Introduction
Colorectal cancer (CRC) is one of the most prevalent
cancers and common causes of cancer-related mortality. The
identification of differentially expressed genes and proteins
between cancer and adjacent non-tumor mucosa provides valuable
information with which to identify the molecular and physiological
changes involved in tumor development and differentiation. In our
previous proteomic studies, we demonstrated that selenium binding
protein 1 (SELENBP1) is one of the significantly downregulated
proteins in CRC (1). SELENBP1 has
been found to be downregulated in multiple cancer types, such as
lung, ovarian, colorectal, esophageal, gastric and liver cancers
(2–8), and its reduced expression has been
associated with poor outcome (4).
However, little is known concerning its function and regulatory
mechanism.
The two well-recognized epigenetic alterations, DNA
methylation and histone modification, are known to play a key role
in the development and progression of various types of cancers,
including CRC (9). DNA methylation
at CpG islands in promoter regions often leads to silencing of
tumor-suppressor genes (10,11).
Histone methylation patterns associated with CRC were uncovered by
Enroth et al (12) in a
genome-wide search in tumor samples. Pohl et al (22) demonstrated that in colon cancer
cells the SELENBP1 promoter is hypermethylated which presumably
leads to downregulation of this protein. They also demonstrated
that overexpression of SELENBP1 prevents cancer cell migration
in vitro and inhibits tumor growth in nude mice. Due to its
patterns of expression in cancer cells, SELENBP1 has also been
suggested as a potential marker for predicting progression
(8,13).
In the present study, we attempted to establish the
role of SELENBP1 in CRC by studying tissue samples from patients
with sporadic colorectal cancer. In addition, SELENBP1 expression
were studied in five different colorectal cancer cell lines
(HCT116, HT29, LS174T, SW480, SW620) and its regulation by
epigenetic modifications was examined in three of these. The
results support the notion that SELENBP1 is associated with CRC
differentiation and shed light on its possible regulation by
histone modification.
Materials and methods
Tissue samples
Tissue samples were obtained from The First Hospital
of China Medical University between 2008 and 2012. The procedures
were approved by the Ethics Committee of the First Hospital of
China Medical University. All tissue specimens were obtained by
experienced surgeons and analyzed by experienced pathologists.
Informed consent was obtained from patients. Samples of cancer
tissues and adjacent normal mucosa were collected from sporadic CRC
patients who had not received any preoperative chemotherapy or
radiotherapy. Normal mucosa was obtained 10 cm from the cancer
tissue with normal visualization. The level of differentiation of
the cancer tissues was determined as either well, moderately or
poorly differentiated, and the TNM stage was estimated.
Fresh samples were obtained by surgical resections,
immediately frozen in liquid nitrogen, and then stored at −80°C
until use. In addition to fresh frozen samples, 10% formalin-fixed
tissues were also prepared at the same time for immunohistochemical
study.
Proteomic analysis
Proteomic analyses were performed as prevously
described (1). Briefly, protein
extracts from cancer, normal mucosa and an internal pool were
extracted from fresh frozen tissues, and then labeled with
different fluorescent dyes. Each paired CRC and normal mucosa
sample together with the internal pool (the mixture of all extracts
of the 6 paired tissues) were resolved by electrophoreses on a
single gel. After isoelectric focusing on immobilized pH gradient
(IPG) and SDS-PAGE, proteins were visualized by fluorescence
scanning. Changes in the amount of each protein were calculated by
DeCyder 6.5 software (GE Healthcare). Proteins showing significant
changes in abundance were excised from the gel, subjected to
trypsin digestion and prepared for MALDI-TOF-MS analysis.
Swiss-Prot database was used for sequence comparisons.
Western blot analysis
After tissue or cell sonication lysis, protein
extracts were quantified using the BCA method. Samples equivalent
to 50–90 μg proteins were resolved by 8–12% SDS-PAGE and
transferred to a PVDF membrane. Primary antibodies for SELENBP1
(Abcam) and carcinoembryonic antigen (CEA) (Sigma-Aldrich) were
diluted 1:1,000 and incubation was carried out overnight at 4°C.
The anti-GAPDH antibody (Santa Cruz Biotechnology) was used as a
loading control. After washing, membranes were incubated for 2 h
with 1:4,000 diluted HRP-conjugated secondary antibodies (Santa
Cruz Biotechnology), washed and developed with ECL-Plus (GE
Healthcare).
Real-time reverse
transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from the tissue samples or
the cells with TRIzol (Invitrogen) according to the manufacturer’s
protocol. cDNA was synthesized from 500 ng total RNA using a
reverse transcription system (Promega). SYBR® Premix Ex
Taq™ II (Takara) was used for real-time quantitative PCR analysis.
The PCR reaction consisted of DNA denaturation (95°C, 30 sec) and
45 cycles of an amplification step (95°C, 5 sec; 62°C, 30 sec). The
dissociation curve was generated for every run to validate the
specificity of amplification. β-actin was chosen as the internal
control. The sequences of the primers (Takara Biotechnology,
Dalian, China) were as follows: SELENBP1-129048F (5′-TGG TGC TGC
CCA GTC TCA TC-3′) and SELENBP1-129048R (5′-AGT TCG CAC TTG GCA TGG
A-3′); β-actin F, 5′-TGG CAC CCA GCA CAA TGA A-3′ and β-actin R,
5′-CTA AGT CAT AGT CCG CCT AGA AGC A-3′. For calculation of the
relative mRNA level, the ΔΔCT method was used as described in the
manufacturer’s technical note (Roto Gene 6000).
Immunohistochemistry
Consecutive paraffin wax-embedded tissue sections
(3–5 μm) were obtained, and the sections were dewaxed and
rehydrated. The slides were pretreated for 2 min with citrate
buffer (pH 6.0) in an autoclave to retrieve the antigen. To quench
endogenous peroxidase activity, the slides were incubated in fresh
3% H2O2 and then washed for 4 min with water.
For the specific staining of SELENBP1, the slides were first
incubated for 1 h at room temperature with normal goat serum and
then incubated at 4°C overnight with rabbit anti-human SELENBP1
(diluted 1:100; Abcam). After rinsing with PBS, the slides were
incubated with 1:1,000 HRP-conjugated goat anti-rabbit secondary
antibodies (Santa Cruz Biotechnology) at 37°C for 30 min, rinsed
with PBS, followed by reaction with diaminobenzidine and
counterstaining with Mayer’s hematoxylin. Slides containing samples
of normal mucosa were incubated with PBS instead of the primary
antibodies to serve as negative controls. Immunostaining was graded
by three pathologists as: 0, lack of staining; 1, weak staining; 2,
moderate staining and 3, strong staining.
Cell culture and treatments
Human colon cancer cell lines, HCT116, HT29, LS174T,
SW480 and SW620, were obtained from the laboratory stocks of the
Department of Cell Biology, China Medical University. All of the
cell lines were cultured in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS)(HyClone®, Thermo Scientific)
and incubated at 37°C in a humidified 5% CO2 atmosphere.
Human colonic epithelial cell line, HCoEpiC (ScienCell, San Diego,
CA, USA), was cultured in high-glucose DMEM medium
(HyClone®, Thermo Scientific) under the same conditions
as above.
The cultures of HT29, SW480, SW620 cells (in two
25-cm2 flasks) were treated with sodium butyrate (NaB)
to induce differentiation. Cells were allowed to adhere for 24 h,
and then the medium was replaced with medium containing 2 mM NaB
(Sigma Aldrich) and incubated for 72 h, with changes of the
NaB-containing medium every 24 h.
For analysis of epigenetic modifications, cells were
treated with 5′-aza-2′-deoxycytidine (5-Aza-dC) and/or Trichostatin
A (TSA; Sigma Aldrich). HT29, SW480 and SW620 cells were incubated
for 24 h prior to treatment with the drugs as follows: i) 5-Aza-dC
(5 μM) for 72 h, medium changes every 24 h; ii) TSA (0.3 μM) for 24
h; ii) 5-Aza-dC (5 μM) for 48 h followed by TSA (0.3 μM) for an
additional 24 h. Control cells were incubated without NaB or
5-Aza-dC or TSA, with medium changes every 24 h. The experiments
were repeated three times.
Alkaline phosphatase (AKP) activity
assays
Following treatment of HT29, SW480 and SW620 cells
with NaB for 72 h, the cells were harvested and lysed. AKP activity
was assayed in the cell lysates using an AKP kit (Pointe Scientific
Inc. Canton, MI, USA) according to the manufacturer’s protocol,
using para-nitrophenol phosphate as a substrate. Reaction
production was detected by the absorbance at 400–415 nm.
Statistical analysis
All data are expressed as means ± SD. Measurement
data that did not follow a Gaussian distribution were transformed
into a logarithm. 2D DIGE spots with intensity changes, Western
blot analysis and RT-PCR results were analyzed by paired two sample
t-test or one-way ANOVA. Western blot data were analyzed by a
paired two-sample t-test. Immunohistochemistry staining intensity
score was analyzed by Wilcoxon signed-rank test or Kruskal-Wallis
test. Statistical significance was defined as P<0.05.
Results
Tissue samples
A total of 83 sporadic CRC patients who had not
received preoperative chemotherapy or radiotherapy were included in
the present study. Forty-two patients were males, 41 were females,
and their mean age was 61.3±12.4 years. All were cases of
adenocarcinoma. From all patients, samples of cancer tissue along
with paired adjacent normal mucosa were collected. There were 33
benign polyps including 20 hyperplastic polyps and 13 adenomatous
polyps, and 26 lymph node metastasis (LNM). Of the 83 tissue
samples, the majority were considered moderately differentiated
(44/83, 53.0%), while 17 (20.5%) were well differentiated and 22
(26.5%) were poorly differentiated. According to TNM stage, 6 were
stage I, 35 were stage II, 38 were stage III and 4 were stage
IV.
Proteomic analysis of CRC tissues and
normal mucosa
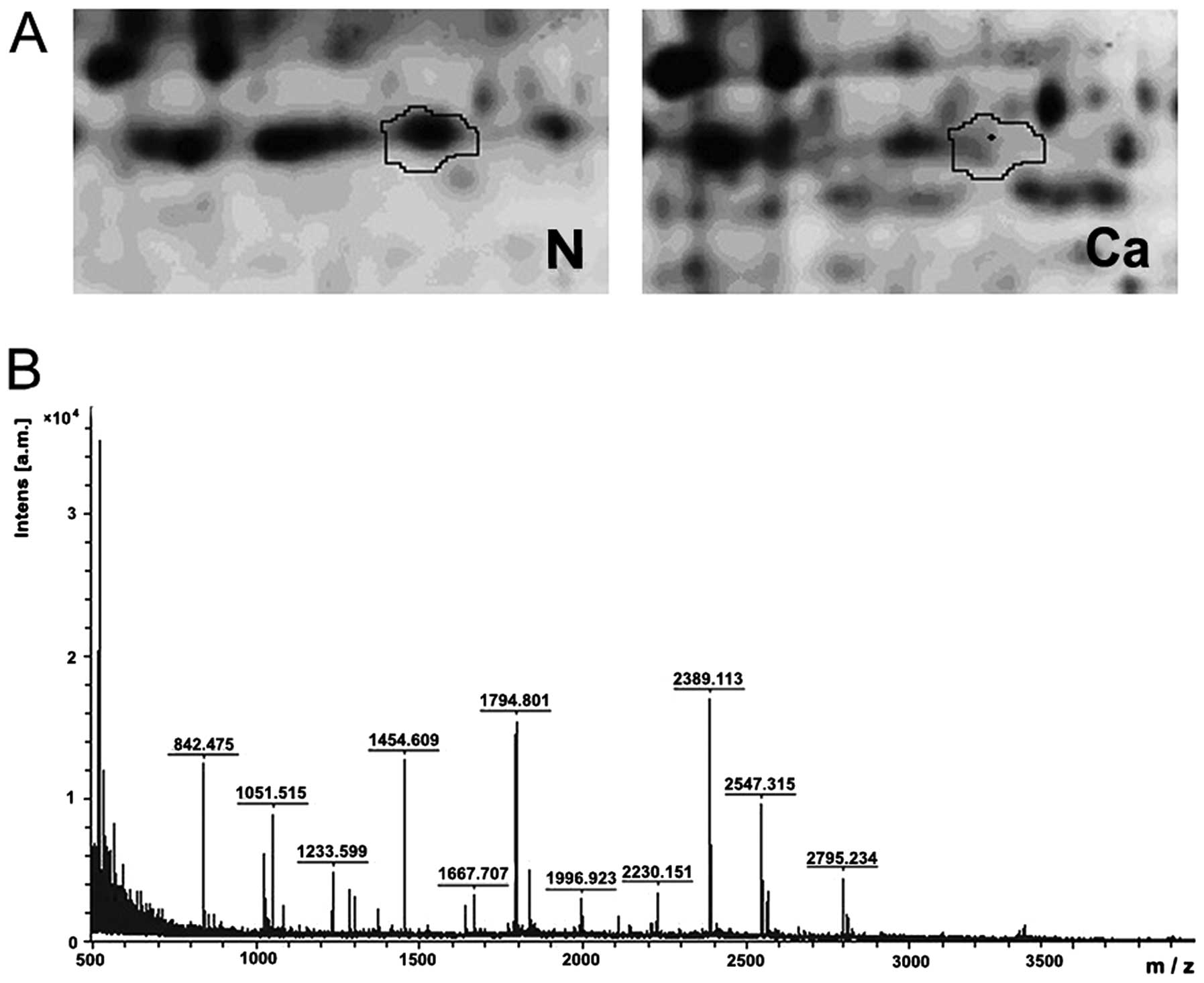
In the 2D-DIGE analysis, cancer tissues and normal
mucosa were compared in the collected samples. The spot intensities
of more than 20 proteins from the CRC samples were found to be
<1.5-fold that of the normal mucosa (P<0.05), and these were
identified by MALDI-TOF-MS analysis and Swiss-Prot database
similarity search with a score of at least 64. In 6 CRC tissue
samples, one particular protein spot was significantly less intense
than this spot in the corresponding normal mucosa samples, by an
average of 2.54-fold (Fig. 1).
Subsequent MALDI-TOF-MS analysis and Swiss-Prot database similarity
search identified it as SELENBP1 with a score of 148 (Fig. 1).
Differential expression of SELENBP1 in
the CRC tissues
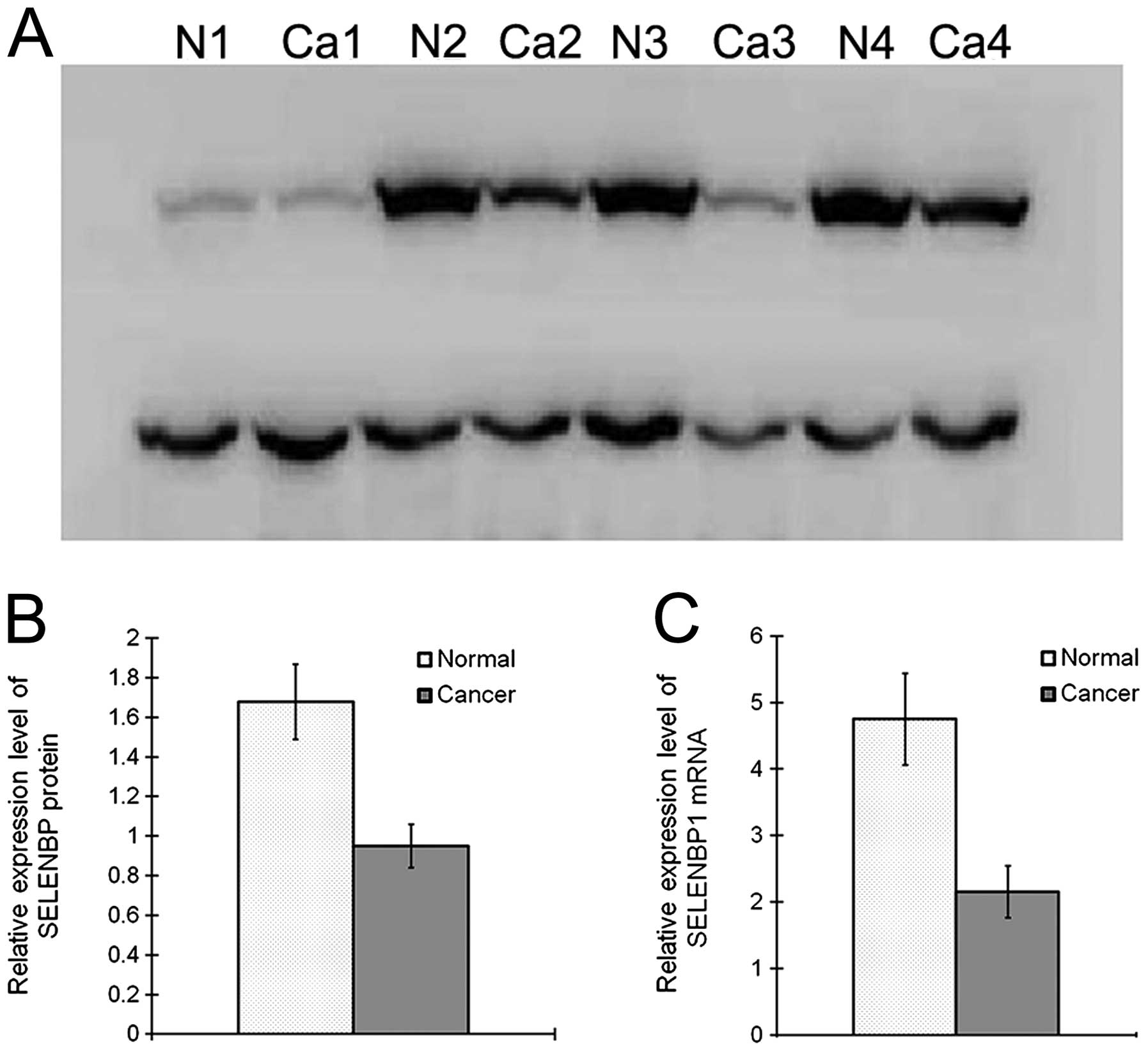
To validate our proteomic analysis, 24 paired fresh
frozen CRC and normal mucosa samples were randomly selected, and
SELENBP1 expression was analyzed by western blot analysis and
real-time RT-PCR. Western blot analysis revealed that SELENBP1
expression relative to the internal control was 1.68±0.41 and
0.95±0.23 in the normal and CRC tissues, respectively (P<0.01),
indicating a 1.77-fold downregulation in CRC. RT-PCR analysis
revealed that SELENBP1 expression relative to the internal control
was 4.75±3.36 and 2.15±1.90 in the normal and CRC tissues,
respectively (P<0.01), indicating a 2.21-fold downregulation in
CRC (Fig. 2).
SELENBP1 expression in human colon
carcinoma cell lines
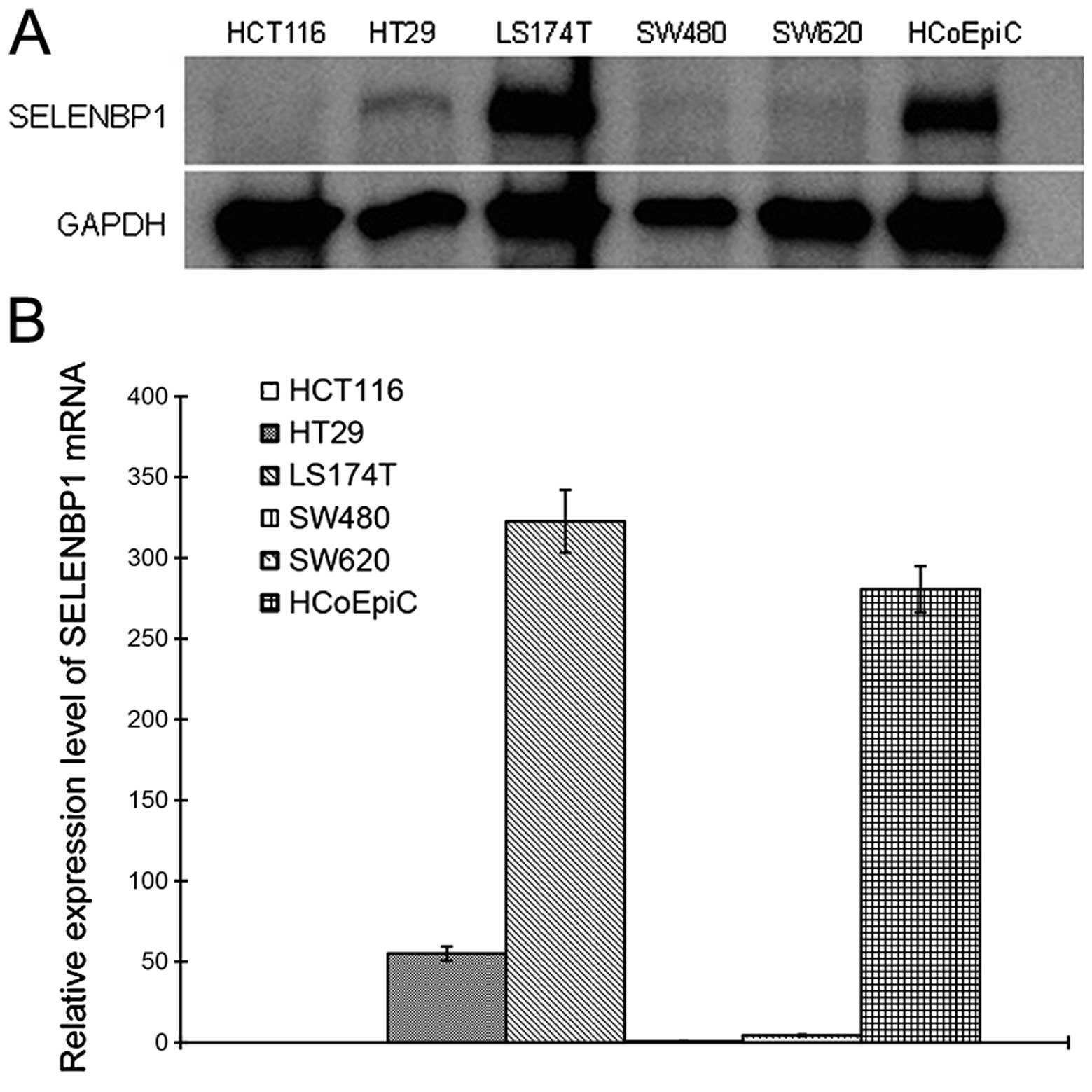
SELENBP1 expression in the HCoEpiC cultured cells
was compared with that in five human colon cancer cultured cell
lines, by western blot analysis and real-time RT-PCR. In all cell
lines except for LS174T, protein and mRNA levels of SELENBP1 were
significantly lower than the levels in the HCoEpiC cells (Fig. 3).
Immunohistochemical staining of SELENBP1
in CRC tissues
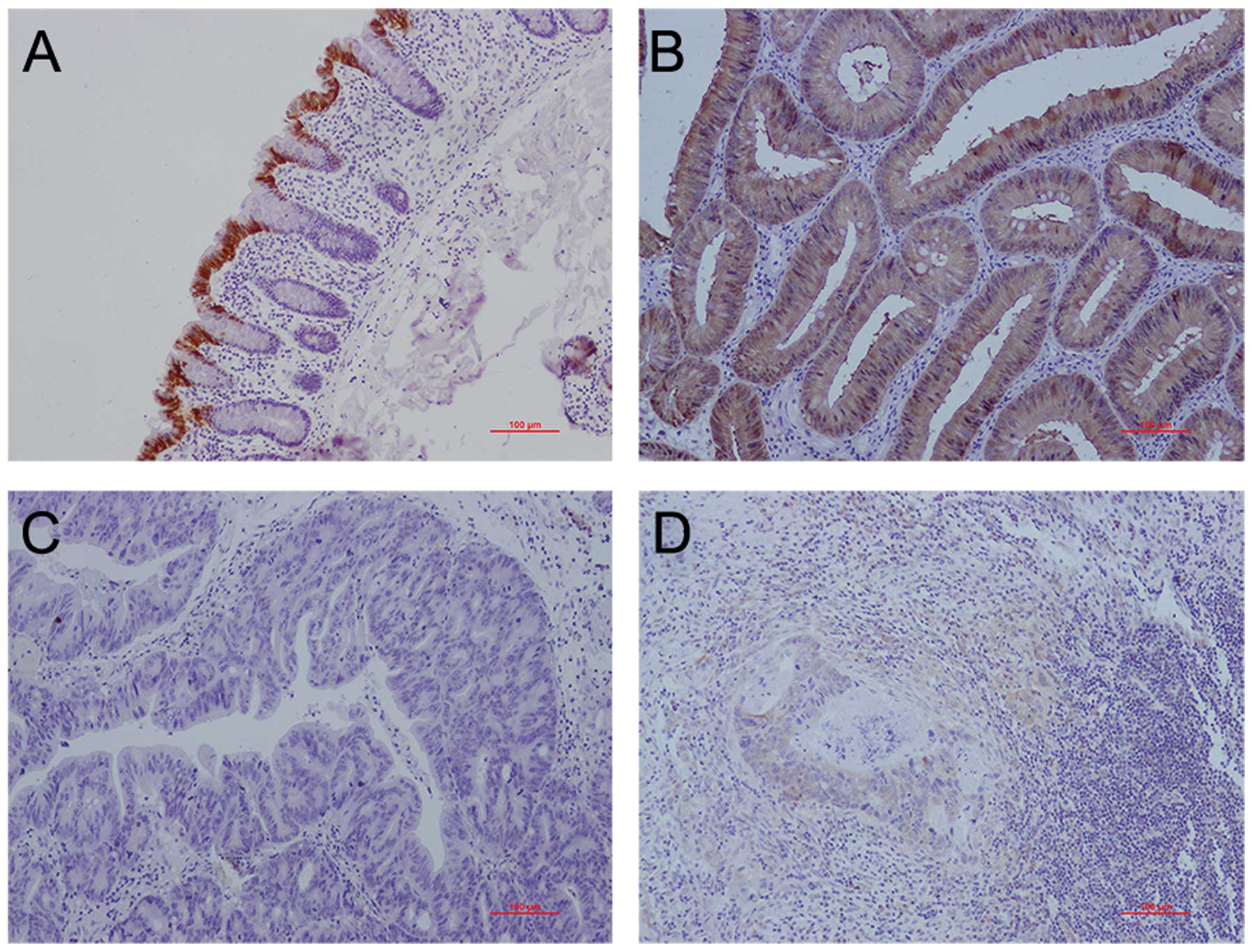
SELENBP1 protein expression was compared between the
samples of CRC tissues and the corresponding normal mucosa by
immunohistochemical staining. SELENBP1 was expressed in both the
cytoplasm and nucleus. SELENBP1 expression scores in CRC tissues
(n=83) and their paired normal mucosa (n=83) were 1.25±0.51 and
2.02±0.57, respectively (P<0.01). Of the 83 patients, in 56
patients (67.5%), SELENBP1 expression scores in CRC were lower than
those in the normal mucosa; while in 18 patients (21.7%) SELENBP1
expression scores in CRC were similar to those in the normal
mucosa.
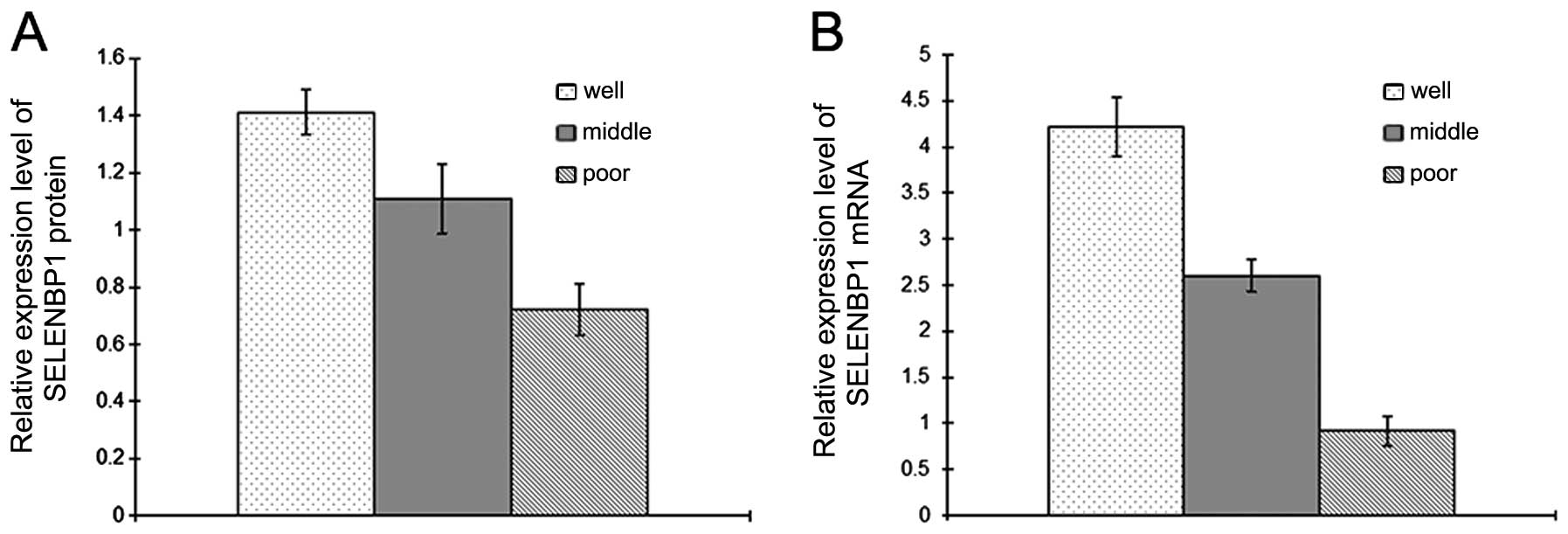
The SELENBP1 staining scores in well-differentiated
(n=17), moderately differentiated (n=44) and poorly differentiated
(n=22) CRC tissues were 1.75±0.53, 1.29±0.41 and 0.89±0.49,
respectively, with a significant difference (P<0.05). Among the
CRC samples analyzed, there were only 6 and 4 that were classified
as TNM stages I and IV respectively; therefore, early (I and II,
n=41) and advanced (III and IV, n=42) stages were pooled for
analysis. However, SELENBP1 staining scores were not significantly
different between TNM stages I/II (1.36±0.92) and III/IV
(1.13±1.03).
The staining scores of SELENBP1 were similar in the
benign polyps (1.97±0.57, n=33) and their paired normal mucosa
(2.06±0.59, n=28). However, the staining score in the benign polyps
was significantly higher than the score in CRC (0.96±0.59, n=28)
(P<0.01).
There was no significant difference between the
SELENBP1 expression score in LNM (1.27±0.67, n=26) and the score in
the primary cancer tissues (0.96±0.63, n=23) (P>0.05) (Fig. 4).
SELENBP1 expression is correlated with
degree of differentiation
SELENBP1 expression was compared between CRC samples
showing different levels of differentiation. Of the fresh frozen
CRC samples analyzed, 10 were well differentiated, while 14 were
moderately differentiated and 10 were poorly differentiated.
Western blot and real-time RT-PCR analyses showed that the
expression levels of SELENBP1 exhibited significant differences
(P<0.01) (Fig. 5).
SELENBP1 expression in NaB-induced
differentiated cells
To analyze the correlation between the degree of
differentiation and SELENBP1 expression, cultured cell lines were
induced to differentiate by NaB treatment. Expression levels of
SELENBP1 and CEA, and enzyme activity levels of AKP were assessed
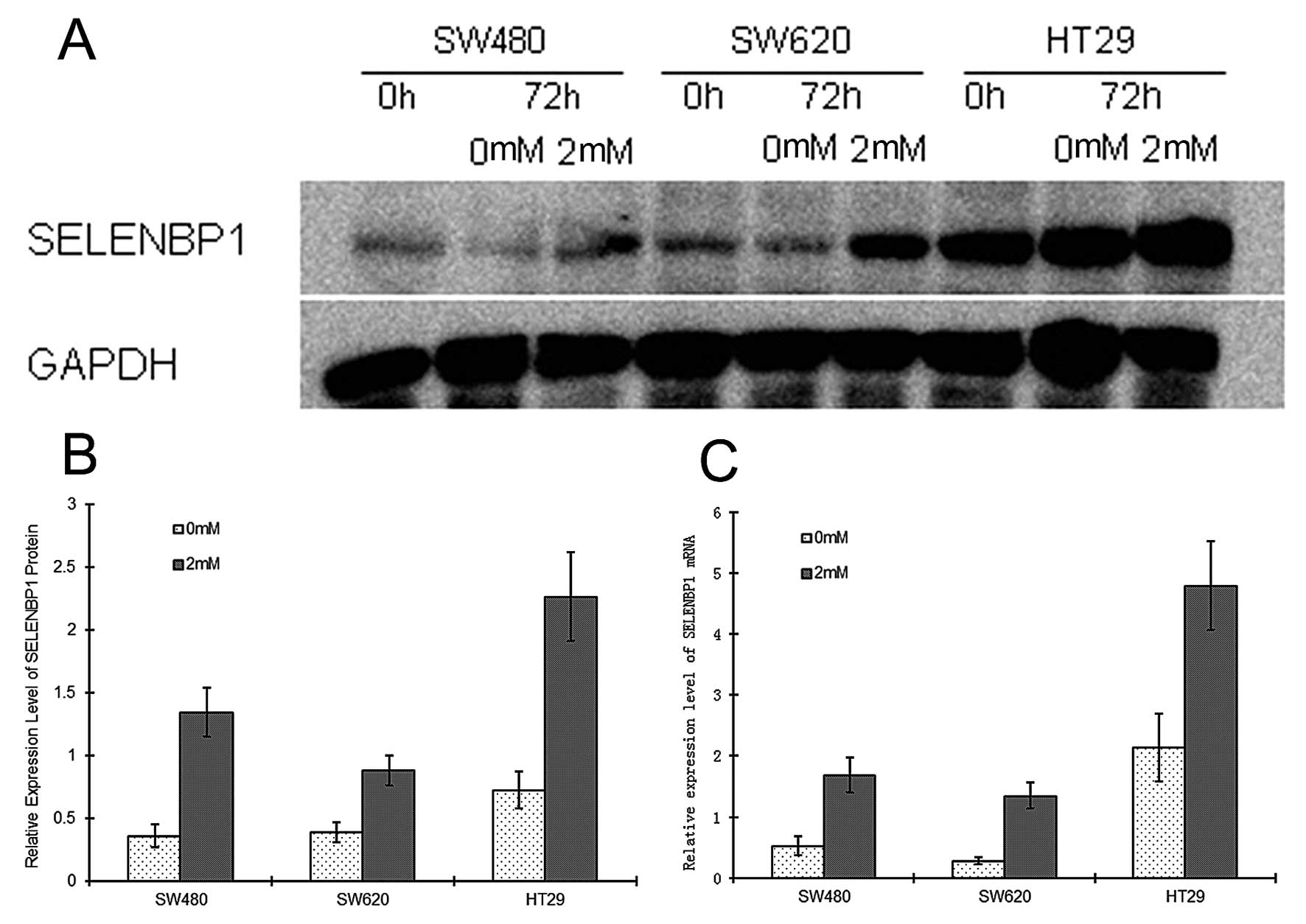
before and following treatment with NaB for 72 h. Western blot
analysis showed that SELENBP1 protein levels were similar in all
cell lines prior to NaB treatment, but were higher by ~3.73-, 2.25-
and 3.12-fold, in the SW480, SW620 and HT29 cells, respectively,
following NaB treatment (P<0.05) (Fig. 6). RT-PCR analysis also revealed that
SELENBP1 mRNA levels were higher by ~3.19-, 4.82- and 2.24-fold,
respectively, in these three cell lines (P<0.05) following NaB
treatment (Fig. 6).
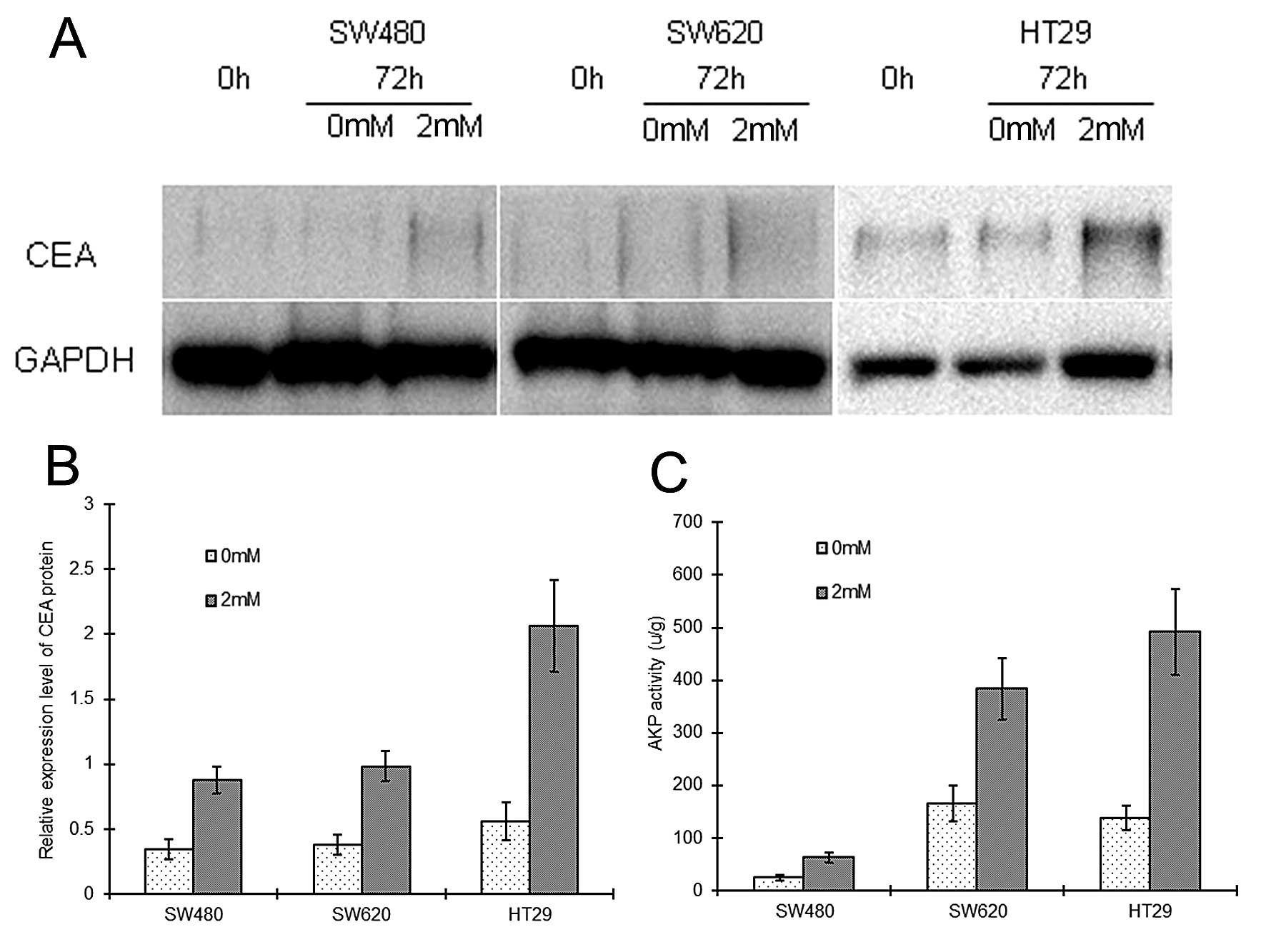
Similarly, following NaB treatment, SW480, SW620 and
HT29 cells showed upregulation of CEA protein by ~2.54-, 2.58- and
3.67-fold, respectively (P<0.05), and upregulation of AKP
activity by ~2.50-, 2.32-, and 3.55-fold, respectively (P<0.05)
(Fig. 7).
Epigenetic modifications and SELENBP1
expression
To investigate whether SELENBP1 is regulated by
epigenetic modification, cultured SW480, SW620 and HT29 cells were
incubated with the DNA demethylating agent 5-Aza-dC and/or the
histone deacetylase inhibitor, TSA.
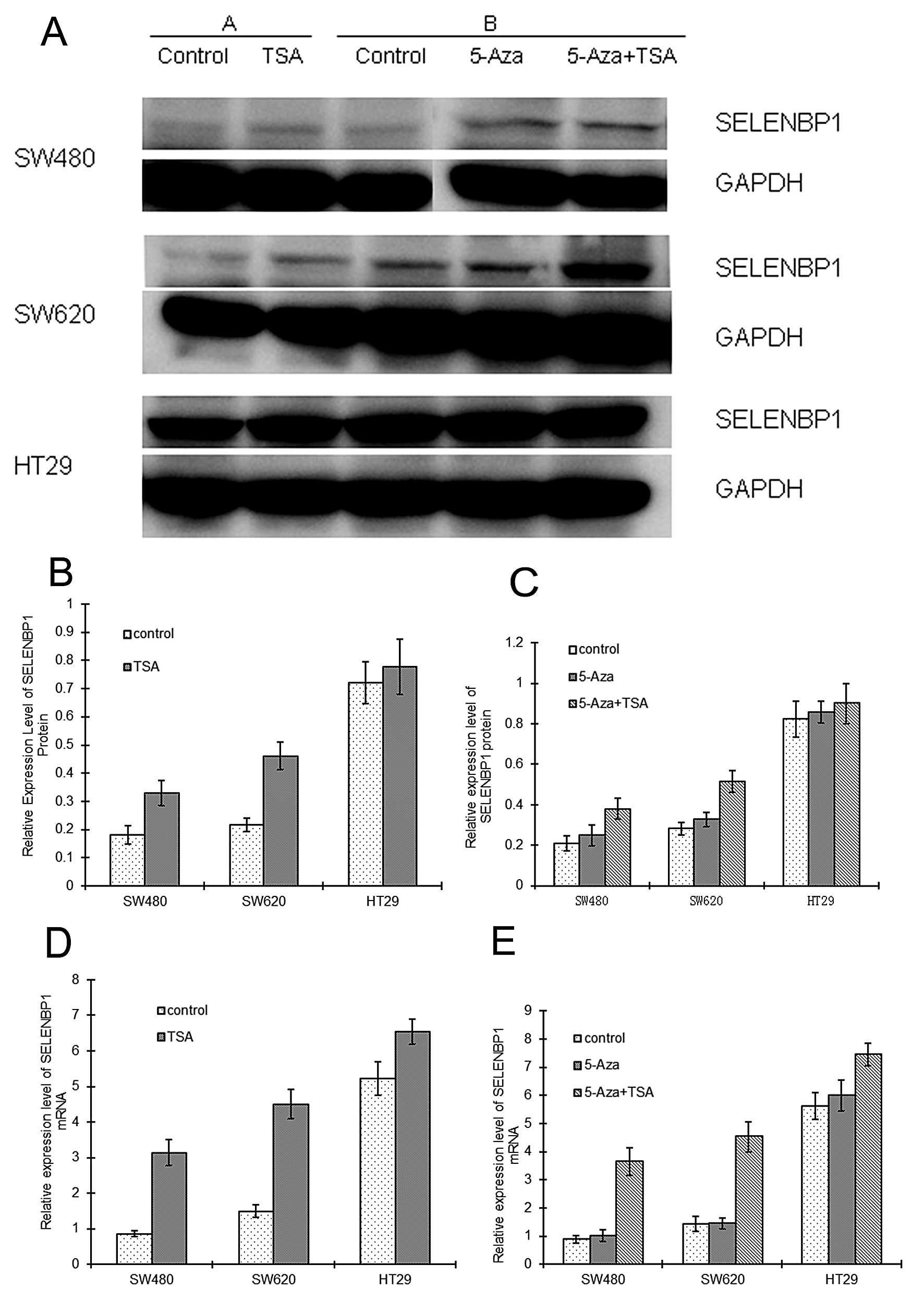
Following treatment with TSA alone, SELENBP1 protein
levels were higher by 1.82- and 2.12-fold on average in the SW480
and SW620 cells, respectively, when compared with these levels in
the corresponding untreated control cells (P<0.05), while in the
HT29 cells the change was not significant (Fig. 8). SELENBP1 mRNA levels were also
higher by 3.64- and 3.01-fold on average in the SW480 and SW620
cells, respectively, when compared with the levels in the untreated
control cells (P<0.05). Although SELENBP1 protein did not
significantly increase in the HT29 cells, the mRNA level was
significantly higher by 1.25-fold following TSA treatment
(P<0.05) (Fig. 8).
Treatment with 5-Aza-dC alone did not significantly
alter SELENBP1 protein and mRNA levels in any of the three cell
lines when compared with these levels in the untreated controls
(P>0.05) (Fig. 8). However,
following the combined treatment of 5-Aza-dC and TSA, SELENBP1
protein levels were higher by 1.82- and 1.83-fold on average in the
SW480 and SW620 cells, respectively, when compared to the levels in
the corresponding untreated control cells (P<0.05). In the HT29
cells, SELENBP1 protein levels increased slightly, but
insignificantly. SELENBP1 mRNA levels were higher by 4.13-, 3.16-
and 1.32-fold on average in the SW480, SW620 and HT29 cells,
respectively, when compared with the levels in the corresponding
untreated control cells (P<0.05) (Fig. 8).
Discussion
CRC is one of the most commonly diagnosed malignant
diseases worldwide. Although great progress has been achieved in
ensuring early diagnosis and treatment, many patients still succumb
to CRC due to cancer progression and metastasis. Understanding the
molecular alterations underlying CRC is critical for identifying
new biomarkers and therapeutic targets that may ultimately lead to
individualized cancer treatment.
Proteomic analysis is one of the most effective and
high-throughput methods by which to screen novel biomarkers for
cancer (3,4,14,15).
In the present study, proteomic analysis showed SELENBP1 to be one
of the significantly downregulated proteins in CRC. Furthermore,
western blot analysis and real-time RT-PCR of CRC patient tissue
samples confirmed this finding. SELENBP1 protein and mRNA levels
were lower in established cancer cell lines when compared with
levels in the HCoEpiC cell line. SELENBP1 was demonstrated to be
significantly downregulated not only in CRC (4,5), but
also in lung, ovarian, esophageal, gastric and liver cancer by
different research groups (2,3,6–8).
Immunohistochemical staining showed slightly higher
expression of SELENBP1 in TNM stages I/II than in stages III/IV,
but the difference was not significant. The difference in
immunohistochemical staining for SELENBP1 was similar between
normal mucosa and benign polyps, but was higher in benign polyps
than in cancer tissues. The difference in SELENBP1 staining between
primary cancer tissues and lymph node metastasis was not
significant. These results are similar to those reported by Kim
et al (4). SW480 and SW620
cells were obtained from the same patient, but from primary and
lymph node metastasis. The similarity of SELENBP1 expression levels
in the two cell lines also confimed the results.
However, in contrast to the report by Kim et
al (4), our IHC analysis showed
significantly differential expression of SELENBP1 among well,
moderately and poorly differentiated CRC tissues from freshly
obtained samples. SELENBP1 had the strongest expression in
terminally differentiated epithelial cells on the luminal surface
of crypts in normal mucosa (Fig.
4). Li et al (5) also
demonstrated that SELENBP1 was upregulated during differentiation
and that its downregulation by small interfering RNA in colonic
cells was associated with reduced expression of the differentiation
marker, CEA.
Sodium butyrate (NaB) has been widely used as a
differentiation inducer in CRC research (16–18).
In the present study, NaB treatment led to reactivation of
expression of SELENBP1 protein and mRNA, concomitant with
upregulation of the differentiation indicators, CEA and AKP
(5,17,18).
One of the differentiation characteristics of colon epithelium is
the appearance of or a significant increase in brush border hydrola
enzyme activities, such as AKP. Cell lines with high expression of
CEA were found to exhibit differentiation-associated morphologic
changes and decreased cell growth and de novo tumor
formation in nude mouse xenografts (18).
In the present study, SELENBP1 expression was not
affected by treatment with 5-Aza-dC alone, but was significantly
upregulated following treatment with TSA alone or combined with
5-Aza-dC. Since NaB and TSA are both HDAC inhibitors (19–21),
we hypothesized that the downregulation of SELENBP1 was mainly due
to histone deacetylation that occurred following treatment with
these two agents and during differentiation. Pohl et al
(22) showed that treatment with 10
or 30 μM 5-Aza-dC for 72 or 96 h, significantly reactivated
SELENBP1 in the cancer cell line HCT116. Further research
concerning the relationship between SELENBP1 and histone
deacetylation is warranted.
Cancer is a type of differentiation disorder
disease. Inducing cancer cells to re-differentiate is a concept
that is being pursued for cancer therapy (23). Although 5-Aza-dC is routinely
applied at a concentration of 5 μM for epigenetic modification
research (24–26), as a cancer therapeutic, the correct
dosage and the assocated side-effects of a differentiation inducer
must be determined.
Collectively, our results indicate that SELENBP1
expression is downregulated in CRC, is associated with
differentiation, and can be reactivated by NaB and TSA. Therefore,
SELENBP1 is a potential new target for pharmacological intervention
for CRC.
Acknowledgements
The present study was supported by the General
Project of Liaoning Province Department of Education
(L2010600).
References
|
1
|
Wang N, Chen Y, Han Y, et al: Proteomic
analysis shows down-regulations of cytoplasmic carbonic anhydrases,
CAI and CAII, are early events of colorectal carcinogenesis but are
not correlated with lymph node metastasis. Tumori. 98:783–791.
2012.PubMed/NCBI
|
|
2
|
Chen G, Wang H, Miller CT, et al: Reduced
selenium-binding protein 1 expression is associated with poor
outcome in lung adenocarcinomas. J Pathol. 202:321–329. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang KC, Park DC, Ng SK, et al: Selenium
binding protein 1 in ovarian cancer. Int J Cancer. 118:2433–2440.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim H, Kang HJ, You KT, et al: Suppression
of human selenium-binding protein 1 is a late event in colorectal
carcinogenesis and is associated with poor survival. Proteomics.
6:3466–3476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li T, Yang W, Li M, et al: Expression of
selenium-binding protein 1 characterizes intestinal cell maturation
and predicts survival for patients with colorectal cancer. Mol Nutr
Food Res. 52:1289–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silvers AL, Lin L, Bass AJ, et al:
Decreased selenium-binding protein 1 in esophageal adenocarcinoma
results from posttranscriptional and epigenetic regulation and
affects chemosensitivity. Clin Cancer Res. 16:2009–2021. 2010.
View Article : Google Scholar
|
|
7
|
Zhang J, Dong WG and Lin J: Reduced
selenium-binding protein 1 is associated with poor survival rate in
gastric carcinoma. Med Oncol. 28:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Stasio M, Volpe MG, Colonna G, et al: A
possible predictive marker of progression for hepatocellular
carcinoma. Oncol Lett. 2:1247–1251. 2011.PubMed/NCBI
|
|
9
|
Khare S and Verma M: Epigenetics of colon
cancer. Methods Mol Biol. 863:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae JM, Kim JH and Kang GH: Epigenetic
alterations in colorectal cancer: the CpG island methylator
phenotype. Histol Histopathol. 28:585–595. 2013.PubMed/NCBI
|
|
11
|
Dai Z and Jin Y: Promoter methylation of
the DLC1 gene and its inhibitory effect on human colon cancer.
Oncol Rep. 30:1511–1517. 2013.PubMed/NCBI
|
|
12
|
Enroth S, Rada-Iglesisas A, Andersson R,
et al: Cancer associated epigenetic transitions identified by
genome-wide histone methylation binding profiles in human
colorectal cancer samples and paired normal mucosa. BMC Cancer.
11:4502011. View Article : Google Scholar
|
|
13
|
Zhang S, Li F, Younes M, Liu H, Chen C and
Yao Q: Reduced selenium-binding protein 1 in breast cancer
correlates with poor survival and resistance to the
anti-proliferative effects of selenium. PLoS One. 8:e637022013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Linge A, Kennedy S, O’Flynn D, et al:
Differential expression of fourteen proteins between uveal melanoma
from patients who subsequently developed distant metastases versus
those who did not. Invest Ophthalmol Vis Sci. 53:4634–4643. 2012.
View Article : Google Scholar
|
|
15
|
Giusti L, Iacconi P, Da Valle Y, et al: A
proteomic profile of washing fluid from the colorectal tract to
search for potential biomarkers of colon cancer. Mol Biosyst.
8:1088–1099. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Luo HS and Xia H: Sodium butyrate
induces human colon carcinoma HT-29 cell apoptosis through a
mitochondrial pathway. J Int Med Res. 37:803–811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orchel A, Dzierzewicz Z, Parfiniewicz B,
Weglarz L and Wilczok T: Butyrate-induced differentiation of colon
cancer cells is PKC and JNK dependent. Dig Dis Sci. 50:490–498.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruan W, Zhu S, Wang H, et al: IGFBP-rP1, a
potential molecule associated with colon cancer differentiation.
Mol Cancer. 9:2812010.PubMed/NCBI
|
|
19
|
Li Q and Chen H: Silencing of Wnt5a during
colon cancer metastasis involves histone modifications.
Epigenetics. 7:551–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Jackson LN, Johnson SM, Wang Q and
Evers BM: Suppression of neurotensin receptor type 1 expression and
function by histone deacetylase inhibitors in human colorectal
cancers. Mol Cancer Ther. 9:2389–2398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krishnan M, Singh AB, Smith JJ, et al:
HDAC inhibitors regulate claudin-1 expression in colon cancer cells
through modulation of mRNA stability. Oncogene. 29:305–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pohl NM, Tong C, Fang W, Bi X, Li T and
Yang W: Transcriptional regulation and biological functions of
selenium-binding protein 1 in colorectal cancer in vitro and in
nude mouse xenografts. PLoS One. 4:e77742009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pierce GB and Johnson LD: Differentiation
and cancer. In Vitro. 7:140–145. 1971. View Article : Google Scholar
|
|
24
|
Meng CF, Zhu XJ, Peng G and Dai DQ: Role
of histone modifications and DNA methylation in the regulation of
O6-methylguanine-DNA methyltransferase gene
expression in human stomach cancer cells. Cancer Invest.
28:331–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brait M, Ling S, Nagpal JK, et al:
Cysteine dioxygenase 1 is a tumor suppressor gene silenced by
promoter methylation in multiple human cancers. PLoS One.
7:e449512012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roy S, Levi E, Majumdar AP and Sarkar FH:
Expression of miR-34 is lost in colon cancer which can be
re-expressed by a novel agent CDF. J Hematol Oncol. 5:582012.
View Article : Google Scholar : PubMed/NCBI
|