Introduction
Tumor invasion and metastasis are the leading causes
of death from cancer. For a tumor cell to metastasize and form
distal metastases, it must initially penetrate the extracellular
matrix (ECM) and invade the vascular system (1).
The CD44 gene, which is located on the short arm of
human chromosome 11, spans ~50 kb of genomic DNA. Theoretically,
over 800 CD44 variants can be expressed in tissues and cells.
However, not all CD44 isoforms can be expressed virtually. Dozens
of CD44 isoforms have been identified to date, and the standard
CD44 (CD44s) isoform is the most common; in CD44s, exon 5 is
directly connected to exon 16, and lacks the entire variant exon
region. In our previous study, the multidrug-resistant human breast
cancer MCF-7/Adr cells were used to clone the short-tail isoform of
CD44 (CD44st), a novel short-tail isoform of CD44, which contains
exons 1–4 and 16–17, and 1–205 base pairs of exon 18. The CD44
family members and their structures have been described previously
(2).
Nuclear factor (NF)-κB, a member of the Rel protein
family, is composed of a group of homodimers or heterodimers of
transcription factors. NF-κB is found to be involved in tumor
occurrence, invasion and metastasis, and it is closely associated
with multidrug resistance (MDR) in tumors. Abnormal NF-κB
expression and activity are found to affect the regulation of
multidrug resistance protein 1 (MDR1) gene expression in
multidrug-resistant tumor cells. Exon 1 at the promoter region of
the MDR1 gene contains an NF-κB-binding sequence
(5′-CCTTTCGGGG-3′), and NF-κB can activate the transcription of the
reporter gene connected to the MDR1 promoter. It is
therefore speculated that the transcription of the MDR1
gene, which may be a downstream gene of NF-κB, is activated by
NF-κB and than transcribes into P-glycoprotein (P-gp) resulting in
tumor cell multidrug resistance.
NF-κB is reported to be involved in regulating the
expression of MDR1 and CD44 genes in multiple cancer
cells (3–7), and it has been found that the
CD44-hyaluronan (HA) interaction induces ankyrin binding to P-gp,
resulting in the efflux of chemotherapeutic drugs and development
of MDR in cancer (8,9).
It has been found that both CD44st mRNA and
CD44st protein are highly expressed in multidrug-resistant
MCF-7/Adr, Lovo/Adr, K562/Adr and HL-60/Adr cell lines, while the
sensitive MCF-7, Lovo, K562 and HL-60 cells do not contain either
CD44st mRNA or CD44st protein. In addition, matrix
metalloproteinase (MMP)-2 and MMP-9 expression can be upregulated
by HA treatment, which can be blocked by pretreatment with CD44
neutralizing antibody. Such findings demonstrate that the HA-CD44st
interaction activates the MAPK signaling pathway, thereby
increasing MMP-2 and MMP-9 secretion (2,10,11).
To investigate the role of the transcription factor NF-κB in
doxorubicin-induced drug resistance and the association with CD44st
expression, we used low-dose doxorubicin to induce MDR in human
breast cancer MCF-7 cells and determined the expression of
MDR1, CD44st and NF-κB mRNA in these
multidrug-resistant MCF-7 cells. In addition, the effects of an
NF-κB inhibitor on MMP-2 and MMP-9 expression and the invasive
ability of MCF-7 cells mediated through the HA-CD44st signaling
pathway were evaluated.
Materials and methods
Cell lines and cell culture
The human breast cancer MCF-7 cell line and the
doxorubicin-resistant human breast cancer MCF-7/Adr cell line were
purchased from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). MCF-7 cells were
cultured as described previously (2). Cells were maintained in RPMI-1640
medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd., Hangzhou, China) at 37°C in a humidified atmosphere
containing 5% CO2, while MCF-7/Adr cells were maintained
in a medium containing 0.2 μg/ml doxorubicin, with drug withdrawal
occurring 1 week prior to the experiment.
Detection of the 50% inhibitory
concentration (IC50) of doxorubicin against MCF-7 cells
by MTT assays
Log phase MCF-7 cells were seeded onto 96-well cell
culture plates at a density of 2×104 cells/well, and
doxorubicin treatment was administered after the cells were
adherent to the plate wall. Doxorubicin was formulated into
concentrations of 0, 0.05, 0.1, 0.2, 0.4, 0.8 and 1.6 μg/ml, and 4
replicate wells were used for each concentration, with a final
volume of 200 μl. Following incubation at 37°C in a humidified
atmosphere containing 5% CO2, 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(5 mg/ml) was added to each well, and the cells were cultured for
another 4 h. The supernatant was then removed, 100 μl of dimethyl
sulfoxide (DMSO) was added to each well, and the plates were then
shaken on a horizontal shaker for 15 min to allow for complete
dissolution. The optical density (OD) was read at 492 nm on an
enzyme-linked immunosorbent assay (ELISA) microplate reader. The
inhibition of cell growth (IR) was calculated using the following
formula: Inhibitory rate (%) = (1 - mean OD value in the
experimental group/mean OD value in the control group) × 100%
(Table I). The IC50
value was estimated from the OD value and was applied to evaluate
the cytostatic efficacy of doxorubicin on MCF-7 cells. Each
experiment was repeated in triplicate.
 | Table IThe 50% inhibitory concentration
(IC50) of doxorubicin against MCF-7 cells (mean ± SD,
n=3). |
Table I
The 50% inhibitory concentration
(IC50) of doxorubicin against MCF-7 cells (mean ± SD,
n=3).
| Concentration of
DOX (μg/ml) | OD value | IR (%) |
|---|
| 0 | 1.25±0.02 | |
| 0.05 | 1.10±0.03 | 12.0 |
| 0.1 | 0.95±0.02 | 24.0 |
| 0.2 | 0.82±0.03 | 34.4 |
| 0.4 | 0.71±0.01 | 43.2 |
| 0.8 | 0.60±0.01 | 52.0 |
| 1.6 | 0.48±0.02 | 61.2 |
Induction of doxorubicin resistance in
MCF-7 cells
MCF-7 cells were seeded into 96-well cell culture
plates at a density of 5×103 cells/well, and after the
cells were adherent to the plate wall, doxorubicin was added to
each well at concentrations of 0, 0.01, 0.03, 0.06 and 0.12 μg/ml.
Four replicate wells were used for each concentration, with a final
volume of 200 μl, and the plates were placed at 37°C in a
humidified atmosphere containing 5% CO2. The medium was
not replaced, and the OD value was read at 492 nm on an ELISA
microplate reader 7 days following the doxorubicin treatment. The
inhibitory rate of MCF-7 cells following doxorubicin treatment for
7 days was estimated according to the OD value, and cell morphology
was observed to aid in the determination of the appropriate
doxorubicin concentration for the induction of drug resistance.
Each experiment was repeated in triplicate.
MCF-7 cells were seeded onto cell culture flasks at
a density of 1×106 cells/well, and drug resistance was
induced starting at a doxorubicin concentration of 0.01 μg/ml after
the cells were adherent to the flask wall. Following incubation for
24 h, the supernatant was removed and the medium was replaced with
complete RPMI-1640 medium until cell recovery. Following trypsin
digestion, cells were harvested and seeded onto cell culture flasks
at a density of 1×106 cells/well. Following incubation
with doxorubicin at a final concentration of 0.02 μg/ml for 24 h,
the supernatant was removed and the medium was replaced with fresh
complete RPMI-1640 medium for the further induction of drug
resistance. The doxorubicin concentration was gradually increased
to 0.06 μg/ml. The MCF-7 cells treated with doxorubicin at final
concentrations of 0.01, 0.03 and 0.06 μg/ml were harvested for the
subsequent experiments.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR), sequencing and
quantitative real-time PCR (qRT-PCR)
The MCF-7 cells treated with doxorubicin at
concentrations of 0.01, 0.03 and 0.06 μg/ml were digested,
centrifuged and harvested. The total RNA was extracted from the
cells using the reverse-transcription PCR reagent (Fermentas, Glen
Burnie, MD, USA) according to the manufacturer’s instructions and
reverse-transcribed into cDNA. The primer sequences containing
EcoRI and KpnI cleavage sites were designed using the
software Primer version 5.0 (Premier Corporation, Canada).
The primers for CD44st-1 (GenBank accession
no: FJ216964; amplification fragment size, 1023 bp, used for RT-PCR
and gene sequencing) were as follows: forward primer,
5′-GGGAATTCATGGACAAGTTTTGGTGGCAC
G-3′ and reverse primer, 5′-GGGGTACCTTACACCCCAAT
CTTCATGTCC-3′. PCR amplification for CD44st-1 was performed
under the following conditions: incubation at 94°C for 5 min; 30
cycles at 94°C for 30 sec, at 65°C for 30 sec and at 72°C for 1
min; and extension at 72°C for 10 min. The MDR1 gene primers
(amplification fragment size, 173 bp) were as follows: forward
primer, 5′-GTTGCCATTGACTGAAAGA AC-3′ and reverse primer,
5′-ACAGGAGATAGGCTGGTTT GA-3′. PCR amplification for MDR1 was
performed under the following conditions: incubation at 94°C for 5
min; 30 cycles at 94°C for 30 sec, at 56°C for 30 sec and at 72°C
for 1 min; and extension at 72°C for 10 min. The NF-κB gene
primers (amplification fragment size, 340 bp) were as follows:
forward primer, 5′-AGCACAGATACCACCAAGACCC-3′ and reverse primer,
5′-CCCACGCTGCTCTTCTATAGGAAC-3′. PCR amplification for NF-κB
was performed under the following conditions: incubation at 94°C
for 5 min; 30 cycles at 94°C for 30 sec, at 61°C for 30 sec and at
72°C for 1 min; and extension at 72°C for 10 min. The MMP-2
gene primers (amplification fragment size, 162 bp) were as follows:
forward primer, 5′-CGG TGCCCAAGAATAGATG-3 and reverse primer,
5′-AAAGGA GAAGAGCCTGAAGTG-3′. PCR amplification for MMP-2
was performed under the following conditions: incubation at 94°C
for 5 min, 30 cycles at 94°C for 30 sec and at 59°C for 30 sec,
incubation at 72°C for 30 sec, and extension at 72°C for 10 min.
The MMP-9 gene primers (amplification fragment size, 682 bp)
were as follows: forward primer, 5′-CGGAGC ACGGAGACGGGTAT-3′ and
reverse primer, 5′-GCCGCC ACGAGGAAAACT-3′. PCR amplification for
MMP-9 was performed under the following conditions:
incubation at 94°C for 5 min; 30 cycles at 94°C for 30 sec, at 62°C
for 30 sec and at 72°C for 45 sec; and extension at 72°C for 10
min. The β-actin gene primers (amplification fragment size, 330
bp), which were used as a control, were as follows: forward primer,
5′-CTCGCGCTACTCTCTCTTTC-3′ and reverse primer,
5′-CATGTCTCGATCCCACTTAAC-3′. PCR amplification was performed under
the following conditions: incubation at 94°C for 5 min, followed by
30 cycles at 94°C for 30 sec, at 58°C for 30 sec, at 72°C for 30
sec and at 72°C for 10 min. The PCR amplification product of CD44st
was extracted and purified using the gel extraction and PCR
purification kit. Following T/A cloning, the purified product was
transformed into Escherichia coli, and the plasmid DNA was
extracted and validated using double digestion with EcoRI
and KpnI (New England Biolabs, Inc., Ipswich, MA, USA). The
positive plasmid was sequenced by Sangon Biotech Co., Ltd.
(Shanghai, China).
The CD44st-2 gene primers used for qRT-PCR
amplification (amplification fragment size, 682 bp) were as
follows: forward primer, 5′-CCCTGCTACCAGACACTCA-3′ and reverse
primer, 5′-TGTTCACCAAATGCACCAT-3′. The sequences of the
MDR1, NF-κB and β-actin gene primers were the same as
described above. qRT-PCR amplification was performed with a total
volume of 50 μl containing 32 μl of ddH2O, 5 μl of 10X
buffer, 1 μl of dNTP, 4 μl of MgCl2 (25 mmol/l), 2 μl of
the forward and reverse primers (10 pmol/l), 2 μl of cDNA template
and 2 μl of Taq DNA polymerase (Takara Bio, Co., Ltd.,
Dalian, China) under the following conditions: incubation at 94°C
for 5 min; 40 cycles at 94°C for 30 sec, at 60°C for 1 min and at
72°C for 45 sec; and incubation at 72°C for 10 min. The relative
level of mRNA expression was normalized to β-actin, and the
difference in mRNA expression was estimated using the
2−ΔΔCt method.
Western blot analysis
CD44 and NF-κB protein expression was determined
using western blot analysis. Briefly, 1×106 MCF-7 cells
treated with doxorubicin at various concentrations were transferred
to Eppendorf tubes, and 100 μl of boiling 2X loading buffer was
added to each tube. The cell lysates were collected and boiled at
100°C for 5 min for protein denaturation. The NF-κB protein was
extracted using the Nuclear/Cytosol Fractionation kit following the
manufacturer’s instructions, electrophoresed, transferred to
membranes, incubated with antibodies, and stained with
3,3′-diaminobenzidine (DAB; Invitrogen).
P-gp expression as detected by flow
cytometry
An estimated 5×105 MCF-7 cells treated
with doxorubicin at various concentrations were harvested and
marked. After being washed three times with phosphate-buffered
saline (PBS), the harvested cells were blown uniformly in PBS
buffer to prepare a single-cell suspension. A volume of 20 μl of
the cell suspension was collected, and 1 μl of FITC-conjugated
mouse anti-human CD44 monoclonal antibody was added (eBioscience,
San Diego, CA, USA). Additionally, 20 μl of PE-labeled P-gp
monoclonal antibody (BD, San Jose, CA, USA) was transferred to the
cell suspension for the determination of P-gp expression. The cells
were incubated in darkness at room temperature for 30 min and
centrifuged at 1,000 rpm for 5 min. The supernatant was then
discarded, and the cells were washed twice with PBS solution and
re-suspended with 500 μl of PBS solution. The relative fluorescence
intensity was detected with a flow cytometer (BD), and data
analyses were performed using the CellQuest software. The
experiment was repeated in triplicate.
P-gp activity as detected by
Rhodamine-123 (Rho-123) retention assay
An estimated 2×105 MCF-7 cells treated
with doxorubicin at various concentrations were harvested, washed
once with aseptic PBS solution at 37°C, and added to fresh medium.
Fluorescent dye Rho-123 was added to each flask to achieve a final
concentration of 1 μg/ml, and the flasks were incubated for another
2 h. Cells were harvested using the conventional method and washed
twice with PBS. After removal of the extracellular Rho-123, 5 μg
(50 μl) of propidium iodide (PI) was added to the cells. The
solution was incubated in darkness at room temperature for 30 min
and centrifuged at 1,000 rpm for 5 min. The supernatant was then
discarded, and the cells were washed twice with PBS solution and
blown gently with PBS solution. The P-gp activity was detected with
a flow cytometer (BD), and data analyses were performed using the
CellQuest software. The experiment was repeated in triplicate.
Electrophoretic mobility shift assay
(EMSA)
An estimated 6×106 MCF-7 cells treated
with doxorubicin at various concentrations were harvested, and 300
μl of plasmosin extraction solution buffer A was added. Then, the
solution was stilled on ice for 15 min and centrifuged at 2,000 rpm
for 10 min at 4°C. The supernatant was discarded, and the sediment
was washed with 100 μl of buffer A and then centrifuged at 5,000
rpm for 10 min at 4°C. After the supernatant was discarded, the
sediment was mixed evenly with 100 μl of buffer B, stilled on ice
for 10 min, mixed evenly, and stilled on ice for 10 min again.
Following centrifugation at 15,000 rpm for 20 min at 4°C, the
supernatant was collected, which served as the nuclear protein
extraction solution. The nuclear protein concentration was
determined using the Bradford method, and 2 μl of the nuclear
protein was stored at −70°C. The sequence of the NF-κB DNA probe
was as follows: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ and
5′-GCCTGGGAAAGTCCCCTACT-3′. Probe labeling, the binding reaction,
electrophoresis and membrane transferring were performed according
to the manufacturer’s instructions (Promega, Madison, WI, USA), and
the DNA was labeled with chemiluminescent biotin. The membrane was
placed in the film cassette and visualized using X-rays. The
exposure time was adjusted to obtain the appropriate result.
Gelatin zymography
Cells were divided into five groups. MCF-7 cells in
group 1 did not receive any treatment; MCF-7 cells in group 2 were
treated with doxorubicin at a concentration of 0.06 μg/ml; MCF-7
cells in group 3 were treated with 0.06 μg/ml doxorubicin and HA
following pretreatment with BMS-345541 (Merck, Whitehouse Station,
NJ, USA); MCF-7 cells in group 4 were treated with 0.06 μg/ml
doxorubicin and HA; and MCF-7/Adr cells in group 5 were treated
with HA. An estimated 1.5×106 cells in each group were
seeded into cell culture flasks, and 1.5 ml of serum-free RPMI-1640
medium was transferred to the flasks. HA (Sigma-Aldrich
Corporation, St. Louis, MO, USA) was boiled at 100°C for 5 min
prior to use to eliminate the potential contamination by other
growth factors. MCF-7 cells in the BMS-345541 pretreatment group
were pretreated with BMS-345541 at a concentration of 5 μmol/l for
3 h, while cells in groups 3, 4 and 5 were treated with HA at a
concentration of 100 μg/ml, and the cells following incubation in
HA for 24 h were harvested for RT-PCR amplification and the
Transwell invasion assay. Following centrifugation, the supernatant
of the cell culture was used for gelatin zymography following the
manufacturer’s protocol (Applygen Technologies Inc., Beijing,
China). A 1:1 ratio of whole human blood and 2X non-denaturing
SDS-PAGE loading buffers (both 100 μl) served as the positive
control.
Invasive ability of MCF-7 cells as
detected by the Transwell invasion assay
Matrigel (BD) was diluted with serum-free RPMI-1640
medium at a ratio of 1:1 and then transferred to a Transwell
(Corning Inc., Corning, NY, USA) at a low temperature at a
concentration of 150 μl/chamber. The Transwell was then placed at
37°C for 1 h. After the Matrigel polymerized, 1 ml of serum-free
RPMI-1640 medium containing 0.1% FBS was transferred to the lower
compartment of the Transwell for a 1-h incubation period. Cells in
each of the aforementioned 5 groups were prepared as cell
suspensions at a concentration of 1×105 cells/ml, and
200 μl of the cell suspension was transferred to the upper
compartment of the Transwell and incubated for 24 h. The Matrigel
on the surface of the polycarbonate membrane was lightly wiped with
cotton buds, and the upper and lower sides of the polycarbonate
membrane were gently flushed with PBS solution. The polycarbonate
membrane was dried in a shady environment, stained with 0.1%
crystal violet for 10 min, destained and visualized under an
optical microscope. The cells that had invaded the polycarbonate
membrane were counted in each of five random fields using optical
microscopy (×200). The invasive capability of the tumor cells was
determined by averaging the number of positively stained cells in
each of the microscopic fields. The experiment was repeated in
triplicate.
Statistical analysis
All data are presented as the means ± standard
deviation (SD), and all statistical analyses were performed using
the statistical software SPSS version 16.0 (SPSS Inc., Chicago, IL,
USA). To test for statistical significance, experimental data were
analyzed by one-way analysis of variance (ANOVA) and the
SNK-q test. A P-value of <0.05 was considered to indicate
a statistically significant result.
Results
IC50 value of doxorubicin
against MCF-7 cells and the doxorubicin concentration for the
induction of drug resistance
Doxorubicin treatment for 48 h significantly
inhibited the growth of the MCF-7 cells, and the IC50
value of doxorubicin against MCF-7 cells was 0.68±0.04 μg/ml. The
OD values and inhibitory rates of MCF-7 cells following doxorubicin
treatment are shown in Table I.
Treatment with doxorubicin at concentrations of 0.01, 0.03, 0.06
and 0.12 μg/ml for 7 days achieved significant inhibition of the
growth of MCF-7 cells compared with the growth of cells that did
not receive doxorubicin treatment (P<0.05). The MCF-7 cells
treated with doxorubicin at concentrations of 0.03 and 0.06 μg/ml
for 7 days only developed mild swelling, with a good cell state,
while the cells treated with 0.12 μg/ml doxorubicin for 7 days
exhibited a poor state with obvious swelling and apparent cell
debris. Therefore, doxorubicin at a concentration of 0.06 μg/ml was
used for the subsequent induction of drug resistance (Table II).
 | Table IIInhibitory effect of doxorubicin on
the growth of MCF-7 cells (mean ± SD, n=3). |
Table II
Inhibitory effect of doxorubicin on
the growth of MCF-7 cells (mean ± SD, n=3).
| Concentration of
DOX (μg/ml) | OD value | IR (%) |
|---|
| 0 | 1.50±0.02 | - |
| 0.01 | 1.44±0.03a | 4.0 |
| 0.03 | 1.37±0.02a | 8.7 |
| 0.06 | 1.31±0.01a | 12.7 |
| 0.12 | 1.18±0.03a | 21.3 |
Changes in MDR1, CD44st and NF-κB mRNA
expression in the MCF-7 cells following doxorubicin-induced drug
resistance
Low expression of MDR1, CD44st and
NF-κB mRNA in MCF-7 cells was revealed by the qRT-PCR assay,
and the mRNA expression of these genes gradually increased in the
MCF-7 cells in a dose-dependent manner following treatment with
doxorubicin at concentrations of 0.01, 0.03 and 0.06 μg/ml. There
were significant differences in the expression of MDR1,
CD44st and NF-κB mRNA between the doxorubicin-treated
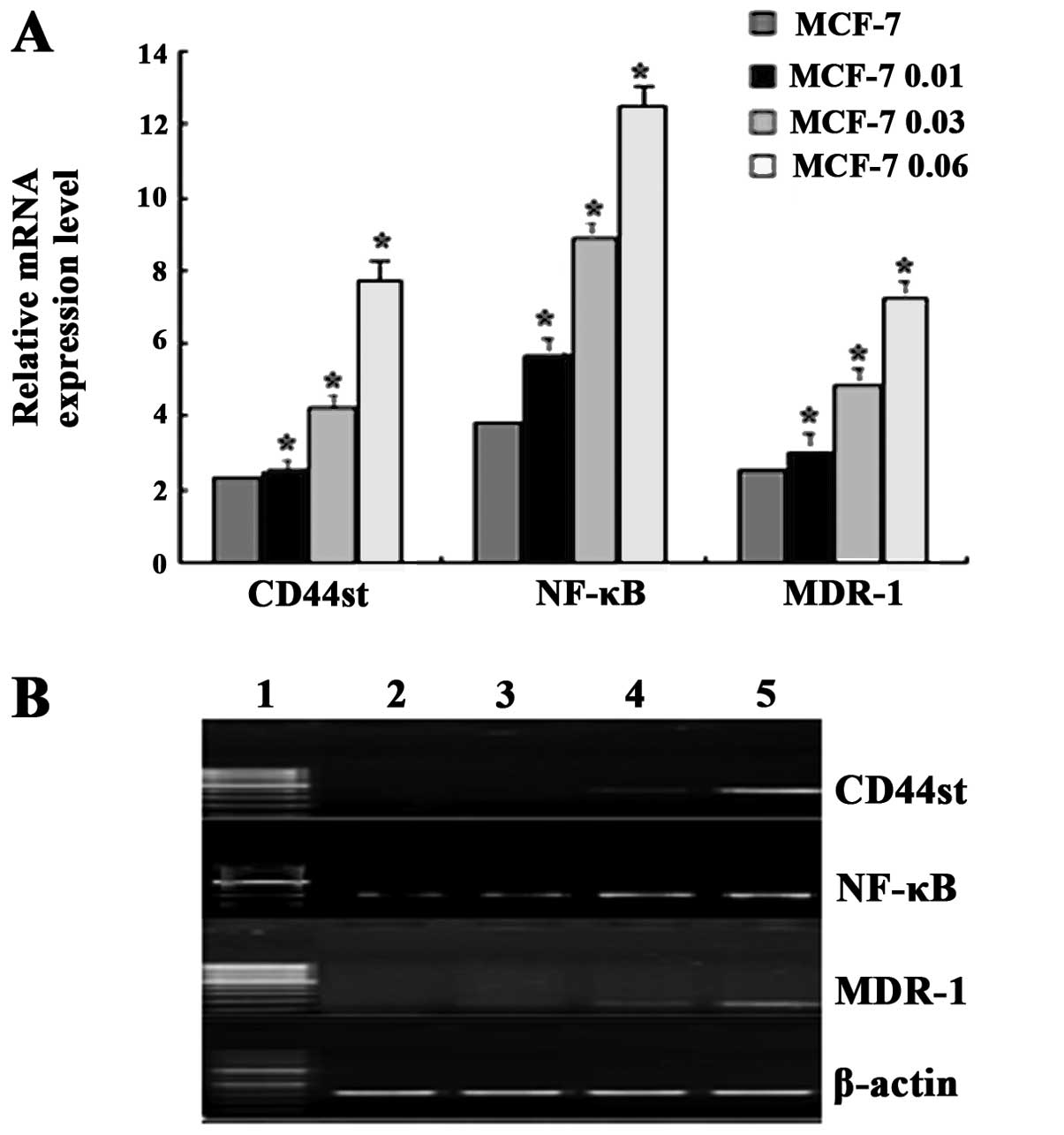
MCF-7 cells and the untreated MCF-7 cells (Fig. 1A) (P<0.05). Semi-quantitative
reverse transcription-PCR amplification results were similar to the
results obtained by qRT-PCR (Fig.
1B). Sequencing revealed that the CD44st gene sequence was
consistent with the sequence of the gene we identified previously
(GenBank accession no: FJ216964).
Changes in the percentage of MCF-7 cells
positive for P-gp following doxorubicin treatment
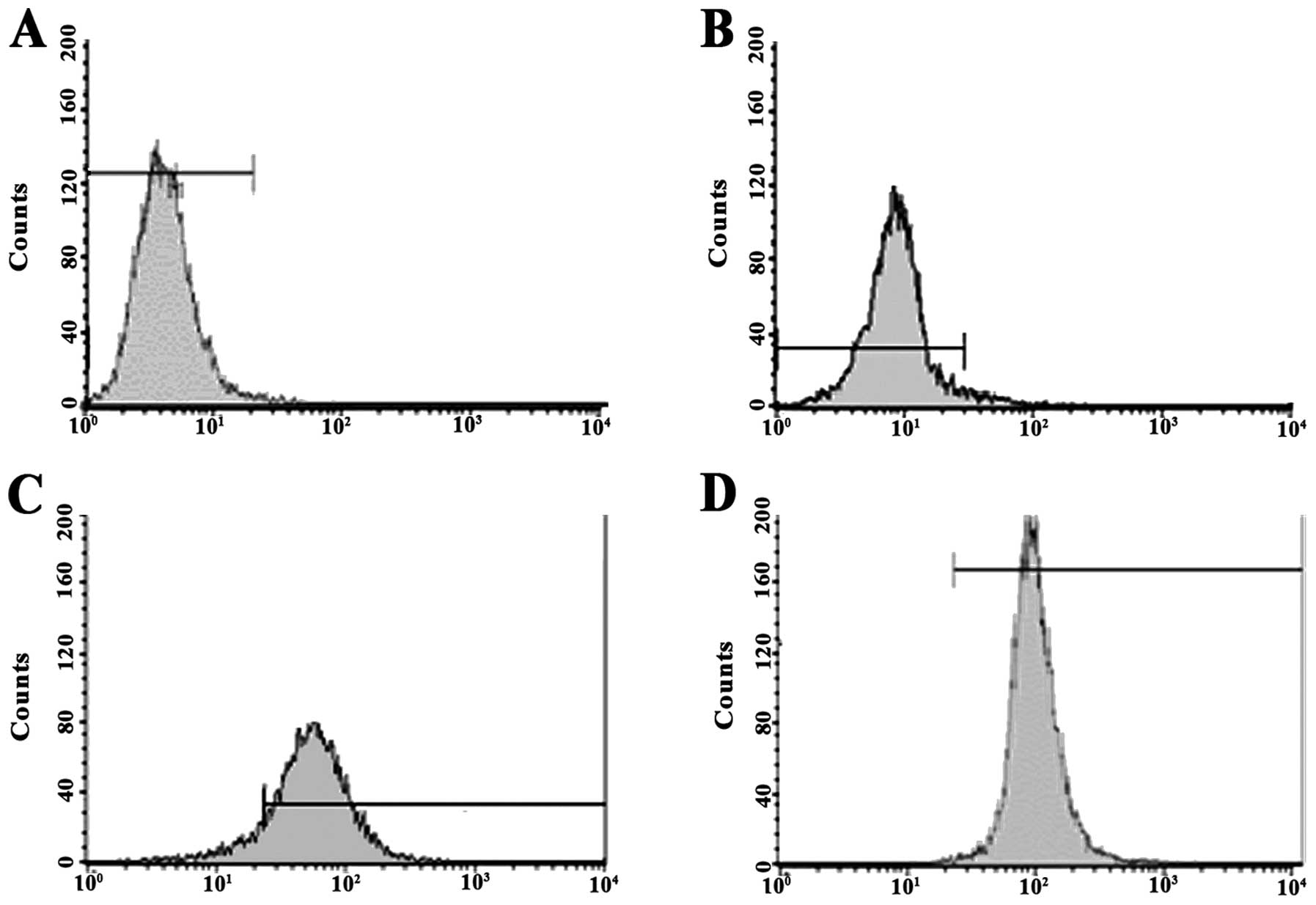
Flow cytometry revealed that the percentages of
P-gp-positive MCF-7 cells were 5.61±0.52, 11.53±0.46, 30.55±1.62
and 61.12±2.05% in the untreated MCF-7 cells and cells treated with
doxorubicin at concentrations of 0.01, 0.03 and 0.06 μg/ml,
respectively. Significant differences were detected in the
percentage of P-gp-positive MCF-7 cells between the groups
(P<0.05) (Fig. 2).
Changes in Rho-123 efflux in MCF-7 cells
following doxorubicin-induced drug resistance
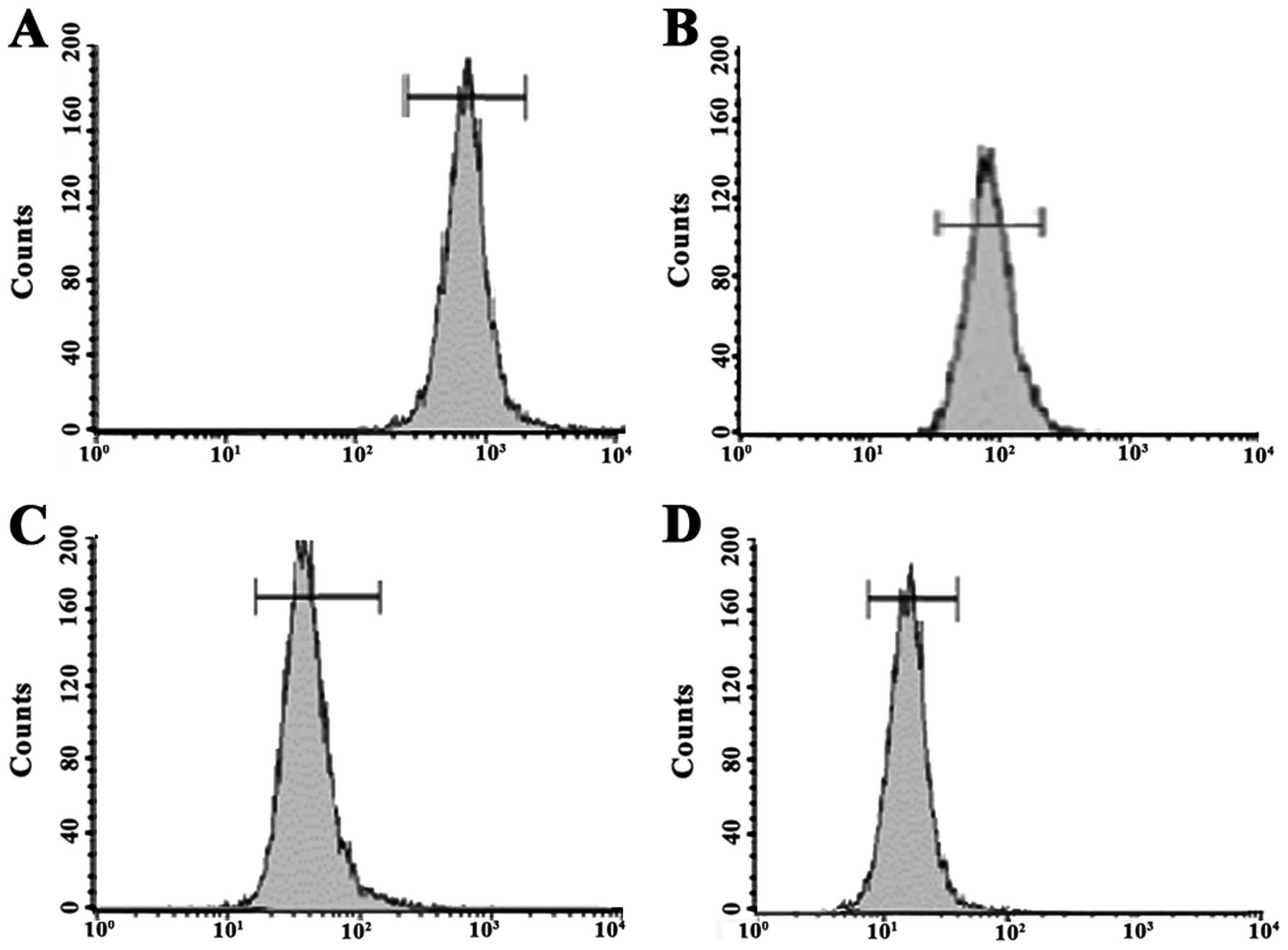
The Rho-123 retention assay revealed that the
fluorescence intensities of Rho-123 were 451.36±15.43,
326.07±10.36, 153.56±7.83 and 78.84±5.61 in the MCF-7 and MCF-7
cells treated with doxorubicin at concentrations of 0.01, 0.03 and
0.06 μg/ml, respectively. There were significant differences
observed in the fluorescence intensity of Rho-123 between groups
(P<0.05). The fluorescence intensity of Rho-123 was gradually
reduced in the MCF-7 cells, with or without doxorubicin treatment,
with increased doxorubicin concentration, and the intracellular
fluorescence intensity exhibited an inverse relationship with the
percentage of P-gp-positive cells (Fig.
3).
Changes in CD44 and NF-κB protein
expression and NF-κB DNA-binding activity in MCF-7 cells following
doxorubicin-induced drug resistance
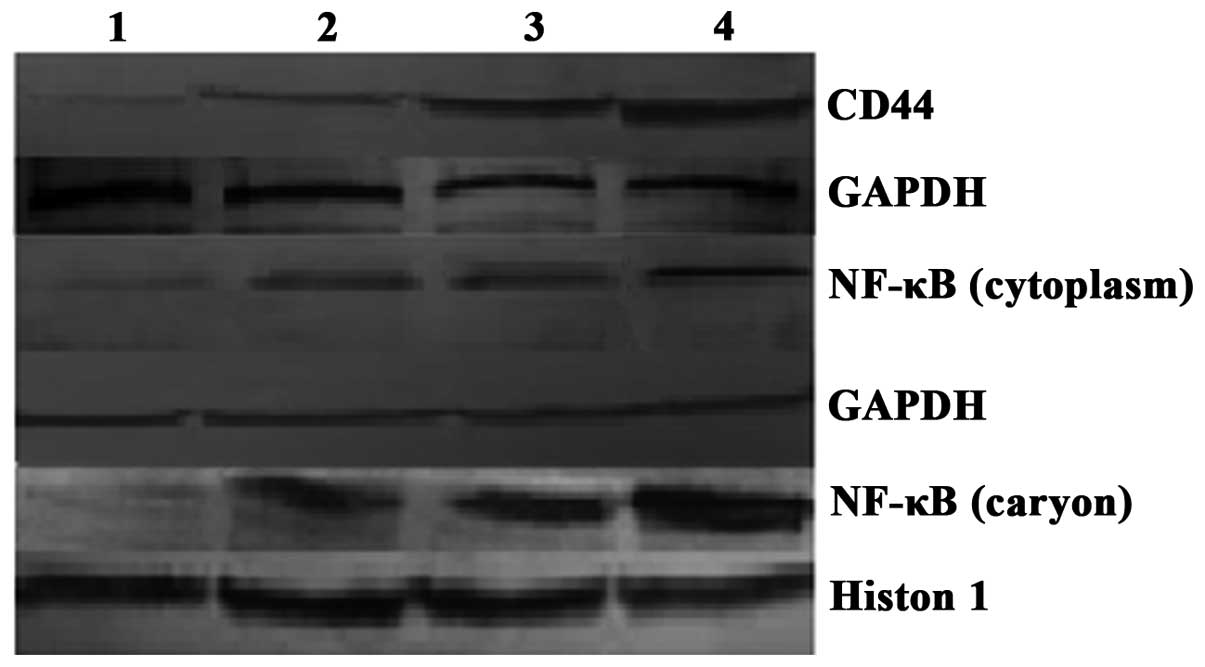
Western blot analysis revealed that CD44 and NF-κB
protein expression was correlated with CD44 and NF-κB
mRNA expression, and dose-dependent CD44 protein expression was
gradually detected in the MCF-7 cells following treatment with
doxorubicin at concentrations of 0.01, 0.03 and 0.06 μg/ml. NF-κB
protein expression was found to gradually increase, and
intranuclear transfer increased (Fig.
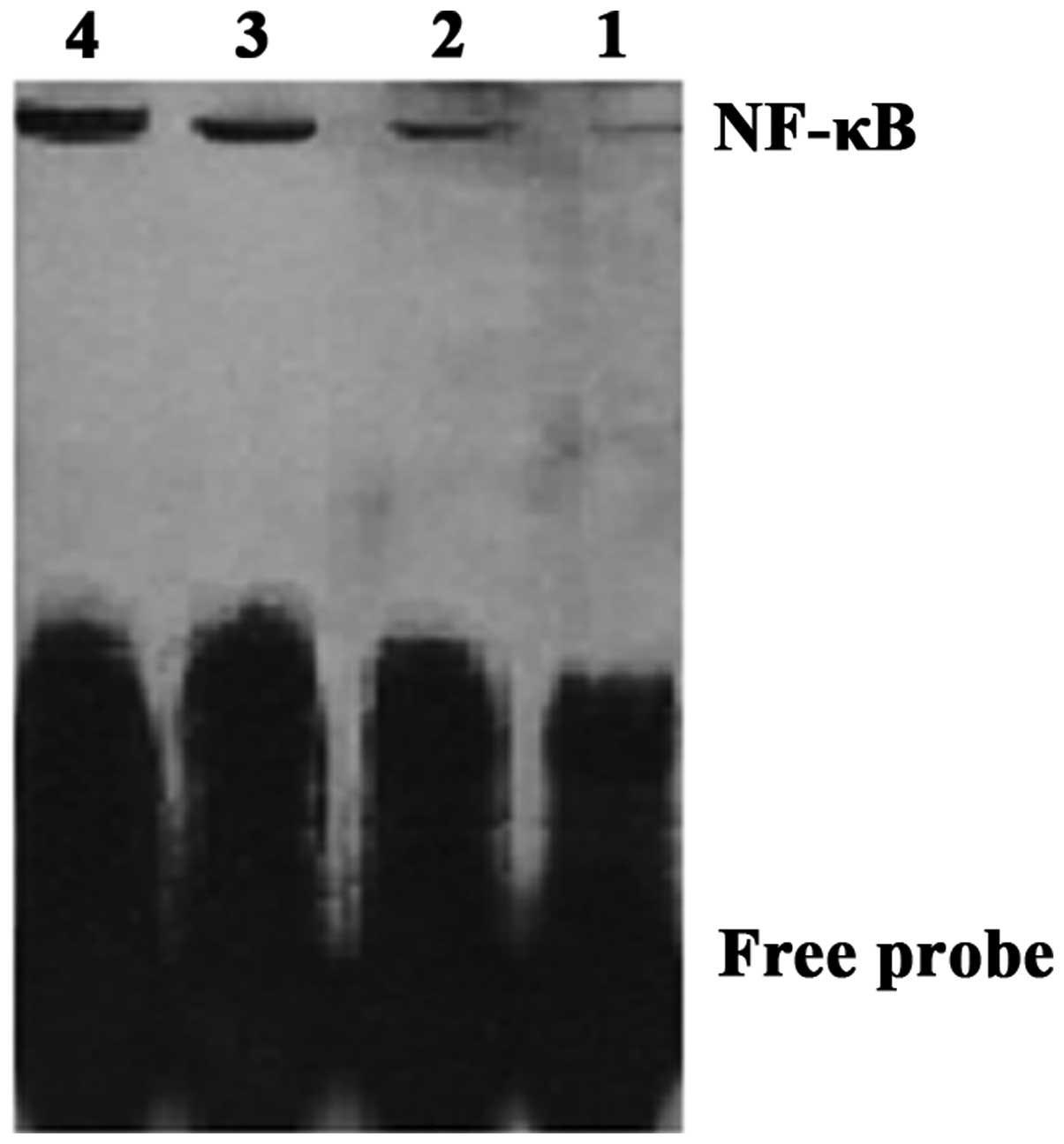
4). EMSA showed that the NF-κB DNA-binding activity was
enhanced in a dose-dependent manner with increasing concentrations
of doxorubicin (Fig. 5).
Changes in MMP-2 and MMP-9 expression in
MCF-7 cells following treatment with 0.06 μg/ml doxorubicin and
HA
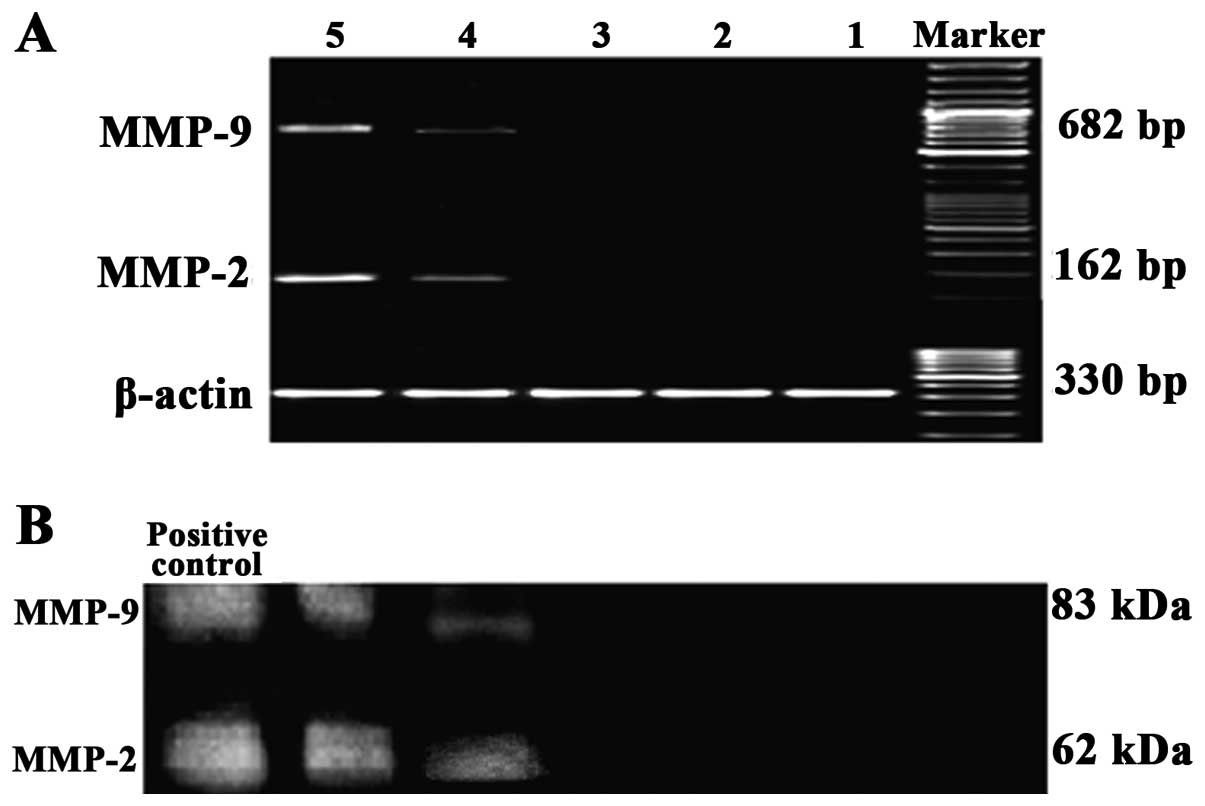
RT-PCR and gelatin zymography revealed no MMP-2 or
MMP-9 expression in the supernatants of the cultures of the MCF-7
cells, the MCF-7 cells treated with 0.06 μg/ml doxorubicin, and the
MCF-7 cells treated with 0.06 μg/ml doxorubicin and HA following
pretreatment with BMS-345541, while MMP-2 and MMP-9 were highly
expressed in the MCF-7 cells treated with 0.06 μg/ml doxorubicin
and HA, as well as in the MCF-7/Adr cells treated with HA. These
findings demonstrated that HA induced the secretion of MMP-2 and
MMP-9 in the MCF-7 cells treated with 0.06 μg/ml doxorubicin, which
could be blocked by BMS-345541 treatment (Fig. 6).
Changes in the invasive ability of MCF-7
cells following treatment with 0.06 μg/ml doxorubicin and HA
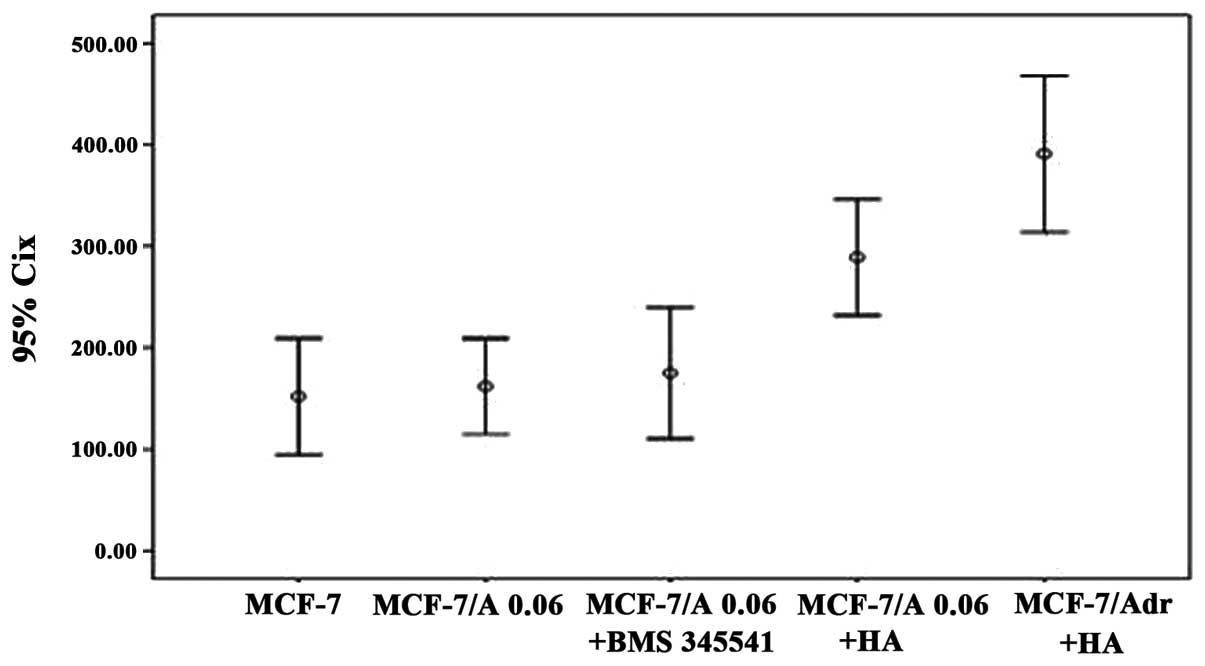
Transwell invasion assays revealed that the number
of MCF-7 cells following treatment with 0.06 μg/ml doxorubicin and
HA (289±23 cells/field) and the number of MCF-7/Adr cells treated
with HA (391±31 cells/field) that invaded the Transwell were
significantly higher than those of the MCF-7 cells (153±23
cells/field), the 0.06 μg/ml doxorubicin-treated MCF-7 cells
(162±19 cells/field), and the MCF-7 cells treated with 0.06 μg/ml
doxorubicin and HA following pretreatment with BMS-345541 (175±26
cells/field) (P<0.05). The number of MCF-7 cells that invaded
the Transwell following treatment with 0.06 μg/ml doxorubicin and
HA was significantly lower than that of the MCF-7/Adr cells treated
with HA in other groups (P<0.05), while no significant
differences were observed in the numbers of MCF-7 cells, the MCF-7
cells treated with 0.06 μg/ml doxorubicin, and the MCF-7 cells
treated with 0.06 μg/ml doxorubicin and HA following pretreatment
with BMS-345541 (P>0.05). These findings showed that HA
increased the invasive ability of the MCF-7 cells treated with 0.06
μg/ml doxorubicin, which could be blocked by pretreatment with
BMS-345541 (Fig. 7).
Discussion
Our findings indicated that MCF-7 cells subjected to
low-dose exposure to doxorubicin may develop multidrug resistance
to doxorubicin, and the expression of MDR1, CD44st
and NF-κB mRNA and MDR1, CD44 and NF-κB protein was
upregulated in a dose-dependent manner during the induction of drug
resistance in MCF-7 cells. In addition, HA pretreatment increased
the secretion of MMP-2 and MMP-9 in the multidrug-resistant MCF-7
cells and affected the invasive ability of MCF-7 cells, which could
be blocked by the NF-κB-specific inhibitor BMS-345541. The present
study demonstrated that the transcription factor NF-κB is involved
in tumor MDR and invasion and plays a critical role in inducing
CD44st expression. It is possible that the detection of this
molecular target may play an important role in predicting tumor
invasion and drug resistance and that the NF-κB-specific inhibitor
plays a critical role in inhibiting tumor invasion and drug
resistance.
The major causes of tumor treatment failure involve
tumor metastasis and MDR. It has been reported that MDR is closely
associated with tumor metastasis and invasion. MMPs were found to
be involved in ECM degradation and remodeling (12–15).
It has been shown that MMP-2 and MMP-9 are involved in the invasion
of breast cancer, and they may be potential cancer markers. In
patients with basal-like breast cancer, the activity of precursor
and active forms of MMP-2 and MMP-9 was found to significantly
increase with each advancing clinical stage of disease, and the
activity of the precursor and active forms of MMP-9 in tumor tissue
showed a positive association with tumor size. In addition,
patients with lymph node involvement had higher activity of
precursor and active forms of MMP-2 and the active form of MMP-9
than node-negative patients, and patients with steroid
receptor-negative tumors had enhanced MMP-2 and MMP-9 activity
compared with patients with luminal A tumors (16).
CD44 is involved in multiple human
pathophysiological processes. The binding of various abnormally
expressed CD44 molecules to the receptor HA promotes the secretion
of MMPs, resulting in ECM degradation, which is closely associated
with axillary lymph node metastasis, increased angiogenesis,
prognosis and drug resistance (17–22).
It has been shown that HA binds to the CD44 variant that is widely
distributed on the surface of the cell membrane during tumor
progression, which induces the activation of ankyrin and small
G-protein Rho, and the activation of the PI3K-AKT signaling
pathway, thereby leading to enhanced tumor cell adhesion, growth,
migration and invasion, and resultant tumor progression (23). HA is found to induce the elevation
of MMP expression in breast cancer cell lines, which confers
enhanced CD44 cleavage and cell migration during the invasion
process (17).
NF-κB was found to be involved in the regulation of
the production of MDR1 and CD44 genes in multiple
types of cancer cells. It was reported that the MAPK/NF-κB pathway
mediates doxorubicin-induced P-gp expression in MCF-7/Adr cells and
subsequently alters the cellular pharmacokinetics of doxorubicin
(3). Paracrine signaling through
the receptor activator of the NF-κB (RANK) pathway was reported to
promote the development of mammary stem cells and breast cancer,
and high levels of RANK were found in human primary breast
adenocarcinomas that lack expression of the hormone receptors
estrogen and progesterone (triple-negative breast adenocarcinomas)
and in tumors with a high pathological grade and proliferation
index; in addition, high RANK/RANKL expression was significantly
associated with metastatic tumors. Together, the data indicate that
RANK promotes tumor initiation, progression and metastasis in human
mammary epithelial cells by increasing the population of
CD44+CD24− cells, thereby affecting the
prognosis of breast cancer patients (24).
Results recently indicate that
CD44+CD24− cells are a type of tumor stem
cells in breast cancer tissues, which are usually termed
triple-negative breast adenocarcinoma cells (25,26).
The CD44+CD24− phenotype was found to be
significantly associated with lymph node involvement, progesterone
receptor status in patients with recurrent or metastatic tumors,
the phenotype of triple-negative breast cancer, and disease-free
and overall survival of patients. It was therefore considered that
the CD44+CD24− phenotype may be an important
factor associated with malignant relapse following surgical
resection and chemotherapy in patients with invasive ductal
carcinoma (22).
It has been shown in our clinical trials that the
CD44st gene was highly expressed in breast cancer tissues,
and it plays a critical role in breast cancer development and
progression, which may be associated with breast cancer invasion,
metastasis and prognosis (27).
Taken together, these findings demonstrated that anti-CD44
treatment may inhibit cancer cell metastasis and improve the
prognosis of breast cancer patients with high CD44st
expression.
The present study revealed that expression levels of
MDR1, CD44st and NF-κB mRNA and MDR1,
CD44 and NF-κB protein were upregulated in a dose-dependent manner
in MCF-7 cells with doxorubicin-induced drug resistance, and HA
pretreatment increased MMP-2 and MMP-9 expression in the
multidrug-resistant MCF-7 cells and affected the invasive ability
of MCF-7 cells, which could be blocked by an NF-κB-specific
inhibitor. In addition, the NF-κB-specific inhibitor was found to
play a critical role in inhibiting tumor invasion and drug
resistance. It is suggested that CD44st, a molecular marker, may
play an important role in MDR and the invasive ability of breast
cancer cells.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang XJ, Jiang H, Zhao XP and Jiang WM:
The role of a new CD44st in increasing the invasion capability of
the human breast cancer cell line MCF-7. BMC Cancer. 11:2902011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Lu M, Zhou F, Sun H, Hao G, Wu X
and Wang G: Key role of nuclear factor-κB in the cellular
pharmacokinetics of adriamycin in MCF-7/Adr cells: the potential
mechanism for synergy with 20(S)-ginsenoside Rh2. Drug Metab
Dispos. 40:1900–1908. 2012.
|
|
4
|
Sun J, Yeung CA, Co NN, et al: Clitocine
reversal of P-glycoprotein associated multi-drug resistance through
down-regulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012.PubMed/NCBI
|
|
5
|
Xia Q, Wang ZY, Li HQ, et al: Reversion of
p-glycoprotein-mediated multidrug resistance in human leukemic cell
line by diallyl trisulfide. Evid Based Complement Alternat Med.
2012:7198052012.PubMed/NCBI
|
|
6
|
Damm S, Koefinger P, Stefan M, et al:
HGF-promoted motility in primary human melanocytes depends on
CD44v6 regulated via NF-kappa B, Egr-1, and C/EBP-beta. J Invest
Dermatol. 130:1893–1903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu Y, Zhang X, Zhou H, et al: Factor VIIa
regulates the expression of caspase-3, MMP-9, and CD44 in SW620
colon cancer cells involving PAR2/MAPKs/NF-κB signaling pathways.
Cancer Invest. 31:7–16. 2013.PubMed/NCBI
|
|
8
|
Bourguignon LY, Peyrollier K, Xia W and
Gilad E: Hyaluronan-CD44 interaction activates stem cell marker
Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cells. J Biol Chem.
283:17635–17651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bourguignon LY, Spevak CC, Wong G, Xia W
and Gilad E: Hyaluronan-CD44 interaction with protein kinase Cɛ
promotes oncogenic signaling by the stem cell marker Nanog and the
production of microRNA-21, leading to down-regulation of the tumor
suppressor protein PDCD4, anti-apoptosis, and chemotherapy
resistance in breast tumor cells. J Biol Chem. 284:26533–26546.
2009.
|
|
10
|
Fang XJ, Xu WL, Gong JL, Chen C, Fang LL
and Chen QY: CD44 variant increases the invasive ability of human
breast cancer cell line MCF-7 cells. Zhonghua Zhong Liu Za Zhi.
32:22–28. 2010.(In Chinese).
|
|
11
|
Fang XJ, Wu XL, Zhang X, Qian H, Chen C,
Fang LL and Chen QY: Construction of human CD44 eukaryotic
expression vector and its expression in mammary carcinoma cells. J
Jiangsu Univ Med Ed. 19:102–106. 2009.
|
|
12
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuang YH, Chen X, Su J, et al: RNA
interference targeting the CD147 induces apoptosis of multi-drug
resistant cancer cells related to XIAP depletion. Cancer Lett.
276:189–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q,
Yang JM and Xu ZD: Up-regulation of CD147 and matrix
metalloproteinase-2, -9 induced by P-glycoprotein substrates in
multidrug resistant breast cancer cells. Cancer Sci. 98:1767–1774.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weinstein RS, Jakate SM, Dominguez JM, et
al: Relationship of the expression of the multidrug resistance gene
product (P-glycoprotein) in human colon carcinoma to local tumor
aggressiveness and lymphnode metastasis. Cancer Res. 51:2720–2726.
1991.PubMed/NCBI
|
|
16
|
Radenkovic S, Konjevic G, Jurisic V, et
al: Values of MMP-2 and MMP-9 in tumor tissue of basal-like breast
cancer patients. Cell Biochem Biophys. 68:143–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kung CI, Chen CY, Yang CC, Lin CY, Chen TH
and Wang HS: Enhanced membrane-type 1 matrix metalloproteinase
expression by hyaluronan oligosaccharides in breast cancer cells
facilitates CD44 cleavage and tumor cell migration. Oncol Rep.
28:1808–1814. 2012.
|
|
18
|
Golshani R, Lopez L, Estrella V, Kramer M,
Iida N and Lokeshwar VB: Hyaluronic acid synthase-1 expression
regulates bladder cancer growth, invasion, and angiogenesis through
CD44. Cancer Res. 68:483–491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu R, Wang X, Chen GY, Dalerba P, Gurney
A, Hoey T, Sherlock G, Lewicki J, Shedden K and Clarke MF: The
prognostic role of a gene signature from tumorigenic breast-cancer
cells. N Engl J Med. 356:217–226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Charafe-Jauffret E, Ginestier C and
Birnbaum D: Breast cancer stem cells: tools and models to rely on.
BMC Cancer. 9:2022009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park D, Kim Y, Kim H, et al: Hyaluronic
acid promotes angiogenesis by inducing RHAMM-TGFβ receptor
interaction via CD44-PKCδ. Mol Cells. 33:563–574. 2012.
|
|
22
|
Lin Y, Zhong Y, Guan H, Zhang X and Sun Q:
CD44+/CD24− phenotype contributes to
malignant relapse following surgical resection and chemotherapy in
patients with invasive ductal carcinoma. J Exp Clin Cancer Res.
31:592012.
|
|
23
|
Bourquiqnon LY: Hyaluronan-mediated CD44
activation of RhoGTPase signaling and cytoskeleton function
promotes tumor progression. Semin Cancer Biol. 18:251–259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palafox M, Ferrer I, Pellegrini P, Vila S,
Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Muñoz P,
Viñals F, Tometsko M, Branstetter D, Dougall WC and González-Suárez
E: RANK induces epithelial-mesenchymal transition and stemness in
human mammary epithelial cells and promotes tumorigenesis and
metastasis. Cancer Res. 72:2879–2888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stem cell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong JL, Xu WL, Li J, Yang J, Wu XJ and
Chen QY: Expression of the new CD44 variant (CD44v17) and its
clinical significance in breast cancer tissues. J Jiangsu Univ Med
Ed. 20:248–252. 2010.
|