Introduction
Glutathione peroxidase 3 (GPX3 or plasma GPX)
is a member of the glutathione peroxidase family of selenoproteins
and is one of the key defensive enzymes against oxidative damages
to host cells (1). A considerable
amount of research has been performed on the role of glutathione
peroxidases on cancer progression. Since the major biochemical role
of a hydroperoxide is to regulate the characteristics of cancer
cells including proliferation, invasion, migration, angiogenesis
and apoptosis, GPX3 is expected to regulate cancer progression by
regulating the level of hydroperoxides inside cells (2). More is now known about the
relationship between the GPX3 expression, methylation and cancer
progression. For example, the GPX3 gene shows a pattern of deletion
or promoter hypermethylation, which is accompanied by gene
silencing in multiple types of cancer, including prostate, gastric
and Barrett’s esophageal cancer, and conversely the overexpression
of GPX3 inhibits tumor growth and metastasis (3–7). GPX3
has also been shown to be responsible for the cisplatin sensitivity
of clear cell adenocarcinoma in ovarian cancer (8), and its negative expression is
significantly correlated with poor prognosis of gallbladder cancer,
multiple myeloma and gastric cancer (7,9,10). In
particular, silencing of GPX3 increases ROS production in colon
cancer cell lines and muscle stem cells (11,12).
Another possible link between cancer suppression and GPX3, although
it remains to be experimentally confirmed, is that GPX3 is a
selenoprotein, an enzyme containing a selenocysteine (Sec) residue
at its active site, and uptake of selenium has a potential to
reduce certain cancers (13,14).
Human selenoproteins encode 25 genes, including glutathione
peroxidases, and are all involved in crucial biological defense
systems such as antioxidant defense (14). On the other hand, there are quite
different patterns of gene expression among GPXs. GPX2, for
example, shows elevated levels of expression, while GPX1, 3 and 4
show decreased expression in multiple types of cancer (2). A recent study also predicted a causal
relationship between changes in GPX3 expression and in
obesity-related traits (15).
Cervical cancer is the second most common cancer in
women and identification and establishment of molecular markers for
cervical cancer is highly necessary for improved diagnoses and
development of therapeutic targets (16).
Based on the information that GPX3 is downregulated
in multiple types of cancer and functions as a tumor suppressor, in
the present study, we examined whether GPX3 is also downregulated
in cervical cancer and, if so, whether promoter methylation is
related to its repression. We investigated further the
clinicopathological significance of GPX3 downregulation in cervical
cancer and demonstrated the prognostic value of GPX3 in cervical
cancer patients.
Materials and methods
RNA extraction and SYBR-Green real-time
PCR
Total RNAs were extracted from 4 cervical cancer
cell lines, 6 normal cervical tissues and 10 cervical cancer
tissues using TRIzol (Invitrogen) and RNeasy Mini kit (Qiagen GmbH,
Hilden, Germany). One microgram each of the quantitated total RNA
was converted into cDNAs using Transcriptor High Fidelity cDNA
Synthesis kit (Roche Applied Science, Netherlands) following the
manufacturer’s protocol. Quantitative real-time PCR was performed
using 1X SYBR-Green Master Mix (Applied Biosystems), 10 pmol of
each primer and 2 μl of the cDNA in an Mx3005P QPCR System (Agilent
Technologies, Santa Clara, CA, USA) under the following conditions:
initial denaturation for 10 min at 95°C, followed by 40 cycles of
95°C for 20 sec, 50°C for 30 sec and 72°C for 45 sec. GPX3 mRNA
expression was also detected by conventional RT-PCR using Glod Taq
Polymerase (Applied Biosystems) with an annealing temperature of
58°C. The GPX3 expression was normalized by the expression of
β-actin. Oligonucleotide primers used for the PCR were:
5′-CAACCAATTTGGAAAACAGG-3′ and 5′-GTGGGAGGACAGGAGTTCTT-3′ for GPX3;
and 5′-ATA GCACAGCCTGGATAGCAACGTAC-3′ and 5′-CACCTTCT
ACAATGAGCTGCGTGTG-3′ for β-actin.
Cell culture and 5-Aza-2′-deoxycytidine
(5-Aza-dC) treatment
Cervical cancer cell lines (HeLa, SiHa, CaSki and
ME-180) were purchased from the Korean Cell Line Bank (Seoul,
Korea). Cells were maintained in minimum essential medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with fetal bovine
serum to a final concentration of 10%, streptomycin (final
concentration, 100 μg/ml) and penicillin (final concentration, 50
U/ml) at 37°C in a humidified incubator containing 5%
CO2. For the demethylation experiment, 5×105
cells were grown for 24 h prior to 5-Aza-dC (Sigma-Aldrich)
treatment. 5-Aza-dC in PBS was filtered and treated to the final
concentration of 1 μM for 3 days, with fresh 5-Aza-dC changes at
every 24 h. PBS-treated cells were grown alongside as untreated
controls.
DNA extraction and methylation-specific
PCR (MSP)
Genomic DNA was extracted from 4 cervical cancer
cell lines (HeLa, SiHa, CaSki and ME-180) using QIAamp DNA mini kit
(Qiagen GmbH, Hilden, Germany). MSP was based on a previous report
(17). Genomic DNA (500 ng) was
used for bisulfite conversion overnight following a protocol
included in the EZ DNA Methylation kit (Zymo Research Corporation,
Orange, CA, USA). Oligonucleotide primers used for amplifying the
CpG islands for GPX3 promoter regions were:
5′-TATGTTATTGTCGTTTCGGGAC-3′ and 5′-GTCCGT CTAAAATATCCGACG-3′ for
methylation-specific amplification; and
5′-TTTATGTTATTGTTTTGGGATG-3′ and 5′-ATCCATCTAAAATATCCAACACTCC-3′
for non-methylation-specific amplification. Amplification was
performed in a cycle of 95°C for 10 min, 40 cycles of 94°C for 30
sec, 59°C for 30 sec and 72°C for 30 sec, followed by the final
incubation at 72°C for 10 min. The amplified DNA products were
resolved with electrophoresis on 1% agarose gels.
Public microarray data acquisition and
analysis
Gene expression data related to cervical cancer were
downloaded from either Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) or Array Express (www.ebi.ac.uk/arrayexpress). Gene expression profiling
data containing cervical cancer cell lines, normal cervices and
cervical cancer tissues (GSE 9750) and non-malignant cervical
tissues and various FIGO stages of carcinomas (GSE 46857) were used
to test the differences in GPX3 mRNA expression levels. A
methylation profiling experiment performed on Illumina Human
Methylation 27 BeadChip (GSE 30760) was used to probe methylation
level of CpG islands of GPX3 in normal cervices and cervical tumor
tissues. Microarray analyses were performed using BRB-Array Tools
developed by Dr Richard Simon and BRB-Array Tools Development Team
or using GeneSpring 12.6.
Immunostaining of GPX3 on surgical
specimens
The present study included 50 cervical cancer
patients who were diagnosed with cervical cancer in Yonsei
University Hospital, Korea, between 2000 and 2004.
Clinicopathological characteristics of the patients are shown in
Table I. The present study was
approved by the Institutional Review Board of Yonsei University
Hospital. Formalin-fixed, paraffin-embedded surgical specimens were
cut into 4 μM tissue sections and deparaffinized with xylene. After
hydrating with graded ethanol, endogenous peroxidase activity was
blocked with a mixture of methanol and H2O2
at a ratio of 40:1. Antigen retrieval was performed with antigen
retrieval buffer (Dako, Carpinteria, CA, USA) and primary antibody
incubation was performed at RT for 1 h (mouse monoclonal anti-human
GPX3 antibody, working dilution; 1:100) (Abcam, Cambridge, MA,
USA). Real™ EnVision™ HRP rabbit/mouse detection system (Dako) was
used as secondary antibody. The sections were developed with
3,3′-diaminobenzidine (DAB) chromogen and counterstaining was
carried out with hematoxylin. For analysis, patients were divided
into two groups based on the expression level of GPX3; negative (no
or <5% positive cells) and positive (>5% positive cells)
group. The survival estimates were based on Kaplan-Meier survival
curves along with 95% CI and the log-rank test was used to compare
survival curves. A chi-square test was used to analyze the
relationship between GPX3 protein expression and various
clinicopathological parameters. Fisher’s exact test was used to
determine the difference between matched normal and cancer tissues
in GPX3 expression.
 | Table IClinicopathological characteristics of
cervical squamous cell carcinoma patients. |
Table I
Clinicopathological characteristics of
cervical squamous cell carcinoma patients.
| Variables | No. of patients
(%) |
|---|
| Total cases | 50 |
| Age (years) |
| Median age
(range) | 53 (32–70) |
| <53 | 24 (48.0) |
| ≥53 | 26 (52.0) |
| Tumor depth |
| Tis | 3 (6.0) |
| T1 | 42 (84.0) |
| T2 | 5 (10.0) |
| LN metastasis |
| N0 | 31 (62.0) |
| N1 | 19 (38.0) |
| Stage |
| 0 | 3 (6.0) |
| I | 27 (54.0) |
| II | 1 (2.0) |
| III | 19 (38.0) |
Results
GPX3 is downregulated in cervical
cancer
The mRNA expression of GPX3 was comparatively
investigated in 6 normal cervix and 10 cervical cancer tissues. We
found that GPX3 mRNA expression was significantly decreased in
cervical cancer tissues compared to normal cervical tissues
(Fig. 1A). We also examined the
GPX3 expression profiles in other published genomic data. In a set
of public microarray data containing normal cervices, cervical
cancer tissues and cell lines (18), GPX3 showed significant
downregulation in cervical cancer tissues and cell lines compared
to normal cervical tissues (Fig.
1B). In other independent microarray data, GPX3 expression was
again significantly repressed in cervical cancer compared to normal
cervical tissues (Fig. 1C).
However, there were no differences in expression levels of GPX3
among cancer tissues obtained from patients with different FIGO
stages (Fig. 1D). Collectively,
these results suggest that GPX3 is significantly downregulated in
cervical cancer compared to its normal counterpart.
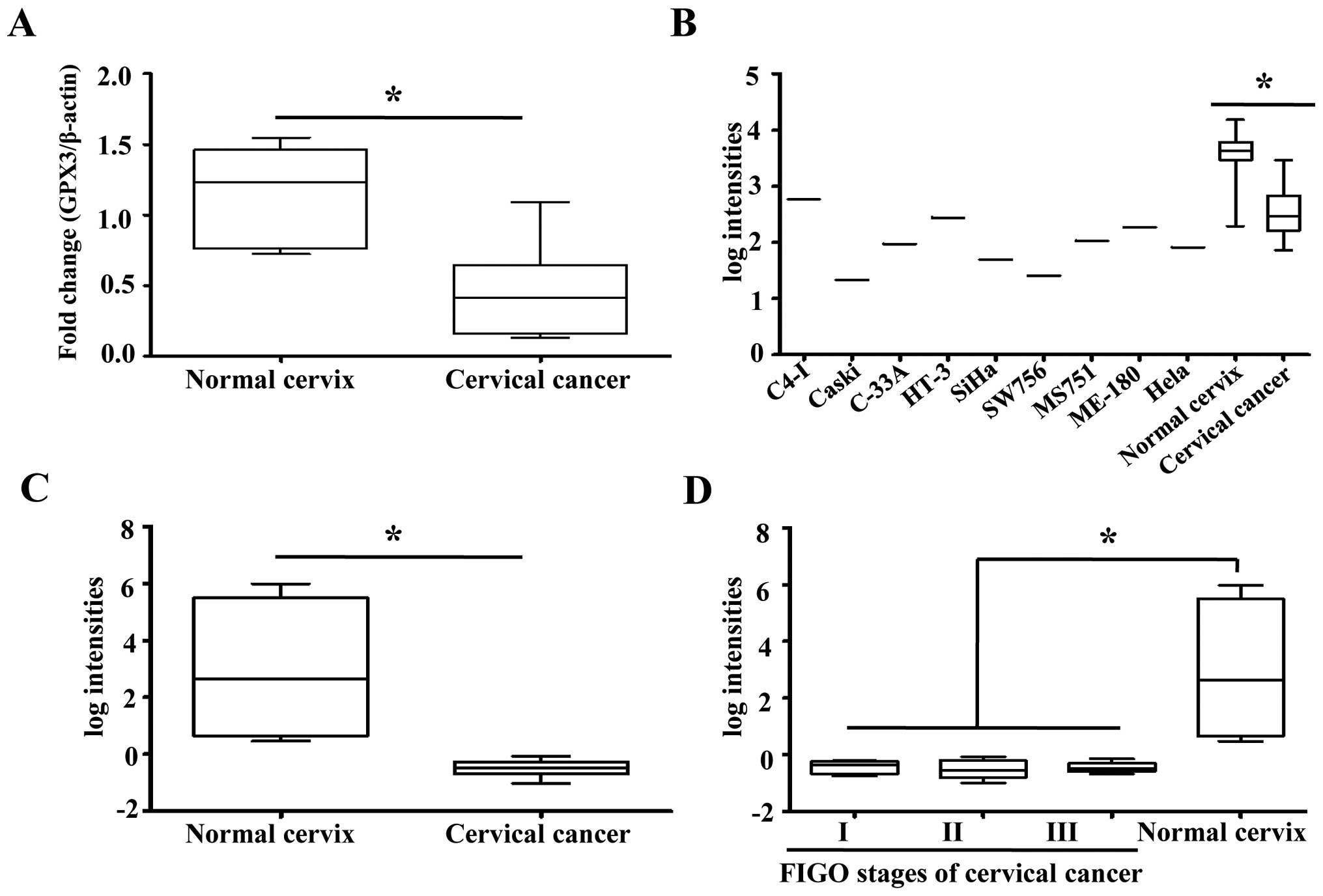 | Figure 1GPX3 mRNA expression in cervical
cancer cell lines and surgical tissue samples. (A) GPX3 mRNA
expression was significantly decreased in cervical cancer tissues
compared to normal cervix (p<0.001). β-actin was used as an
internal control in all measurements. (B) In a gene expression
profiling of cervical cancer cell lines, cervical cancer tissues
and normal cervical tissues, performed on an Affymetrix Human
Genome U133A Array format (GSE 9750), GPX3 showed ~11 and 35-fold
downregulation in cervical tumor tissues and cancer cell lines,
respectively, compared with GPX3 expression in normal cervix
tissues (p<0.001). (C) In a global gene expression profiling
study that compared normal cervical tissues and various FIGO stages
of cervical cancer tissues (GSE 46857), GPX3 showed an average of
11-fold downregulation in cervical cancer tissues compared with
normal cervical tissues (p<0.001). (D) However, when the cancer
samples in Fig. 2C were analyzed in
detail, there were no differences in expression levels of GPX3
among cancer tissues obtained from patients with different FIGO
stages. Data in Fig. 2C and D are
from a common dataset, the only difference being the cancer samples
are shown in detailed FIGO groups in Fig. 2D. GPX3, glutathione peroxidase
3. |
In silico analysis of methylation profile
of GPX3 in cervical cancer
DNA methylation is one of the most prominent
epigenetic mechanisms that control gene expressions and, therefore,
disease progression (19–21). Previous results showed GPX3
downregulation in gastric cancer, Barrett’s adenocarcinomas and
prostate cancer and their concurrent promoter hypermethylation
(1,3,5–7). We
searched the epigenome analysis database of normal and cancer
tissues from the uterine cervix to examine the differential
methylation degrees of GPX3. In a methylation profiling study
comparing 152 normal uterine cervical tissues and 63 cervical
cancer tissues (GSE 30760), GPX3 showed β values close to zero (no
methylation in known CpG islands) in almost all normal tissues,
whereas there was a significant trend of increase in β values in
tumor samples, indicating an elevated degree of methylation in CpG
islands of GPX3 (Fig. 2A). This is
consistent with previous reports that GPX3 repression in cancer is
due to hypermethylation or deletion. This finding shows that there
is a higher degree of CpG methylation in GPX3 promoter region in
cervical cancer tissues compared to normal cervical tissues.
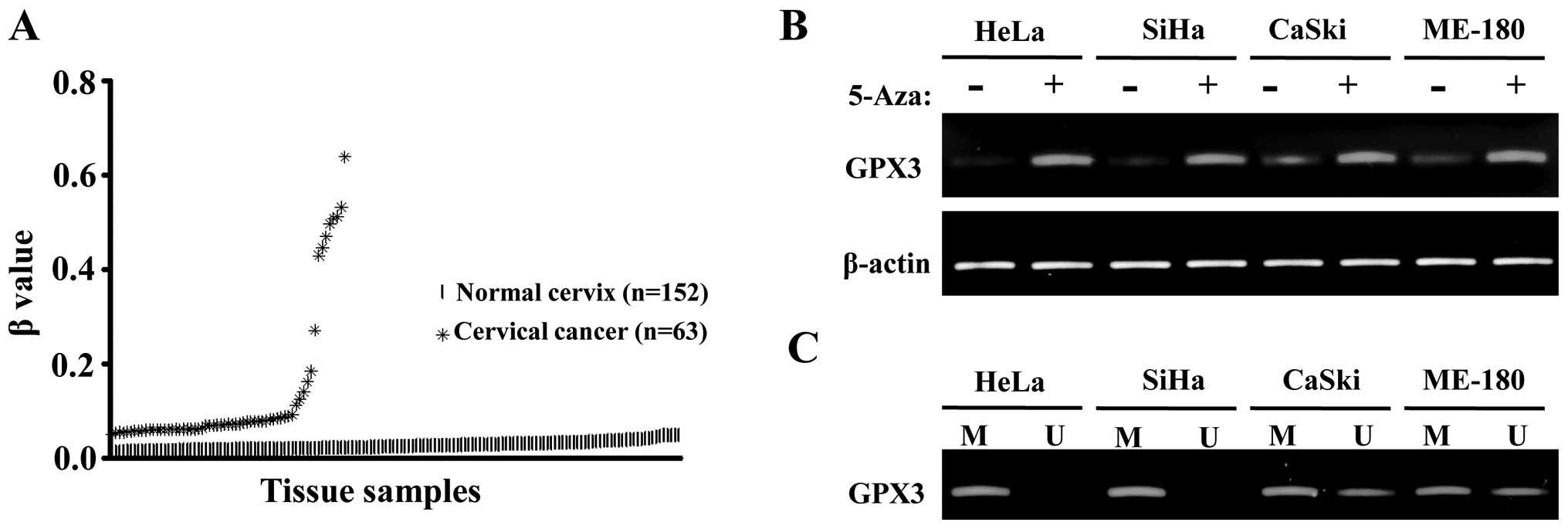 | Figure 2CpG hypermethylation is partly
responsible for the downregulation of GPX3 in cervical cancer
cells. (A) In silico analysis of GPX3 promoter methylation.
Methylation profiling data, performed on Illumina Human Methylation
27 BeadChip (GSE 30760), were downloaded (as described in Materials
and methods) and β values for GPX3 were analyzed. The β value
represents the proportion of methylated signal intensities among
all intensities (methylated and unmethylated intensities) in each
probe, and it is always a number between 0 and 1, with 0 being
non-methylation among CpG islands analyzed and 1 representing full
methylation. The mean, median and standard deviations were 0.027,
0.025 and 0.009 for normal cervical tissues; and 0.1327, 0.072 and
0.148 for cervical cancer tissues, respectively. (B) RT-PCR
analysis in cervical cancer cell lines with or without 5-Aza-dC (1
μM) treatment; restoration of GPX3 mRNA expression was found in all
cervical cancer cell lines with 5-Aza-dC (1 μM) treatment. (C) MSP
analysis in cervical cancer cell lines. Full methylations for GPX3
were found in HeLa and SiHa cell lines and partial methylations
were found in CaSki and ME-180 cell lines. GPX3, glutathione
peroxidase 3; 5-Aza-dC, 5-Aza-2′-deoxycytidine. |
Reversion of GPX3 downregulation by a
demethylating agent in cervical cancer cells
To directly test whether GPX3 repression in cervical
cancer is correlated with promoter methylation, we compared the
GPX3 mRNA expression levels in cervical cancer cells with or
without 5-Aza-dC treatment, a potent demethylating agent (22). In HeLa and SiHa, significant
upregulation of GPX3 mRNAs, following a 5-Aza-dC treatment, were
observed, whereas in CaSki and ME-180, relatively mild but still
clear upregulation of GPX3 mRNAs were evident in the same
conditions (Fig. 2B). To directly
test the promoter methylation, bisulfite-converted genomic DNAs
from the same cervical cancer cells were subjected to MSP. In HeLa
and SiHa, only methylation-specific amplified DNA fragments were
visible, indicating full methylation of CpG islands, whereas in
CaSki and ME-180, both of the methylation-specific and
unmethylation-specific amplified DNA fragments were visible,
indicating partial methylation of CpG islands in these two cell
lines. The degree and pattern of CpG methylation in different
cervical cancer cell lines are consistent with those of
upregulation of GPX3 following a 5-Aza-dC treatment. Therefore,
GPX3 is downregulated in cervical cancer due to CpG
hypermethylation, although methylation may not be the only
mechanism suppressing its transcription.
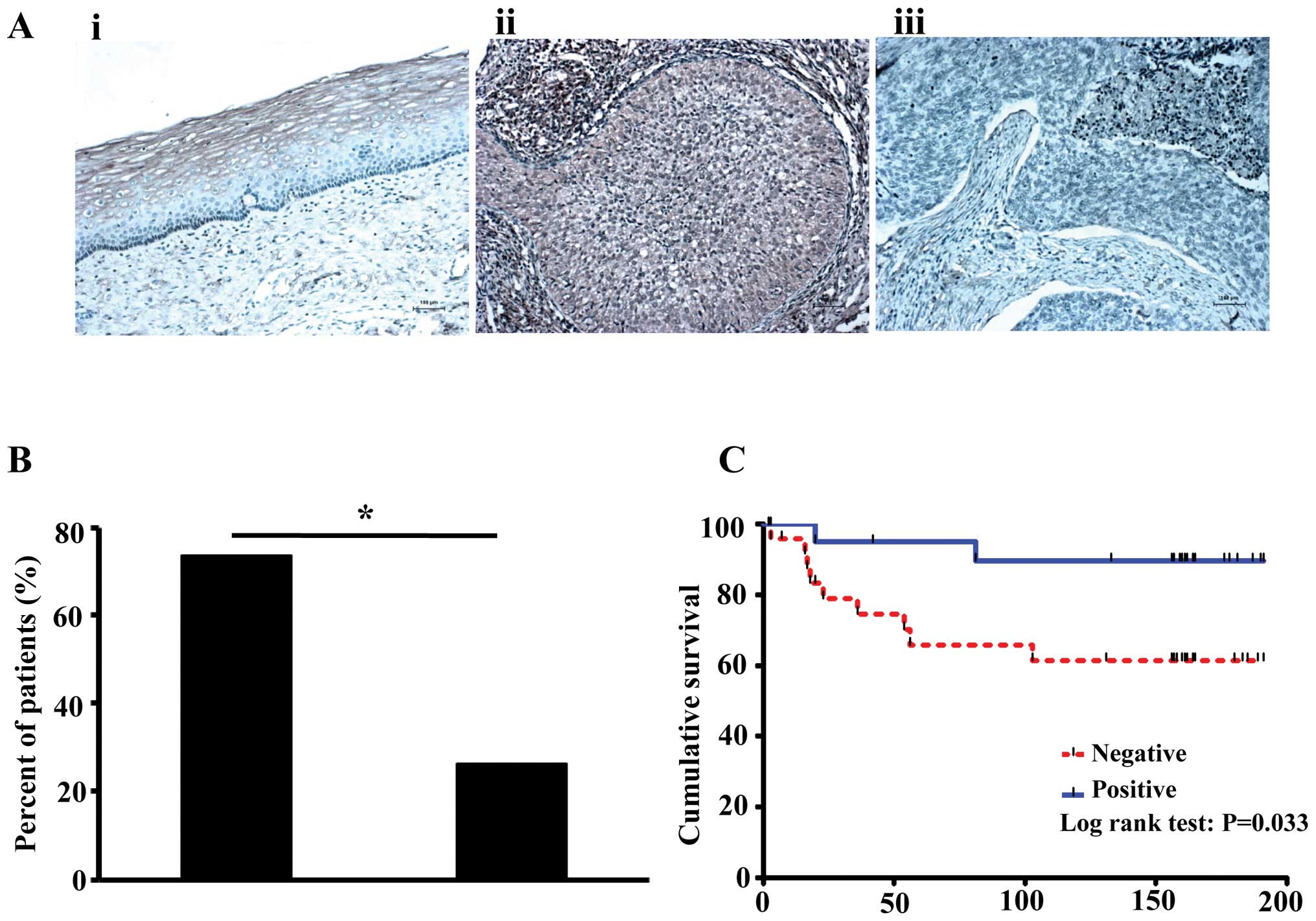
Immunostaining for GPX3 in normal cervix
and cervical cancer tissues
GPX3 was mainly expressed in cytoplasm of the cells,
and its expression was significantly increased in normal cervix
(6/6, 100%) compared to cervical cancer tissues (22/50, 44.0%,
p=0.023; Fig. 3A). Furthermore,
lymph node metastases were observed more often in patients without
GPX3 expression (73.7%) than in patients with GPX3 expression
(26.3%, p=0.049; Fig. 3B). Patients
without GPX3 expression also showed poorer prognosis than patients
with GPX3 expression (p=0.033, median survival duration was 117
months for low GPX3 vs. 160.5 months for high GPX3; Fig. 3C).
Discussion
In humans, there are 5 selenocysteine-containing
glutathione peroxidases (GPX1–4 and GPX6), and they all promote the
reduction of hydroperoxides by means of glutathione (2 glutathiones
+ H2O2 ↔ glutathione disulfide +
2H2O), thereby acting as antioxidant enzymes that remove
hydroperoxides (23). GPX3, in
particular, is known to function as a transcription factor in the
form of a homotetramer. Selenium uptake is correlated with
decreased cancer incidence and the chemopreventive function is
evident especially to those with low selenium level (23,24).
The relationship between the selenium level and cancer development,
GPX3 being the selenoprotein and downregulated in certain types of
cancer, might suggest that the in vivo selenium level
determines the tumor suppressor activity of GPX3, which functions
to regulate the in vivo hydroperoxide level and therefore
oxidative damages to the cancer cell. However, to date, no direct
experimental evidence for these links has been provided (2).
Epigenetic modulation of gene expression is a
significant complement to other genetic alterations including gene
amplifications and deletions, where a significant amount of DNA
sequence changes are accompanied. Several reports have detailed the
relationship between GPX3 expression and its correlation with
cancer progression (25). For
example, in prostate cancer, GPX3 is methylated at its promoter in
93% of the cancer tissues examined and its mRNA expression is
concurrently repressed, whereas robust mRNA expression was observed
in normal prostate epithelial cells (1). In the present study, we showed that
both mRNA and protein expression levels of GPX3 are significantly
reduced in cervical cancer tissues compared to normal cervical
tissues. In addition, in 5 matched tissue samples, GPX3 protein
expression showed a tendency to gradually decrease from normal
cervical tissues (100%, 5/5), primary cervical tissues (20.0%, 1/5)
to lymph node metastases (0%, 0/5), indicating that the
downregulation of GPX3 may also be involved in cervical cancer
progression. Furthermore, as in other types of cancer (3), downregulation of GPX3 expression is
closely related to lymph node metastasis and prognosis in cervical
cancer, indicating the importance of GPX3 expression in cervical
cancer. Downregulation of GPX3 induced by promoter methylation has
also been observed in gastric carcinoma (26) and Barrett’s adenocarcinoma (6). In the present study, we also found
that promoter methylation was detected frequently in cervical
cancer cell lines and 5-Aza-dC treatment can restore the mRNA
expression of GPX3. Hypermethylation of CpG islands is one of the
causes of GPX3 downregulation in cervical cancer.
Sustained level of reactive oxygen species (ROS)
generates oxidative stress and results in damages to host cell DNA
protein and lipids. ROS-induced DNA can be the most deleterious
since it is not easily replaced and, when the DNA repair system
does not function properly to prevent the passage of damage to
progeny cells, in the most severe case, human disease such as
cancer develops (27). From yeast
to higher eukaryotes, GPXs and other antioxidant enzymes function
to protect cells against oxidative damages. It remains to be
examined whether GPX3 functions to suppress cervical cancer
promotion in vitro and in vivo.
In conclusion, GPX3 could be a novel predictive
molecular biomarker for lymph node metastasis and cancer prognosis
in cervical cancer patients.
Acknowledgements
This research was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT & Future Planning
(No. 2013R1A2A2A04015894). This work was also partly supported by
the Priority Research Centers Program through the NRF funded by the
Ministry of Education, Science and Technology (2009-0094027).
References
|
1
|
Lodygin D, Epanchintsev A, Menssen A,
Diebold J and Hermeking H: Functional epigenomics identifies genes
frequently silenced in prostate cancer. Cancer Res. 65:4218–4227.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brigelius-Flohé R and Kipp A: Glutathione
peroxidases in different stages of carcinogenesis. Biochim Biophys
Acta. 1790:1555–1568. 2009.PubMed/NCBI
|
|
3
|
Yu YP, Yu G, Tseng G, et al: Glutathione
peroxidase 3, deleted or methylated in prostate cancer, suppresses
prostate cancer growth and metastasis. Cancer Res. 67:8043–8050.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mikata R, Yokosuka O, Fukai K, et al:
Analysis of genes upregulated by the demethylating agent
5-aza-2′-deoxycytidine in gastric cancer cell lines. Int J Cancer.
119:1616–1622. 2006.
|
|
5
|
Lee OJ, Schneider-Stock R, McChesney PA,
Kuester D, Roessner A, Vieth M, Moskaluk CA and El-Rifai W:
Hypermethylation and loss of expression of glutathione peroxidase-3
in Barrett’s tumorigenesis. Neoplasia. 7:854–861. 2005.PubMed/NCBI
|
|
6
|
Peng DF, Razvi M, Chen H, et al: DNA
hypermethylation regulates the expression of members of the
Mu-class glutathione S-transferases and glutathione peroxidases in
Barrett’s adenocarcinoma. Gut. 58:5–15. 2009.PubMed/NCBI
|
|
7
|
Zhang X1, Yang JJ, Kim YS, Kim KY, Ahn WS
and Yang S: An 8-gene signature, including methylated and
down-regulated glutathione peroxidase 3, of gastric cancer.
Int J Oncol. 36:405–414. 2010.PubMed/NCBI
|
|
8
|
Saga Y, Ohwada M, Suzuki M, et al:
Glutathione peroxidase 3 is a candidate mechanism of anticancer
drug resistance of ovarian clear cell adenocarcinoma. Oncol Rep.
20:1299–1303. 2008.PubMed/NCBI
|
|
9
|
Yang ZL, Yang L, Zou Q, et al: Positive
ALDH1A3 and negative GPX3 expressions are biomarkers for poor
prognosis of gallbladder cancer. Dis Markers. 35:163–172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaiser MF, Johnson DC, Wu P, et al: Global
methylation analysis identifies prognostically important
epigenetically inactivated tumour suppressor genes in multiple
myeloma. Blood. 122:219–226. 2013. View Article : Google Scholar
|
|
11
|
El Haddad M, Jean E, Turki A, et al:
Glutathione peroxidase 3, a new retinoid target gene, is crucial
for human skeletal muscle precursor cell survival. J Cell Sci.
125:6147–6156. 2012.PubMed/NCBI
|
|
12
|
Barrett CW, Ning W, Chen X, et al: Tumor
suppressor function of the plasma glutathione peroxidase gpx3 in
colitis-associated carcinoma. Cancer Res. 73:1245–1255. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fitzpatrick JM, Schulman C, Zlotta AR and
Schröder FH: Prostate cancer: a serious disease suitable for
prevention. BJU Int. 103:864–870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu J and Holmgren A: Selenoproteins. J
Biol Chem. 284:723–727. 2009. View Article : Google Scholar
|
|
15
|
Yang X, Deignan JL, Qi H, et al:
Validation of candidate causal genes for obesity that affect shared
metabolic pathways and networks. Nat Genet. 41:415–423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bachtiary B, Boutros PC, Pintilie M, et
al: Gene expression profiling in cervical cancer: an exploration of
intratumor heterogeneity. Clin Cancer Res. 12:5632–5640. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Y, Wang Y, Li P, Zhu S, Wang J and
Zhang S: Identification of GPX3 epigenetically silenced by
CpG methylation in human esophageal squamous cell carcinoma. Dig
Dis Sci. 56:681–688. 2011.
|
|
18
|
Scotto L, Narayan G, Nandula SV, et al:
Identification of copy number gain and overexpressed genes on
chromosome arm 20q by an integrative genomic approach in cervical
cancer: potential role in progression. Genes Chromosomes Cancer.
47:755–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baylin SB and Ohm JE: Epigenetic gene
silencing in cancer - a mechanism for early oncogenic pathway
addiction? Nat Rev Cancer. 6:107–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008. View Article : Google Scholar
|
|
21
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
22
|
Christman JK: 5-Azacytidine and
5-aza-2′-deoxycytidine as inhibitors of DNA methylation:
mechanistic studies and their implications for cancer therapy.
Oncogene. 21:5483–5495. 2002.
|
|
23
|
Brigelius-Flohé R: Glutathione peroxidases
and redox-regulated transcription factors. Biol Chem.
387:1329–1335. 2006.PubMed/NCBI
|
|
24
|
Clark LC, Combs GF Jr, Turnbull BW, et al:
Effects of selenium supplementation for cancer prevention in
patients with carcinoma of the skin. A randomized controlled trial.
Nutritional Prevention of Cancer Study Group. JAMA. 276:1957–1963.
1996. View Article : Google Scholar
|
|
25
|
Dobosy JR, Roberts JL, Fu VX and Jarrard
DF: The expanding role of epigenetics in the development, diagnosis
and treatment of prostate cancer and benign prostatic hyperplasia.
J Urol. 177:822–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jee CD, Kim MA, Jung EJ, Kim J and Kim WH:
Identification of genes epigenetically silenced by CpG methylation
in human gastric carcinoma. Eur J Cancer. 45:1282–1293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maynard S, Schurman SH, Harboe C, de
Souza-Pinto NC and Bohr VA: Base excision repair of oxidative DNA
damage and association with cancer and aging. Carcinogenesis.
30:2–10. 2009. View Article : Google Scholar : PubMed/NCBI
|