Introduction
Endometrial carcinoma (EC) is one of the most common
malignancies that occur in the female reproductive system. In the
US, it is estimated that ~49,560 new cases of EC with 8,190 deaths
occurred in 2013 (1). In Korea, its
incidence has markedly increased, accounting for ~16% of total
gynecologic malignancies (2). There
are two types of EC depending on the clinicopathological
characteristics (3,4). Type I ECs are characterized by the
endometrioid histology, accounting for ~80% of total ECs. It is
known that type I ECs are hormone dependent, show a predilection in
younger patients and are associated with a favorable prognosis. In
addition, they are also characterized by a high incidence of
loss-of-function alterations in the PTEN tumor suppressor gene as
well as defects in DNA mismatch repair genes. By contrast, type II
ECs usually have non-endometrioid histology, are not estrogen
dependent, are seen in older patients and are associated with a
poor prognosis. In addition, they are likely to harbor p53
mutation. The prognosis of EC is dependent on several factors,
including the stage, histologic grade, histopathologic subtype and
invasion of myometrium (5).
The S100 gene family located on chromosome 1q21,
comprises >20 members whose protein sequences encompass at least
one EF-hand Ca++ binding motif. It has been reported to
be involved in a variety of physiological functions, such as cell
proliferation, extracellular signal transduction, intercellular
adhesion and motility as well as cancer metastasis (6–8).
S100A4 belongs to the S100 calcium binding protein family and may
be characterized as a cytoplasmic protein that promotes cellular
motility via direct interaction with myosin-IIA and also has
functions in cell cycle progression. Its overexpression has been
documented in breast (9–12), gastric (13), colorectal (14–16),
esophageal squamous cell (17) and
gallbladder carcinoma (18),
ovarian (19) and bladder cancer
(20), papillary thyroid carcinoma
(21,22) and non-small cell lung cancer
(23). In addition, its
upregulation has been associated with disease progression,
metastasis and decreased patient survival. However, little is known
about the exact mechanisms of its action.
In human ECs, however, there have been few findings
on the expression and subcellular localization of S100A4. We found
only one report on its expression in EC (24). This showed that the level of S100A4
mRNA on quantitative real-time reverse transcription-polymerase
chain reaction (qRT-PCR) was significantly higher in the grade 3
EC, uterine papillary serous carcinoma and malignant mixed
Müllerian tumor (MMMT) compared with grade 1 or 2 EC and normal
endometrium. This was also demonstrated in immunohistochemistry. It
has also been reported that the positive immunoreactivity for
S100A4 was seen in the cytoplasm of cancer cells and overexpressed
in the grade 3 ECs, uterine papillary serous carcinoma and uterine
MMMT.
Based on the above background, we conducted the
present study to evaluate the prognostic value of S100A4 and to
examine its correlation with the clinicopathological parameters. To
perform this, we carried out immunohistochemistry to determine the
expression and localization of S100A4 protein and quantified its
mRNA levels using qRT-PCR.
Materials and methods
Patients and tissue samples
Formalin-fixed paraffin-embedded specimens were
selected from 135 patients with EC who were diagnosed with EC and
underwent surgical resection between January 1998 and December 2009
at Pusan National University Hospital, Busan, Korea. Of these,
eleven cases of tumor and normal endometrial tissue were collected
immediately after surgery, cut into small pieces, frozen in liquid
nitrogen and stored at −70°C until use for qRT-PCR. The
biospecimens for this study were provided by the Pusan National
University Hospital, a member of the National Biobank of Korea,
which is supported by the Ministry of Health, Welfare and Family
Affairs. All samples derived from the National Biobank of Korea
were obtained with informed consent under institutional review
board-approved protocols.
Based on the primary pathology reports and the
medical records of the patients, we collected clinicopathological
data such as age, gender, tumor grading, histologic type, stage,
lymphovascular invasion and lymph node metastasis. Surgical staging
was performed based on the International Federation of Gynecology
and Obstetrics (FIGO) criteria for EC; the stage I and II–IV were
considered the early- and advanced-stage EC. Moreover, the
histologic types and grades of EC were determined based on the
World Health Organization (WHO) criteria. The overall survival (OS)
was calculated from the date of surgery to the date of mortality or
the last follow-up visit. The progression-free survival (PFS) was
calculated from the date of surgery to the date of relapse or
progression of EC.
The present study was approved by the Institutional
Review Board (IRB) at Pusan National University Hospital after
obtaining informed consent.
Immunohistochemistry
Each slide was deparaffinized and rehydrated
according to the standard procedure and then treated with 0.01 M
sodium citrate buffer (pH 6.0) in a microwave for 5 min. This was
followed by a 5-min cooling. This process was performed three
times. Then, the slides were incubated for 1 h at room temperature
using rabbit polyclonal anti-S100A4 (1:100; Dako, Carpinteria, CA,
USA), rabbit monoclonal anti-estrogen receptor (ER) (SP1; 1:200),
rabbit monoclonal anti-progesterone receptor (PR) (SP2; 1:200) and
rabbit monoclonal anti-p53 (SP5; 1:100) (all from Lab Vision,
Fremont, CA, USA). The EnVision Detection System (Dako) was used to
detect the antibody responses according to the manufacturer’s
recommended protocol. The reaction products were visualized with
diaminobenzidine (DAB) as a chromogen and then counterstained with
Mayer’s hematoxylin. For negative controls, we replaced the primary
antibody with the phosphate-buffered saline (PBS).
Assessment of immunohistochemical
staining
Evaluation of immunohistochemical staining was
performed by two independent pathologists. S100A4 immunoreactivity
was characterized by cytoplasmic and/or nuclear staining in tumor
cells. The results of the immunohistochemical staining for S100A4
were evaluated based on the intensity of immunohistochemistry
(none, 0; weak, 1+; moderate, 2+; or strong, 3+) as well as the
percentage of positively stained tumor cells (0, none; 1, <10%;
2, 10–49%; and 3, 50–100%). These two values were multiplied and
the results served as the immunoreactive score; the negative and
positive immunoreactivity were defined as 0 or 1 point and ≥2
points, respectively.
The degree of the expression of ER, PR and p53 was
evaluated according to the percentage of tumor cells with nuclei
showing positive immunoreactivity. When >10% of tumor cells were
positively stained, the tumor was considered positive expression.
On the other hand, the tumor was considered negative expression
when <10% of tumor cells were positively stained.
Quantitative real-time PCR (qRT-PCR)
Total tissue RNA was extracted from frozen tissue
samples using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). cDNA
synthesis was performed using the QuantiTect Reverse Transcription
kit (Qiagen). qRT-PCR analysis was performed according to the
manufacturer’s instructions (QuantiSpeed SYBR kit; PKT, Seoul,
Korea). β-actin was applied as an internal control. The primers for
β-actin (205 bp) were: 5′-TGACGTGGACATC CGCAAAG-3′ (sense) and
5′-CTGGAAGGTGGACAGCG AGG-3′ (antisense). The primers for S100A4
(185 bp) were: 5′-GCCCTGGATGTGATGGTGT-3′ (sense) and 5′-TCGTT
GTCCCTGTTGCTGTC-3′ (antisense). cDNA was amplified with an initial
denaturation at 95°C for 3 min followed by the sequential cycles of
denaturation at 95°C for 5 sec, annealing at 60°C for 10 sec, and
extension at 72°C for 10 sec for 45 cycles, with final extension at
72°C for 5 min. Each assay was carried out in triplicate and
results were averaged. For relative quantification,
2−ΔΔCt was calculated and used as an indication of the
relative expression levels.
Statistical analysis
We used the Pearson’s Chi-square test to analyze the
correlation between the expression of S100A4 and various
clinicopathological parameters. In addition, we also analyzed the
OS and PFS using the Kaplan-Meier method. Furthermore, we performed
the Cox regression analysis to determine prognostic factors.
Statistical analysis was carried out using SPSS (version 14; SPSS,
Inc., Chicago, IL, USA). A P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Clinicopathological features are represented in
Table I. Based on the FIGO
criteria, there were 95 cases of stage I EC, 19 cases of stage II
EC, 18 cases of stage III EC and three cases of stage IV EC.
Histopathologic grading showed that there were 44 cases of G1, 58
cases of G2 and 33 cases of G3.
 | Table IClinicopathological features of
endometrial carcinoma patients (n=135). |
Table I
Clinicopathological features of
endometrial carcinoma patients (n=135).
| Parameters | n (%) |
|---|
| Histologic type |
| Endometrioid | 123 (91.1) |
| Serous | 8 (5.9) |
| Clear cell | 3 (2.2) |
|
Undifferentiated | 1 (0.8) |
| Histologic grade |
| 1 | 44 (32.6) |
| 2 | 58 (43.0) |
| 3 | 33 (24.4) |
| Myometrial
invasion |
| <1/2 of
myometrium | 88 (65.2) |
| ≥1/2 of
myometrium | 47 (34.8) |
| FIGO stage |
| I | 95 (70.4) |
| II | 19 (14.1) |
| III | 18 (13.3) |
| IV | 3 (2.2) |
| LN metastasis |
| Absent | 117 (86.7) |
| Present | 18 (13.3) |
| Disease
progression |
| Progression
free | 107 (79.3) |
| Progression | 28 (20.7) |
| Overall survival |
| Alive | 124 (91.9) |
| DOD | 11 (8.1) |
| ER expression |
| No | 58 (43.0) |
| Yes | 77 (57.0) |
| PR expression |
| No | 41 (30.4) |
| Yes | 94 (69.6) |
| p53 expression |
| No | 100 (74.1) |
| Yes | 35 (25.9) |
In our series, the median follow-up period was 43
months (range, 0.25–131 months). Based on the disease progression,
we classified the patients into two groups: the progression group
(n=28) (20.7%), comprising those who developed either recurrence or
metastasis, and the progression-free group (n=107) (79.3%),
comprising those who were free of progression. In addition, there
were 124 (91.9%) survivors at the last follow-up.
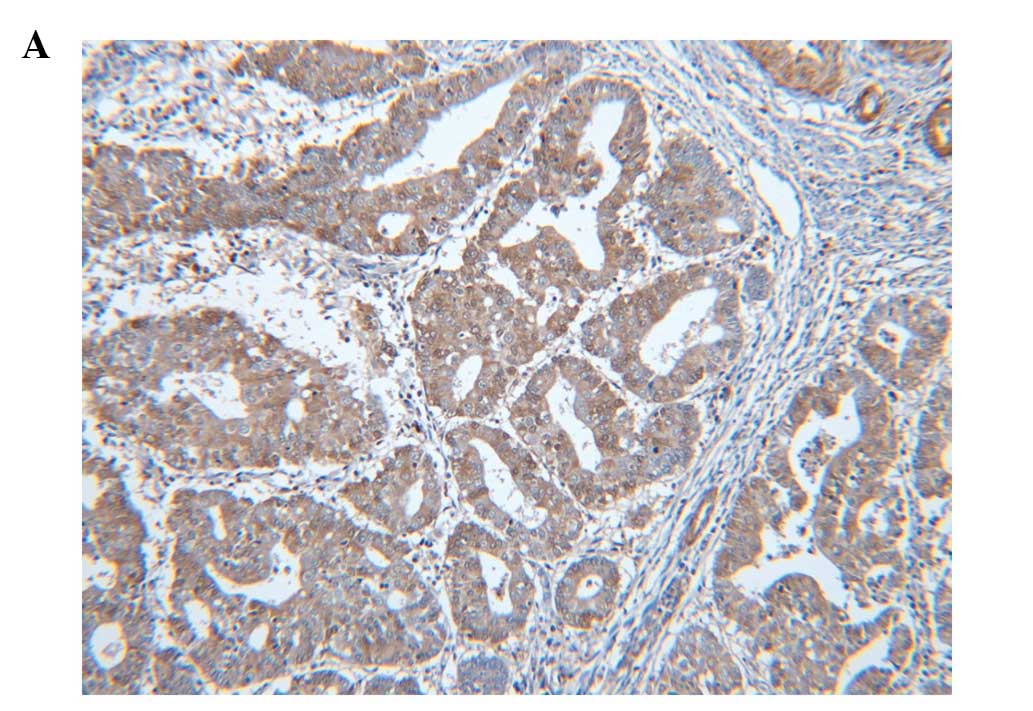
The positive immunoreactivity for S100A4 was
commonly seen in the cytoplasm of tumor cells. However, there were
some cases in which cytoplasm and nucleus showed a positive
immunoreactivity. S100A4 protein was also expressed in histiocytes,
lymphocytes, fibroblasts and endothelial cells as well as tumor
cells. In the normal endometrium, S100A4 protein immunoreactivity
was clearly absent in both cytoplasm and nucleus. Of the total
cases (n=135), 35 (25.9%) showed positive immunoreactivity for
S100A4 (Fig. 1). The positive
immunoreactivity for S100A4 was associated with histologic type and
grade, the FIGO stage and lymph node metastasis. Non-endometrioid
histologic type exhibited S100A4 expression more frequently than
endometrioid type. Reduced expression of PR was significantly
correlated with S100A4 protein expression. There was no significant
correlation between the expression of S100A4 and that of ER.
Moreover, there was no significant correlation between the
expression of S100A4 and p53. Correlations between the expression
of S100A4 and clinicopathological findings are summarized in
Table II.
 | Table IIAssociation between positive
expression of S100A4 and clinicopathological features (n=135). |
Table II
Association between positive
expression of S100A4 and clinicopathological features (n=135).
| S100A4
expression | |
|---|
|
| |
|---|
| Parameters | No (%) | Yes (%) | P-value |
|---|
| Histologic
type | | | 0.002 |
| Endometrioid | 96 (78.0) | 27 (22.0) | |
|
Non-endometrioid | 4 (33.3) | 8 (66.7) | |
| Histologic
grade | | | 0.000 |
| Low (grade 1) | 38 (86.4) | 6 (13.6) | |
| High (grade
2/3) | 62 (68.1) | 29 (31.9) | |
| Myometrial
invasion | | | 0.149 |
| <1/2 | 69 (78.4) | 19 (21.6) | |
| ≥1/2 | 31 (66.0) | 16 (34.0) | |
| FIGO stage | | | 0.002 |
| I | 78 (82.1) | 17 (17.9) | |
| II–IV | 22 (55.0) | 18 (45.0) | |
| LN metastasis | | | 0.007 |
| Absent | 92 (78.6) | 25 (21.4) | |
| Present | 8 (44.4) | 10 (55.6) | |
| Disease
progression | | | 0.040 |
| Progression
free | 86 | 24 | |
| Progression | 14 | 11 | |
| Overall
survival | | | 0.001 |
| Alive | 97 | 27 | |
| DOD | 3 | 8 | |
| ER expression | | | 0.552 |
| No | 41 (70.7) | 17 (29.3) | |
| Yes | 59 (71.4) | 18 (28.6) | |
| PR expression | | | 0.003 |
| No | 23 (56.1) | 18 (43.9) | |
| Yes | 77 (81.9) | 17 (18.1) | |
| p53 expression | | | 0.262 |
| No | 77 (77.0) | 23 (23.0) | |
| Yes | 23 (65.7) | 12 (34.3) | |
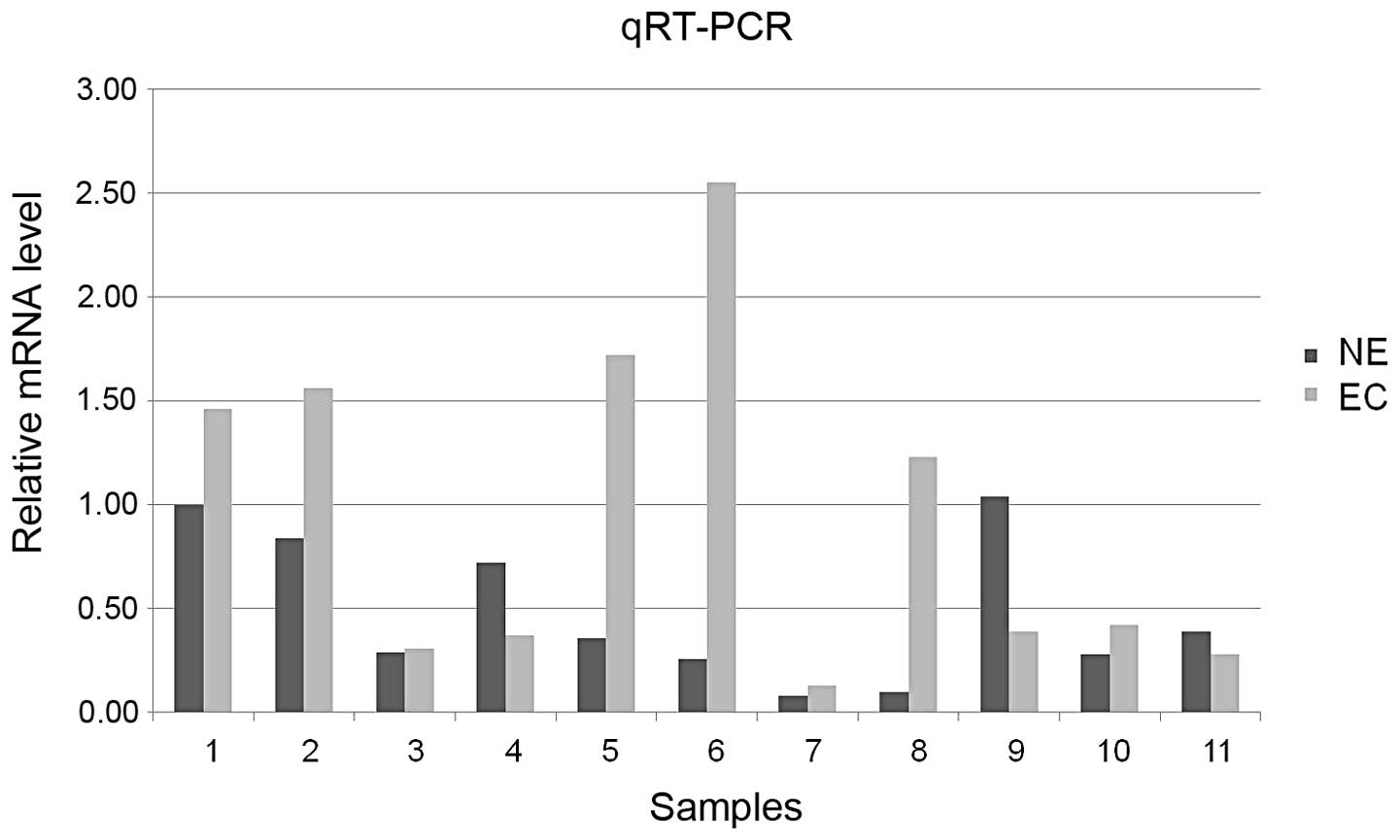
qRT-PCR revealed that the level of S100A4 mRNA was
higher in EC as compared with normal endometrium (7/11, 63.6%),
which is consistent with the degree of expression of S100A4 protein
(Fig. 2).
The Kaplan-Meier survival analysis showed that poor
OS and PFS were associated with histologic grade, the FIGO stage,
myometrial invasion, lymph node metastasis, ER and PR expression
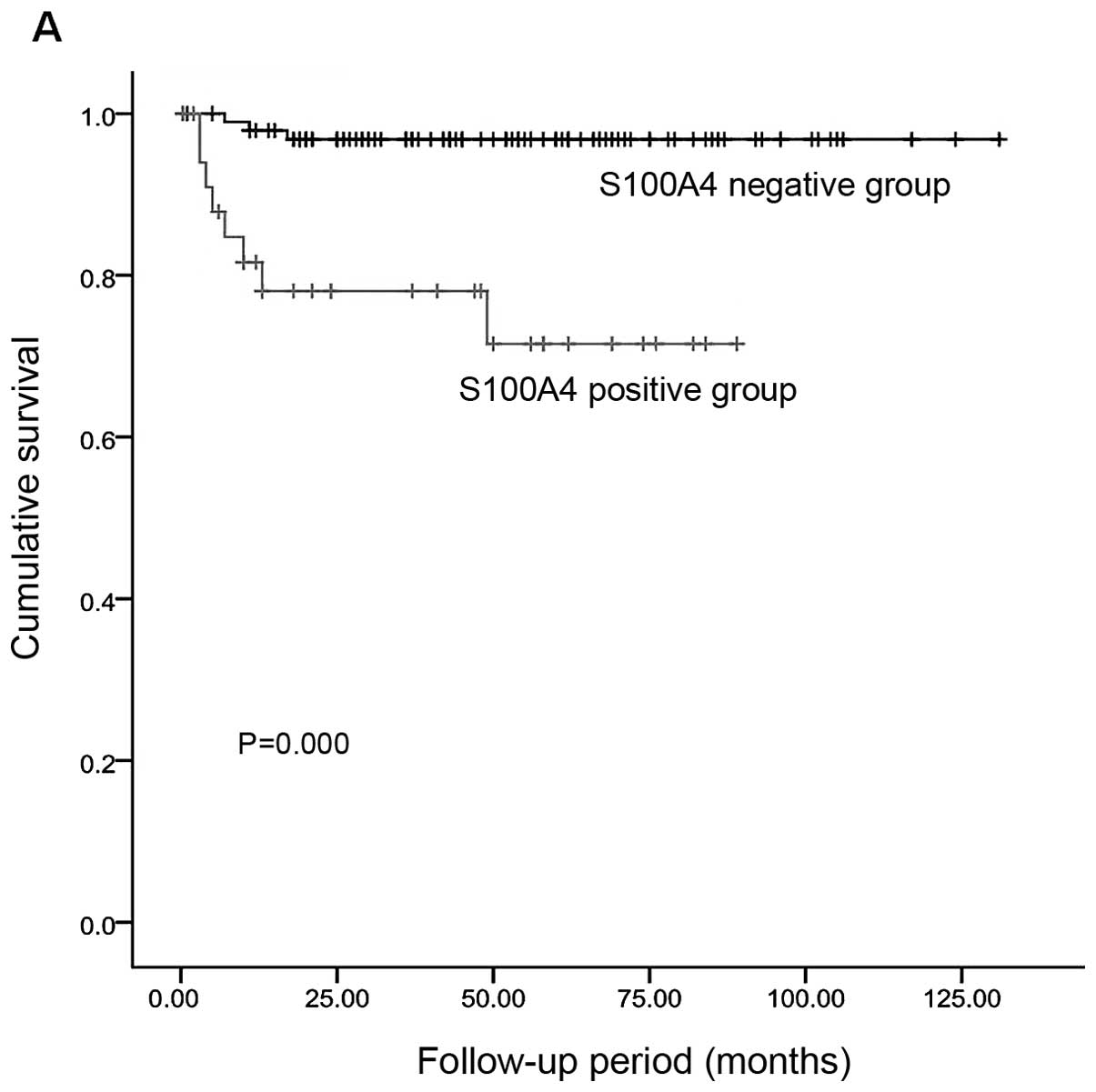
and positive immunoreactivity for S100A4 (Table III). Disease progression was
observed in 37.1% (13/35) of the patients with expression of S100A4
and in 15.0% (15/100) of those with no S100A4 expression. This
difference reached a statistical significance (P=0.000). Moreover,
the proportion of disease-related deaths was 22.9% (8/35) in the
patients with S100A4 expression and 3.0% (3/100) in those with no
S100A4 expression (P=0.000) (Fig.
3). There was a negative correlation between the expression of
ER and PR and the OS and PFS. In multivariate analysis of the
variables defined in Table IV,
there was a significant correlation between the cytoplasmic
expression of S100A4 and shorter OS after the adjustment for the
FIGO stage, lymph node metastasis, myometrial invasion and the
expression of ER and PR, all of which were significant variables on
univariate analysis. The cytoplasmic expression of S100A4 was not
significantly correlated to PFS in multivariate analysis (Table V).
 | Table IIIUnivariate analysis of
clinicopathological features, including S100A4 immunoreactivity and
OS and PFS (n=135). |
Table III
Univariate analysis of
clinicopathological features, including S100A4 immunoreactivity and
OS and PFS (n=135).
| Overall
survival | Progression-free
survival |
|---|
|
|
|
|---|
| Alive (n=124) | DOD (n=11) | P-value | Progression-free
(n=107) | Progression
(n=28) | P-value |
|---|
| Histologic
type | | | 0.278 | | | 0.287 |
| Endometrioid | 114 (92.7) | 9 (7.3) | | 99 (80.5) | 24 (19.5) | |
|
Non-endometrioid | 10 (83.3) | 2 (16.7) | | 8 (66.7) | 4 (33.3) | |
| Histologic
grade | | | 0.078 | | | 0.012 |
| Low (grade 1) | 43 (97.7) | 1 (2.3) | | 40 (90.9) | 4 (9.1) | |
| High (grade
2/3) | 81 (89.0) | 10 (11.0) | | 67 (73.6) | 24 (26.4) | |
| Myometrial
invasion | | | 0.004 | | | 0.000 |
| <1/2 | 85 (96.6) | 3 (3.4) | | 78 | 10 | |
| ≥1/2 | 39 (83.0) | 8 (17.0) | | 29 | 18 | |
| FIGO stage | | | 0.000 | | | 0.000 |
| I | 95 (100) | 0 (0) | | 86 (90.5) | 9 | |
| II–IV | 29 (72.5) | 11 (27.5) | | 21 | 19 | |
| LN metastasis | | | 0.000 | | | 0.000 |
| Absent | 113 (96.6) | 4 (3.4) | | 100 | 17 | |
| Present | 11 (61.1) | 7 (38.9) | | 7 | 11 | |
| S100A4
expression | | | 0.000 | | | 0.000 |
| No | 97 (97.0) | 3 (3.0) | | 85 (85.0) | 15 (15.0) | |
| Yes | 27 (77.1) | 8 (22.9) | | 22 (62.9) | 13 (37.1) | |
| ER expression | | | 0.006 | | | 0.002 |
| No | 49 (84.5) | 9 (15.5) | | 39 | 19 | |
| Yes | 75 (97.4) | 2 (2.6) | | 68 | 9 | |
| PR expression | | | 0.001 | | | 0.000 |
| No | 33 (80.5) | 8 (19.5) | | 25 | 16 | |
| Yes | 91 (96.8) | 3 (3.2) | | 82 | 12 | |
| p53 expression | | | 0.120 | | | 0.066 |
| No | 94 (94.0) | 6 (6.0) | 83 | 17 | | |
| Yes | 30 (85.7) | 5 (14.3) | 24 | 11 | | |
 | Table IVMultivariate analysis of prognostic
factors for OS in EC. |
Table IV
Multivariate analysis of prognostic
factors for OS in EC.
| Variables | Grouping | P-value | Ratio of risk | 95% of CI |
|---|
| FIGO stage | I vs. II–IV | 0.016 | 0.898 | 0.000–1.136 |
| Myometrial
invasion | <1/2 vs.
≥1/2 | 0.250 | 0.435 | 0.105–1.800 |
| LN metastasis | Absent vs.
present | 0.032 | 0.180 | 0.038–0.859 |
| ER expression | No vs. yes | 0.051 | 25.676 | 0.980–67.286 |
| PR expression | No vs. yes | 0.083 | 0.095 | 0.007–1.354 |
| S100A4
expression | No vs. yes | 0.037 | 0.162 | 0.029–0.893 |
 | Table VMultivariate analysis of prognostic
factors for PFS in EC. |
Table V
Multivariate analysis of prognostic
factors for PFS in EC.
| Variables | Grouping | P-value | Ratio of risk | 95% of CI |
|---|
| Histologic
grade | Low vs. high | 0.244 | 0.502 | 0.157–1.602 |
| FIGO stage | I vs. II–IV | 0.040 | 3.374 | 1.059–10.744 |
| Myometrial
invasion | <1/2 vs.
≥1/2 | 0.023 | 0.357 | 0.147–0.869 |
| LN metastasis | Absent vs.
present | 0.034 | 0.366 | 0.144–0.925 |
| ER expression | No vs. yes | 0.076 | 0.392 | 0.139–1.102 |
| PR expression | No vs. yes | 0.207 | 0.423 | 0.111–1.611 |
| S100A4
expression | No vs. yes | 0.056 | 0.424 | 0.176–1.021 |
Discussion
In the present study, we immunohistochemically
examined whether S100A4 has a prognostic value in cases of EC. It
has been reported that the high degree of S100A4 expression is
correlated with a shorter prognosis in cases of cancer. In breast
cancer, the expression of S100A4 was increased in the metastatic
lesion (9,11,12).
Moreover, it has also been reported that the expression of S100A4
is an indicator of lymph node metastasis and tumor recurrence in
colorectal cancer. Thus, its prognostic value has been suggested
(14–16). There are several recent published
studies on the correlation between the survival rate and the
increased expression of S100A4 in other types of human malignancies
(17–23). These reports suggest that S100A4 is
associated with the aggressiveness of cancer and it may play a role
in the pathogenesis of advanced-stage cancer. However, there are
also some contradictory reports in this series. According to some
studies, there is no significant correlation between the expression
of S100A4 and OS in colon cancer, non-small cell lung carcinoma
(NSCLC) and melanoma (25–27).
There is a limited amount of data available on the
prognostic value and clinical implication of the expression of
S100A4 in patients with EC. Xie et al reported that the
level of S100A4 mRNA and the positive cytoplasmic immunoreactivity
of S100A4 were increased in patients with high grade EC (24). Consistent with the previous report,
we observed that the cytoplasmic expression of S100A4 and the level
of S100A4 mRNA were increased in EC compared to the normal
endometrium. We also analyzed the correlation between the
expression of S100A4 and the clinicopathological parameters. The
present study is of significance in that we first analyzed the
correlation between the immunohistochemical properties of S100A4
and survival in patients with EC. Our results showed that 25.9% of
the patients with EC had positive immunoreactivity for S100A4. In
our series, the positive immunoreactivity for S100A4 had a
significant correlation with higher histologic grade, FIGO stage
and lymph node metastasis. This suggests that it plays a role in
progression in EC. The OS and PFS were significantly shorter in the
patients with positive immunoreactivity for S100A4 as compared with
their negative counterparts. In multivariate analysis, the positive
immunoreactivity for S100A4 had a significant correlation with
shorter OS after the adjustment of the FIGO stage, the depth of
myometrial invasion, lymph node metastasis, and ER and PR
expression, all of which were significant variables in univariate
analysis. These results suggest that the expression of S100A4 may
be an indicator of tumor progression as well as poor prognosis.
The loss of steroid receptors has been considered an
indicator of the aggressiveness of ‘hormone-dependent cancers’ such
as breast cancer and EC. It has been previously shown that the
expression of S100A4 was associated with the loss of ER in breast
cancer (11,12). On the other hand, Xie et al
failed to demonstrate an inverse correlation between the expression
of S100A4 and that of the steroid hormone receptors, thus
suggesting that the expression of S100A4 was not subject to the
hormonal status (24). Our results
showed that the expression of S100A4 had a significant correlation
with loss of PR rather than that of ER. Further studies are
therefore warranted to clarify the correlation between the
expression of S100A4 and that of steroid hormone receptors in
patients with EC.
There have been attempts to clarify the mechanisms
of action of S100A4. To explain this, it has been hypothesized that
the hypomethylation of the S100A4 gene leads to the increased
expression of S100A4. It has been reported that hypomethylation of
the S100A4 gene is associated with gene activation and
overexpression of S100A4 in human malignancies (28,29).
Moreover, it has also been reported that the methylation of the
S100A4 gene was detected in normal endometrium and grade 1 EC with
the decreased expression of S100A4. In grade 3 EC with increased
level of S100A4 mRNA and the increased expression of S100A4,
however, there was no methylation of the gene. These findings
indicated that hypomethylation may play a role in the progression
and aggressive behavior of EC (24). S100A4 binds to actin, non-muscle
myosin and the p53 tumor suppressor protein (7,30).
These interactions may increase the cell motility and modulate the
function of p53. Of note, S100A4 binds to p53 protein and thereby
inhibits its phosphorylation (16,30).
Presumably, S100A4 may be involved in the expression of p53 and the
inhibition of its activity to regulate the G1/S checkpoint pathway.
It has therefore been speculated that S100A4 may be involved in the
inhibition of the function of p53. In the present study, we also
examined the possible correlation between the expression of S100A4
and that of p53, considered a possible target of S100A4, in
patients with EC. As shown in the present study, however, we failed
to demonstrate such correlation using immunohistochemistry.
It has been reported that the positive cytoplasmic
expression of S100A irrespective of its nuclear expression has a
prognostic value in many human malignancies (9,11,12,14,16,21,22).
Kicuchi et al reported that S100A4 could translocate between
the cytoplasm and nucleus in ovarian cancer cells (19). Although little is known about the
exact mechanisms by which S100A4 translocates between the cytoplasm
and nucleus and its nuclear expression has a prognostic value in
carcinomas only in a limited scope, its nuclear localization has
been considered an indicator of poor prognosis in patients with
ovarian or colorectal cancer (19,31).
In conclusion, the positive cytoplasmic
immunoreactivity for S100A4 is closely associated with the
progression of EC. However, there is no correlation between the
expression of S100A4 and that of p53. The OS and PFS were
significantly shorter in the patients with positive
immunoreactivity for S100A4 as compared with their negative
counterparts. Based on the above results, it can be concluded that
the expression of S100A4 may be an indicator of poor prognosis in
patients with EC. Therefore, these patients should be given more
intensive care and meticulous monitoring of the clinical course.
Finally, our results indicate that S100A4 may be a biological
marker indicating the recurrence and poor prognosis in patients
with EC. However, further large-scale studies are warranted to
establish its prognostic value and clinical implications in
patients with EC.
Acknowledgements
This study was supported by a grant from the
National R&D Program for Cancer Control, the Ministry for
Health, and the Welfare and Family Affairs, Republic of Korea
(0920050). The biospecimens for this study were provided by the
Pusan National University Hospital, a member of the National
Biobank of Korea, which is supported by the Ministry of Health,
Welfare and Family Affairs. All samples derived from the National
Biobank of Korea were obtained with informed consent under
institutional review board-approved protocols.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Lee HP: Annual report of gynecologic
cancer registry program in Korea: 1991–2004. Korean J Obstet
Gynecol. 51:1411–1420. 2008.
|
|
3
|
Lax SF: Molecular genetic pathways in
various types of endometrial carcinoma: from a phenotypical to a
molecular-based classification. Virchows Arch. 444:213–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zannoni GF, Scambia G and Gallo D: The
dualistic model of endometrial cancer: the challenge of classifying
grade 3 endometrioid carcinoma. Gynecol Oncol. 127:262–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rose PG: Endometrial carcinoma. N Engl J
Med. 335:640–649. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schafer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: functions and
pathology. Trends Biochem Sci. 21:134–140. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mazzucchelli L: Protein S100A4: too long
overlooked by pathologists? Am J Pathol. 160:7–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt-Hansen B, Klingelhöfer J,
Grum-Schwensen B, Christensen A, Andresen S, Kruse C, Hansen T,
Ambartsumian N, Lukanidin E and Grigorian M: Functional
significance of metastasis-inducing S100A4(Mts1) in tumor-stroma
interplay. J Biol Chem. 279:24498–24504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ismail NI, Kaur G, Hashim H and Hassan MS:
S100A4 overexpression proves to be independent marker for breast
cancer progression. Cancer Cell Int. 8:122008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trujillo KA, Heaphy CM, Mai M, Vargas KM,
Jones AC, Vo P, Butler KS, Joste NE, Bisoffi M and Griffith JK:
Markers of fibrosis and epithelial to mesenchymal transition
demonstrate field cancerization in histologically normal tissue
adjacent to breast tumors. Int J Cancer. 129:1310–1321. 2011.
View Article : Google Scholar
|
|
11
|
Platt-Higgins AM, Renshaw CA, West CR,
Winstanley JH, De Silva Rudland S, Barraclough R and Rudland PS:
Comparison of the metastasis-inducing protein S100A4 (p9ka) with
other prognostic markers in human breast cancer. Int J Cancer.
89:198–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pedersen KB, Nesland JM, Fodstad Ø and
Maelandsmo GM: Expression of S100A4, E-cadherin, α- and β-catenin
in breast cancer biopsies. Br J Cancer. 87:1281–1286. 2002.
|
|
13
|
Wang YY, Ye ZY, Zhao ZS, Tao HQ and Chu
YQ: High-level expression of S100A4 correlates with lymph node
metastasis and poor prognosis in patients with gastric cancer. Ann
Surg Oncol. 17:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang LY, Xu Y, Cai GX, Guan ZQ, Sheng WQ,
Lu HF, Xie LQ, Lu HJ and Cai SJ: S100A4 over-expression underlies
lymph node metastasis and poor prognosis in colorectal cancer.
World J Gastroenterol. 17:69–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang YG, Jung CK, Lee A, Kang WK, Oh ST
and Kang CS: Prognostic significance of S100A4 mRNA and protein
expression in colorectal cancer. J Surg Oncol. 105:119–124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwak JM, Lee HJ, Kim SH, Kim HK, Mok YJ,
Park YT, Choi JS and Moon HY: Expression of protein S100A4 is a
predictor of recurrence in colorectal cancer. World J
Gastroenterol. 16:3897–3904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen D, Zheng XF, Yang ZY, Liu DX, Zhang
GY, Jiao XL and Zhao H: S100A4 silencing blocks invasive ability of
esophageal squamous cell carcinoma cells. World J Gastroenterol.
18:915–922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Ajiki T, Murao S, Kamigaki T,
Maeda S, Ku Y and Kuroda Y: Prognostic significance of S100A4
expression in gallbladder cancer. Int J Oncol. 20:937–941.
2002.PubMed/NCBI
|
|
19
|
Kikuchi N, Horiuchi A, Osada R, Imai T,
Wang C, Chen X and Konishi I: Nuclear expression of S100A4 is
associated with aggressive behavior of epithelial ovarian
carcinoma: an important autocrine/paracrine factor in tumor
progression. Cancer Sci. 97:1061–1069. 2006. View Article : Google Scholar
|
|
20
|
Davies BR, O’Donnell M, Durkan GC, Rudland
PS, Barraclough R, Neal DE and Mellon JK: Expression of S100A4
protein is associated with metastasis and reduced survival in human
bladder cancer. J Pathol. 196:292–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Min HS, Choe G, Kim SW, Park YJ, Park do
J, Youn YK, Park SH, Cho BY and Park SY: S100A4 expression is
associated with lymph node metastasis in papillary microcarcinoma
of the thyroid. Mod Pathol. 21:748–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou M, Al-Baradie RS, Al-Hindi H, Farid NR
and Shi Y: S100A4 (Mts1) gene overexpression is associated with
invasion and metastasis of papillary thyroid carcinoma. Br J
Cancer. 93:1277–1284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuna M, Kageyama S, Fukuoka J, Kitano H,
Doki Y, Tezuka H and Yasuda H: Significance of S100A4 as a
prognostic marker of lung squamous cell carcinoma. Anticancer Res.
29:2547–2554. 2009.PubMed/NCBI
|
|
24
|
Xie R, Loose DS, Shipley GL, Xie S,
Bassett RL Jr and Broaddus RR: Hypomethylation-induced expression
of S100A4 in endometrial carcinoma. Mod Pathol.
20:1045–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho YG, Kim CJ, Nam SW, Yoon SH, Lee SH,
Yoo NJ, Lee JY and Park WS: Overexpression of S100A4 is closely
associated with progression of colorectal cancer. World J
Gastroenterol. 11:4852–4856. 2005.PubMed/NCBI
|
|
26
|
Bolander A, Agnarsdóttir M, Wagenius G,
Strömberg S, Pontén F, Ekman S, Brattström D, Larsson A, Einarsson
R, Ullenhag G, Hesselius P and Bergqvist M: Serological and
immunohistochemical analysis of S100 and new derivatives as markers
for prognosis in patients with malignant melanoma. Melanoma Res.
18:412–419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung EA, Cho HD, Lee JH and Oh MH:
Clinicopathological significance of S100A4 expression in non-small
cell lung carcinomas. Korean J Pathol. 44:477–482. 2010. View Article : Google Scholar
|
|
28
|
Li Y, Liu ZL, Zhang KL, Chen XY, Kong QY,
Wu ML, Sun Y, Liu J and Li H: Methylation-associated silencing of
S100A4 expression in human epidermal cancers. Exp Dermatol.
18:842–848. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rehman I, Goodarzi A, Cross SS, Leiblich
A, Catto JW, Phillips JT and Hamdy FC: DNA methylation and
immunohistochemical analysis of the S100A4 calcium binding protein
in human prostate cancer. Prostate. 67:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grigorian M, Andresen S, Tulchinsky E,
Kriajevska M, Carlberg C, Kruse C, Cohn M, Ambartsumian N,
Christensen A, Selivanova G and Lukanidin E: Tumor suppressor p53
protein is a new target for the metastasis-associated Mts1/S100A4
protein: functional consequences of their interaction. J Biol Chem.
276:22699–22708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Flatmark K, Pedersen KB, Nesland JM,
Rasmussen H, Aamodt G, Mikalsen SO, Bjørnland K, Fodstad Ø and
Maelandsmo GM: Nuclear localization of the metastasis-related
protein S100A4 correlates with tumour stage in colorectal cancer. J
Pathol. 200:589–595. 2003. View Article : Google Scholar : PubMed/NCBI
|