Introduction
Histone modifications have been reported to include
acetylation, phosphorylation, methylation, ADP ribosylation and
ubiquitination (1). Histone
methylation indicates the methylation on lysine (K) and arginine
residues on the N-terminal of histones H3 and H4. Histone tail
modifications can be markers for both active and repressed
chromatin and are also interdependent. For histone lysine
methylation, there are currently five described positions in the
N-terminal of histone H3 (K4, K9, K27 and K36) and histone H4K20.
Methylation of histone H3 lysine 4 (H3K4), H3K36 and H3K79 are
mainly correlated with transcriptional stimulation, whereas
methylation of H3 lysine 9 (H3K9), H3K27 and H4K20 comprise
imprints for transcriptionally silent chromatin (2,3).
Histone methylation, especially tri-methyl modification, was once
believed to be irreversible, and to have an impact on long-term
epigenetic memory. Yet, recent studies have discovered several
demethylases, such as LSD-1, LSD-2 and JHDM1, which can demethylate
histone and cause epigenetic changes (4–6).
Histone methylation on lysines is important for
transcriptional silence. Suv39H1 is the first described histone
methyltransferase (HMT) which is the mammalian homologues of S.
pombe Clr4 and Drosophila Su (Var) 39, two proteins that
are involved in silencing by pericentric heterochromatin (7–10). The
Suv39H1 can be classified as the major H3K9 tri-methylating enzyme
which appears to use an H3K9 0 monomethylated residue as the in
vivo substrate. This interpretation is supported by in
vitro HMT assays indicating a much higher preference for H3K9
monomethylated substrates (11).
Suv39H1 may be involved in tumorigenesis. The
PML-RARα fusion protein causes acute promyelocytic leukemia (PML),
presumably via redistribution of the Suv39H1 to PML bodies and
perturbed histone lysine methylation at RARα target promoters
(12). Other examples include
association of heterochromatic HMTs with the tumor suppressor
retinoblastoma protein (RB) (13).
Furthermore, the SET domain genes EZH2 and MLL1 are involved in the
progression of prostate cancer and mixed-lineage leukemia (14,15).
Chakraborty et al reported that Suv39H1 methylated H3K9 and
affected DNA transcriptional activity through interaction with the
nut region of AML protein, leading to the development of acute
myeloid leukemia (16).
Aberrant histone methylation has not been well
characterized in human disease, especially in gastric carcinoma.
The function of Suv39H1 is still not well known. In the present
study, we investigated the expression of Suv39H1 and histone
methylation in gastric carcinoma, and evaluated the correlation
between Suv39H1, histone methylation and clinicopathological
features, and prognosis in gastric carcinoma. Furthermore, we
applied the siRNA technique to deplete Suv39H1 and measure
consequent histone modification, gene transcription, cell
proliferation and apoptosis in MGC-803 cells.
Materials and methods
Collection of patient samples and
data
Ethics statement
The present study consisted of a total of 175
gastric carcinoma patients who underwent curative surgical
resection at the Department of Surgery of Zhangzhou Affiliated
Hospital of Fujian Medical University between January 2001 and
December 2011. The surgically removed tissue samples were collected
from all participants who had signed an informed consent form
indicating their willingness to participate. Experimental
procedures were approved by the Ethics Committee of Zhangzhou
Affiliated Hospital of Fujian Medical University.
Pathological stage was determined by the
tumor-node-metastasis (TNM) classification. Gastric carcinoma was
classified according to the degree of differentiation, depth of
infiltration, lymphatic invasion and TNM stage as described in
Table I. Patient samples from
chronic superficial gastritis (n=30), chronic atrophic gastritis
(n=30) and moderate-severe atypical hyperplasia (n=30) were used as
control.
 | Table IClinical significance of Suv39H1
expression in the gastric carcinoma cases (n=175). |
Table I
Clinical significance of Suv39H1
expression in the gastric carcinoma cases (n=175).
| Suv39H1 protein
expression | | |
|---|
|
| | |
|---|
| Group | Positive | Negative |
χ2/t | P-value |
|---|
| Gender
(Male/Female) | 97/30 | 36/12 | 0.036 | >0.05 |
| Body mass index
(BMI) | 20.1±3.4 | 21.4±3.6 | 2.09 | 0.37 |
| Age (years) | 60.5±12.3 | 57.1±9.8 | 2.04 | 0.34 |
| Pathological
type | | | 0.493 | >0.05 |
| Papillary
adenocarcinoma | 14 | 6 | | |
| Tubular
adenocarcinoma | 67 | 25 | | |
| Poorly
differentiated adenocarcinoma | 20 | 8 | | |
| Signet-ring cell
carcinoma | 6 | 3 | | |
| Mucinous
adenocarcinoma | 20 | 6 | | |
| Level of
differentiation | | | 11.27 | <0.05 |
|
Intermediate-highly differentiated | 65 | 38 | | |
| Poorly
differentiated | 62 | 10 | | |
| Depth of
infiltration | | | 28.43 | <0.05 |
| T1+T2 | 14 | 23 | | |
| T3+T4 | 113 | 25 | | |
| Lymphatic
metastasis | | | 10.71 | <0.05 |
| No | 12 | 14 | | |
| Yes | 115 | 34 | | |
|
Tumor-node-metastasis stage | | | 0.071 | >0.05 |
| I+II | 58 | 23 | | |
| III+IV | 69 | 25 | | |
Tissue microarray construction and
immunohistochemistry
A representative tumor section paraffin block (donor
block) was collected from each case, and two tissue cores (2 mm in
diameter) were obtained using a trephine apparatus. Since gastric
carcinoma frequently shows histological heterogeneity, we sampled
duplicate tissue cores from separate areas in individual
paraffin-embedded gastric tumors for better representation of the
tumor. Trephinated paraffin tissue cores were then arranged in a
new recipient paraffin block (tissue array block). Cores containing
tumor in >50% of the area were considered adequate.
Immunohistochemical staining was performed using commercially
polyclonal rabbit anti-histone antibodies to histone
methyltransferase Suv39H1, tri-methyl-histone H3K9 and H3K4
(Upstate Biotechnology, Lake Placid, NY, USA). Tissue array blocks
were sectioned at a thickness of 4 μm and mounted on pre-coated
glass slides. The sections were deparaffinized and hydrated prior
to immunohistochemistry. The immunohistochemical S-P method was
performed according to the manufacturer’s protocol. Tissues
positive for all of the purchased antibodies were used as positive
controls; sections prepared with phosphate-buffered saline instead
of the primary antibody were used as negative controls. Positive
controls exhibited a brown color in the nuclei. Chevallier’s
semi-quantity system analysis was used for the calculation of the
immunohistochemistry results. Results are presented as the sum of
scores presenting color density and the percentage of stained
cells. According to color density, non-stained cells were scored as
0; slightly stained as 1; intermediate-stained as 2 and strongly
stained as 3. When the number of positive cells was <25, 25–50,
>50–75, or >75%, the immunoreactivity was scored as 1+, 2+,
3+ and 4+, respectively. The two scores for color density and the
number of positive cells were added for each slide. A sum of 0 was
consider negative; 1–2, +; 3–4, ++; 5–6, +++ and 7 as ++++. If the
sum of the two scores was ≤2, it was considered negative staining;
>2 was considered positive staining. The scores for each patient
group were averaged.
Cell line and culture
Human gastric carcinoma MGC-803 cells were purchased
from the American Type Culture Collection. Cells were cultured in
10% fetal bovine serum and RPMI-1640 containing L-glutamine under
37°C, saturated humidity and 5% CO2. Cells were
subcultured every 3–5 days. The subculture of cells was performed
using 0.25% trypsin to digest the attached cells for 2–3 min.
RNA interference
The approach by transient transfection using
Lipofectamine™ 2000 was employed to deplete the Suv39H1 gene in the
MGC-803 cell line. Suv39H1 siRNA sense: 5′-CGUGGAUUGUCUCAGGGAATT-3′
and antisense 5′-UUCCCUGAGACAAUCCACGTG-3′ were synthesized by
Shanghai GenePharma Co., Ltd. (China). Transfection with siRNA was
performed according to the Lipofectamine™ 2000 manufacturer’s
protocol (Invitrogen, Carlsbad, CA, USA). Inducible MGC-803 cells
(1×105 cells/well) were seeded onto 24-well plates,
(Costar Life Science, Acton, MA, USA) and transiently transfected
with 0, or 60, 120 or 240 nmol/l Suv39H1 siRNA. All experiments
were conducted in triplicate using independent cultures. Both total
RNA and protein were extracted after a 24-h transfection.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from samples of
1×106 cells using TRIzol (Invitrogen). The quantity and
quality of RNA samples were measured by absorbance at 260 and 280
nm. RNA samples with an A260:A280 ratio 1.8–2.0 were stored at
−80°C until use. cDNA synthesis was performed using an Avian
Myeloblastosis Virus Reverse Transcriptase kit, according to the
manufacturer’s protocol (Promega, Madison, WI, USA). β-actin was
used as the internal control. Primers used in the PCR
amplifications were: Suv39H1 forward, 5′-TGCGTATCCTCAAGCAGTTCC-3′
and Suv39H1 reverse, 5′-CCGTCCAGGTCCACCTCATTC-3′; β-actin forward,
5′-CTCCTTAATGTCAC GCACGATTTC-3′ and β-actin reverse,
5′-CTACAATGAGCTGCGTGTGGC-3′. Amplicon size was 217 base pairs (bp)
and 517 bp for Suv39H1 and β-actin, respectively. PCR reaction
conditions were: 94°C for 45 sec, 56°C for 1 min, 72°C for 80 sec
which was repeated for 30 cycles. The PCR-amplified products were
electrophoresed on 1.0% (w/v) agarose gels with 1 μg/ml ethidium
bromide. Experiments were repeated twice.
Cell proliferation as measured by MTT
assay
The MGC-803 cells were seeded at a density of
1×105/well in 96-well culture dishes (Costar Life
Science). After 0, 24, 48, 72 and 96 h transfection (n=5) with 0,
30, 60, 120, 240 or 480 nmol/l Suv39H1 siRNA, cell proliferation
was measured using MTT assays (0.5 mg/ml; Sigma). The
spectrophotometric absorbance of the samples was determined by
using an Ultra Multifunctional Microplate Reader (Tecan, Durham,
NC, USA) at 492 and 630 nm. Suppression ratio was also calculated.
The experiment was repeated in triplicate.
Apoptosis as detected by TUNEL assay
Cells at a logarithmic phase of growth were
transfected with negative control siRNA and 60, 120 and 240 nmol/l
Suv39H1 siRNA, and were seeded in 6-well plates (1×106
cells/well; Costar Life Science) with a sterile cover glass placed
at the bottom of each well. Cells were used for the detection of
apoptosis 24 h after transfection by TUNEL, according to the
manufacturer’s protocol (DeadEnd™ Fluorometric TUNEL System;
Promega).
Western blot analysis
Total protein lysate and western blot analysis were
performed as previously described (17). Briefly, MGC-803 cells were plated on
culture dishes and transfected with Suv39H1 siRNA at 60, 120 and
240 nmol/l for 24 h. Control cells were incubated in the medium
with Na2CO3 using the same time points. After
incubation, total proteins were prepared from each culture
condition with a lysis buffer containing freshly prepared protease
inhibitors. The protein concentration was then measured using the
BCA protein assay (Pierce, Rockford, IL, USA). Cell extracts were
subjected to 12% SDS-PAGE and electrophoretically transferred to
nitrocellulose membranes. The membranes were incubated with
monoclonal anti-acetyL-HistoneH3, anti-acetyL-Histone H4,
tri-methyl-Histone H3K9, Suv39H1 (Upstate, USA), BCL-2,
pro-caspase-9, pro-caspase-3 and C-myc antibodies (Santa Cruz,
Santa Cruz, CA, USA). After being washed with TBS, the membranes
were incubated with the secondary antibody conjugated with
peroxidase. The signal was then detected using the
chemiluminescence detection system (Pierce) and analyzed by a color
image analysis system (AlphaDigiDoc; Alpha Innotech).
Statistical analysis
The Student’s t-test for mean and standard deviation
or the Mann-Whitney test for median and range were performed for
comparisons between the groups in regards to continuous data.
Comparisons among groups regarding categorical data were analyzed
by performing the Chi-square test. All data were analyzed using the
SPSS, version 16.0 computer program (SPSS, Inc., Chicago, IL, USA),
and the significance of these differences was defined as
p<0.05.
Results
Overexpression of Suv39H1 and histone
tri-methylation of H3K9 in gastric carcinoma
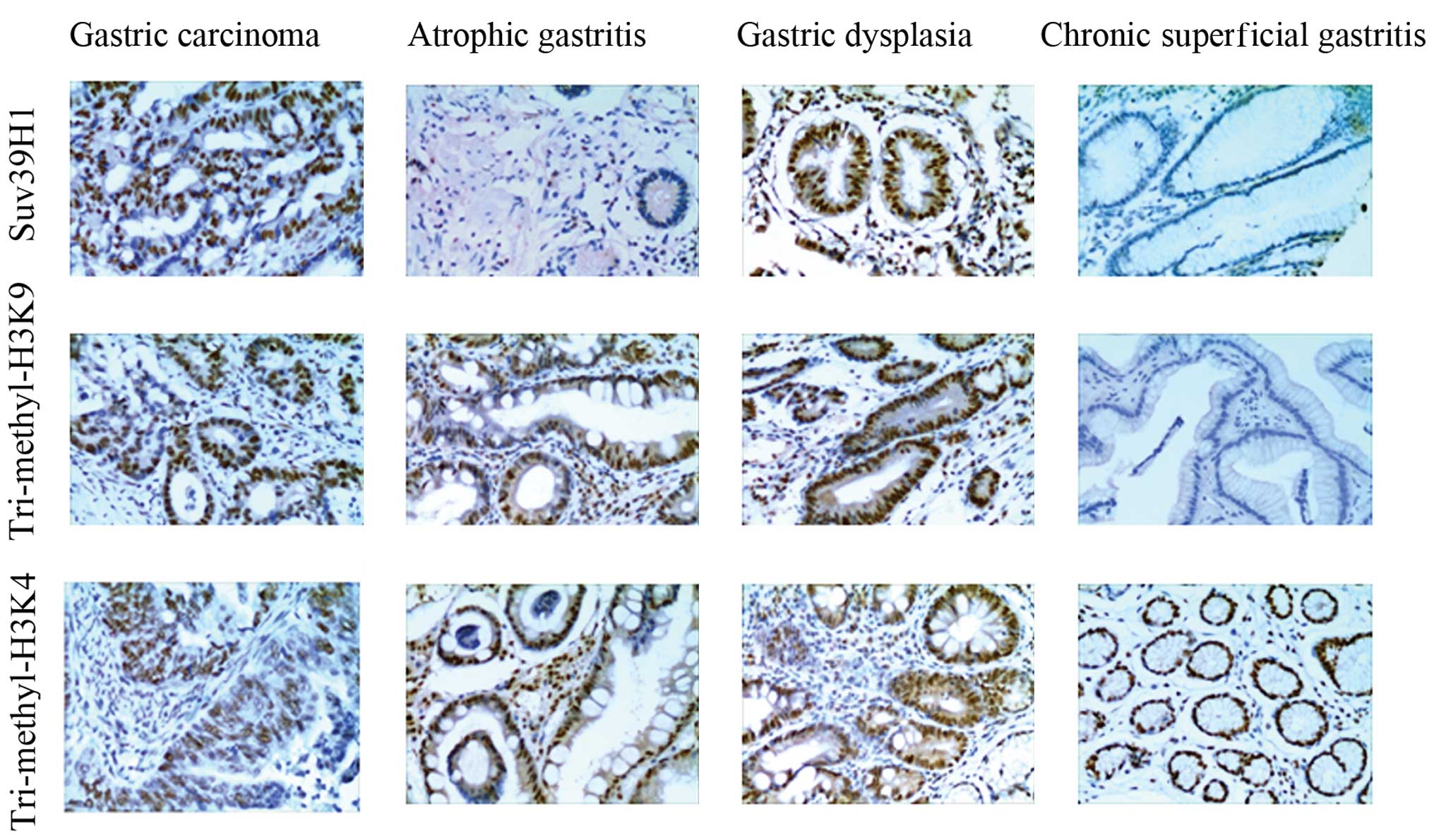
We assessed the staining score for Suv39H1,
tri-methylation of H3K9 and H3K4 in 175 cases of gastric carcinoma.
The expression of Suv39H1 in gastric carcinoma was 72.57%, higher
than that in the chronic superficial gastritis (33.33%, p<0.05),
chronic atrophic gastritis (30.00%, p<0.05) and moderate-severe
dysplasia (46.67%, p<0.05) (Fig.
1). The expression of histone tri-methylation of H3K9 in
gastric carcinoma was 85.47%, higher than that in the chronic
superficial gastritis (36.67%, p<0.05), chronic atrophic
gastritis (33.33%, p<0.05) and moderate-severe dysplasia
(53.33%, p<0.05) (Fig. 1). The
histone tri-methylation of H3K4 in gastric carcinoma was 59.43%,
which was similar to that of chronic superficial gastritis (46.67%,
p>0.05), chronic atrophic gastritis (60.00%, p>0.05) and
moderate-severe dysplasia (50.00%, p>0.05) (Fig. 1).
Correlation of Suv39H1 and histone
tri-methylated H3K9 with clinicopathological variables in the
gastric carcinoma cases
To evaluate the correlation between the histone
methylation status and various clinicopathological data, we divided
the level of staining scores into two groups: negative (score ≤2)
and positive (score >2). The data showed that the Suv39H1 status
was positively correlated with the degree of differentiation
(χ2=11.27, p<0.05), depth of infiltration
(χ2=28.43, p<0.05) and lymphatic invasion
(χ2=10.71, p<0.05). The Suv39H1 status was not
correlated with TNM stage (χ2=0.071, p>0.05), age,
(t=2.04, p>0.05), gender (χ2=0.036, p>0.05), body
mass index (BMI) (t=2.09, p>0.05) or pathological type
(χ2=0.493, p>0.05) (Table
I).
The tri-methylated H3K9 status was positively
correlated with the degree of differentiation (χ2=7.46,
p<0.05), depth of infiltration (χ2=16.65, p<0.05),
lymphatic invasion (χ2=6.33, p<0.05) or TNM stage
(χ2=20.84, p<0.05). It was not correlated with age
(t=2.20, p>0.05), gender (χ2=0.381, p>0.05), BMI
(t=2.13, p>0.05) or pathological type (p=0.865) (Table II).
 | Table IIClinical significance of histone H3K9
methylation in the gastric carcinoma cases (n=175). |
Table II
Clinical significance of histone H3K9
methylation in the gastric carcinoma cases (n=175).
| Histone H3K9
protein expression | | |
|---|
|
| | |
|---|
| Group | Positive | Negative |
χ2/t | P-value |
|---|
| Gender
(Male/Female) | 113/34 | 20/8 | 0.381 | >0.05 |
| Body mass index
(BMI) | 20.3±3.6 | 21.3±3.8 | 2.13 | 0.39 |
| Age (years) | 59.3±11.3 | 58.1±10.7 | 2.20 | 0.56 |
| Pathological
type | | | Fisher’s precise
probability | 0.865 |
| Papillary
adenocarcinoma | 16 | 4 | | |
| Tubular
adenocarcinoma | 78 | 14 | | |
| Poorly
differentiated adenocarcinoma | 23 | 5 | | |
| Signet-ring cell
carcinoma | 7 | 2 | | |
| Mucinous
adenocarcinoma | 23 | 3 | | |
| Level of
differentiation | | | 7.46 | <0.05 |
|
Intermediate-highly differentiated | 80 | 23 | | |
| Poorly
differentiated | 67 | 5 | | |
| Depth of
infiltration | | | 16.65 | <0.05 |
| T1+T2 | 23 | 14 | | |
| T3+T4 | 124 | 14 | | |
| Lymphatic
metastasis | | | 6.33 | <0.05 |
| No | 17 | 9 | | |
| Yes | 130 | 19 | | |
|
Tumor-node-metastasis stage | | | 20.84 | <0.05 |
| I+II | 57 | 24 | | |
| III+IV | 90 | 4 | | |
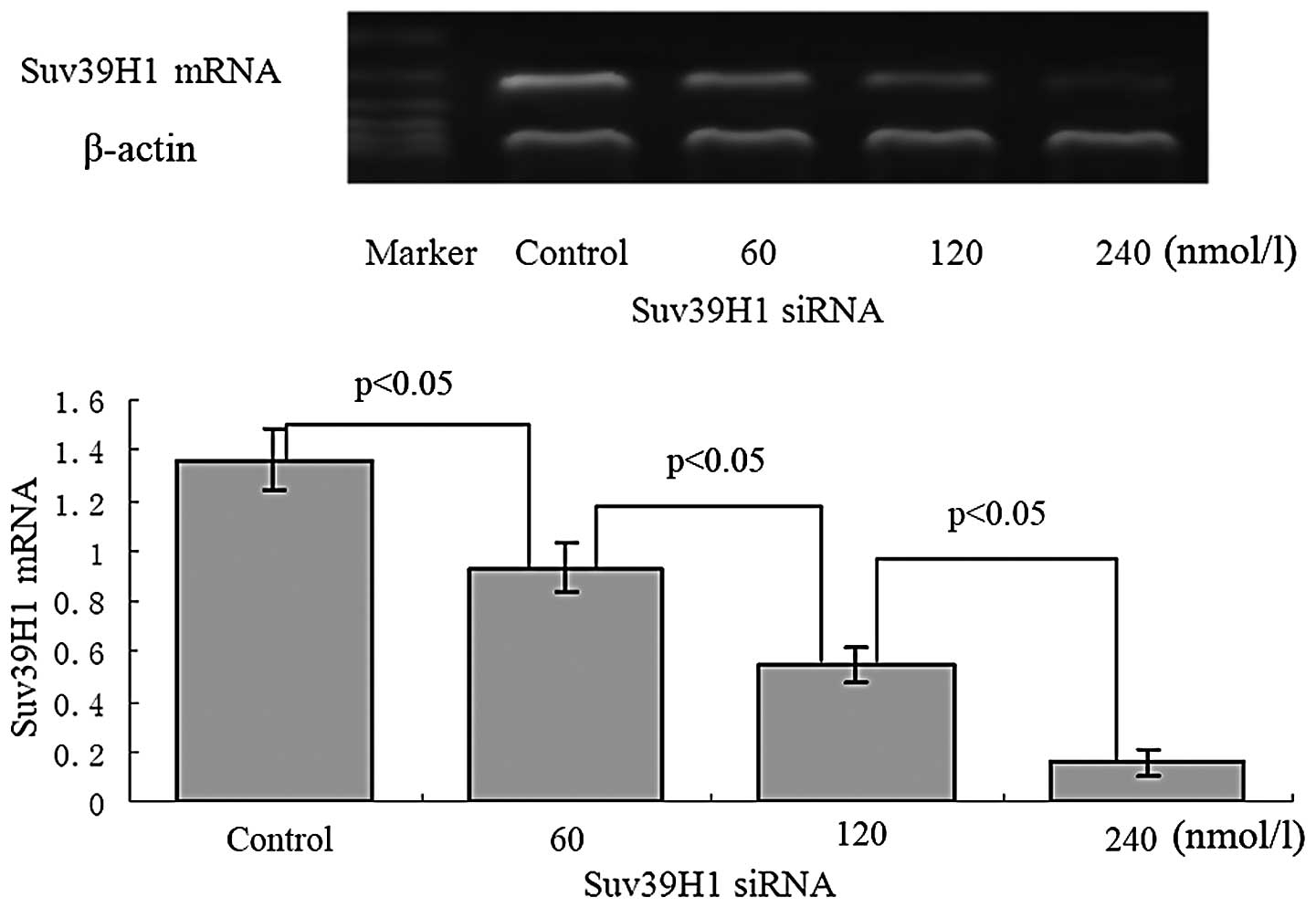
Silencing efficiency of the Suv39H1 gene
following transfection of Suv39H1 siRNA in MGC-803 cells
After 24 h of transfection of MGC-803 cells with
Suv39H1 siRNA at 60, 120 and 240 nmol/l, the amplification of
Suv39H1 mRNA was attenuated in a concentration-dependent manner.
Gray value (to β-actin) showed that the amplification of Suv39H1
was 0.93±0.10 at 60 nmol/l, 0.55±0.07 at 120 nmol/l and 0.16±0.05
at 240 nmol/l respectively, compared to the control (1.36±0.12)
(p<0.05) (Fig. 2).
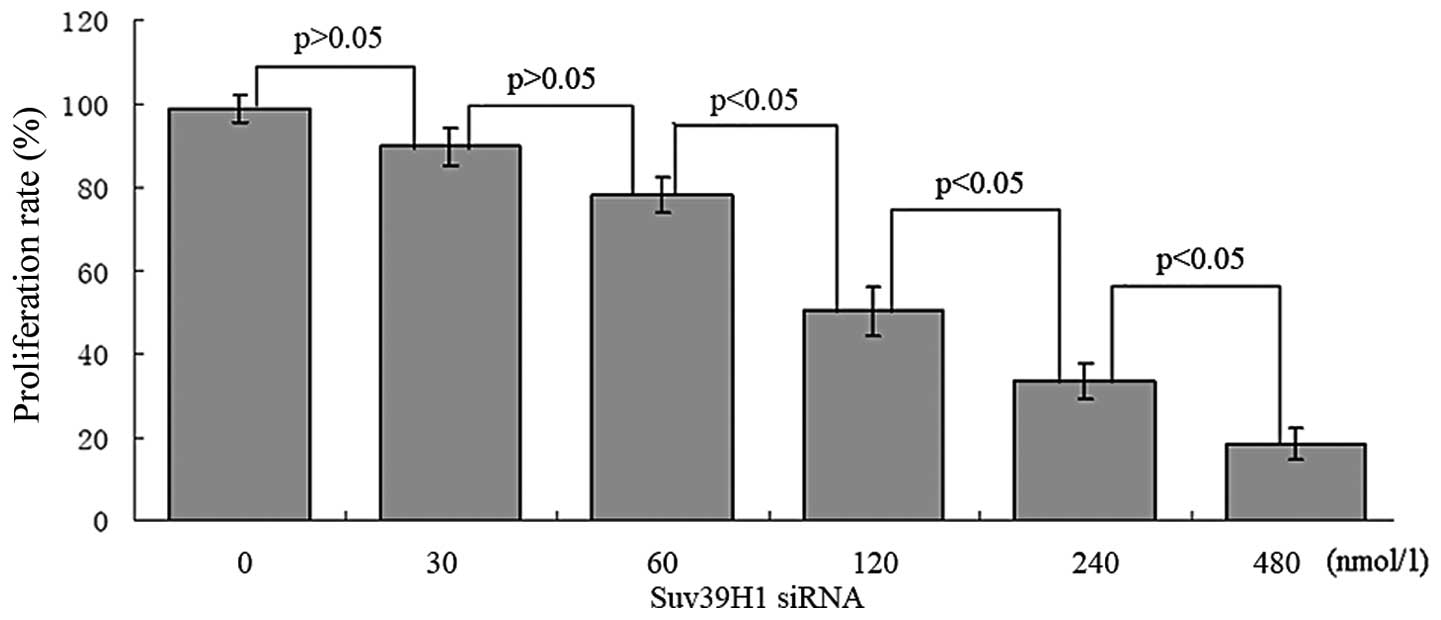
Suv39H1 siRNA inhibits cell proliferation
and promotes apoptosis in MGC-803 cells
Suv39H1 siRNA significantly suppressed cell
proliferation. Twenty-four hours after transfection with Suv39H1
siRNA, ~50.12±5.98% reduction in cell density was noted at 120
nmol/l, 33.37±4.13% at 240 nmol/l and 18.35±3.96% at 480 nmol/l
(Fig. 3).
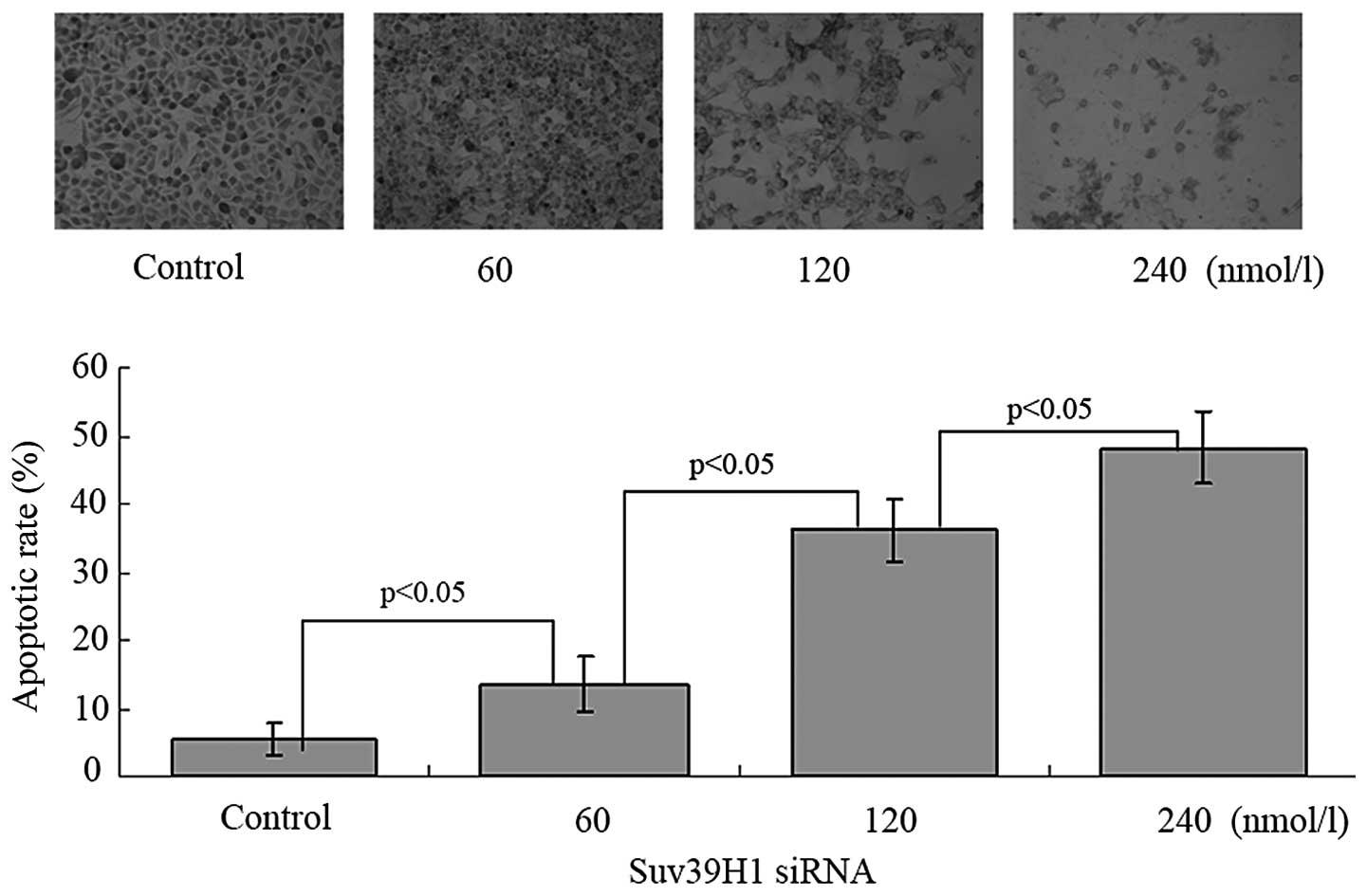
The apoptotic cells exhibited brown staining in the
nucleus. The apoptotic rate was 5.4±2.3, 13.5±4.1, 36.3± 4.6 and
48.3±5.4% after 24 h of transfection with Suv39h1 siRNA at 0, 60,
120 and 240 nmol/l, respectively. The apoptotic rate was increased
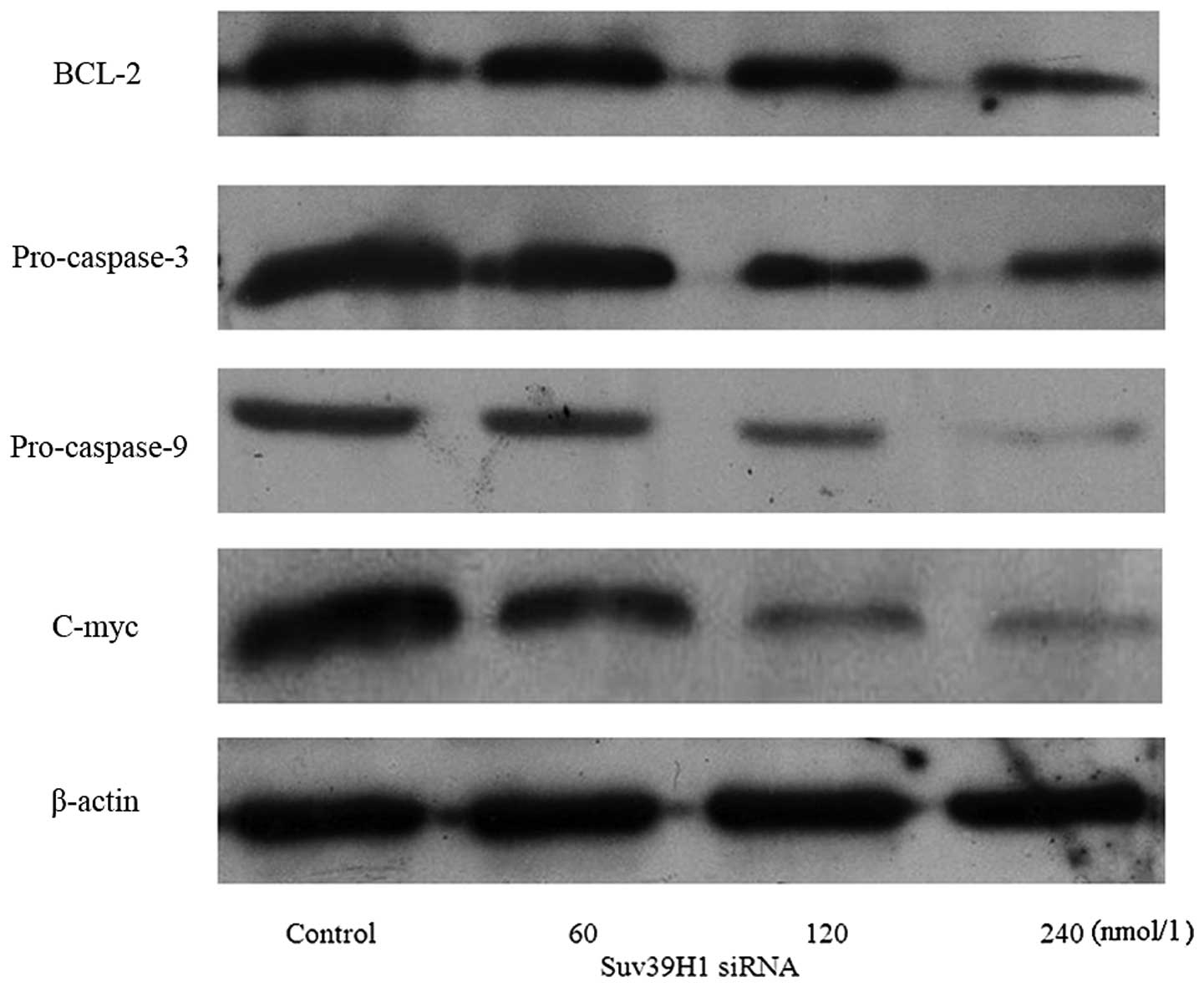
in a concentration-dependent manner (p<0.05) (Fig. 4). We further assessed expression of
the apoptosis-associated proteins: BCL-2, pro-caspase-3,
pro-caspase-9 and C-myc. Suv39H1 siRNA significantly inhibited
BCL-2, pro-caspase-3, pro-caspase-9 and C-myc (Fig. 5).
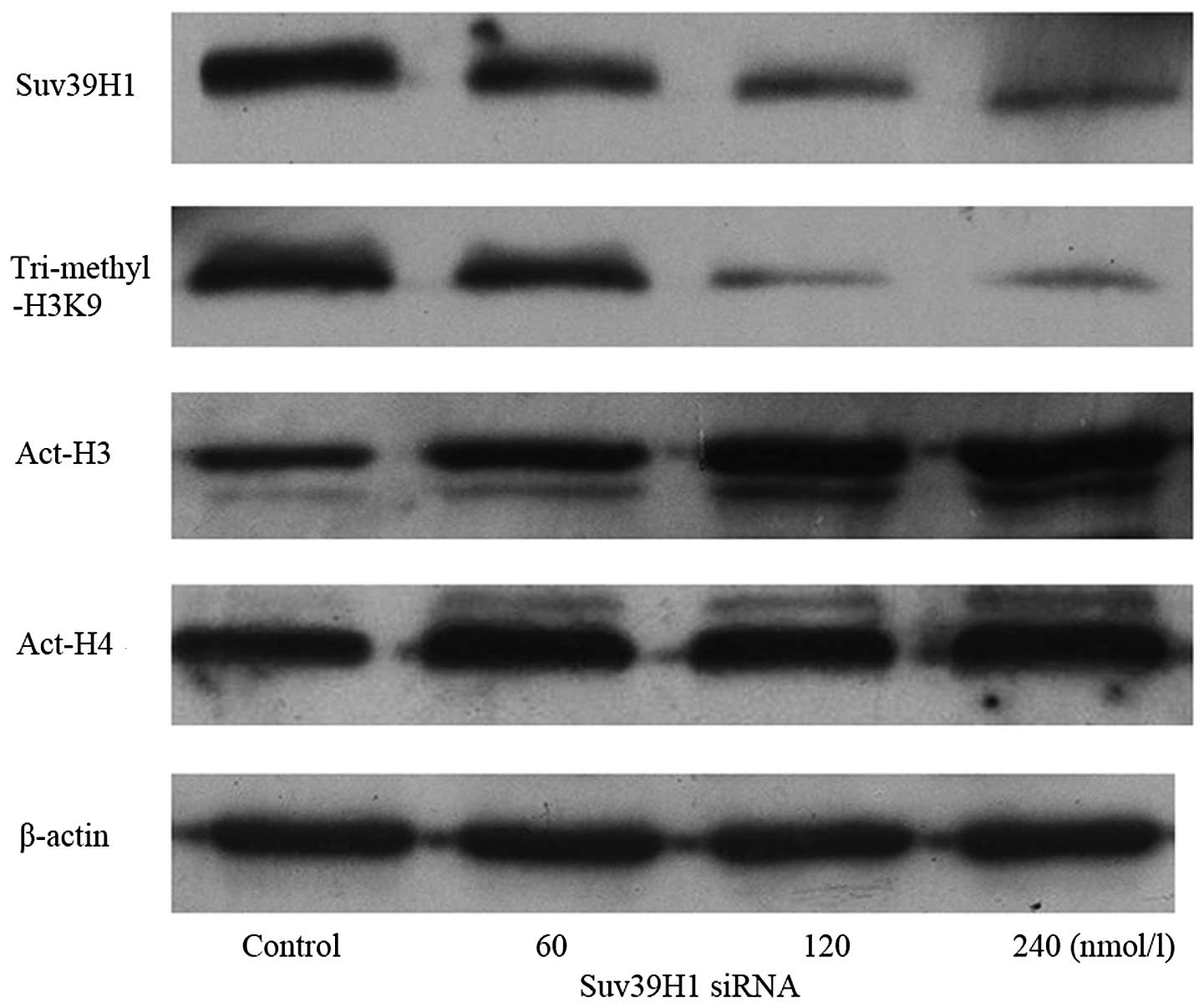
Suv39H1 siRNA modulates histone
tri-methylation of H3K9 and acetylation of H3 and H4
Protein of Suv39H1, and histone tri-methylated H3K9
were downregulated, while acetylated H3 was upregulated after a 24
h transfection with Suv39H1 siRNA, when compared with the control.
The relative (to β-actin) gray value showed that Suv39H1 was
1.18±0.1, 0.82±0.08, 0.43±0.12, 0.4±0.11 and tri-methylated H3K9
was 1.21±0.11, 0.89±0.09, 0.26±0.12 and 0.25±0.10; Act-H3 was
0.6±0.05, 0.91±0.04, 1.22±0.11 and 1.61±0.10 in the control, and at
60, 120, 240 nmol/l Suv39H1 siRNA, respectively (p<0.05). There
was no change in histone H4 acetylation. The expression of Act-H4
was 1.22±0.05, 1.31±0.10, 1.25±0.06, 1.33±0.11 in the control and
at 60, 120, 240 nmol/l Suv39H1 siRNA, respectively (p>0.05)
(Fig. 6).
Discussion
H3K9 methylation is a major component of gene
regulation and chromatin organization. Suv39H1 methylates H3K9 at
the pericentric heterochromatin region and participates in the
maintenance of genome stability. In the present study, we described
the overexpression of Suv39H1 and histone tri-methylated H3K9 in
gastric carcinoma. Suv39H1 and histone tri-methylated H3K9 were
positively correlated with the degree of differentiation, depth of
infiltration and lymphatic invasion in gastric carcinoma. The
expression of histone methylated H3K9 was correlated with TNM
stage. This indicates that overexpression of Suv39H1 and histone
tri-methylated H3K9 may be involved in the initiation and
development of gastric carcinoma. This is an initial study of
Suv39H1 in gastric carcinoma and its clinicopathological
characteristics.
The role of Suv39H1 in pericentric heterochromatin
has been extensively investigated, but only a few studies have
suggested the involvement of Suv39H1 in tumorigenesis.
Overexpression of Suv39H1 is associated with colon cancer (18). We previously found that aberrant
histone methylation occurs in acute leukemia and lymphoma (19). Park et al studied 261 gastric
adenocarcinoma samples and concluded that tri-methylation of H3K9
was positively correlated with tumor stage, lymphovascular
invasion, cancer recurrence, and a higher level of H3K9
tri-methylation was correlated with a poor survival rate (20). Multivariate survival analysis showed
that H3K9 tri-methylation status is an independent prognostic
factor. Differential expression of Suv39H1 was reported in
different types of cancer, and a prominent increase in the
expression of Suv39H1 was observed in preneoplastic nodules and
liver tumors induced by methyl deficiency in rats (21,22).
The mechanism of Suv39H1’s tumorigenesis involves
Suv39H1-associating partner molecule HP1 which is able to interact
with RB (23), raising the
possibility that Suv39H1 could be directly linked to specific
tumor-suppressor proteins (24,25).
This may be the reason why overexpression of Suv39H1 and H3K9 is
involved in carcinogenesis.
We further silenced the Suv39H1 gene by transfection
of Suv39H1 siRNA into gastric carcinoma MGC-803 cells to study the
effect on cell growth and apoptosis. The results demonstrated that
silencing of the Suv39H1 gene inhibited cell proliferation and
induced cell apoptosis in the MGC-803 cells, along with
downregulation of pro-caspase-3 and -9, BCL-2 and C-myc, which are
related to the apoptosis pathway. The escape from replicative
senescence in Suv39H1 transgenic erythroblasts was previously found
to be correlated with reduced amounts of the ‘antiproliferative’
cell cycle regulator p21, deregulated abundance of RB, aberrant
cytoplasmic levels of p53 and elevated expression of the
p53-destabilizing protein mdm (26,27).
Suv39H1 is targeted to promoters of cell-cycle control genes by RB
and also induces the silencing of S-phase genes through H3K9
methylation in differentiating cells (13,28).
H3K9 methylation is a prerequisite for DNA methylation to occur
(29,30). Loss of Suv39H1/2 in knockout mouse
cells also altered the DNA methylation pattern of their pericentric
heterochromatin (31).
The present study revealed that suppression of the
expression of Suv39H1 not only downregulates H3K9 methylation, but
also upregulates histone H3 acetylation, which is consistent with
previous results (32). We
previously found that silencing of Suv39H1 upregulated histone
acetylated H3K9, H3 and P15 in HL60 cells. It did not affect H3K27,
H3K14 and H4. Vaute et al found that interaction with
histone deacetylase could be mediated by the N-terminus of Suv39H1,
which contains a chromodomain (33). Histone deacetylases (HDACs)
associated with Suv39H1 may include HDAC1, HDAC2 and HDAC3.
Multiple residues on each of the four core histones have been
identified as potential modification sites, and some lysine
residues, such as H3K9, can be either methylated or acetylated.
Recent studies have shown that switching acetylation to methylation
on H3K9 contributes to euchromatin gene silencing in many organisms
(34).
In summary, utilizing a large number of specimens
from patients with gastric carcinoma, we demonstrated for the first
time that Suv39H1 is positively correlated with initiation,
development and migration of gastric carcinoma. Suv39H1 is also
positively correlated with the level of H3K9 methylation in these
tissues. In addition, we confirmed that Suv39H1 affects H3K9
methylation and H3 acetylation, and inhibits cell growth and
induces cell apoptosis in vitro. The results from this study
highlight the potential of H3K9 methyltransferases as therapeutic
targets for gastric carcinoma.
Acknowledgements
This study was partly supported by grant-in-aid from
the Foundation of Science and Technology of Zhangzhou, Fujian,
China (No. Z07014), the Foundation of Science and Technology of
Fujian Medical University, Fujian, China (No. FZS08018), the
Science Research Foundation of Ministry of Health, United Fujian
Provincial Health, and the Education Project for Tackling the Key
Research, China (WKJ2008-2-55).
Abbreviations:
|
H3K9
|
histone H3 lysine 9
|
|
H3K4
|
histone H3 lysine 4
|
|
K
|
lysine
|
|
HMT
|
histone methyltransferase
|
|
PML
|
promyelocytic leukemia
|
|
RB
|
retinoblastoma protein
|
|
TNM
|
tumor-node-metastasis
|
|
PCR
|
polymerase chain reaction
|
|
HDAC
|
histone deacetylase
|
References
|
1
|
Jenuwein T and Allis CD: Translating the
histone code. Science. 293:1074–1080. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fischle W, Wang Y and Allis CD: Histone
and chromatin cross-talk. Curr Opin Cell Biol. 15:172–183. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lachner M, O’Sullivan RJ and Jenuwein T:
An epigenetic road map for histone lysine methylation. J Cell Sci.
116:2117–2124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klose RJ, Kallin EM and Zhang Y:
JmjC-domain-containing proteins and histone demethylation. Nat Rev
Genet. 7:715–727. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cang S, Ma Y and Liu D: New clinical
developments in histone deacetylase inhibitors for epigenetic
therapy of cancer. J Hematol Oncol. 2:222009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan J, Cang S, Ma Y, Petrillo RL and Liu
D: Novel histone deacetylase inhibitors in clinical trials as
anti-cancer agents. J Hematol Oncol. 3:52010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rea S, Eisenhaber F, O’Carroll D, et al:
Regulation of chromatin structure by site-specific histone H3
methyltransferases. Nature. 406:593–599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allshire RC, Nimmo ER, Ekwall K, Javerzat
JP and Cranston G: Mutations derepressing silent centromeric
domains in fission yeast disrupt chromosome segregation. Genes Dev.
9:218–233. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tschiersch B, Hofmann A, Krauss V, Dorn R,
Korge G and Reuter G: The protein encoded by the Drosophila
position-effect variegation suppressor gene Su(var)3–9 combines
domains of antagonistic regulators of homeotic gene complexes. EMBO
J. 13:3822–3831. 1994.
|
|
10
|
Aagaard L, Laible G, Selenko P, et al:
Functional mammalian homologues of the Drosophila PEV-modifier
Su(var)3–9 encode centromere-associated proteins which complex with
the heterochromatin component M31. EMBO J. 18:1923–1938.
1999.PubMed/NCBI
|
|
11
|
Peters AH, Kubicek S, Mechtler K, et al:
Partitioning and plasticity of repressive histone methylation
states in mammalian chromatin. Mol Cell. 12:1577–1589. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Croce L, Raker VA, Corsaro M, et al:
Methyltransferase recruitment and DNA hypermethylation of target
promoters by an oncogenic transcription factor. Science.
295:1079–1082. 2002.PubMed/NCBI
|
|
13
|
Nielsen SJ, Schneider R, Bauer UM, et al:
Rb targets histone H3 methylation and HP1 to promoters. Nature.
412:561–565. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Milne TA, Briggs SD, Brock HW, et al: MLL
targets SET domain methyltransferase activity to Hox gene
promoters. Mol Cell. 10:1107–1117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chakraborty S, Sinha KK, Senyuk V and
Nucifora G: SUV39H1 interacts with AML1 and abrogates AML1
transactivity. AML1 is methylated in vivo. Oncogene. 22:1107–1117.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Fang Y, Beklemisheva A, et al:
Phenylhexyl isothiocyanate inhibits histone deacetylases and
remodels chromatins to induce growth arrest in human leukemia
cells. Int J Oncol. 28:1287–1293. 2006.PubMed/NCBI
|
|
18
|
Kang MY, Lee BB, Kim YH, et al:
Association of the SUV39H1 histone methyltransferase with the DNA
methyltransferase 1 at mRNA expression level in primary colorectal
cancer. Int J Cancer. 121:2192–2197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou Y, Ma X, Huang Y, Hong L and Chiao JW:
Effect of phenylhexyl isothiocyanate on aberrant histone H3
methylation in primary human acute leukemia. J Hematol Oncol.
5:362012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park YS, Jin MY, Kim YJ, Yook JH, Kim BS
and Jang SJ: The global histone modification pattern correlates
with cancer recurrence and overall survival in gastric
adenocarcinoma. Ann Surg Oncol. 15:1968–1976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozdağ H, Teschendorff AE, Ahmed AA, et al:
Differential expression of selected histone modifier genes in human
solid cancers. BMC Genomics. 7:902006.PubMed/NCBI
|
|
22
|
Pogribny IP, Ross SA, Tryndyak VP,
Pogribna M, Poirier LA and Karpinets TV: Histone H3 lysine 9 and H4
lysine 20 trimethylation and the expression of Suv4-20h2 and
Suv-39h1 histone methyltransferases in hepatocarcinogenesis induced
by methyl deficiency in rats. Carcinogenesis. 27:1180–1186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams L and Grafi G: The retinoblastoma
protein - a bridge to heterochromatin. Trends Plant Sci. 5:239–240.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dunaief JL, Strober BE, Guha S, et al: The
retinoblastoma protein and BRG1 form a complex and cooperate to
induce cell cycle arrest. Cell. 79:119–130. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trouche D, Le Chalony C, Muchardt C, Yaniv
M and Kouzarides T: RB and hbrm cooperate to repress the activation
functions of E2F1. Proc Natl Acad Sci USA. 94:11268–11273. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng M, Olivier P, Diehl JA, et al: The
p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators
of cyclin D-dependent kinases in murine fibroblasts. EMBO J.
18:1571–1583. 1999.
|
|
27
|
Czvitkovich S, Sauer S, Peters AH, et al:
Over-expression of the SUV39H1 histone methyltransferase induces
altered proliferation and differentiation in transgenic mice. Mech
Dev. 107:141–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ait-Si-Ali S, Guasconi V, Fritsch L, et
al: A Suv39h-dependent mechanism for silencing S-phase genes
in differentiating but not in cycling cells. EMBO J. 23:605–615.
2004.PubMed/NCBI
|
|
29
|
Tamaru H and Selker EU: A histone H3
methyltransferase controls DNA methylation in Neurospora
crassa. Nature. 414:277–283. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson JP, Lindroth AM, Cao X and
Jacobsen SE: Control of CpNpG DNA methylation by the KRYPTONITE
histone H3 methyltransferase. Nature. 416:556–560. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lehnertz B, Ueda Y, Derijck AA, et al:
Suv39h-mediated histone H3 lysine 9 methylation directs DNA
methylation to major satellite repeats at pericentric
heterochromatin. Curr Biol. 13:1192–1200. 2003. View Article : Google Scholar
|
|
32
|
Czermin B, Schotta G, Hülsmann BB, et al:
Physical and functional association of SU(VAR)3–9 and HDAC1 in
Drosophila. EMBO Rep. 2:915–919. 2001.PubMed/NCBI
|
|
33
|
Vaute O, Nicolas E, Vandel L and Trouche
D: Functional and physical interaction between the histone methyl
transferase Suv39H1 and histone deacetylases. Nucleic Acids Res.
30:475–481. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lachner M and Jenuwein T: The many faces
of histone lysine methylation. Curr Opin Cell Biol. 14:286–298.
2002. View Article : Google Scholar : PubMed/NCBI
|