Introduction
Non-small cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality worldwide and is associated with
a high rate of metastasis (1).
Although metastasis is responsible for as much as 90% of
cancer-associated deaths, it remains the most poorly understood
aspect of cancer pathogenesis (2).
Forkhead box M1 (FOXM1), a member of the Fox family
of transcriptional factors, belongs to a group of evolutionarily
conserved transcriptional regulators that are characterized by the
presence of a DNA-binding domain called the Forkhead box or the
winged helix domain (3,4). Generally speaking, FOXM1 plays a
critical role in cell cycle progression by regulating G1/S and G2/M
phase transitions of the cell cycle and ensuring the proper
execution of mitotic cell division (3–5). It
has been reported to be overexpressed in a variety of tumors,
including lung, liver, breast and bladder cancer, clear cell renal
cell carcinoma and early stage cervical cancer (6–11).
Furthermore, increased expression of FOXM1 has been associated with
poor prognosis in cancer patients and is considered to be an
independent predictor of poor survival in many solid cancers
(6,7,10–14).
Importantly, downregulation of FOXM1 leads to the inhibition of
invasion of pancreatic cancer (15), prostate cancer (16), clear cell renal cell carcinoma
(14) and NSCLC (12) cells.
Epithelial-mesenchymal transition (EMT) is an
essential phenotypic event during embryonic development, tissue
remodeling and wound healing, and plays an essential role in tumor
invasion and metastasis (17–19).
EMT is also a reversible process that often occurs at the invasive
front of many metastatic cancers (20). Loss of E-cadherin and relocalization
of β-catenin from the membrane to the nucleus are frequently
observed in tumor cells undergoing EMT (17,21).
Additionally, expression of the intermediate filament protein
Vimentin, which is known to induce changes in cell motility, is a
classic marker of EMT (22).
Although both FOXM1 and EMT play important roles in the metastasis
of cancer, the interaction between them remains unclear.
The PI3K/AKT pathway regulates EMT, which is a
fundamental event that is thought to predict tissue invasion and
metastatic potential (23,24). PI3K and AKT also promote tissue
invasiveness and downregulate expression of E-cadherin, a key
marker of EMT (25–28). Additionally, Park et al found
that FOXM1b induced an EMT-like phenotype by activating the
AKT-Snail pathway in hepatocellular carcinoma (HCC) (29). However, there is no report on the
important role of AKT in FOXM1-induced EMT in NSCLC.
In the present study, we sought to determine whether
FOXM1 expression is associated with EMT in NSCLC specimens and
whether these factors can predict the clinical outcome and survival
of these cancer patients. In addition, we examined whether a strong
correlation exists between FOXM1 expression and the EMT process in
NSCLC cells and whether the AKT signaling pathway is involved in
FOXM1-regulated EMT.
Materials and methods
Tissue samples and tissue microarray
construction
The preparation of tissue specimens and the
construction of the tissue microarray (TMA) were performed as
described previously (30).
Immunohistochemistry
Immunohistochemical analysis of TMA slides was
carried out as previously reported (30). The following primary monoclonal
antibodies were used: FOXM1 rabbit mAb (1:100; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), E-cadherin and Vimentin rabbit
mAb (1:100; Cell Signaling Technology, Beverly, MA, USA). The
intensity score was defined as follows: no signal, 0; weak, 1;
moderate, 2 and intense, 3. The score was based on the fraction of
immunoreactive cells (0–100%). The total score was calculated by
multiplying the intensity score and the fraction score, yielding a
total score ranging from 0 to 300%. For statistical analyses,
scores of 0–100% were considered low expression, and scores of
101%–300% were considered high expression. Two of the pathologists,
without prior knowledge of the clinical data, independently graded
the staining intensity in all cases.
Cell culture, reagents and
treatments
The lung cancer cell lines A549 and H1299 were
cultured in Dulbecco’s modified Eagle’s minimum (DMEM) and H1650,
H1975 in RPMI-1640 medium (both from Gibco-BRL), respectively,
supplemented with 10% fetal bovine serum (FBS) (HyClone Inc., USA).
These cell lines were incubated under standard conditions at 37°C
in a humidified atmosphere containing 5% CO2. The PI3K
inhibitor LY294002 (10 μmol/l) and the activator epidermal growth
factor (EGF) (0.1 ng/ml) were obtained from Sigma-Aldrich (St.
Louis, MO, USA).
Western blotting and antibodies
Equal amounts of protein (50–200 μg/lane) were
extracted from whole-cell lysates and were subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
After being transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore, USA), the proteins were incubated with
primary antibodies [FOXM1 rabbit mAb (1:500; Santa Cruz
Biotechnology), E-cadherin, Vimentin, AKT, p-AKT (Ser473),
p70S6K or p-p70S6K (Thr389) rabbit mAb
(1:1,000; Cell Signaling Technology)], followed by appropriate
HRP-conjugated secondary antibodies. Immunoreactive proteins were
detected using the enhanced chemiluminescence detection kit (Thermo
Scientific, Rockford, IL, USA).
RT-PCR
Total RNA of the cultured cells was isolated with
TRIzol reagent according to the manufacturer’s protocol
(Invitrogen, Carlsbad, CA, USA) and was then resuspended in
diethylpyrocarbonate (DEPC)-treated water. Reverse transcription
was performed according to the manufacturer’s instructions (Takara,
Dalian, China). The primer sequences utilized were: FOXM1 sense,
5′-AGCGACAGGTTAAGGTTGAG-3′ and antisense,
5′-GTGCTGTTGATGGCGAATTG-3′; E-cadherin sense,
5′-TGCCCAGAAAATGAAAAAGG-3′ and antisense,
5′-GTGTATGTGGCAATGCGTTC-3′; Vimentin sense, 5′-GA
GAACTTTGCCGTTGAAGC-3′ and antisense, 5′-CTCAAT GTCAAGGGCCATCT-3′;
GAPDH sense, 5′-ACGGATTTG GTCGTATTGGGCG-3′ and antisense,
5′-CTCCTGGAA GATGGTGATGG-3′. The PCR products were separated by
1.5% agarose gel electrophoresis.
Transwell migration and invasion
assays
In vitro, migration assays were performed in
a Transwell chamber (Corning Costar, USA) containing a membrane
filter (8-μm pore size). To measure cell invasion overnight,
Transwell filters were coated on the lower chamber with 5 μg/ml of
BioCoat Matrigel (BD Biosciences, USA) to reconstitute the matrix
of the basement membrane. Cells were seeded at a density of
2.0×104/insert in 200 μl serum-free medium and
transferred to wells filled with 600 μl culture medium containing
10% FBS as a chemoattractant. After 24 h of incubation,
non-invading cells on the top of the membrane were removed by
scraping. Invading cells on the bottom of the membrane were fixed
in 4% paraformaldehyde and stained with 0.05% crystal violet. The
number of invading cells was then counted in nine random high power
fields/well at ×200 magnification under a light microscope.
Transient transfection of FOXM1
siRNA
FOXM1-specific small interfering RNA (siRNA)
oligonucleotides were purchased from GenePharma (Shanghai
GenePharma Co., Shanghai). The sequences of the double-stranded
siRNA oligonucleotides utilized were: 5′GGACCACUUUCCCUA CUUUTT3′
(sense) and 5′AAAGUAGGGAAAGUGGUC CTT3′ (antisense). The negative
control siRNA sequences were: 5′UUCUCCGAACGUGUCACGUTT3′ (sense) and
5′ACGUGACACGUUCGGAGAATT3′ (antisense). Human lung carcinoma H1975
and H1299 cells which were tested to have high FOXM1 expression
were transfected with siRNA (100 nM) using the DharmaFECT4 siRNA
transfection reagent (Dharmacon, Chicago, IL, USA) according to the
manufacturer’s protocol.
Stable transfection of FOXM1
Plasmid (EX-Z5438-LV135) and Lenti-Pac HIV
Expression Packaging kit were purchased from GeneCopoeia Inc.
(Guangzhou, China). Human lung carcinoma H1650 and A549 cells which
were tested to have low FOXM1 expression were stably transfected
according to the manufacturer’s instructions. The presence of
stable transfectants was confirmed by RT-PCR and western
blotting.
Statistical analyses
A comparison of clinicopathologic characteristics of
the patients was evaluated using the Chi-square test. Overall
survival was defined as the length of time from the date of
diagnosis to the date of death or the date at which patients were
last known to be alive. Survival analysis data were collected until
the 1st of January 2012, and the Kaplan-Meier method was used to
calculate overall survival. The differences between the survival
curves were analyzed using the log-rank test. Spearman rank
correlation coefficients were used to quantify the correlations
between the expression of FOXM1 and EMT indicator proteins.
Univariate analysis was performed using the log-rank test.
Multivariate analysis was performed using the Cox proportional
hazards model. All factors with a value of p<0.05 by univariate
analysis were included in the multivariate analysis. In the
analyses, p<0.05 was considered to indicate a statistically
significant result. Statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Increased expression of FOXM1 is
associated with EMT in NSCLC tissues
The clinicopathological characteristics of the
patients were previously reported in detail (30) and are summarized in Table I. The overall duration of the
follow-up of these patients ranged from 1 to 60 months.
 | Table ICorrelation between expression of
FOXM1 and EMT indicator proteins and clinicopathological factors of
the NSCLC patients. |
Table I
Correlation between expression of
FOXM1 and EMT indicator proteins and clinicopathological factors of
the NSCLC patients.
|
Characteristics | Total (%) | High FOXM1
expression, n (%) | High E-cadherin
expression, n (%) | High Vimentin
expression, n (%) |
|---|
| Gender |
| Female | 30 (44.1) | 18 (60.0) | 15 (50.0) | 10 (33.3) |
| Male | 38 (55.9) | 25 (65.8) | 12 (31.6) | 7 (18.4) |
| Age (years) |
| Mean | 59.44 | | | |
| <60 | 29 (42.6) | 17 (58.6) | 14 (48.3) | 5 (17.2) |
| ≥60 | 39 (57.4) | 26 (66.7) | 13 (33.3) | 12 (30.8) |
| Smoking status |
| No | 30 (44.1) | 15 (50.0) | 14 (46.7) | 5 (16.7) |
| Yes | 38 (55.9) | 28 (73.7)a | 13 (34.2) | 12 (31.6) |
| Histologic
characterisation |
|
Adenocarcinoma | 33 (48.5) | 20 (60.6) | 14 (42.4) | 6 (18.2) |
| Squamous
carcinoma | 21 (30.9) | 12 (57.1) | 9 (42.9) | 3 (14.2) |
| Adenosquamous
carcinoma | 14 (20.6) | 11 (78.6) | 4 (28.6) | 8 (57.1)b |
|
Differentiation |
| High/moderate | 39 (57.3) | 21 (53.8) | 17 (43.6) | 10 (25.6) |
| Low | 29 (42.7) | 22 (75.9) | 10 (34.5) | 7 (24.1) |
| TNM stage |
| I+II | 27 (39.7) | 13 (48.1) | 15 (55.6) | 2 (7.4) |
| III+IV | 41 (60.3) | 30 (73.2)a | 12 (29.3)a | 15 (36.6)a |
| Lymph node
metastasis |
| Negative | 27 (39.7) | 12 (44.4) | 16 (59.3) | 2 (7.4) |
| Positive | 41 (60.3) | 31 (75.6)a | 11 (26.8)a | 15 (36.6)a |
We sought to investigate the expression levels of
the FOXM1 and EMT indicator proteins in NSCLC specimens and
correlate these results with the patient clinical and pathologic
findings. Immunohistochemical (IHC) analyses of tissue array slides
were used to determine the expression levels of FOXM1, E-cadherin
and Vimentin proteins. Either nuclear expression or mixed nuclear
and cytoplasmic expression of FOXM1 in tumor cells was defined as
positive immunoreactivity for FOXM1. Positive immunostaining for
E-cadherin was localized to the cell membrane, whereas Vimentin was
observed in the cytoplasm of cancer cells. Representative patterns
of IHC staining are illustrated in Fig.
1.
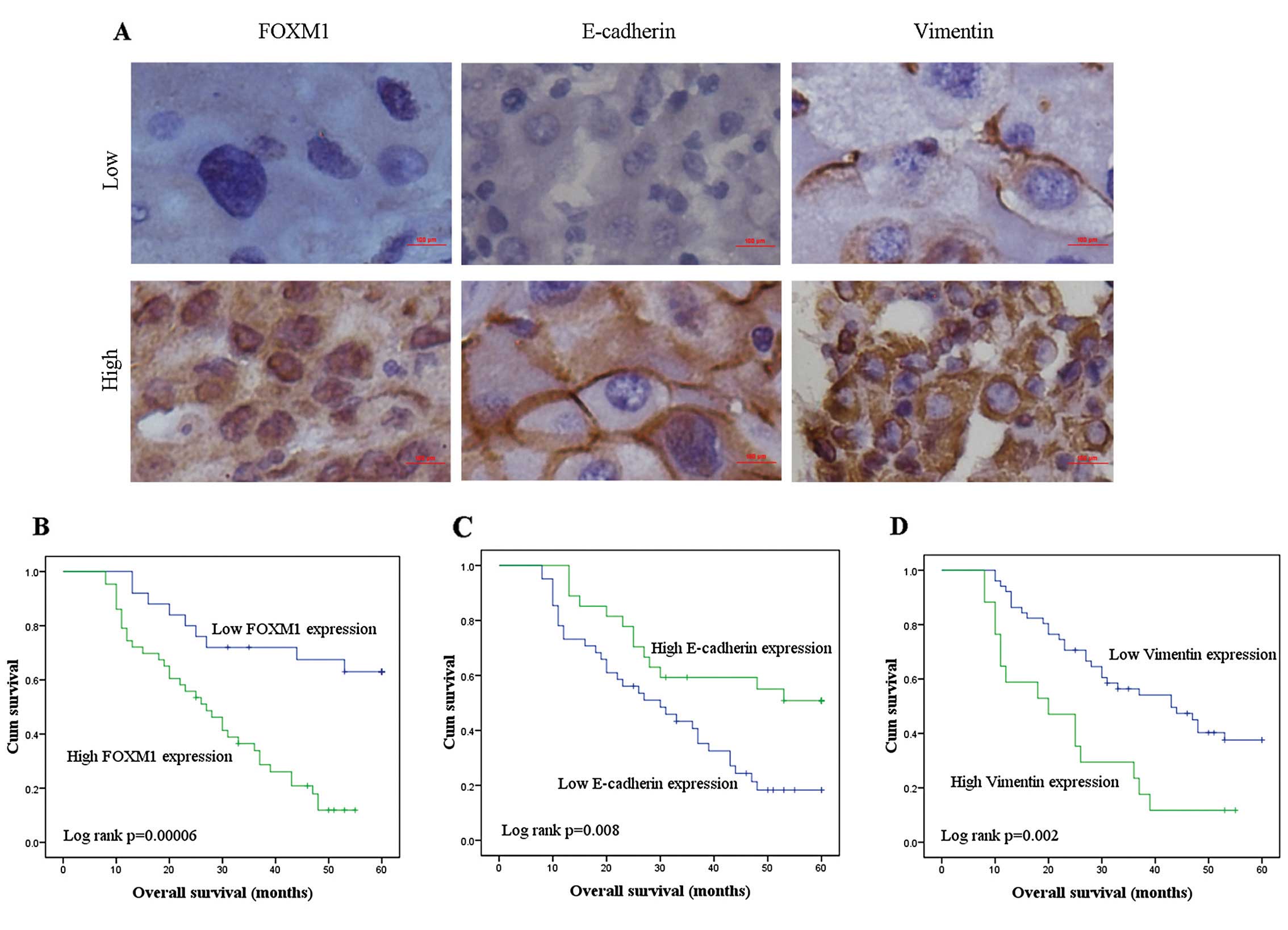 | Figure 1Representative IHC images showing
expression of FOXM1 and EMT-related biomarkers in TMA sections of
NSCLC and corresponding Kaplan-Meier survival curves. (A) The image
shows representative NSCLC tissues exhibiting low and high levels
of expression for FOXM1, E-cadherin and Vimentin. Scale bar, 100
μm. (B-D) Kaplan-Meier survival analyses of overall survival of
NSCLC patients with respect to their level of expression of FOXM1,
E-cadherin and Vimentin (B, FOXM1; C, E-cadherin and D, Vimentin).
FOXM1, Forkhead box M1; EMT, epithelial-mesenchymal transition;
TMA, tissue microarray; NSCLC, non-small cell lung cancer. |
As shown in Table I,
high levels of expression of FOXM1, E-cadherin and Vimentin were
observed in 43/68 (63.2%), 27/68 (39.7%) and 17/68 (25.0%)
specimens of NSCLC, respectively. Notably, statistically
significant correlations were demonstrated between FOXM1 and the
smoking history of the patients (p=0.044), TNM stage (p=0.036) and
lymph node metastasis status (p=0.009). Tumors from patients with a
positive smoking history, advanced TNM stage and lymph node
metastasis were characterized by higher FOXM1 expression compared
with those without these features. Expression levels of E-cadherin
and Vimentin were significantly associated with advanced TNM stage
and lymph node metastasis. In addition, Vimentin expression was
found to be significantly greater in lung adenosquamous carcinoma
than that in squamous cell carcinoma.
Increased expression of FOXM1 is
correlated with EMT indicator proteins E-cadherin and Vimentin in
NSCLC tissues
To investigate the association between the
expression of FOXM1 and EMT indicator proteins in NSCLC specimens,
Spearman correlation analysis was used. As shown in Table II, a high expression level of FOXM1
was found to correlate with a loss of E-cadherin expression
(r=−0.69, p<0.001) and anomalous immunoreactivity of Vimentin
(r=0.370, p=0.002) in the NSCLC samples. Therefore, FOXM1
expression was significantly correlated with EMT in the NSCLC
tissues.
 | Table IISpearman correlation analysis between
expression of FOXM1 and EMT indicator proteins in NSCLC. |
Table II
Spearman correlation analysis between
expression of FOXM1 and EMT indicator proteins in NSCLC.
| FOXM1
expression | | |
|---|
|
| | |
|---|
| EMT indicator
proteins | Low | High | P-value | R-value |
|---|
| E-cadherin
expression |
| Low | 4 | 37 | <0.001 | −0.690 |
| High 21 | 6 | | | |
| Vimentin
expression |
| Low | 24 | 27 | 0.002 | 0.370 |
| High | 1 | 16 | | |
The expression levels of FOXM1,
E-cadherin and Vimentin predict overall survival time of NSCLC
patients
Overall survival analysis was used to determine the
existence of a correlation between the expression of FOXM1 and EMT
indicator proteins and patient prognosis. Kaplan-Meier survival
curves for overall survival with respect to FOXM1 and EMT indicator
protein expression are shown in Fig.
1. These plots demonstrated that NSCLC patients expressing low
levels of FOXM1 and Vimentin but high levels of E-cadherin survived
significantly longer than patients with NSCLC expressing high FOXM1
and Vimentin but low E-cadherin levels.
In addition, a Cox proportional hazards model was
applied to estimate the effect of FOXM1 and EMT indicator proteins
on patient survival. Univariate Cox regression analysis identified
tumor differentiation, TNM stage, lymph node metastasis, and
expression of FOXM1, E-cadherin and Vimentin as significant
prognostic factors (Table III).
The crude hazard ratio (HR) of NSCLC with high FOXM1 expression
compared with low FOXM1 expression was 4.099 (95% confidence
interval 1.934–8.688, p=0.00006). In other words, high FOXM1
expression in NSCLC patients increased the likelihood of lung
cancer-related death 4-fold above that of a low FOXM1 status. Using
multivariable analysis, lymph node metastasis and FOXM1 were found
to be statistically significant independent prognostic factors for
overall survival (p=0.042 and p=0.043, respectively). These
findings suggest that FOXM1 expression behaves as an independent
and statistically significant predictor for poor patient
survival.
 | Table IIIUnivariate and multivariate analyses
of overall survival. |
Table III
Univariate and multivariate analyses
of overall survival.
|
Characteristics | Univariate
analysis | Median survival
(months) | Multivariate
analysis |
|---|
|
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender | 0.897 | 0.496–1.622 | 0.716 | | - | - | - |
| Female | | | | 36.733 | | | |
| Male | | | | 35.531 | | | |
| Age (years) | 1.811 | 0.980–3.348 | 0.058 | | - | - | - |
| <60 | | | | 42.151 | | | |
| ≥60 | | | | 31.498 | | | |
| Smoking status | 1.152 | 0.639–2.076 | 0.637 | | - | - | - |
| No | | | | 37.5 | | | |
| Yes | | | | 34.93 | | | |
| Histologic
characterisation | 0.900 | 0.613–1.322 | 0.593 | | - | - | - |
|
Adenocarcinoma | | | | 33.391 | | | |
| Squamous
carcinoma | | | | 40.899 | | | |
| Adenosquamous
carcinoma | | | | 35.714 | | | |
|
Differentiation | 0.540 | 0.300–0.972 | 0.040 | | 0.606 | 0.320–1.148 | 0.124 |
| High or
moderate | | | | 40.812 | | | |
| Low | | | | 29.610 | | | |
| TNM stage | 2.996 | 1.532–5.858 | 0.001 | | 1.247 | 0.495–3.143 | 0.639 |
| I+II | | | | 45.591 | | | |
| III+IV | | | | 29.733 | | | |
| Lymph node
metastasis | 3.395 | 1.732–6.654 | 0.0001 | | 2.595 | 1.034–6.510 | 0.042 |
| Negative | | | | 47.113 | | | |
| Positive | | | | 28.548 | | | |
| FOXM1
expression | 4.099 | 1.934–8.688 | 0.00023 | | 2.950 | 1.036–8.397 | 0.043 |
| Low | | | | 47.645 | | | |
| High | | | | 28.610 | | | |
| E-cadherin
expression | 0.429 | 0.223–0.824 | 0.001 | | 1.181 | 0.500–2.789 | 0.704 |
| Low | | | | 31.297 | | | |
| High | | | | 43.344 | | | |
| Vimentin
expression | 2.596 | 1.386–4.864 | 0.003 | | 1.287 | 0.642–2.580 | 0.477 |
| Low | | | | 40.012 | | | |
| High | | | | 23.882 | | | |
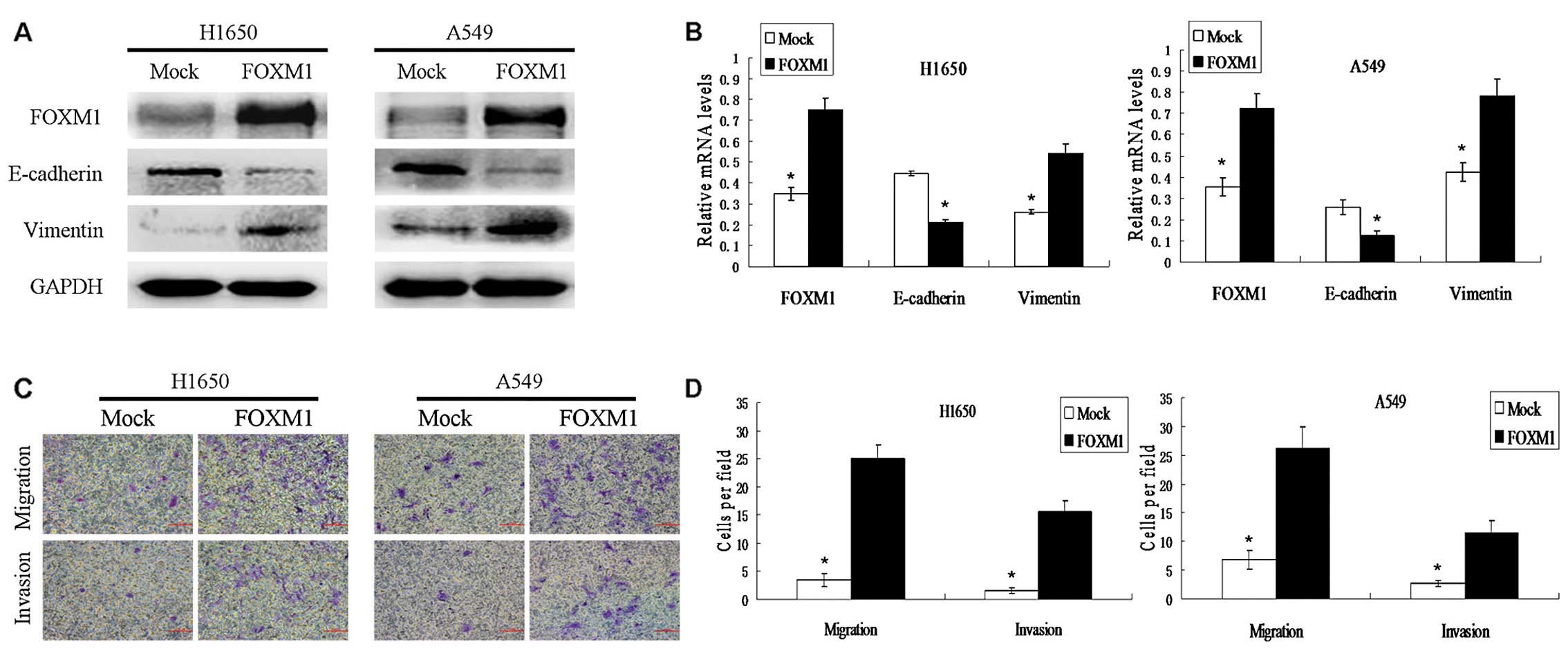
Stable overexpression of FOXM1 protein
induces EMT in NSCLC cells
Our data provide clinical evidence supporting the
hypothesis that high FOXM1 expression is associated with EMT and
poor survival of patients with NSCLC. Furthermore, FOXM1 has been
implicated in the regulation of the EMT process in pancreatic
cancer cells (31). To test whether
FOXM1 expression induces EMT in NSCLC cells, human lung carcinoma
H1650 and A549 cells were stably transfected with a lentiviral
vector expressing FOXM1. Following transfection, protein and mRNA
levels of E-cadherin were found to be decreased while those of
Vimentin were increased in these cells (Fig. 2A and B). To further characterize the
EMT process in FOXM1-expressing cells, the migration capacity of
FOXM1-transfected cells and control lentivirus-transfected (mock)
cells were compared using Transwell chambers. The elevated
expression of FOXM1 promoted the migration and invasion of
transfected cells when compared with the mock cells (Fig. 2C and D). These data suggest that
FOXM1 may promote metastasis by inducing the EMT process in NSCLC
cell lines.
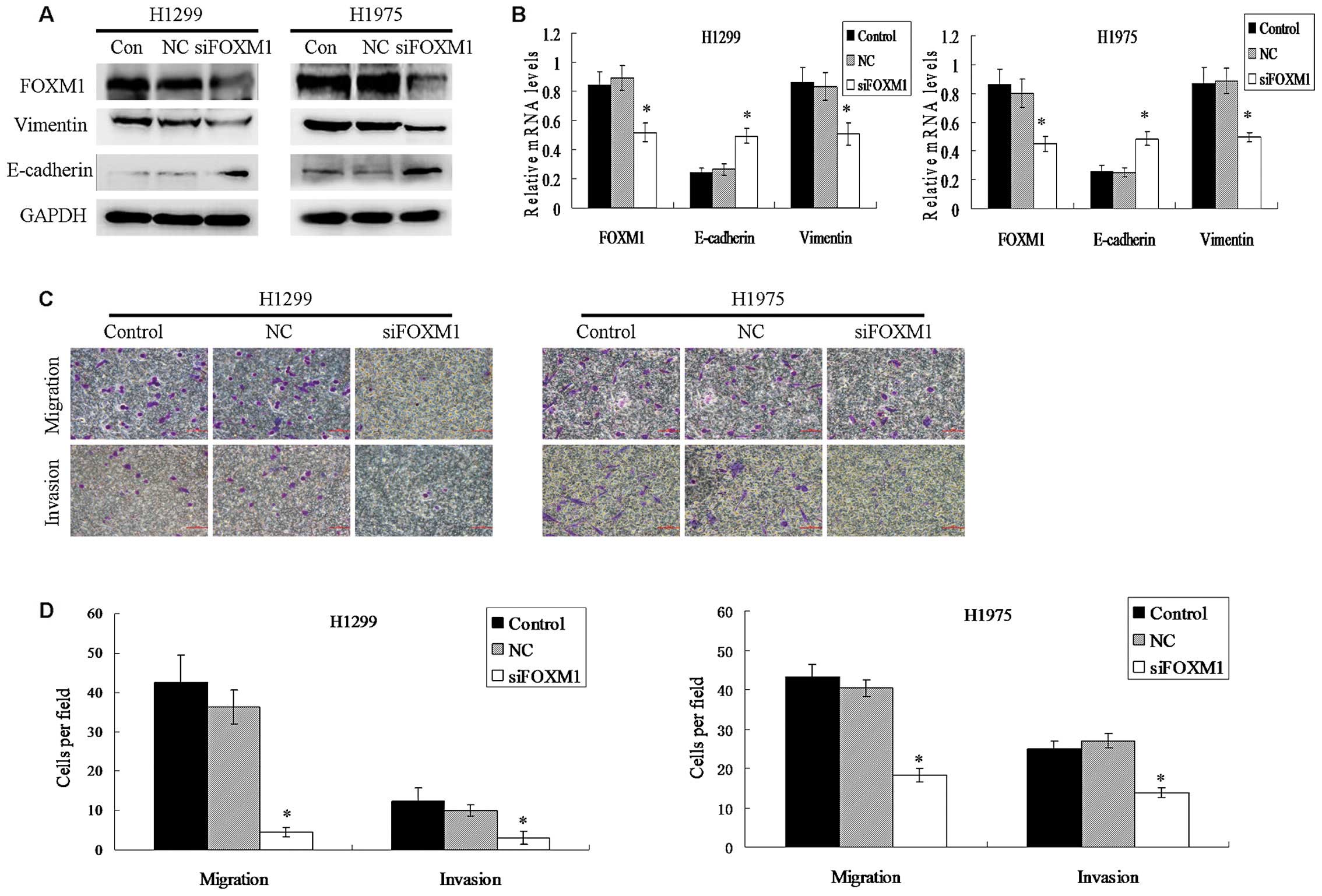
Knockdown of FOXM1 expression inhibits
the EMT process of NSCLC cells
To test whether FOXM1 expression was necessary for
the EMT process of NSCLC cells, the human NSCLC cell lines H1299
and H1975 were transiently transfected with negitive control siRNA
or FOXM1-specific siRNA to suppress the expression of FOXM1.
Knockdown of FOXM1 gene expression increased the expression of
E-cadherin while decreasing the expression of Vimentin both at the
protein and mRNA levels in the transfected cells (Fig. 3A and B). As shown in Fig. 3C and D, knockdown of FOXM1 markedly
reduced the migration and invasion of the transfected cells. These
results suggest that FOXM1 expression is critical for the EMT
process in lung cancer cells.
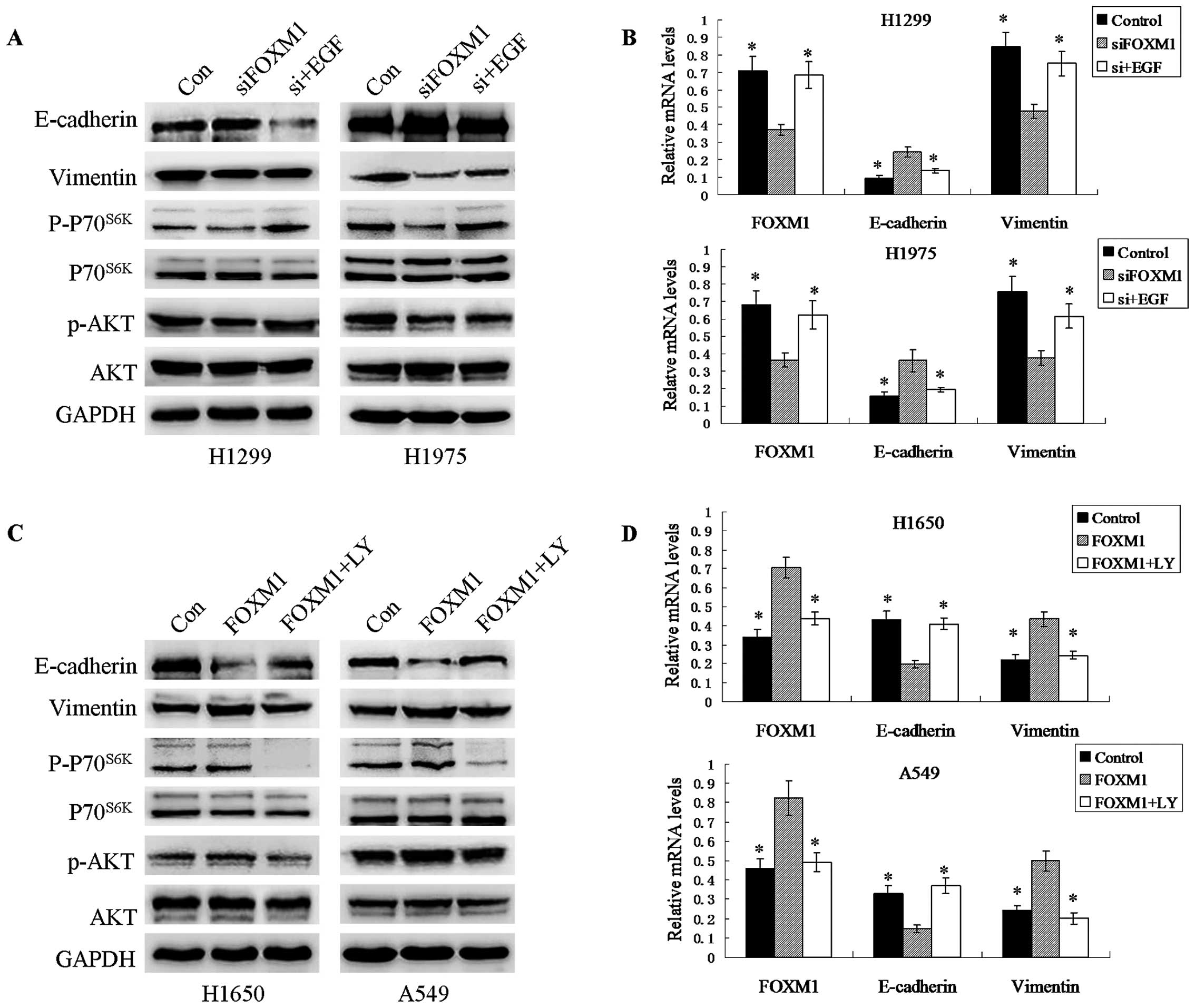
Overexpression and knockdown of FOXM1
expression regulate EMT through activation and inhibition of the
AKT/p70S6K signaling pathway
To explore the mechanism whereby FOXM1 expression
enhances EMT, we examined the effects of FOXM1 on the expression
and phosphorylation of AKT and p70S6K. We found that
knockdown of endogenous FOXM1 inhibited the phosphorylation levels
of AKT and p70S6K but not total AKT/p70S6K.
Conversely, overexpression of FOXM1 induced the opposite
results.
To further investigate whether the activation of AKT
pathway is responsible for the FOXM1-induced EMT in NSCLC, an
inhibitor (LY294002) and an activator (EGF) of the PI3K/AKT pathway
were used in experiments based on the observation that
overexpression and knockdown of FOXM1 can activate and inhibit this
pathway, respectively. As shown in Fig.
4, FOXM1-expressing cells treated with the inhibitor LY294002
showed reduced expression of Vimentin but increased E-cadherin
expression. In contrast, EMT was promoted in siRNA-transfected
cells incubated with EGF for 48 h. These results suggest that
activation of the AKT/p70S6K pathway plays an important
role in FOXM1-induced EMT in NSCLC.
Discussion
In the present study, we demonstrated that high
levels of expression of FOXM1 and EMT indicator proteins are
significantly associated with several clinicopathologic variables.
We also demonstrated, for the first time, the existence of a
significant association between FOXM1 and EMT indicator protein
expression. Additionally, the present study demonstrated that FOXM1
expression may be an independent prognostic indicator for overall
survival of patients with NSCLC. Furthermore, our results suggest
that FOXM1 expression may promote NSCLC metastasis by inducing EMT
of tumors cells via the activation of the AKT/p70S6K
pathway in lung cancer cells.
As previously mentioned, the existence of a
significant association between FOXM1 expression and poor prognosis
has been reported in many human types of cancers, including lung
cancer (6,12,32).
In agreement with these reports, we found that FOXM1 is an
independent prognostic indicator for overall survival of NSCLC
patients. Our results also suggest that FOXM1 expression in tumor
specimens is positively associated with TNM stage and the presence
of lymph node metastasis. However, Liu et al (32) reported no association between FOXM1
expression and the clinical parameters studied, which raises
questions about how tumor specimens were obtained and how IHC
expression was analyzed.
There is considerable evidence that the presence of
EMT signifies reduced survival of lung cancer patients (33–36).
Previous studies reported that negative or low E-cadherin
expression ranged from 46 to 75.21% in NSCLC specimens (37–40),
which is similar to the low E-cadherin expression level of 60.3%
found in the present study. Our results also showed that Vimentin
expression was significantly associated with the presence of lymph
node metastasis, TNM stage and the histologic type of the tumor. In
contrast with our results, Dauphin et al (41) did not identify any association
between Vimentin expression and lymph node metastasis. These
differences may reflect the different study populations and varied
clinical data quality.
To the best of our knowledge, this is the first
report that high FOXM1 expression is significantly associated with
EMT in NSCLC specimens. To extend the above results, we studied
overexpression or knockdown of FOXM1 in lung cancer cell lines and
found that FOXM1-mediated EMT results in the invasion and migration
in vitro. Notably, Xu et al (12) reported that levels of E-cadherin and
ZO-1 were reduced and that Vimentin and N-cadherin expression
levels were elevated in NSCLC cells expressing high levels of
FOXM1. These results suggest that FOXM1 expression is associated
with poor prognosis by regulating EMT in NSCLC.
Until recently, the precise molecular regulation of
FOXM1 on EMT and metastasis was unclear. Previous research has
shown that AKT has a distinct role in regulating EMT and cell
migration (42). Furthermore, FOXM1
has been shown to activate the AKT-Snail pathway to promote cell
migration and invasion in hepatocellular carcinoma (29) and early stage cervical cancer
(11), while its role in NSCLC has
not been reported. The present study demonstrated that FOXM1
activates the AKT/p70S6K signaling pathway thereby
promoting EMT transition by enhancing cell migration and the
invasive capacity in NSCLC. Furthermore, our research team is
currently exploring the possible mechanisms involved in the
regulation of PI3K/AKT by FOXM1.
In summary, our results suggest that FOXM1
expression is associated with a poor prognosis and EMT in NSCLC. In
cell culture experiments, FOXM1 overexpression promoted migration
and invasion by inducing the EMT of lung cancer cells via
activation of the AKT/p70S6K pathway. The present study
raises the possibility that FOXM1 may be a potential target for
cancer therapy as it plays a crucial role in tumor migration and
invasion.
Acknowledgements
This study was supported by the Shanghai Jiaotong
University School of Medicine Science Fund Project (11XJ22014), the
Hospital Foundation of No. 3 People’s Hospital, which is affiliated
with the Shanghai Jiaotong University School of Medicine
(syz2011-05), the Education Fund for Outstanding Young Teachers of
Shanghai (zzjdyx12111), and the Science and Technology Commission
of Shanghai Municipality (10JC1409200).
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
Cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laoukili J, Kooistra MR, Brás A, et al:
FoxM1 is required for execution of the mitotic programme and
chromosome stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laoukili J, Stahl M and Medema RH: FoxM1:
at the crossroads of ageing and cancer. Biochim Biophys Acta.
1775:92–102. 2007.PubMed/NCBI
|
|
5
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang DK, Son CH, Lee SK, Choi PJ, Lee KE
and Roh MS: Forkhead box M1 expression in pulmonary squamous cell
carcinoma: correlation with clinicopathologic features and its
prognostic significance. Hum Pathol. 40:464–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun HC, Li M, Lu JL, et al: Overexpression
of Forkhead box M1 protein associates with aggressive tumor
features and poor prognosis of hepatocellular carcinoma. Oncol Rep.
25:1533–1539. 2011.PubMed/NCBI
|
|
8
|
Bektas N, Haaf At, Veeck J, et al: Tight
correlation between expression of the Forkhead transcription factor
FOXM1 and HER2 in human breast cancer. BMC Cancer. 8:422008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu D, Zhang Z and Kong CZ: High FOXM1
expression was associated with bladder carcinogenesis. Tumour Biol.
34:1131–1138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu XR, Chen YH, Liu DM, Sha JJ, Xuan HQ,
Bo JJ and Huang YR: Increased expression of forkhead box M1 protein
is associated with poor prognosis in clear cell renal cell
carcinoma. Med Oncol. 30:3462013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He SY, Shen HW, Xu L, et al: FOXM1
promotes tumor cell invasion and correlates with poor prognosis in
early-stage cervical cancer. Gynecol Oncol. 127:601–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu N, Jia D, Chen W, et al: FoxM1 is
associated with poor prognosis of non-small cell lung cancer
patients through promoting tumor metastasis. PLoS One.
8:e594122013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu XY, Zhu ZM, Chen LB, et al: FOXM1
expression correlates with tumor invasion and a poor prognosis of
colorectal cancer. Acta Histochem. 114:755–762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xue YJ, Xiao RH, Long DZ, et al:
Overexpression of FoxM1 is associated with tumor progression in
patients with clear cell renal cell carcinoma. J Transl Med.
10:2002012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lynch TP, Ferrer CM, Jackson SR, Shahriari
KS, Vosseller K and Reginato MJ: Critical role of O-linked
β-N-acetylglucosamine transferase in prostate cancer
invasion, angiogenesis, and metastasis. J Biol Chem.
287:11070–11081. 2012.
|
|
17
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu YD and Zhou BP: Snail: more than EMT.
Cell Adh Migr. 4:199–203. 2010. View Article : Google Scholar
|
|
20
|
Christofori G: New signals from the
invasive front. Nature. 441:444–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eriksson JE, Dechat T, Grin B, Helfand B,
Mendez M, Pallari HM and Goldman RD: Introducing intermediate
filaments: from discovery to disease. J Clin Invest. 119:1763–1771.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng GZ, Park S, Shu S, et al: Advances
of AKT pathway in human oncogenesis and as a target for anti-cancer
drug discovery. Curr Cancer Drug Targets. 8:2–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onoue T, Uchida D, Begum NM, Tomizuka Y,
Yoshida H and Sato M: Epithelial-mesenchymal transition induced by
the stromal cell-derived factor-1/CXCR4 system in oral squamous
cell carcinoma cells. Int J Oncol. 29:1133–1138. 2006.PubMed/NCBI
|
|
25
|
Grille SJ, Bellacosa A, Upson J, et al:
The protein kinase Akt induces epithelial mesenchymal transition
and promotes enhanced motility and invasiveness of squamous cell
carcinoma lines. Cancer Res. 63:2172–2178. 2003.
|
|
26
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005.
|
|
27
|
Schramek H, Feifel E, Marschitz I,
Golochtchapova N, Gstraunthaler G and Montesano R: Loss of active
MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in
transdifferentiated MDCK cells. Am J Physiol Cell Physiol.
285:C652–C661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park HJ, Gusarova G, Wang Z, et al:
Deregulation of FoxM1b leads to tumour metastasis. EMBO Mol Med.
3:21–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao M, Gao FH, Wang JY, Liu F, Yuan HH,
Zhang WY and Jiang B: JAK2/STAT3 signaling pathway activation
mediates tumor angiogenesis by upregulation of VEGF and bFGF in
non-small-cell lung cancer. Lung Cancer. 73:366–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao B, Wang Z, Ali S, Kong D, et al:
Over-expression of FoxM1 leads to epithelial-mesenchymal transition
and cancer stem cell phenotype in pancreatic cancer cells. J Cell
Biochem. 112:2296–2306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu YQ, Guo RH, Liu LK, Gao W, Zhu CJ, Wei
J and Shu YQ: Correlation between expression of forkhead box M1
(FOXM1) and clinicopathological features and prognosis in patients
with non-small cell lung cancer (NSCLC). Zhonghua Zhong Liu Za Zhi.
33:426–430. 2011.(In Chinese).
|
|
33
|
Li LP, Lu CH, Chen ZP, et al: Subcellular
proteomics revealed the epithelial-mesenchymal transition phenotype
in lung cancer. Proteomics. 11:429–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang G, Dong W, Shen H, Mu X, Li Z, Lin X,
Liu Y and Du J: A comparison of Twist and E-cadherin protein
expression in primary non-small-cell lung carcinoma and
corresponding metastases. Eur J Cardiothorac Surg. 39:1028–1032.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pirozzi G, Tirino V, Camerlingo R, et al:
Epithelial to mesenchymal transition by TGFβ-1 induction increases
stemness characteristics in primary non small cell lung cancer cell
line. PLoS One. 6:e215482011.
|
|
36
|
Soltermann A, Tischler V, Arbogast S, et
al: Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao JQ, Sun FJ, Liu SS, et al: Expression
of connexin 43 and E-cadherin protein and mRNA in non-small cell
lung cancers in Chinese patients. Asian Pac J Cancer Prev.
14:639–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Wang Z, Kang Y, Li X, Ma X and Ma
L: MCAM expression is associated with poor prognosis in non-small
cell lung cancer. Clin Transl Oncol. 16:178–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin S, Zhu W, Shi Q, Zhang Z and Guo R:
Clinicopathological significance of lymphatic vessel density marked
by D2–40 and E-cadherin expression in non-small-cell lung cancer.
Med Oncol. 29:3157–3161. 2012.
|
|
40
|
Ucvet A, Kul C, Gursoy S, Erbaycu AE, Kaya
SO, Dinc ZA and Yucel N: Prognostic value of epithelial growth
factor receptor, vascular endothelial growth factor, E-cadherin,
and p120 catenin in resected non-small cell lung carcinoma. Arch
Bronconeumol. 47:397–402. 2011.(In Spanish).
|
|
41
|
Dauphin M, Barbe C, Lemaire S, et al:
Vimentin expression predicts the occurrence of metastases in non
small cell lung carcinomas. Lung Cancer. 81:117–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Irie HY, Pearline RV, Grueneberg D, et al:
Distinct roles of Akt1 and Akt2 in regulating cell migration and
epithelial-mesenchymal transition. J Cell Biol. 171:1023–1034.
2005. View Article : Google Scholar : PubMed/NCBI
|