Introduction
Despite preventative and therapeutic advances, the
5-year survival outcome of oral cancer patients still remains at
50% with no significant improvement over the past several decades
(1). This is mainly due to the high
metastatic rates to cervical lymph nodes (2,3),
particularly in oral squamous cell carcinoma (OSCC), which accounts
for more than 90% of all oral cancer cases (4). In addition, nearly 45% of OSCC
patients present with lymph node metastasis (LNM) (5) and their overall survival decreases to
28% (6).
Lymphatic metastasis progresses insidiously.
Migration and invasion are essential stages underlying metastasis
(7). Matrix metalloproteinases
(MMPs) promote tumor cell migration and invasion to normal tissues
by destructing the extracellular matrix and by influencing the
tumor microenvironment (8). Among
the MMP family members, MMP-2 is closely related to the development
and progression of tumors (9).
Human MMP-21, which was expressed higher in LNM foci than that in
the primary tumor (PTs) in the lymphatic metastasis animal model of
OSCC established in the present study, is a new member of the MMP
family (10). MMP-21 expression has
been associated with embryogenesis and tumor progression (11). MMP-21 has been reported to be
related to cell differentiation such that its high expression has
been suggested as a marker of highly differentiated pancreatic
cancer cells (12). In gastric
(13) and colorectal cancer
(14) patients, high MMP-21
expression has been associated with poor overall survival. In
esophageal squamous cell cancer, MMP-21 expression has been
associated with tumor invasion, inflammation, apoptosis and highly
differentiated cells (15). VEGF-C
and VEGFR-3 have also been closely correlated with tumor
progression and LNM (16).
Currently, the PT is considered important in
investigating lymphatic metastasis and predicting the outcome of
cancer patients whereas metastatic foci are ignored. The present
study is the first to investigate LMN foci in assessing the
prognosis of OSCC patients with LMN.
Materials and methods
Cells and cell culture
CAL-27 cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine
serum (FBS; HyClone) in a humidified 5% CO2 incubator at
37°C. Cells in mid-logarithmic growth (~75% confluence) were used
for the following experiments.
Establishment of the LNM animal
model
The present study was approved by the Medical Ethics
Committee of the Peking University School and Hospital of
Stomatology. Six-week-old male BALB/c nude mice (Vital River
Laboratory Animal Technology Co., Ltd., Beijing, China) were placed
under general anesthesia with 1% pentobarbital sodium (Sigma).
CAL-27 cells (5×106) were injected into the tongue of
the nude mice. After 40 days, the PTs and LNM foci were dissected
to generate tumor cells named CAL-27-PT and CAL-27-LNM,
respectively via primary culture. Both of the two types of cells
were identified by short tandem repeat (STR) profiling. DNA was
extracted from CAL-27-PT and CAL-27-LNM cells using STR profiling.
PCR was performed in the ABI 3100 genetic analyzer machine with the
use of 10 ng of DNA as a template and the Goldeneye TM16C STR
detection kit (Fig. 1).
Real-time PCR
mRNA was extracted using TRIzol reagent
(Invitrogen). The GoScript™ reverse transcription system (Promega)
was used to make complementary DNA. Relative quantitative PCR was
carried out using SYBR-Green Master (Roche Diagnostics). Reactions
were examined using the ABI 7500 real-time PCR machine (Applied
Biosystems) coupled with SYBR-Green chemistry. All PCR reactions
were in 20 μl of total volume containing 10 μl of SYBR-Green PCR
Master Mix, 50 ng cDNA, 250 nM of each primer (MMP-2 forward
primer, GCCCCAGACAGGTGATCTTG and reverse primer,
GCTTGCGAGGGAAGAAGTTGT; MMP-21 forward primer, TCGACATCAAGCTGGGCTTT
and reverse primer, ACCTTGAGAAGGCTGATGCC; VEGF-C forward primer,
ACGTTCCCTGCCAGCAACAC and reverse primer, TCA TCCAGCTCCTTGTTTGGTCC;
VEGFR-3 forward primer, CAGGATGAGCGGCTCATCTAC and reverse primer,
ACA GGTTGAGGCGGTACCAG; GAPDH forward primer, ATG GGGAAGGTGAAGGTCG
and reverse primer: GGGGTC ATTGATGGCAACAATA). All amplifications
were conducted in triplicate for each sample and repeated three
times independently. The thermal cycling consisted of 10 min at
95°C, followed by 40 cycles at 95°C for 15 sec, and at 60°C for 60
sec. The specificity of amplification was monitored using the
dissociation curve of the amplified product. Relative expression of
the target genes was determined using the 2−ΔΔCt
method.
Western blot analysis
CAL-27-PT and CAL-27-LNM cells were lysed in RIPA
buffer (Applygen Technologies, Beijing, China) with protease
inhibitors and phosphatase inhibitors (Applygen Technologies).
Protein concentration was determined using the BCA protein assay
(Thermo Fisher Scientific). Proteins (40 μg of each sample) were
separated using sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE; Applygen Technology) and transferred to
polyvinylidene difluoride membranes. The membranes were blocked in
5% non-fat dry milk for 1 h and probed with antibodies against
MMP-2 (1:1,000; Epitomics), MMP-21 (1:5,000; Epitomics, Q8N119),
VEGF-C (1:1,000 dilution; Abcam), VEGFR-3 (1:50; Abcam) and GAPDH
(1:1,000; Santa Cruz Biotechnology; sc-25778) separately at 4°C
overnight. After incubation with HRP-linked secondary antibodies,
immunoreactive proteins were visualized by enhanced
chemiluminescence (ECL) reagent (Applygen Technology).
Patients
The present study was approved by the Medical Ethics
Committee of the Peking University School and Hospital of
Stomatology. From 2008 to 2010, 63 OSCC patients with
pathologically confirmed cervical lymph node metastasis at the time
of diagnosis who were surgically treated at the Department of Oral
and Maxillofacial Surgery, Peking University School of Stomatology,
were enrolled after providing informed consent. Patients with
recurrent cancer or distant metastasis were excluded. The
metastatic area was divided into 6 levels according to the American
Academy of Otolaryngology Head and Neck Surgery Foundation
(17). The pathological stage was
diagnosed by pathologists at the Department of Pathology, Peking
University School of Stomatology. The 63 patients comprised 40 men
and 23 women (median age, 59 years). The tumor sites were the
tongue (27 patients), gingiva (17 patients), palate (10 patients),
buccal mucosa (5 patients) and floor of the mouth (4 patients). All
patients had regular follow-up visits every 2 months for the first
year, every 3 months for the second year, and every 6 months
thereafter.
Immunohistochemical staining
Tissue sections were embedded in paraffin. Sections
(4-μm) were mounted on slides for immunohistochemical staining.
After dewaxing, slides were dipped in 3% hydrogen peroxide for 10
min to block endogenous peroxidase activity. After washing thrice
in phosphate-buffered saline, antigen retrieval (citrate, 0.01 M,
pH 6.0) was performed using a 3-min-long, high-pressure protocol.
After washing, the slides were incubated with antibodies against
MMP-2 (used as supplied, Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China; ZM-0330), MMP-21 (1:200;
Epitomics; Q8N119), VEGF-C (used as supplied, Beijing Zhongshan
Golden Bridge Biotechnology; ZA-0266), and VEGFR-3 (1:100; Abcam;
ab27278) in a humidified chamber at 4°C overnight. After washing,
the slides were treated with a horseradish peroxidase-conjugated
secondary antibody for 1 h at ambient temperature. Following
washing, immunoreaction was detected using 3,3′-diaminobenzidine
(DAB; Beijing Zhongshan Golden Bridge Biotechnology; ZLI-9017)
incubation for 30 sec. Negative controls were performed using
phosphate-buffered solution instead of the primary antibodies.
Slides were counterstained with hematoxylin and visualized using an
Olympus DP controller (Olympus, Tokyo, Japan). Immunostaining
results were semi-quantitatively evaluated by three independent
researchers who were blinded to information concerning the
specimens. Target protein expression was determined in 10 random
fields of the microscope in tumor tissue of every section. The
labeling index was defined as the intensity of staining (strong,
moderate, weak and negative, scored as 4, 3, 2 and 1, respectively)
and multiplied by the percentage of positive cells. If the results
were not consistent, a discussion was held in order to reach a
consensus. High or low expression levels were determined based on
the median of the labeling index.
Statistical analysis
SPSS 13.0 software was used for analyzing the
results. Data are expressed as means ± SD and were compared using
the Wilcoxon paired test and the Pearson’s and Spearman’s
correlation coefficients as indicated (in Results). Patient
survival times were defined from the day of surgery to October 2013
or death. The product-limit method and Kaplan-Meier curves were
used to calculate survival times. The log-rank test was used to
compare the survival curves among the patient groups. Cox
proportional hazards model was used to assess the risk factors.
P<0.05 was considered to indicate a statistically significant
result.
Results
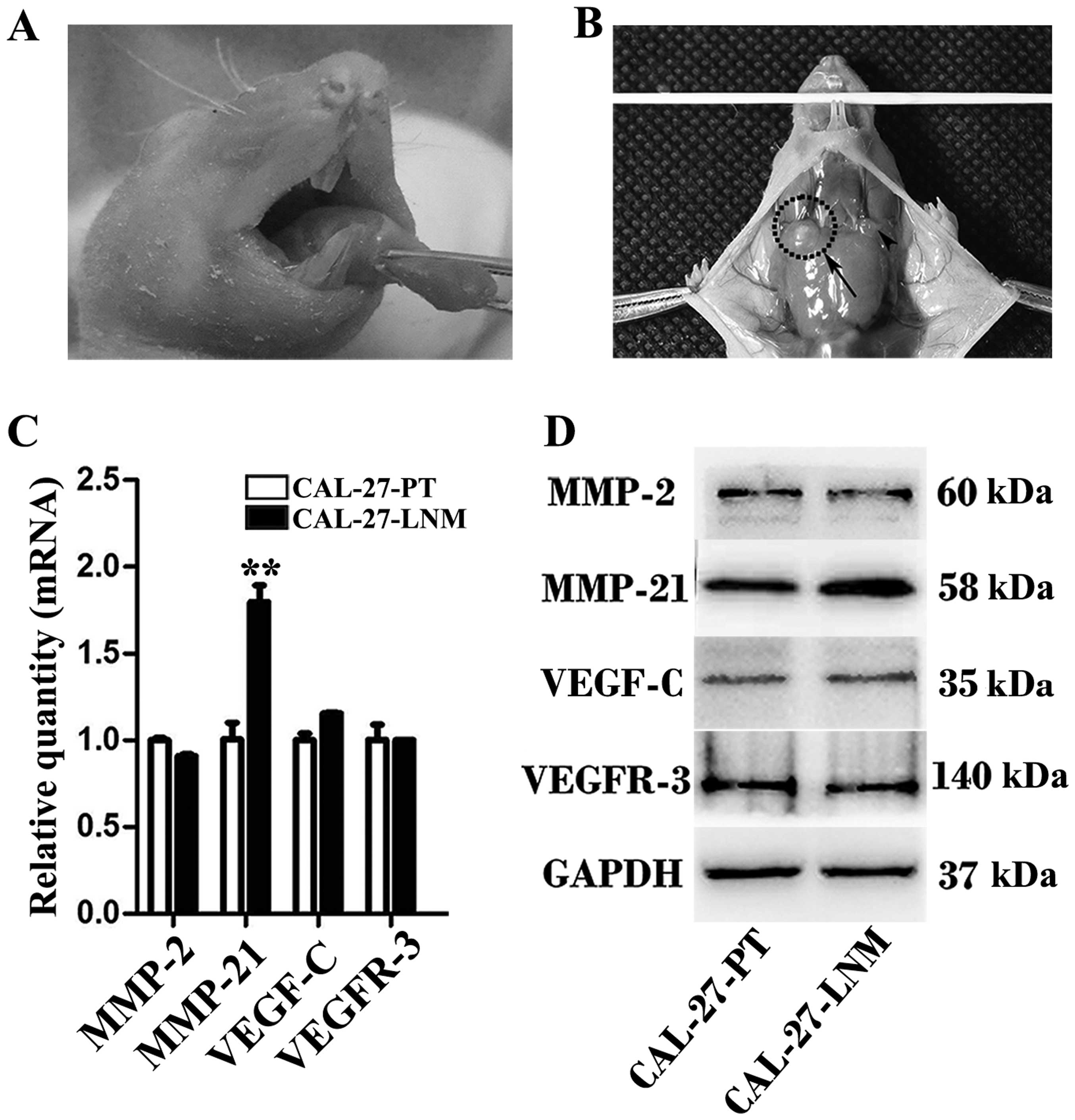
MMP-21 was screened from the LNM animal
model
After 40 days, the PTs and LNM foci were clearly
identifiable (Fig. 2A and B).
Real-time PCR and western blot analysis revealed that mRNA and
protein levels of MMP-21 were higher in the LNM foci than levels in
the PTs. The results of real-time PCR and western blot analysis
were consistent (Fig. 2C and
D).
Characteristics of patients and
relationship with overall survival
Among all the characteristics, the metastatic area
showed a significant relationship with overall survival of the
patients (P=0.044). Others including gender, age, pathological
stage, neck dissection, number of metastatic lymph node and
metastatic side demonstrated no association with overall survival
of the 63 patients based on the Kaplan-Meier and log-rank test
(Table I). The number of metastatic
lymph nodes in every patient ranged from 1 to 9 (1 in 27 patients,
2 in 14 patients, 3 in 8 patients, 4 in 4 patients, 5 in 2
patients, 6 in 5 patients, 7 in 0 patients, 8 in 2 patients and 9
in 1 patient, respectively). The metastatic areas were determined
to be 1 in 38 patients, 2 in 13 patients, 3 in 8 patients, 4 in 3
patients and 5 in 1 patient, respectively. Among all the metastatic
areas, level I (the submental and submandibular triangles)
accounted for 44%, level II (the upper jugular nodal group)
accounted for 36%, level III (the middle jugular nodal group)
accounted for 12%, level IV (the lower jugular nodal group)
accounted for 5%, level V (the posterior triangle of the neck)
accounted for 3%, level VI (the pre-laryngeal, pre-tracheal and
paratracheal nodes) accounted for 0%.
 | Table IAssociation between characteristics
and overall survival of the 63 OSCC patients with lymphatic
metastasis. |
Table I
Association between characteristics
and overall survival of the 63 OSCC patients with lymphatic
metastasis.
| Characteristics | No. of patients
(%) | P-value |
|---|
| Gender |
| Female | 23 (37) | 0.91 |
| Male | 40 (63) | |
| Age (years) |
| ≤59 | 32 (51) | 0.123 |
| >59 | 31 (49) | |
| Pathological
stage |
| High
differentiation | 20 (32) | 0.454 |
| Moderate
differentiation | 35 (56) | |
| Poor
differentiation | 8 (12) | |
| Neck dissection |
| Unilateral | 48 (76) | 0.816 |
| Bilateral | 15 (24) | |
| No. of metastatic
lymph nodes |
| 1 | 27 (43) | 0.185 |
| >1 (2–9) | 36 (57) | |
| Metastatic side |
| Unilateral | 55 (87) | 0.28 |
| Bilateral | 8 (13) | |
| Metastatic area |
| 1 | 38 (60) | 0.044 |
| >1 (2–5) | 25 (40) | |
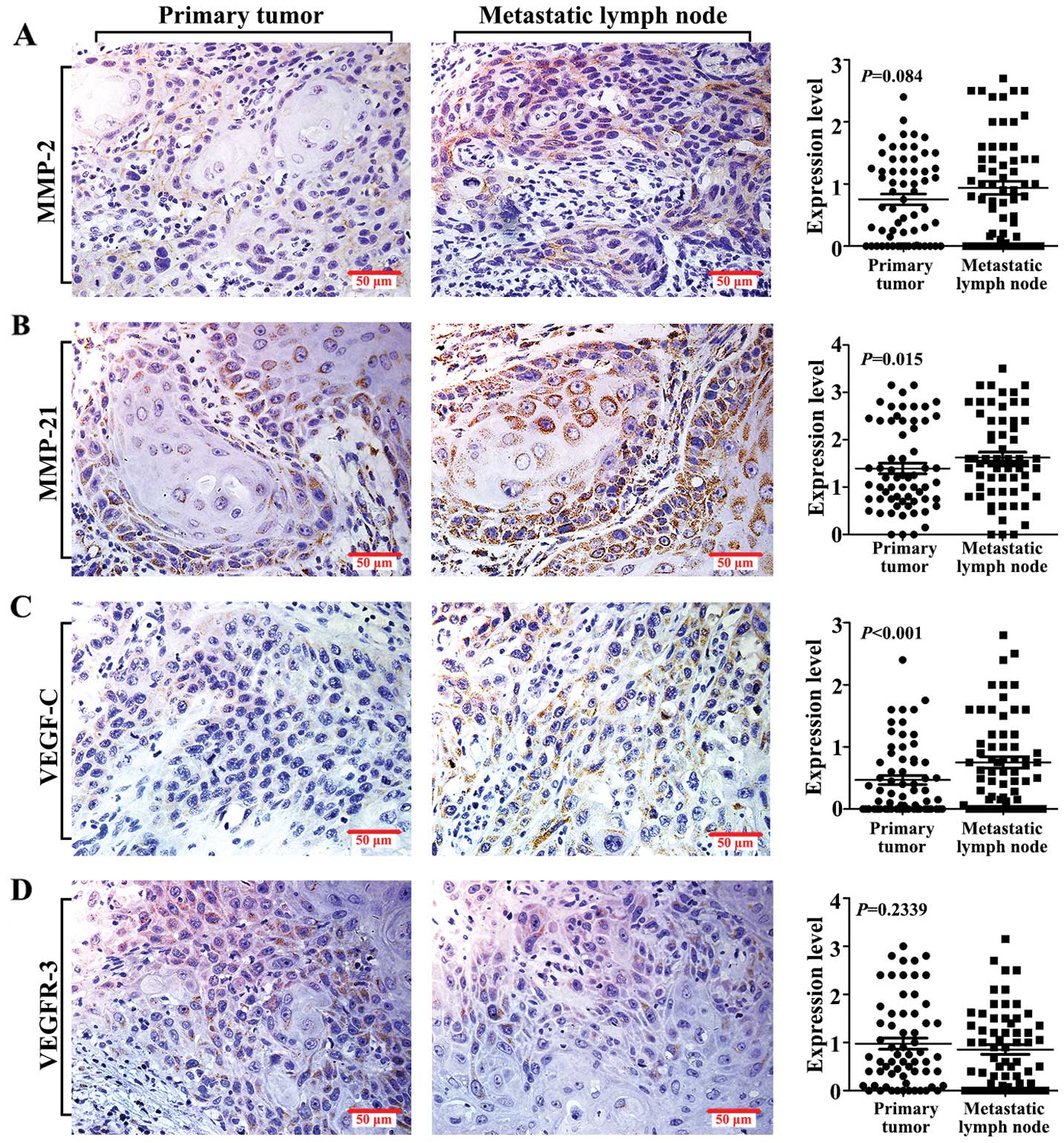
Expression of the tested proteins in the
clinical specimens
MMP-2, MMP-21, VEGF-C and VEGFR-3 expression
patterns were assessed in clinical specimens, including the PTs and
the corresponding LNM foci. All of the tested proteins were
expressed in the tumor cell cytoplasm. The rates of MMP-2, MMP-21,
VEGF-C and VEGFR-3 positive staining were 73.02%, 95.24%, 63.49%,
and 84.13%, respectively in the PTs. The corresponding positive
expression rates were 76.19%, 95.24%, 63.49% and 76.19% in the LNM
specimens. Protein expression levels were assessed using the
labeling index as described above. Our results showed that MMP-21
and VEGF-C expression levels were higher in the LNM foci than
levels in the PTs (Fig. 3B and C),
while MMP-2 and VEGFR-3 showed no significant expression between
the paired specimens (Fig. 3A and
D). These data were analyzed using the Wilcoxon paired
test.
Relationship between protein expression
and clinicopathological characteristics
Relationships between expression of the tested
protein and the clinicopathological characteristics were assessed
using Spearman’s correlation coefficients. VEGF-C and VEGFR-3
expression levels in the LNM foci were associated with the OSCC
pathological stage. However, the pathological cancer stage showed
no correlation with expression of the four proteins in the PTs or
MMP-2 and MMP-21 expression in the LNM foci (Table II). Expression levels of the four
tested proteins in the PTs were not correlated with patient age,
gender, neck dissection, number of metastatic lymph nodes,
metastatic side or metastatic area (data not shown).
 | Table IIRelationships between pathological
stage and the expression of the tested proteins in the 63 OSCC
patients with lymphatic metastases. |
Table II
Relationships between pathological
stage and the expression of the tested proteins in the 63 OSCC
patients with lymphatic metastases.
| Pathological
stage | |
|---|
|
| |
|---|
| Characteristics | 1 | 2 | 3 | P-value |
|---|
| No. of patients | 20 | 35 | 8 | |
| Expression in
PTsa |
| MMP-2 | 76±73 | 81±67 | 29±40 | 0.40 |
| MMP-21 | 113±82 | 165±89 | 96±82 | 0.45 |
| VEGF-C | 42±54 | 52±62 | 38±48 | 0.82 |
| VEGFR-3 | 71±77 | 110±91 | 111±113 | 0.24 |
| Expression in
LNM |
| MMP-2 | 76±85 | 92±86 | 104±99 | 0.30 |
| MMP-21 | 147±76 | 172±92 | 160±114 | 0.46 |
| VEGF-C | 59±61 | 72±73 | 123±72 | 0.04 |
| VEGFR-3 | 59±64 | 90±85 | 132±81 | 0.03 |
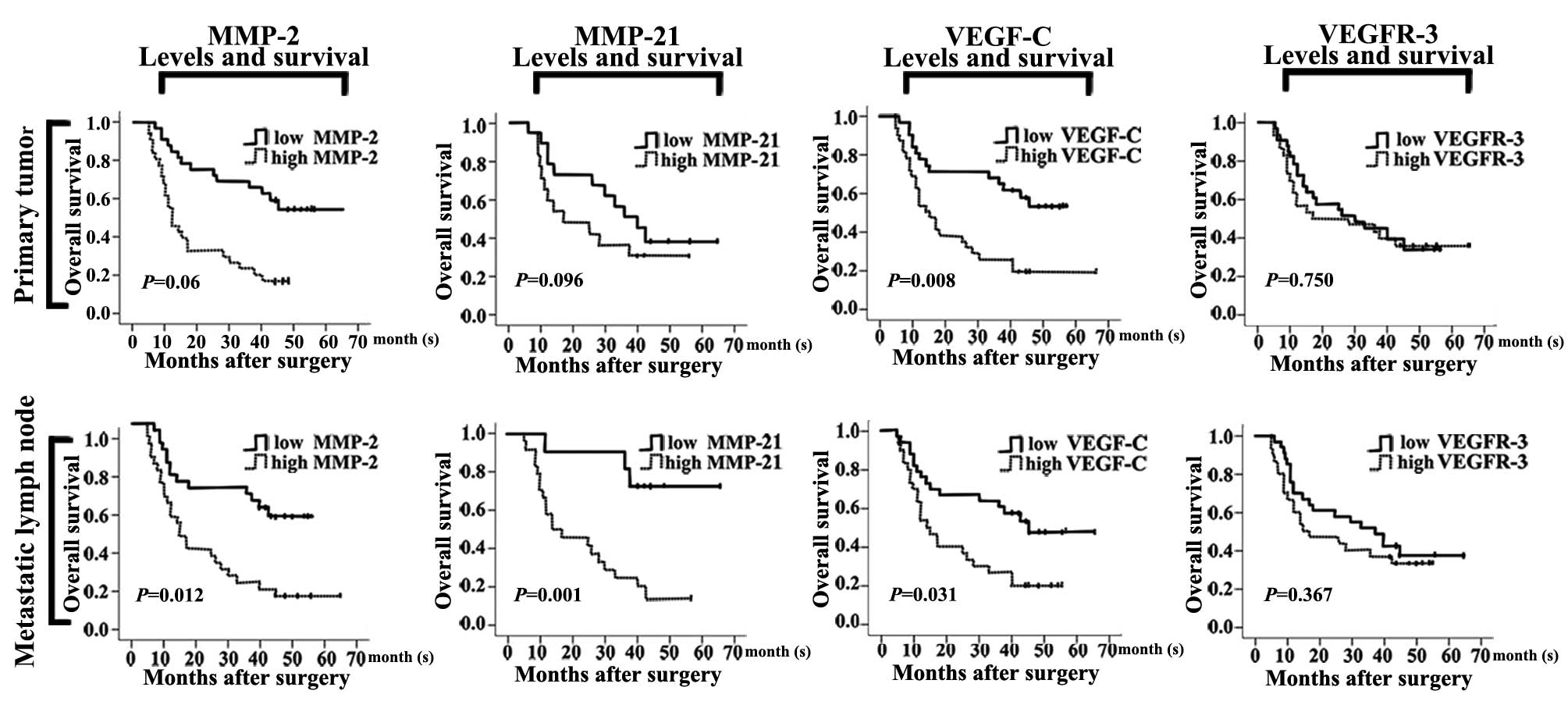
Relationships between protein expression
and overall survival
MMP-2, MMP-21, VEGF-C and VEGFR-3 expression levels
in the PTs and LNM specimens were assessed in relation to patient
overall survival. Patients with high MMP-21 (P=0.001) expression in
the LNM foci had an unfavorable overall survival when compared with
those with low MMP-21 expression. Patients with PTs and LNM foci
that had high MMP-2 (P=0.06, P=0.012) and VEGF-C (P=0.008; P=0.031)
expression levels presented with significantly poor overall
survival. However, the MMP-21 expression level in the PTs and the
VEGFR-3 expression level in the PTs and LNM foci displayed no
significant relationship with overall survival (P>0.05; Fig. 4). The results were analyzed using
the Kaplan-Meier method and the log-rank test.
MMP-21 expression in LNM foci is a
significant predictor of overall survival
MMP-2, MMP-21, VEGF-C and VEGFR-3 expression levels
in the PT and LNM specimens were analyzed using the Cox
proportional hazards model. Three parameters, including ‘overall
score’, ‘change from the previous step’, and ‘change from the
previous block’, were used to assess the significance of the model.
Results by the omnibus testing of model coefficients were
statistically significant (P=0.002; data not shown). Among the
markers, MMP-21 expression in the LNM foci was most closely
associated with the overall survival (P=0.03; Table III). The associated relative risk
was 2.56, which meant that the mortality rate of OSCC patients
would likely increase by 2.56 times in patients with a high MMP-21
expression level. In contrast to MMP-21, the expresion levels of
the other proteins in the LNM foci were not closely correlated with
overall survival (Table III).
 | Table IIICox proportional hazards analysis was
performed to select the relative risk factors for the 63 OSCC
patients with lymphatic metastasis. |
Table III
Cox proportional hazards analysis was
performed to select the relative risk factors for the 63 OSCC
patients with lymphatic metastasis.
| | 95% CI | |
|---|
| |
| |
|---|
| Target
proteins | RR | Lower | Upper | P-value |
|---|
| Primary tumor |
| MMP-2 | 1.465 | 0.682 | 3.147 | 0.328 |
| MMP-21 | 1.307 | 0.669 | 2.554 | 0.433 |
| VEGF-C | 1.767 | 0.815 | 3.830 | 0.149 |
| VEGFR-3 | 1.121 | 0.555 | 2.263 | 0.750 |
| Lymphatic
metastasis |
| MMP-2 | 1.466 | 0.675 | 3.183 | 0.333 |
| MMP-21 | 2.560 | 1.093 | 5.998 | 0.030 |
| VEGF-C | 1.150 | 0.511 | 2.591 | 0.735 |
| VEGFR-3 | 1.491 | 0.722 | 3.079 | 0.280 |
Discussion
Lymphatic metastasis follows a complex course of
progression (18). At present,
researchers have focused on the relationships between the
microenvironment of the PT and mechanisms underlying LNM.
Therefore, expression of a large number of metastasis-related genes
in the PT has been found to correlate with cancer patient overall
survival. However, metastatic foci may be more crucial for
predicting the prognosis of patients with LNM, particularly OSCC
patients with an ~45% chance of suffering metastasis to the neck
lymph nodes (5).
OSCC tends to metastasize to the cervical lymph
nodes (19). PT and LNM cancer
cells have been compared by two opposing hypotheses. First, it is
hypothesized that PT and LNM cells differ characteristically
(20). The second hypothesis
suggests that these differences are negligible (21). Since the identification of cancer
stem cells, it is generally suggested that metastatic cells are
identical to cancer stem cells (22). LNM tumor cells are believed to
express a higher proportion of cancer stem cell markers than PT
cells since stem cell markers are found to be overexpressed in
circulating tumor cells originating from metastatic breast cancer
(23).
In the 63 paired specimens, levels of MMP-21 and
VEGF-C expression, but not MMP-2 or VEGFR-3, were higher in the LNM
foci than levels in the PT cells. This observation suggests that
some tumor cells with higher expression of MMP-21 and VEGF-C are
strongly capable of invasion and metastasis and these tumor cells
metastasize to lymph nodes to grow and accumulate a higher
proportion of tumor cells expressing the two tested proteins.
To the best of our knowledge, the relationship
between protein expression levels in the LNM foci and patient
overall survival has not been extensively studied. MMP-2 and VEGF-C
expression in the PT has been proven to correlate with the overall
survival of OSCC patients (24,25).
Here, we found that high MMP-2 and VEGF-C expression in the PT
specimens or in the corresponding LNM foci was significantly
correlated with overall survival of the 63 OSCC patients. Previous
studies have shown that VEGFR-3 can potentially promote lymphatic
metastasis (26), but cannot
predict OSCC patient overall survival (27). Here, we also found that VEGFR-3 did
not correlate with patient overall survival. A high MMP-21
expression level in the PTs was previously found to be associated
with metastasis and poor overall survival in colorectal cancer
(14,21) as well as gastric cancer patients
(13). In the present study, we
found that high MMP-21 expression in the LNM foci but not in the
PTs was associated with poor overall survival of OSCC patients with
metastatic lesions. This finding may suggest that investigation of
metastatic lesions could provide convincing information for
predicting the overall survival of OSCC patients with LNM. The
underlying mechanisms warrant further investigation.
Among all the tested proteins, the positive MMP-21
expression rate was the highest. Thus, MMP-21 may be used to
identify OSCC cells. Meanwhile, MMP-21 expression in the LNM foci
was most closely associated with patient overall survival as found
using the Cox proportional hazards model. Although MMP-2 and VEGF-C
have been recognized as significant predictors of the overall
survival of OSCC patients (24,25),
MMP-21 expression in the LNM lesions was a more sensitive predictor
of overall survival in the OSCC patients with lymphatic metastasis.
This suggests that investigation of LNM foci is important for OSCC
patients with lymphatic metastasis.
In conclusion, our results may further strengthen
the hypothesis that cellular differences between PT and LNM foci
are characteristically specific. Inhibiting tumorigenesis and
metastasis is the key to curing cancer patients; however, this is a
long and complicated process, which requires tremendous scientific
research. While we are facing the ever-increasing number of cancer
patients with LNM, investigation of LNM foci may help explain the
mechanisms underlying lymphatic metastasis.
Acknowledgements
The authors would like to thank Dr Yan Gao for the
technical support in pathology and Professor Zhong Chen for
critical reading of the manuscript. The present study was supported
by the Natural Science Foundation of China (grant no.
81341062).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Amornphimoltham P, Rechache K, Thompson J,
et al: Rab25 regulates invasion and metastasis in head and neck
cancer. Clin Cancer Res. 619:1375–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Liu Y, Gilcrease MZ, et al: A
lymph node metastatic mouse model reveals alterations of
metastasis-related gene expression in metastatic human oral
carcinoma sublines selected from a poorly metastatic parental cell
line. Cancer. 95:1663–1672. 2002. View Article : Google Scholar
|
|
4
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: clinical features. Oral Oncol. 46:414–417. 2010. View Article : Google Scholar
|
|
5
|
Feng Z, Li JN, Wang L, Pu YF, Wang Y and
Guo CB: The prognostic value of glycerol-3-phosphate dehydrogenase
1-like expression in head and neck squamous cell carcinoma.
Histopathology. 64:348–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lian IB, Tseng YT, Su CC and Tsai K:
Progression of precancerous lesions to oral cancer: results based
on the Taiwan National Health Insurance Database. Oral Oncol.
49:427–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eklund L, Bry M and Alitalo K: Mouse
models for studying angiogenesis and lymphangiogenesis in cancer.
Mol Oncol. 7:259–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahokas K, Lohi J, Lohi H, et al: Matrix
metalloproteinase-21, the human orthologue for XMMP, is expressed
during fetal development and in cancer. Gene. 301:31–41. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahokas K, Lohi J, Illman SA, et al: Matrix
metalloproteinase-21 is expressed epithelially during development
and in cancer and is up-regulated by transforming growth
factor-beta1 in keratinocytes. Lab Invest. 83:1887–1899. 2003.
View Article : Google Scholar
|
|
12
|
Bister V, Skoog T, Virolainen S, Kiviluoto
T, Puolakkainen P and Saarialho-Kere U: Increased expression of
matrix metalloproteinases-21 and -26 and TIMP-4 in pancreatic
adenocarcinoma. Mod Pathol. 20:1128–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu T, Li Y, Lu J, et al: Increased MMP-21
expression is associated with poor overall survival of patients
with gastric cancer. Med Oncol. 30:3232013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Y, Li W, Chu D, et al:
Overexpression of matrix metalloproteinase-21 is associated with
poor overall survival of patients with colorectal cancer. J
Gastrointest Surg. 15:1188–1194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahokas K, Karjalainen-Lindsberg ML, Sihvo
E, Isaka K, Salo J and Saarialho-Kere U: Matrix metalloproteinases
21 and 26 are differentially expressed in esophageal squamous cell
cancer. Tumour Biol. 27:133–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Overall CM and Dean RA: Degradomics:
systems biology of the protease web. Pleiotropic roles of MMPs in
cancer. Cancer Metastasis Rev. 25:69–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robbins KT: Pocket Guide to Neck
Dissection Classification and TNM Staging of Head and Neck Cancer.
2nd edition. American Academy of Otolaryngology-Head and Neck
Surgery Foundation; Alexandria: 2001
|
|
18
|
Hoon DS, Ferris R, Tanaka R, Chong KK,
Alix-Panabieres C and Pantel K: Molecular mechanisms of metastasis.
J Surg Oncol. 103:508–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beltramini GA, Massarelli O, Demarchi M,
et al: Is neck dissection needed in squamous-cell carcinoma of the
maxillary gingiva, alveolus, and hard palate? A multicentre Italian
study of 65 cases and literature review. Oral Oncol. 48:97–101.
2012. View Article : Google Scholar
|
|
20
|
Weiss L, Holmes JC and Ward PM: Do
metastases arise from pre-existing subpopulations of cancer cells?
Br J Cancer. 47:81–89. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao X, Sun B, Hu L, et al: Differential
gene and protein expression in primary breast malignancies and
their lymph node metastases as revealed by combined cDNA microarray
and tissue microarray analysis. Cancer. 100:1110–1122. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aktas B, Tewes M, Fehm T, Hauch S, Kimmig
R and Kasimir-Bauer S: Stem cell and epithelial-mesenchymal
transition markers are frequently overexpressed in circulating
tumor cells of metastatic breast cancer patients. Breast Cancer
Res. 11:R462009. View
Article : Google Scholar
|
|
24
|
Hirota K, Wakisaka N, Sawada-Kitamura S,
et al: Lymph-angiogenesis in regional lymph nodes predicts nodal
recurrence in pathological N0 squamous cell carcinoma of the
tongue. Histopathology. 61:1065–1071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yorioka CW, Coletta RD, Alves F, Nishimoto
IN, Kowalski LP and Graner E: Matrix metalloproteinase-2 and -9
activities correlate with the disease-free survival of oral
squamous cell carcinoma patients. Int J Oncol. 20:189–194.
2002.PubMed/NCBI
|
|
26
|
Matsumoto M, Roufail S, Inder R, et al:
Signaling for lymphangiogenesis via VEGFR-3 is required for the
early events of metastasis. Clin Exp Metastasis. 30:819–832. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu T, Li Y, Liu X, et al: Identification
of high-risk stage II and stage III colorectal cancer by analysis
of MMP-21 expression. J Surg Oncol. 104:787–791. 2011. View Article : Google Scholar : PubMed/NCBI
|