in Fig. 2A and B, the gene expression was
markedly knocked down as determined by qRT-PCR. Similar results
were observed in the western blot analysis (Fig. 9C). These data indicated that
Notch1-specific siRNA clearly and effectively suppressed the
expression of Notch1 in RBE and HCCC-9810 cells.
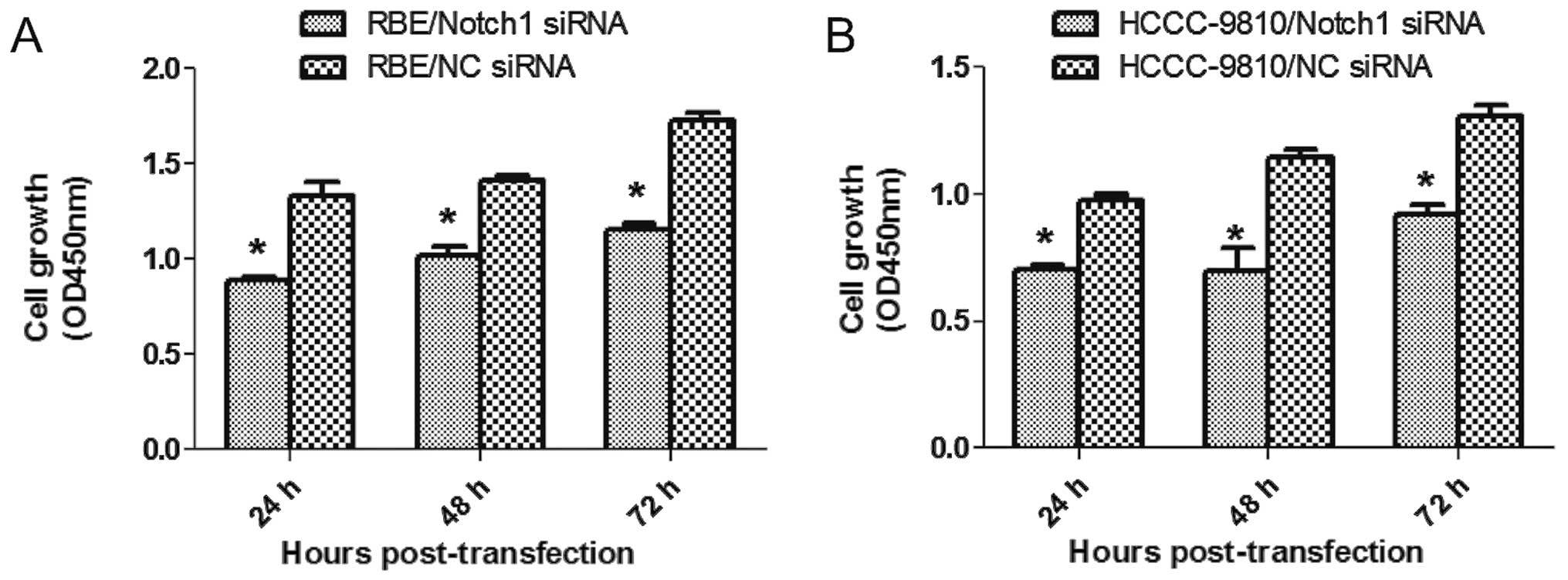
Depletion of Notch1 suppresses ICC cell
proliferation
To this end, CCK-8 assay was performed 24, 48 and 72
h after transfection. Compared to the NC-siRNA, Notch1-siRNA
transfection inhibited the growth of RBE and HCCC-9810 cells in
vitro (P<0.05; Fig. 3A and
B). Also, fewer numbers were formed in Notch1-depletion cells
from colony formation assay (P<0.001; Fig. 4). Our results suggested that
depletion of Notch1 inhibited proliferation and growth of ICC.
Notch1-siRNA diminishes the invasive
ability of ICC cells
To examine whether targeted downregulation of Notch1
in RBE and HCCC-9810 cells affects the invasive ability of tumor
cells, in vitro Transwell invasive assays were performed.
The number of tumor cells migrating through the filter in the
Notch1-siRNA group was markedly lower than that in the NC-siRNA
group (P<0.01; Fig. 5 ). Thus,
Notch1-siRNA silencing markedly diminished the invasiveness of RBE
and HCCC-9810 cells in vitro.
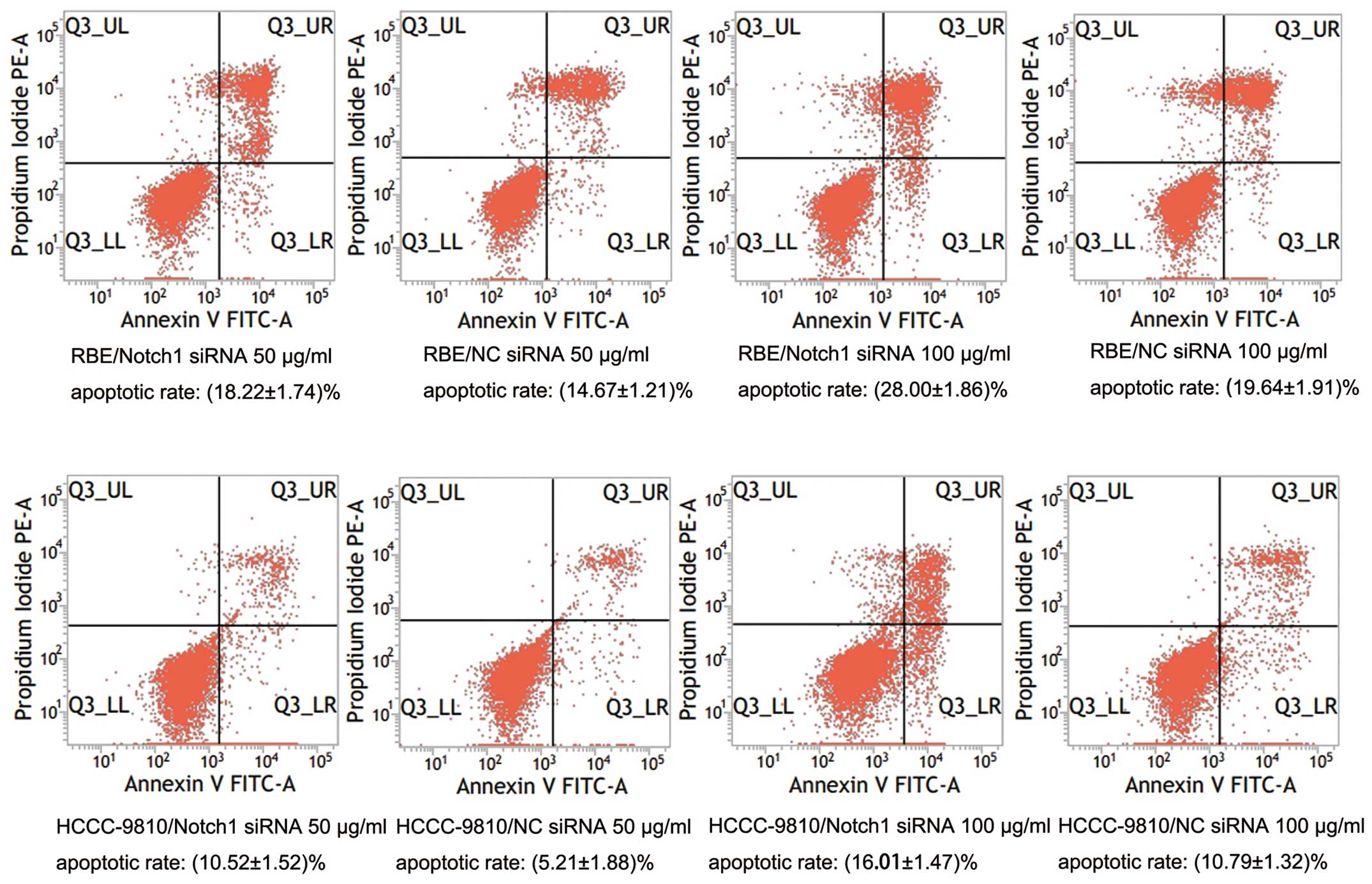
Knockdown of Notch1 sensitizes ICC cells
to 5-FU-induced apoptosis
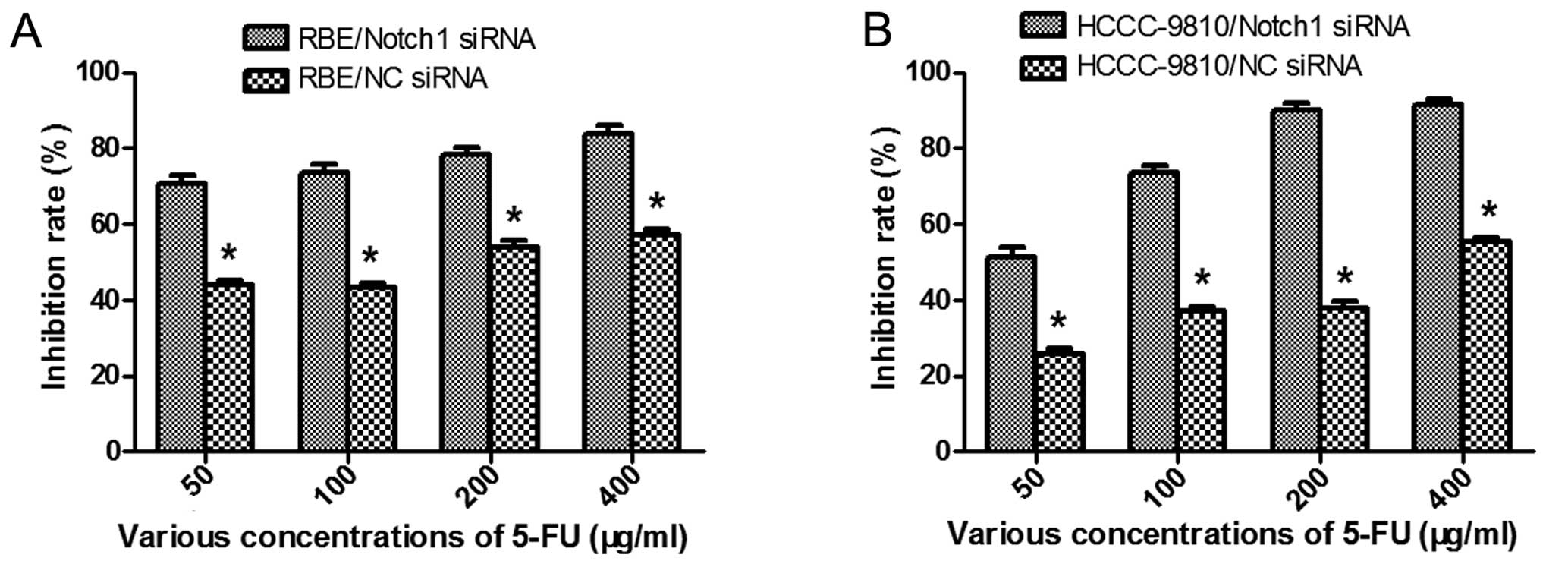
To further explore the role of Notch1 in ICC, we
tested whether downregulation of Notch1 by RNAi sensitizes ICC
cells to 5-FU chemotherapy. After transfection and treatment with
various concentrations of 5-FU, cell viability was determined by
CCK-8 assay. The results showed that both the Notch1-siRNA
transfected cells showed lower cell viability than the control
(P<0.05; Fig. 6). The
IC50 values of 5-FU in the RBE-NC-siRNA and
RBE-Notch1-siRNA cells were 148.74 ± 0.72 μg/ml and 5.37 ± 0.28
μg/ml, respectively, and the corresponding values for
HCCC-9810-NC-siRNA and HCCC-9810-Notch1-siRNA cells were 326.92 ±
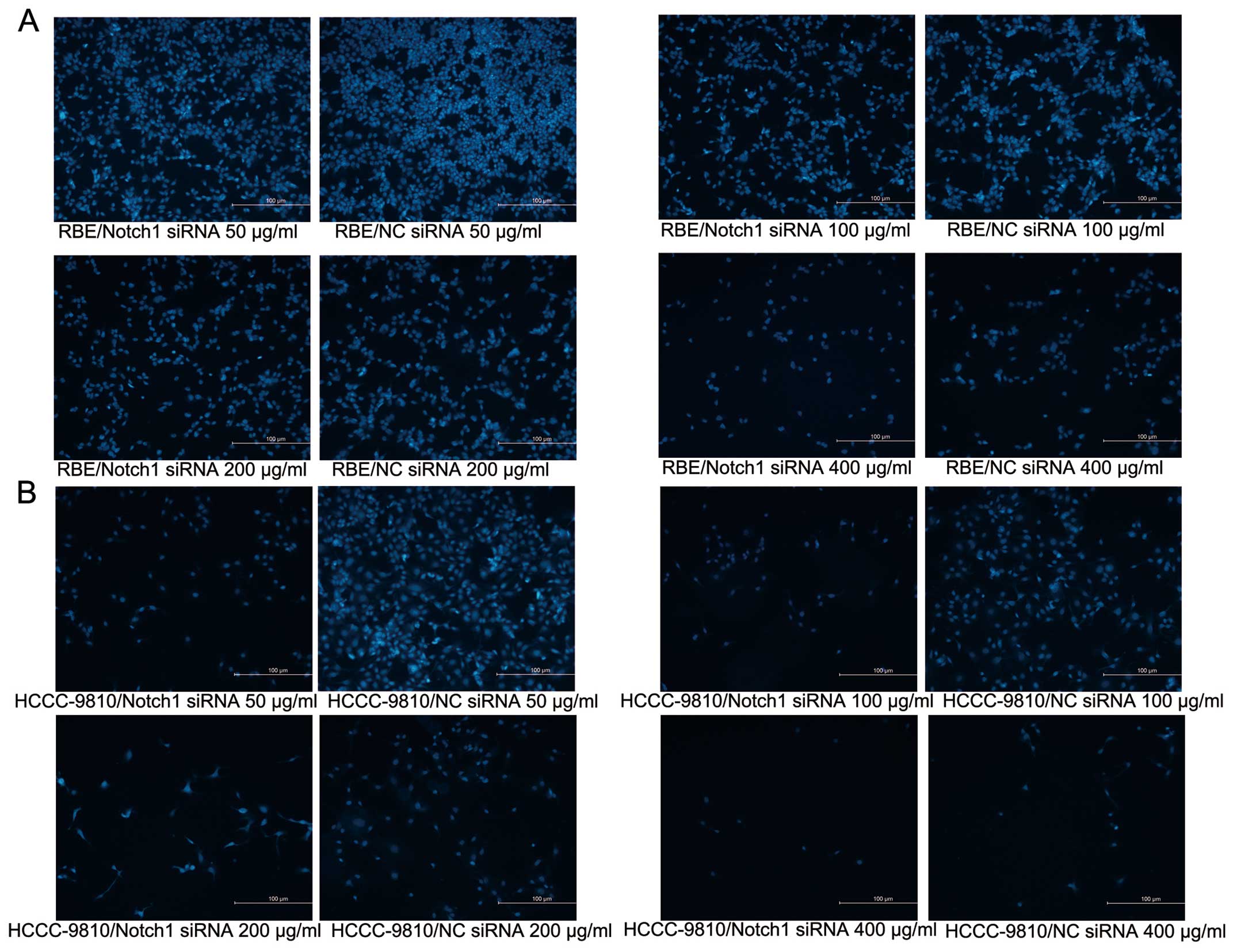
0.87 and 42.60 ± 0.35 μg/ml, respectively. In order to confirm
these results, the cells were subjected to DAPI staining. Both
RBE-Notch1-siRNA and HCCC-9810-Notch1-siRNA cells showed lower cell
numbers than the control cells (Fig. 7A
and B). We also examined the apoptotic rate of tumor cells by
flow cytometry. The results showed that the apoptotic rates of the
RBE-Notch1-siRNA and HCCC-9810-Notch1-siRNA cells were much higher
than those of the RBE-NC-siRNA and HCCC-9810-NC-siRNA cells,
respectively (P<0.01; Fig. 8).
These results indicated that knockdown of Notch1 sensitized ICC
cells to 5-FU treatment.
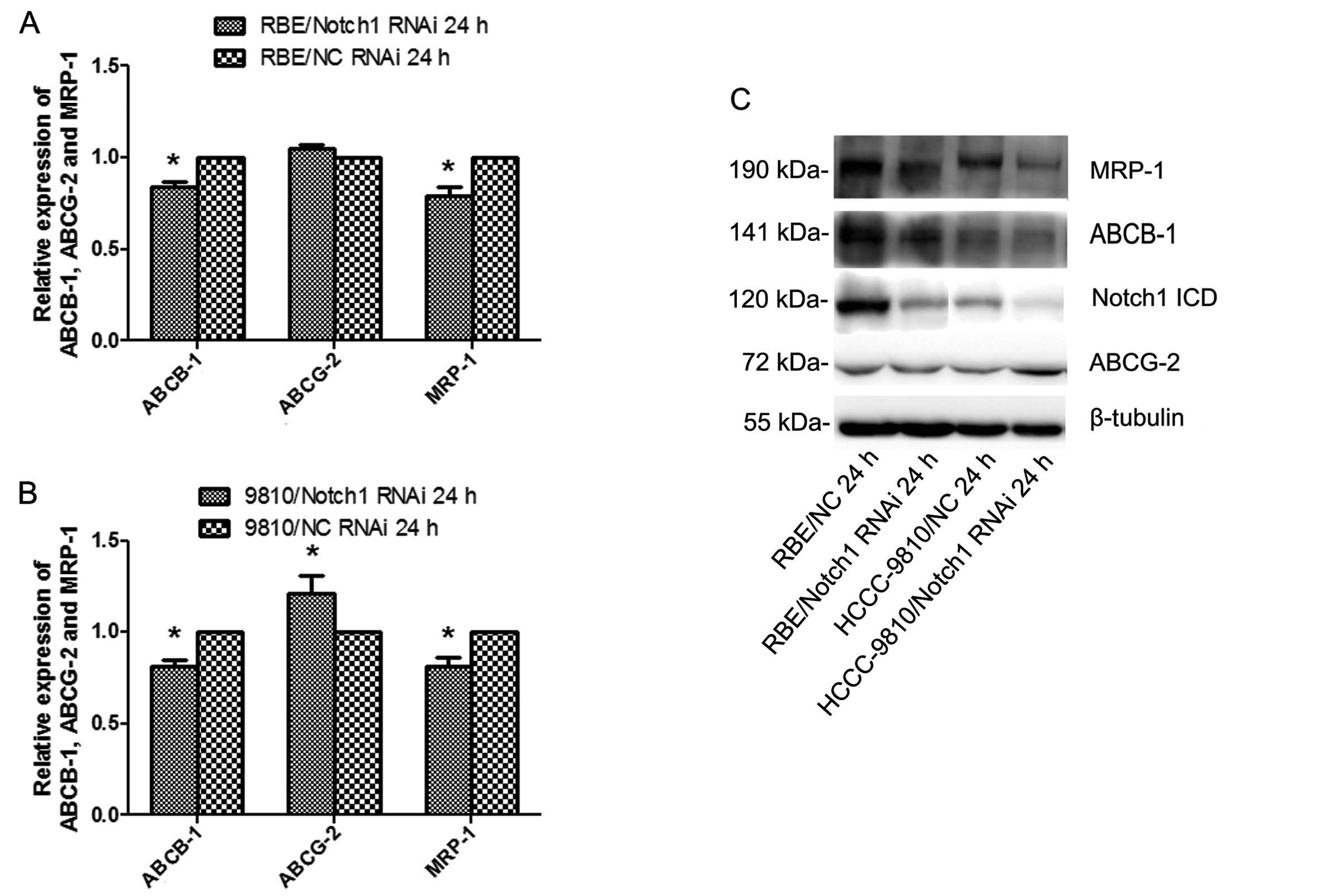
Knockdown of Notch1 diminishes the
expression of ABCB-1 and MRP-1
To further explore the mechanism underlying the
suppression of ICC cell resistance to 5-FU treatment by the
silencing of Notch1, the expression levels of the MDR-related
genes, ABCB-1, ABCG-2 and MRP-1, were examined 24 h after
transfection by qRT-PCR and western blotting. The results showed
that knockdown of Notch1 diminished the expression of ABCB-1 and
MRP-1 in both cell lines (P<0.05; Fig. 9). Silencing Notch1 in RBE cells did
not affect the expression of ABCG-2. However, ABCG-2 was increased
in HCCC-9810-Notch1-siRNA cells but not in the control.
Discussion
A majority of ICC patients who undergo chemotherapy
show MDR, which is often responsible for therapy failure and poor
outcome. To identify signaling pathways that could be targeted to
enhance ICC sensitivity to drug-based therapies is of outmost
importance. However, the precise molecular mechanisms of MDR remain
obscure. One explanation for MDR is overexpression of membrane
transport proteins such as ABCB-1, ABCG-2 and MRP-1, which act as
efflux pumps for anticancer agents (17). In addition, resistance to apoptosis
also contributes to chemoresistance (18).
The Notch signaling pathway plays a critical role in
cell fate decision, tissue patterning, morphogenesis and is hence
regarded as a developmental pathway. If it goes awry, it would lead
to cellular transformation and tumorigenesis (19). It is generally accepted that the
Notch signaling pathway is deregulated in a variety of malignancies
and can behave as either an oncogene or a tumor suppressor gene
depending on cell type. In the study of liver cancer, Wang et
al reported that Notch1/Jagged1 were frequently low expressed
in hepatocellular carcinoma and downregulation of Notch1/Jagged1
may sustain tumor progression (20). It has also been demonstrated that
Notch1 signaling inhibits growth of human hepatocellular carcinoma
through induction of cell cycle arrest and apoptosis. However, in
extrahepatic cholangiocarcinoma and gallbladder carcinoma, Notch1
was overexpressed and correlated with cancer progression (16). Furthermore, regarding the
relationship between Notch and HBV infection, Trehanpati et
al found that HBV infection drives increased Notch1, TGF-β and
FoxP3 expression on intrahepatic T-cells in cirrhosis, resulting in
fibrogenesis and disease progression (21). Pei et al confirmed that
blockage of Notch1 signaling could regulate the immune balance of
Th1/Th2 in chronic hepatitis B patients (22). Moreover, there is mounting evidence
that Notch1 plays an important role in the process of MDR. Nefedova
et al verified that Notch-1 signaling maybe a primary
mechanism mediating the bone marrow stroma influence on hematologic
malignant cell growth and survival from drug-induced apoptosis
(23). Zou et al affirmed
that in hypoxic conditions Notch1 signaling is required to activate
genes regulating cellular proliferation, invasion and
chemoresistance, increasing the aggressiveness of T-ALL and its
likelihood for progression (24).
In the present study, immunohistochemical analysis
showed that Notch1 was overexpressed in the cell membrane and
cytoplasm of human ICC (35/44, 79.5%) compared with the adjacent
liver tissue and was more common in cases with tumor size ≥5 cm and
HBs-Ag positive, suggesting that their overexpression may be linked
to cancer initiation (hepatitis B virus infection) and progression.
To examine the role of Notch1 expression in ICC, cell culture
studies were performed. The Notch1 gene was silenced by
Notch1-siRNA in 2 ICC cell lines, RBE and HCCC-9810. Then, the
proliferation and invasiveness of these cells were detected by
CCK-8, colony formation and Transwell assays. The results showed
that the proliferation and invasion ability of tumor cells in the
Notch1-silenced group were significantly lower than those in the
control group. Loss of Notch1 in ICC specifically resulted in their
enhanced 5-FU mediated death as revealed by multiple criteria:
(1) Annexin V-FITC/PI staining,
(2) CCK-8 analysis, (3) DAPI staining. We observed that Notch1
gene silencing significantly increased the apoptotic rate of tumor
cells treated by 5-FU and significantly decreased the
IC50 values of 5-FU. These results showed that knockdown
of endogenous Notch1 expression of ICC cells inhibited their
proliferation and invasiveness, contributed to sensitization of ICC
cells to 5-FU and increased their apoptotic rates, which suggest
that the combination of conventional chemotherapy and Notch1-gene
target therapy may be a potential clinical strategy for ICC
therapy. To further investigate the mechanism of how Notch1
ablation enhances apoptosis in response to 5-FU treatment, we
examined ABCB-1, ABCG-2 and MRP-1 levels in the presence and
absence of Notch1 by qRT-PCR and western blot analysis. The results
showed that knockdown of endogenous Notch1 expression led to
significantly downregulated ABCB-1 and MRP-1 expression levels at
both mRNA and protein levels in these two cell lines; this
indicated that Notch1-RNAi enhanced apoptosis in response to 5-FU
treatment cells, which may partly be mediated through MDR-related
genes. Since the expression of ABCG-2 was beyond our expectation,
there may be complicated regulatory mechanisms of Notch1 and
MDR-related genes that remain undefined. This requires further
elucidation. In conclusion, we have shown in the present study that
Notch1 is upregulated in ICC and promotes tumor proliferation,
invasiveness and chemoresistance in vitro, indicating that
Notch1 may be involved in ICC carcinogenesis and progression. These
findings suggest that Notch1 could serve as a novel therapeutic
target in patients with ICC and further investigations on the
signaling network of Notch1 may provide new insight into this fatal
disease.
Acknowledgements
This study was supported by the Special Research
Foundation of the National Natural Science Foundation of China
(81172068).
References
|
1
|
Guglielmi A, Ruzzenente A, Campagnaro T,
et al: Intrahepatic cholangiocarcinoma: prognostic factors after
surgical resection. World J Surg. 33:1247–1254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodman ZD: Neoplasms of the liver. Mod
Pathol. 20(Suppl 1): S49–S60. 2007. View Article : Google Scholar
|
|
3
|
Halappa VG, Bonekamp S, Corona-Villalobos
CP, et al: Intrahepatic cholangiocarcinoma treated with
local-regional therapy: quantitative volumetric apparent diffusion
coefficient maps for assessment of tumor response. Radiology.
264:285–294. 2012.
|
|
4
|
Poggi G, Amatu A, Montagna B, et al:
OEM-TACE: a new therapeutic approach in unresectable intrahepatic
cholangiocarcinoma. Cardiovasc Intervent Radiol. 32:1187–1192.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan JC, Coburn NG, Baxter NN, Kiss A and
Law CH: Surgical management of intrahepatic cholangiocarcinoma - a
population-based study. Ann Surg Oncol. 15:600–608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thongprasert S: The role of chemotherapy
in cholangiocarcinoma. Ann Oncol. 16(Suppl 2): i93–i96. 2005.
View Article : Google Scholar
|
|
7
|
Liapi E and Geschwind JF:
Chemoembolization for primary and metastatic liver cancer. Cancer
J. 16:156–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dumont AG, Yang Y, Reynoso D, Katz D,
Trent JC and Hughes DP: Anti-tumor effects of the Notch pathway in
gastrointestinal stromal tumors. Carcinogenesis. 33:1674–1683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brennan K and Clarke RB: Combining Notch
inhibition with current therapies for breast cancer treatment. Ther
Adv Med Oncol. 5:17–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jonusiene V, Sasnauskiene A, Lachej N, et
al: Down-regulated expression of Notch signaling molecules in human
endometrial cancer. Med Oncol. 30:4382013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu H, Zhou X, Redfield S, Lewin J and
Miele L: Elevated Jagged-1 and Notch-1 expression in high grade and
metastatic prostate cancers. Am J Transl Res. 5:368–378.
2013.PubMed/NCBI
|
|
12
|
Yu S, Zhang R, Liu F, Wang H, Wu J and
Wang Y: Notch inhibition suppresses nasopharyngeal carcinoma by
depleting cancer stem-like side population cells. Oncol Rep.
28:561–566. 2012.PubMed/NCBI
|
|
13
|
Villanueva A, Alsinet C, Yanger K, et al:
Notch signaling is activated in human hepatocellular carcinoma and
induces tumor formation in mice. Gastroenterology. 143:1660–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yabuuchi S, Pai SG, Campbell NR, et al:
Notch signaling pathway targeted therapy suppresses tumor
progression and metastatic spread in pancreatic cancer. Cancer
Lett. 335:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ
and He LY: siRNA-mediated silencing of Notch-1 enhances docetaxel
induced mitotic arrest and apoptosis in prostate cancer cells.
Asian Pac J Cancer Prev. 13:2485–2489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon HA, Noh MH, Kim BG, et al:
Clinicopathological significance of altered Notch signaling in
extrahepatic cholangiocarcinoma and gallbladder carcinoma. World J
Gastroenterol. 17:4023–4030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Munoz M, Henderson M, Haber M and Norris
M: Role of the MRP1/ABCC1 multidrug transporter protein in cancer.
IUBMB Life. 59:752–757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baguley BC: Multidrug resistance in
cancer. Methods Mol Biol. 596:1–14. 2010. View Article : Google Scholar
|
|
19
|
Shao H, Huang Q and Liu ZJ: Targeting
Notch signaling for cancer therapeutic intervention. Adv Pharmacol.
65:191–234. 2012. View Article : Google Scholar
|
|
20
|
Wang M, Xue L, Cao Q, et al: Expression of
Notch1, Jagged1 and β-catenin and their clinicopathological
significance in hepatocellular carcinoma. Neoplasma. 56:533–541.
2009.
|
|
21
|
Trehanpati N, Shrivastav S, Shivakumar B,
et al: Analysis of Notch and TGF-β signaling expression in
different stages of disease progression during hepatitis B virus
infection. Clin Transl Gastroenterol. 3:e232012.
|
|
22
|
Pei J, Tang Z, Zang G and Yu Y: Blockage
of Notch1 signaling modulates the T-helper (Th)1/Th2 cell balance
in chronic hepatitis B patients. Hepatol Res. 40:799–805. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nefedova Y, Cheng P, Alsina M, Dalton WS
and Gabrilovich DI: Involvement of Notch-1 signaling in bone marrow
stroma-mediated de novo drug resistance of myeloma and other
malignant lymphoid cell lines. Blood. 103:3503–3510. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou J, Li P, Lu F, et al: Notch1 is
required for hypoxia-induced proliferation, invasion and
chemoresistance of T-cell acute lymphoblastic leukemia cells. J
Hematol Oncol. 6:32013. View Article : Google Scholar : PubMed/NCBI
|