Introduction
Osteosarcoma is the most common primary bone tumor
in children and adolescents, with a 5-year disease-free survival
rate of 70%. The clinical features of osteosarcoma include its
strong local infiltration and rapid metastasis to the lungs.
Neoadjuvant chemotherapy accompanied by large doses of doxorubicin
(DXR) has greatly improved the survival rate, yet 20–40% of the
patients still die of metastasis and recurrence. The primary cause
of treatment failure is the resistance of tumor cells to
chemotherapeutic medicine. DXR is commonly used in clinical
chemotherapy and is important in the chemotherapeutic treatment of
osteosarcomas. However, clinical practice has shown that drug
resistance to DXR is easily acquired. The clinical applications of
anthracyclines such as adriamycin (ADM) have been limited by their
unacceptable toxicity to the heart at the required treatment
doses.
Multidrug resistance (MDR) is mainly attributed to
the increased drug efflux mediated by the P-glycoprotein (P-gp)
product of the multidrug resistance protein 1 (MDR1) gene. P-gp is
a 170-kDa ATP-dependent transmembrane transporter that acts as a
drug efflux pump to decrease intracellular drug accumulation and
consequently reduce intracellular drug efficacy (1,2).
Compounds such as verapamil have been reported to overcome MDR
in vitro by decreasing the expression of MDR1 (3). However, these compounds have side
effects that hinder their clinical applications. Therefore, low
toxicity and high activity reversal agents are needed to be
identified.
Recently, several researchers have focused on
screening for natural product-derived drugs to reverse MDR
(4–6). Epidemiologic data support the premise
that the dietary intake of Allium vegetables, including
garlic, may protect against the risk of various malignancies
(7,8). Diallyl trisulfide (DATS) is the main
sulfur-containing compound in garlic. Engdal et al (9) proposed that garlic compounds can
inhibit the expression of P-gp in vitro and in vivo.
The capacity of DATS to reverse the drug resistance of osteosarcoma
cells is still unknown. Similarly, the signaling pathway for the
inhibition of osteosarcoma cell proliferation by DATS remains
unclear.
In the present study, we aimed to determine the
effect of DATS on the reversal of drug resistance in human
osteosarcoma cells in vitro and to investigate its potential
mechanisms of action. The study also explored whether the
suppression of the nuclear factor κ light-chain enhancer of
activated B cells (NF-κB) is involved in the DATS-induced
inhibition of osteosarcoma cell proliferation.
Materials and methods
Cell culture and experimental
reagents
Human osteosarcoma U2-OS cells (The Institute of
Basic Medicine of the Shandong Academy of Medical Sciences, China)
were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Los Angeles, CA, USA) supplemented with 10% (v/v) heat-inactivated
newborn calf serum (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., China) and 100 U/ml penicillin and 100 μg/ml
streptomycin in a 5% CO2 atmosphere at 37°C. DATS was
purchased from Shandong Lukang Xin Chen Pharmaceutical Co.
(Shandong, China), while ADM was obtained from Sigma (St. Louis,
MO, USA). Annexin V-FITC/propidium iodide (PI) were purchased from
JingMei Co. (Shanghai, China). Primary antibodies for P-gp, NF-κB
and IκBα were obtained from Cell Signaling Technology (Beverly, MA,
USA).
In vitro drug sensitivity assay
U2-OS cells were seeded at 1×104 cells/ml
per well into a 96-well plate. After 24 h, the medium was replaced
with DMEM supplemented with DATS at a dose of 10, 50 and 100 μM.
U2-OS cells were incubated in drug-free culture media as the blank
controls. After treatment for 20, 44 and 68 h, 20 μl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution was added into each well, and the cells were further
incubated for 4 h. The medium was then removed, and 150 μl DMSO was
added to each well to dissolve the formazan crystals. The
absorbance (A) of each sample was measured using a
spectrophotometer at a wavelength of 570 nm. IC50 was
calculated according to the results of the MTT assay. Inhibition of
cell viability = [1 - (average A value of the experimental
group/average A value of the control group)] × 100%.
To determine the capacity of DATS to sensitize U2-OS
cells to ADM cytotoxicity, U2-OS cells were seeded into 96-well
culture plates at a density of 1×104 cells/ml and
incubated for 24 h. ADM was added to a final concentration of 1
μg/ml. The experimental group was treated combined with 10 μM DATS
for 24, 48 and 72 h. The U2-OS cells incubated in DMEM supplemented
with ADM alone were used as controls.
Expression of P-gp by flow cytometry
(FCM)
The U2-OS cells were cocultured with 10, 50 and 100
μM DATS for 48 h. The cells were then washed twice with PBS and
suspended in phosphate-buffered saline (PBS). The cell suspensions
were incubated with phycoerythrin-conjugated UIC2, mouse anti-human
P-gp monoclonal antibody (P-gp-PE), and the homotype control
IgG2a-PE. The mixture was incubated at room temperature and away
from light for 30 min, washed twice, and then detected by FCM. The
results were analyzed using Cell Quest software (BD Pharmingen Co.,
USA).
Morphological changes by light
microscopy
U2-OS cells were grown in complete DMEM for 24 h on
24-well plates that had a coverslip at the bottom of each well.
When a cell density of ~1×104 cells/ml was reached, the
cells were treated with either 50 μM DATS alone or 10 μM DATS
combined with 1 μg/ml ADM and incubated for another 48 h. Treatment
with 1 μg/ml ADM alone was applied to the control group. The
coverslips were removed from each well, and stained with
hematoxylin and eosin (H&E). The contents of the coverslips
were fixed in 95% ethanol for 20 min, and then a series of washing
steps was performed. The coverslips were air-dried, mounted on
slides with neutral gum, and observed under a light microscope.
Apoptosis assay by statistical FCM
After incubation at 37°C for 24 h in DMEM with the
different drug doses (i.e., 10, 50 and 100 μM DATS or 10 μM DATS
with 1 μg/ml ADM or 1 μg/ml ADM alone), the cell suspensions were
washed twice with PBS and centrifuged at 550 × g for 5 min. The
cells were resuspended in 500 μl of binding buffer, 5 μl of Annexin
V-FITC, and 10 μl of PI (20 μg/ml). The samples were then incubated
at room temperature for 15 min in the dark. The fluorescence
intensities of the samples were measured by a flow cytometer with
the FACS software (BD Pharmingen Co.).
Semi-quantitative RT-PCR assay
The total mRNA was extracted from the cells with the
TRIzol reagent (Invitrogen Co., Carlsbad, CA, USA) according to the
manufacturer’s instructions. Single-stranded cDNA was synthesized
by the reverse transcription of 1 μg total RNA using RNAse-free
M-MLV reverse transcriptase (Invitrogen Co.) and the oligo-dT
primer. Amplification was carried out in a thermal cycler. The
following cycling conditions were used for each PCR run: initial
denaturation at 94°C for 1 min, followed by 26 cycles of 58°C for 1
min and 72°C for 1 min, and then the final extension at 72°C for 7
min. The PCR products were separated by electrophoresis on 1.5%
agarose gels. The products were further analyzed using the UVP
bioimaging system (UVP, Upland, CA, USA), with β-actin as the
internal reference. The PCR primers are listed in Table I.
 | Table IPrimer sequences used for PCR
analysis. |
Table I
Primer sequences used for PCR
analysis.
| Genes | | Primer (5′-3′) | Product length
(bp) |
|---|
| β-actin | Forward |
5′-GTGGGGCGCCCCAGGCACCA-3′ | 540 |
| Reverse |
5′-CTCCTTAATGTCACGCACGATTTC-3′ | |
| NF-κB/p65 | Forward |
5′-TGCACCTAGCTGCCAAAGAAGGA-3′ | 293 |
| Reverse |
5′-TCTGCTCCTGCTGCTTTGAGAA-3′ | |
| IκBα | Forward |
5′-GCAGAGGATTACGAGCAGAT-3′ | 634 |
| Reverse |
5′-CCTGGTAGGTTACTCTGTTG-3′ | |
Protein expression by western blot
analysis
The total protein content was isolated and subjected
to SDS-PAGE and transferred to a polyvinylidene difluoride
membrane. The blots were incubated overnight at 4°C with the rabbit
anti-human NF-κB (p65) or the mouse anti-human IκBα primary
antibodies (1:1,000 dilution), and then further incubated for 1 h
at room temperature with the HRP-conjugated goat anti-rabbit IgG
secondary antibody (1:5,000 dilution). The fluorescent signals were
detected with an ECL western blotting detection kit (Zhongshan Co.,
Beijing, China). After normalization according to the corresponding
level of β-actin expression, the protein expression levels were
determined by densitometry scans and calculated using Quantity One
software (Bio-Rad Co., USA).
Statistical analysis
The results are expressed as the means ± standard
deviation (SD) of three independent experiments. The Student’s
t-test was used for the statistical analyses, and P<0.05 was
considered to be significant. Statistical calculations were carried
out using the Student’s t-test with SPSS 13.0 for Windows software
package.
Results
Drug sensitivity
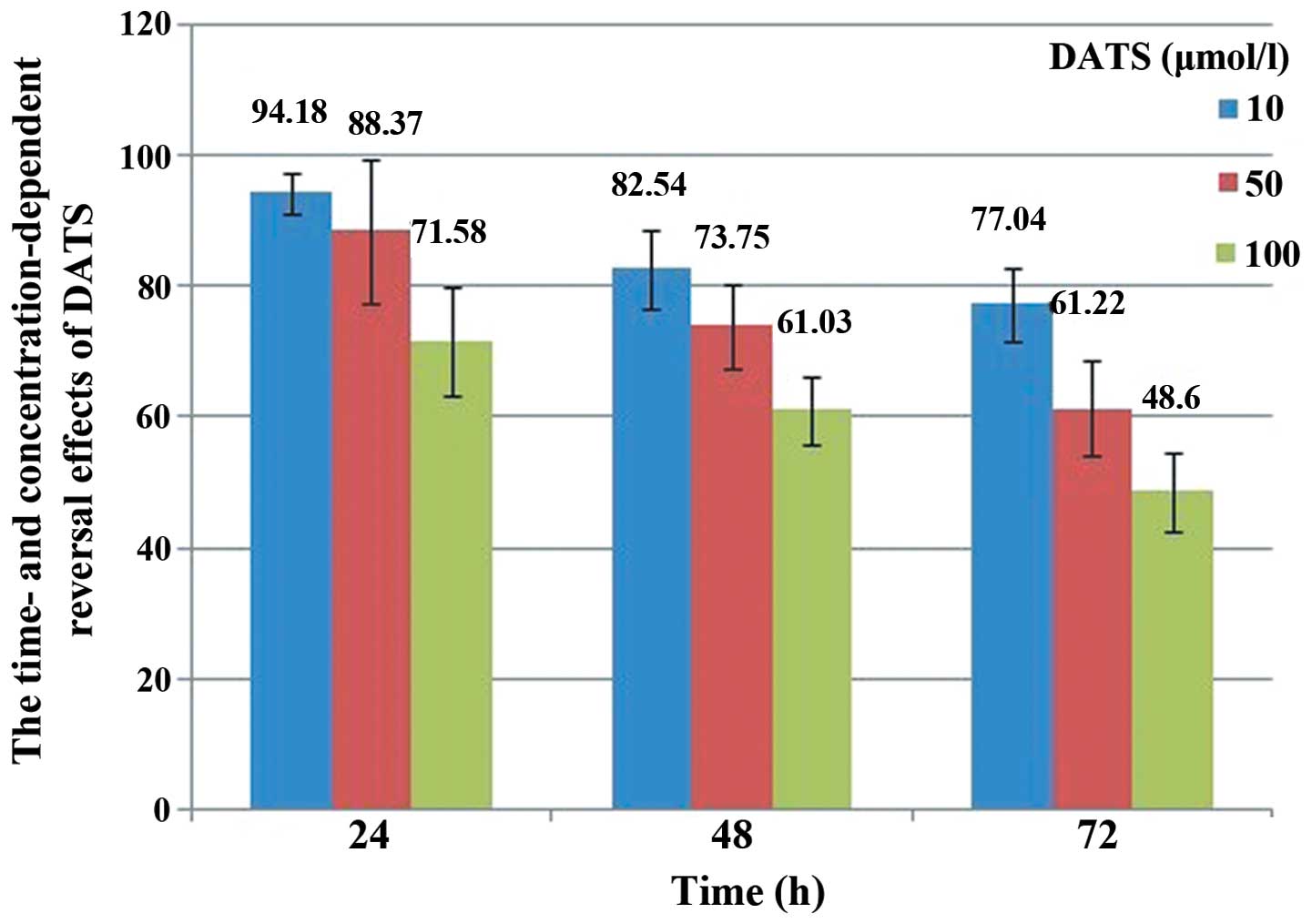
We determined the cytotoxic effect of DATS on
osteosarcoma cells by MTT assay. Treatment of U2-OS cells with DATS
resulted in the inhibition of cell viability in a dose-and
time-dependent manner (Fig. 1). As
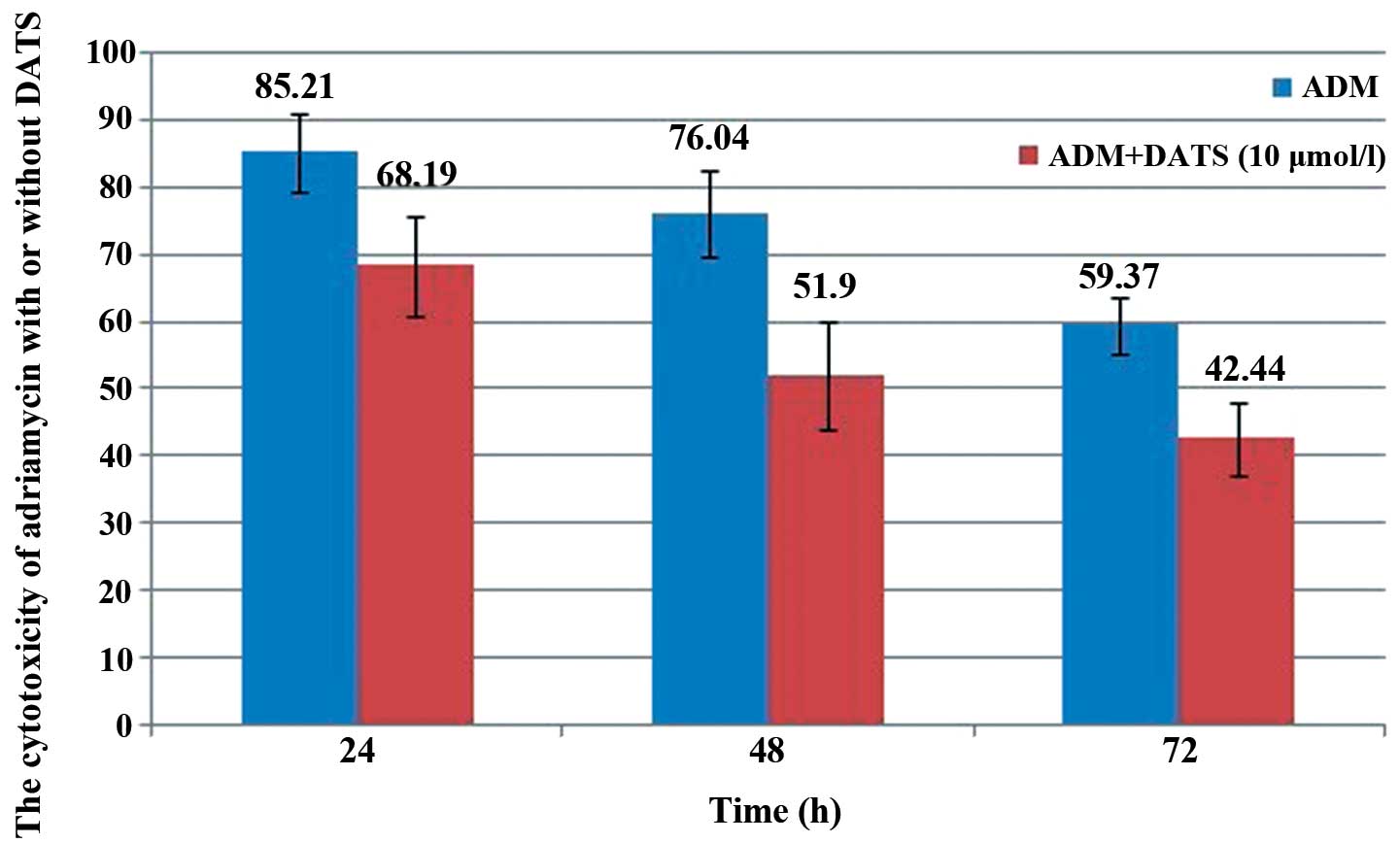
shown in Fig. 2, the survival rate
of the U2-OS cells significantly decreased to 68.19, 51.9 and
42.44% at 24, 48 and 72 h, respectively, after treatment using 10
μM DATS with 1 μg/ml ADM as compared with treatment using ADM alone
(85.21, 76.04 and 59.37% at 24, 48 and 72 h, respectively;
P<0.05).
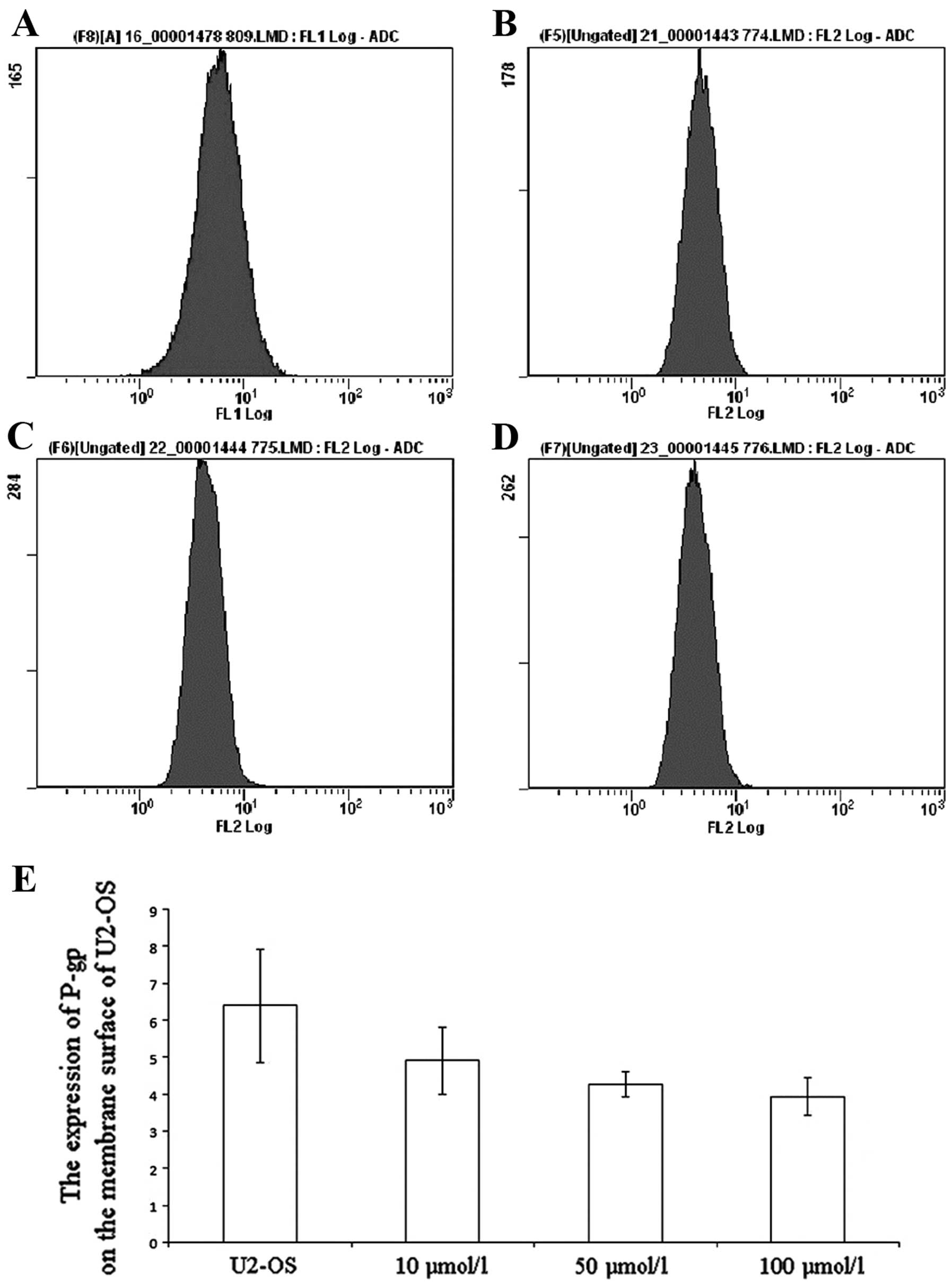
Alteration of P-gp expression
The DATS-treated U2-OS cells were incubated with
phycoerythrin-conjugated UIC2 and then P-gp expression was detected
by FCM. After treatment with different concentrations of DATS (10,
50 and 100 μM), the P-gp expression in the U2-OS cells was
significantly decreased to 4.91, 4.28 and 3.94, as compared with
the untreated group (6.4; P<0.01; Fig. 3).
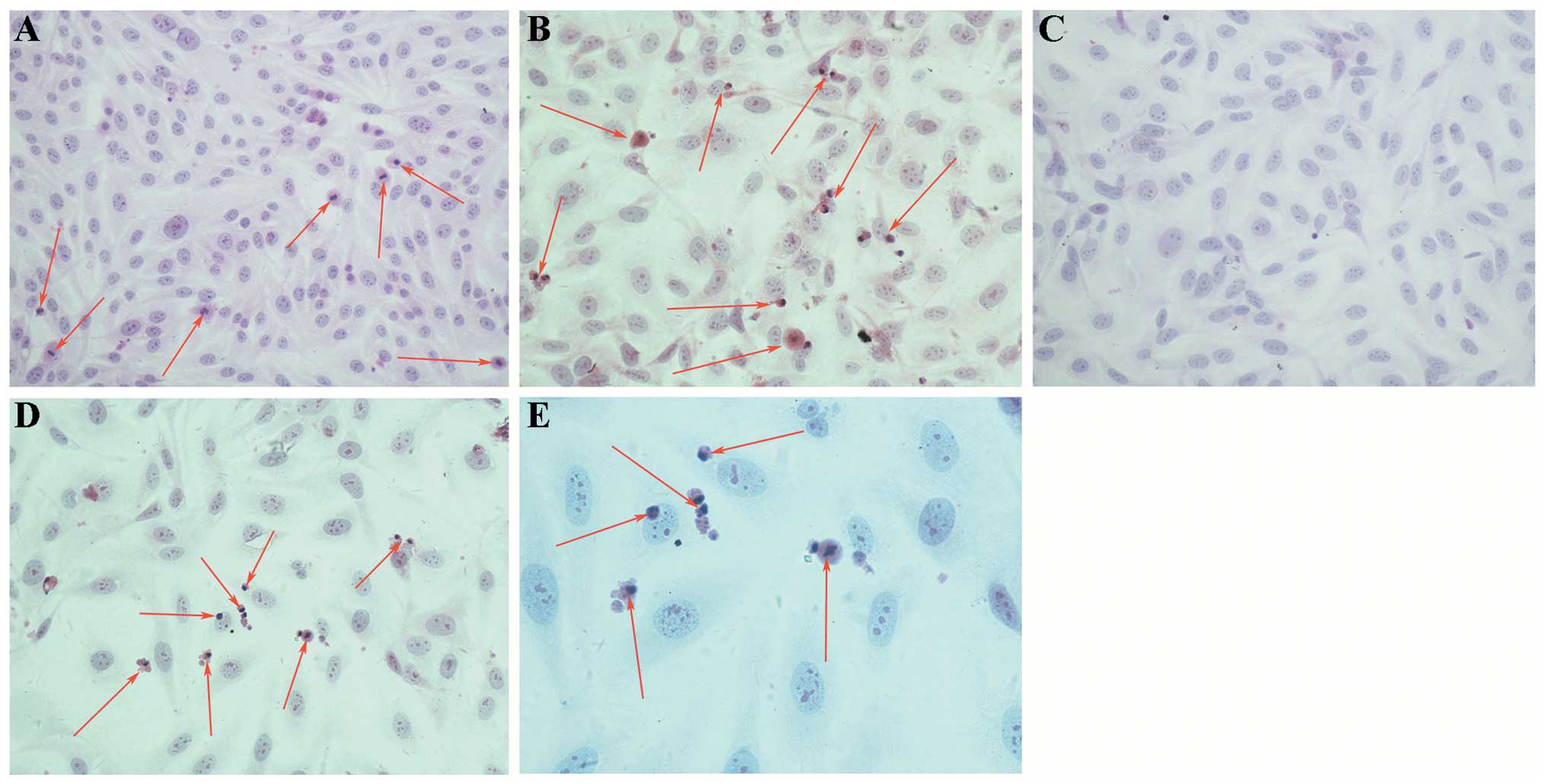
Apoptosis as observed by light
microscopy
The untreated U2-OS cells proliferated actively, and
had the typical morphological characteristics of malignant cells,
such as uneven cell size, deeply stained nucleus, reduced cytoplasm
and an enlarged nucleolus (Fig.
4A). After treatment with 50 μM DATS, proliferation of the
U2-OS cells was reduced, and the cells shrank into rounded shapes
with abundant cytoplasm and vacuoles, chromatin condensation and
margination, nuclear fragmentation, apoptotic bodies, as well as
other typical apoptotic cytomorphological features (Fig. 4B). However, the proliferation of the
U2-OS cells treated with ADM (1 μg/ml) alone was slightly inhibited
and apoptotic cells were rare (Fig.
4C). As shown in Fig. 4D and E,
the apoptotic cytomorphological features appeared at 24 h after
simultaneous treatment with ADM (1 μg/ml) and DATS (10 μM).
Apoptosis assay by statistical flow
cytometry
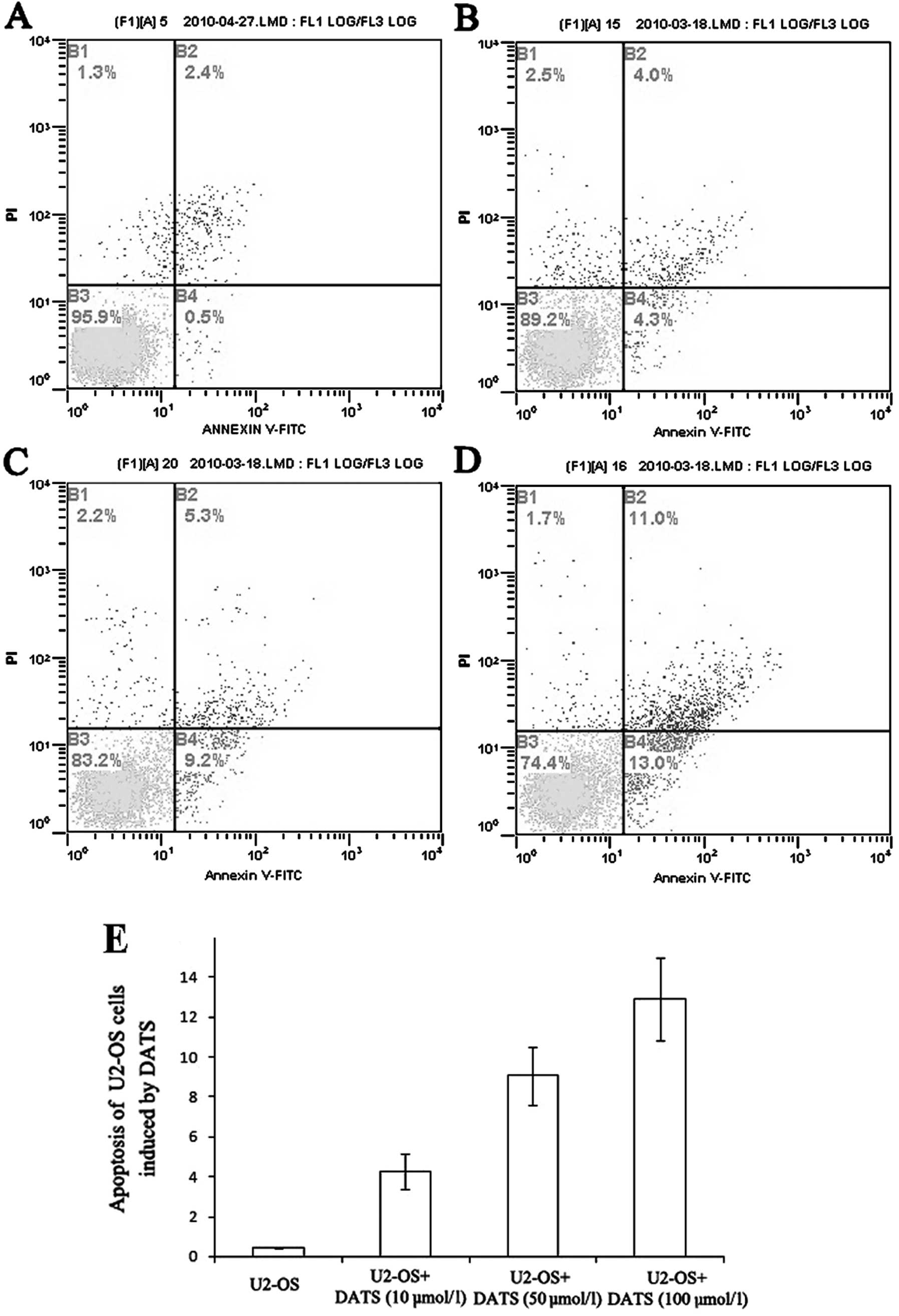
Annexin V and PI were used to further discriminate
apoptotic from necrotic cell death in the cell cycle. The
percentages of early apoptotic cells were significantly increased
at 24 h after treatment with 10, 50 and 100 μM of DATS as compared
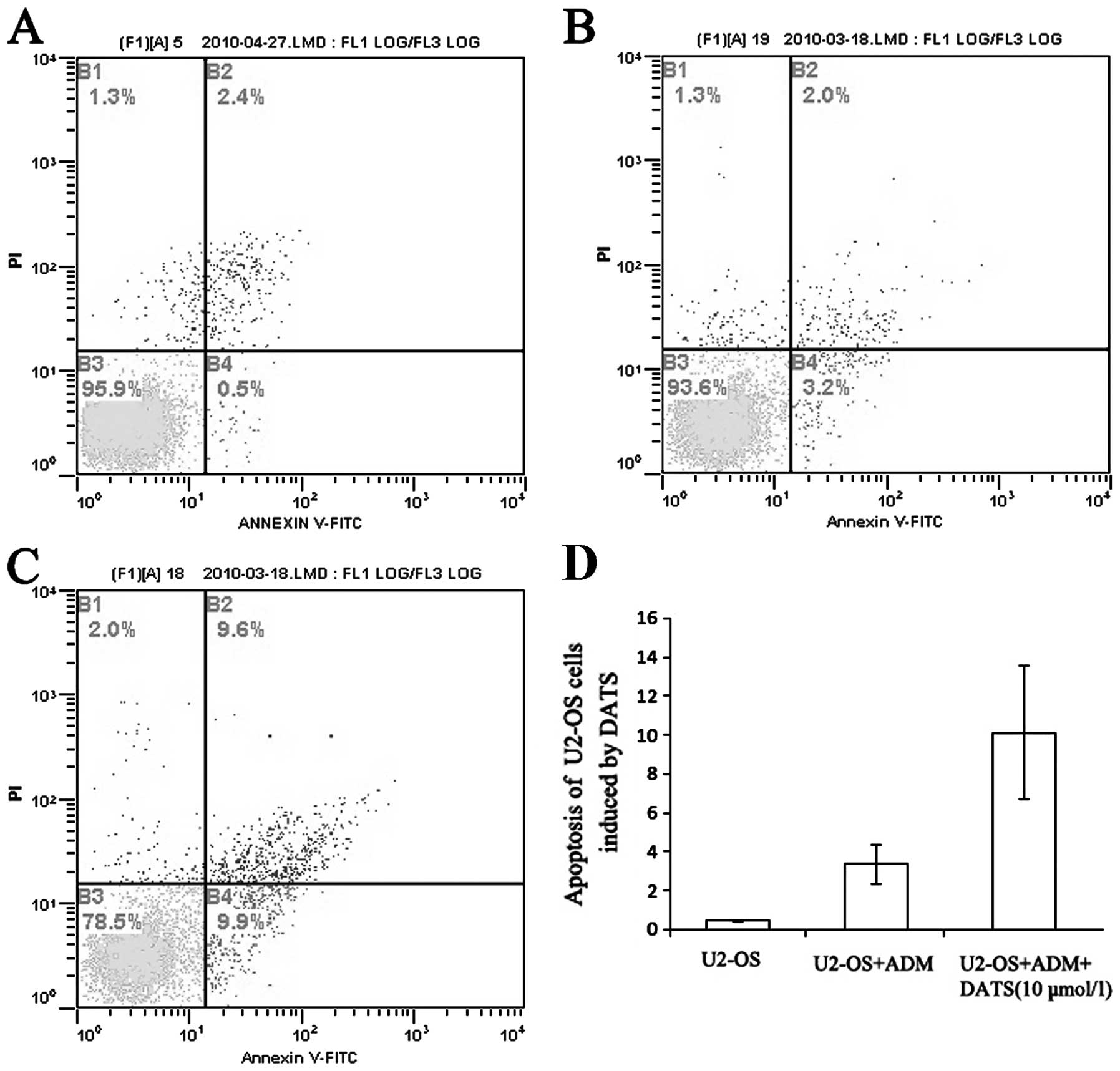
with that of the controls (P<0.01; Fig. 5). Moreover, the percentage of
apoptotic U2-OS cells treated with both ADM and DATS was much
higher than the percentage of apoptotic cells treated with ADM
alone (P<0.01; Fig. 6).
Detection of NF-κB and IκB
expression
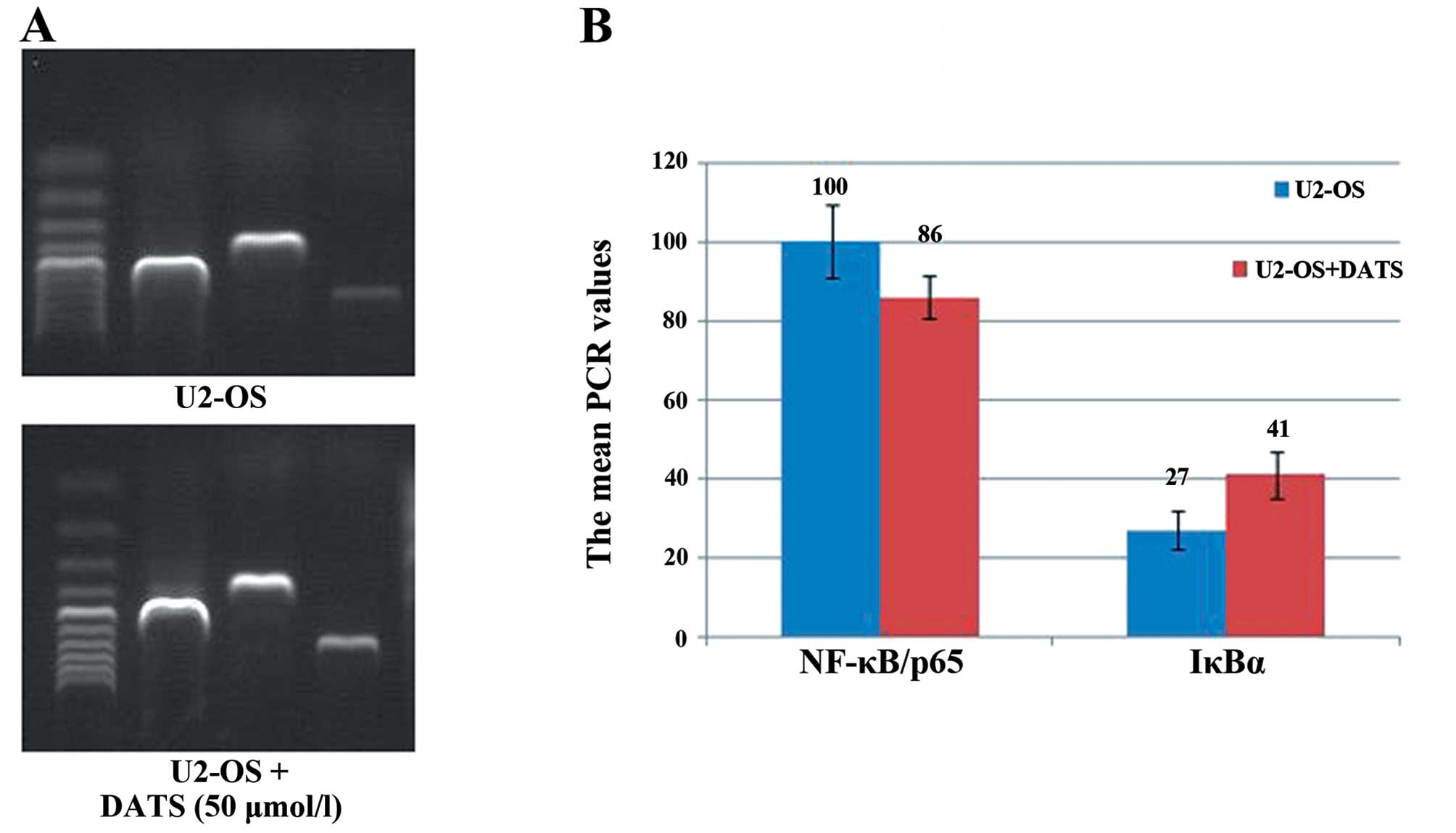
As demonstrated in Fig.
7, semi-quantitative RT-PCR revealed the gray value of
NF-κB/p65 expression to be 100±9.21. After treatment with 50 μM
DATS, an evident decrease in the NF-κB/p65 levels was observed
(86±5.57, P<0.05). Conversely, the IκBα mRNA expression in the
U2-OS cells treatment with 50 μM DATS was increased as compared
with that of the control group (27±4.80 vs. 41±5.94,
P<0.05).
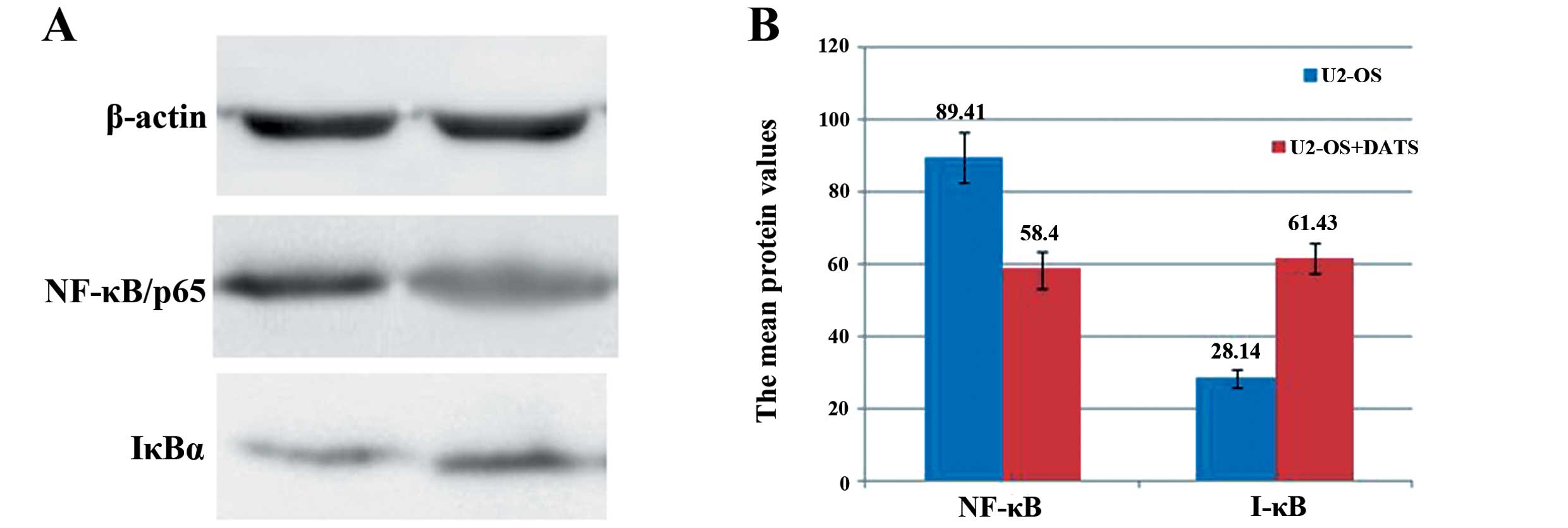
As shown in Fig. 8,
western blot analysis revealed that the NF-κB/p65 protein
expression was decreased by DATS (50 μM) in the U2-OS cells from
89.41±6.98 to 58.40±5.03, whereas the IκBα protein expression was
increased from 28.14±2.58 to 61.43±4.22 (P<0.05).
Discussion
Allicin is the general term that refers to the main
bioactive ingredient of garlic, which is actually a complex variety
of allyl organic sulfides that include the diallyl disulfides
(20–50%) and the diallyl sulfides (DATS, 50–80%). A large number of
studies (10–15) have confirmed that allicin functions
against infection as well as it prevents and treats acute and
chronic liver injury, atherosclerosis, reperfusion injury and
hypoglycemia. DATS also lowers total cholesterol and blood
pressure, inhibits platelet activity and regulates immune
functions. Since the 1980s, research on the anticancer effects of
allicin has increasingly attracted the interest of researchers. The
in vitro and in vivo preclinical studies (16–23)
have implicated DATS as an important mediator of cyclins and cell
cycle arrest as well as apoptosis, cell adhesion and angiogenesis.
In 2008, Shankar et al (23)
confirmed that DATS improves the TNF-related apoptosis-inducing
ligand (TRAIL) treatment effect in prostate cancer in vitro
and in orthotopic androgen-independent prostate cancer PC-3 cells.
These results implicate DATS as a promising reversal agent of drug
resistance.
Our previous study (24) showed that DATS effectively reversed
the P-gp-mediated MDR of K562/AO2 cells in vitro. Similarly,
DATS inhibited the growth of osteosarcoma cell lines in
vitro. Zhang et al (25)
found that the expression of 27 proteins was significantly altered
in osteosarcoma Saos-2 cells following DATS treatment. The
expression levels of 18 proteins were increased, whereas those of 9
proteins were decreased. Approximately half of these proteins
(13/27) were relevant to the cell cycle or to apoptosis. However,
the capacity of DATS to reverse the drug resistance of osteosarcoma
cells remains unknown.
The present study used the MTT assay to detect the
survival rate of U2-OS cells after treatment with different DATS
concentrations. The survival rates of the U2-OS cells were
significantly reduced. The time- and concentration-dependent
inhibition effects of DATS on the U2-OS cells were likewise
observed. Moreover, the survival rate of U2-OS cells that were
treated with a combination of both DATS and ADM was significantly
lower than treatment with ADM alone. Subsequent research is needed
to confirm the specific mechanism by which DATS inhibits the growth
of U2-OS cells.
An extensively characterized MDR mechanism involves
the overexpression of P-gp, which reduces the cellular accumulation
of cytotoxic drugs in tumor cells. P-gp is a transmembrane
glycoprotein that functions as an adenosine triphosphatase
energy-dependent transporter (26,27).
The drug intake capacity of cells with high MDR1 gene
expression levels is not significantly different as compared with
those of sensitive cells, while the drug efflux capacity of the
former is significantly increased (28,29).
Therefore, the modulation of MDR transporters is a promising
approach to overcome MDR. In the present study, FCM was used to
detect the P-gp protein expression on the U2-OS cell surface, which
subsequently showed whether DATS could reduce P-gp expression.
After treatment of the U2-OS cells with different concentrations of
DATS, the P-gp expression was decreased. The significant difference
between the treated and untreated groups supported the hypothesis
that DATS is a candidate for the development of a new MDR reversal
agent.
In addition to P-gp overexpression, various other
mechanisms could cause MDR. In recent years, studies have shown
that apoptosis is closely related to MDR. Several anticancer drugs
with different structures and different targets can induce
apoptosis in tumor cells. The mechanisms of apoptosis may probably
be involved in MDR. Thus, apoptosis is the common pathway of
various drugs. The inhibition of apoptosis makes the tumor cells
simultaneously resistant to several drugs, which eventually leads
to MDR. The results showed that the apoptosis rate of U2-OS cells
was inherently very low but increased after treatment with
different concentrations of DATS. The combined ADM and DATS
treatment caused a significantly higher apoptosis rate in U2-OS
cells as compared with ADM treatment alone. This effect of DATS was
supported by observation of the morphology of the treated cells.
Thus, the induction of apoptosis by DATS is one of the important
mechanisms for inhibiting the proliferation of U2-OS cells and
increasing the response of tumor cells to ADM.
The deregulated activation of NF-κB has been
causally linked to the development of several human pathologies,
including cancers (30,31). The hyperactivation of NF-κB
signaling contributes to tumorigenicity and chemoresistance.
Several other studies have reported that NF-κB can inhibit
apoptosis via various mechanisms (32–34).
NF-κB may participate in tumor chemoresistance, which is mediated
by the expression of MDR proteins. As previously confirmed, NF-κB
can increase the expression of MDR1 genes in tumor cells
(35,36). A purified NF-κB binding sequence
(5′-CCTTTCGGGG-3′) was identified in the first exon of the
MDR1 promoter region, which confirmed the presence of
binding sites of NF-κB in the MDR1 gene. It was further
confirmed that MDR1 may be a downstream gene regulated by
NF-κB (37). Furthermore, previous
literature has confirmed that anticancer drugs such as DXR can
damage tumor cell DNA, which consequently leads to the activation
of NF-κB. The activated NF-κB then promotes the transcription of
MDR1 via the NF-κB binding sites. Therefore, if the
expression of NF-κB can be inhibited, the sensitivity of tumor
cells to chemotherapy can be increased (38–41).
The NF-κB pathway has been associated with the proliferation and
differentiation of osteosarcoma cells (42). However, the molecular mechanisms of
NF-κB in chemoresistance of osteosarcoma still remain poorly
understood.
Semi-quantitative RT-PCR and western blot analysis
in the present study demonstrated that the NF-κB level was
decreased by DATS treatment in U2-OS cells. Furthermore, the
expression levels of IκB mRNA in U2-OS cells were increased by DATS
treatment as compared with those of the control group. Therefore,
the inhibition of NF-κB activation in U2-OS cells may be involved
in the reversal of MDR by DATS.
In conclusion, the present study demonstrated that
DATS can serve as a novel modulator of MDR in vitro by
downregulating P-gp expression and inducing the apoptosis of U2-OS
cells. For the first time, we demonstrated that DATS blocks NF-κB
activation, which subsequently produces downstream inhibitory
effects on the sensitivity to chemotherapy and apoptosis of U2-OS
cells. Thus, DATS could be a highly feasible candidate for the
development of a new MDR reversal agent.
References
|
1
|
Gillet JP, Efferth T and Remacle J:
Chemotherapy-induced resistance by ATP-binding cassette transporter
genes. Biochim Biophys Acta. 1775:237–262. 2007.PubMed/NCBI
|
|
2
|
Mimeault M, Hauke R and Batra SK: Recent
advances on the molecular mechanisms involved in the drug
resistance of cancer cells and novel targeting therapies. Clin
Pharmacol Ther. 83:673–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inaba M, Fujikura R, Tsukagoshi S and
Sakurai Y: Restored in vitro sensitivity of adriamycin- and
vincristine-resistant P388 leukemia with reserpine. Biochem
Pharmacol. 30:2191–2194. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian W, Deng Y, Li L, He H, Sun J and Xu
D: Honokiol synergizes chemotherapy drugs in multidrug resistant
breast cancer cells via enhanced apoptosis and additional
programmed necrotic death. Int J Oncol. 42:721–732. 2013.
|
|
5
|
Xu D, Tian W and Shen H: P-gp upregulation
may be blocked by natural curcuminoids, a novel class of
chemoresistance-preventing agents. Mol Med Rep. 7:115–121.
2013.PubMed/NCBI
|
|
6
|
Sun J, Yeung CA, Co NN, et al: Clitocine
reversal of P-glycoprotein associated multi-drug resistance through
down-regulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012.PubMed/NCBI
|
|
7
|
Gao CM, Takezaki T, Ding JH, Li MS and
Tajima K: Protective effect of allium vegetables against both
esophageal and stomach cancer: a simultaneous case-referent study
of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer
Res. 90:614–621. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fleischauer AT, Poole C and Arab L: Garlic
consumption and cancer prevention: meta-analyses of colorectal and
stomach cancers. Am J Clin Nutr. 72:1047–1052. 2000.PubMed/NCBI
|
|
9
|
Engdal S, Klepp O and Nilsen OG:
Identification and exploration of herb-drug combinations used by
cancer patients. Integr Cancer Ther. 8:29–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen GW, Chung JG, Ho HC and Lin JG:
Effects of the garlic compounds diallyl sulphide and diallyl
disulphide on arylamine N-acetyltransferase activity in
Klebsiella pneumoniae. J Appl Toxicol. 19:75–81. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ankri S and Mirelman D: Antimicrobial
properties of allicin from garlic. Microbes Infect. 1:125–129.
1999. View Article : Google Scholar
|
|
12
|
Ackermann RT, Mulrow CD, Ramirez G,
Gardner CD, Morbidoni L and Lawrence VA: Garlic shows promise for
improving some cardiovascular risk factors. Arch Intern Med.
161:813–824. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung LY: The antioxidant properties of
garlic compounds: allyl cysteine, alliin, allicin, and allyl
disulfide. J Med Food. 9:205–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siddique YH and Afzal M: Antigenotoxic
effect of allicin against SCEs induced by methyl methanesulphonate
in cultured mammalian cells. Indian J Exp Biol. 42:437–438.
2004.PubMed/NCBI
|
|
15
|
Ohaeri OC and Adoga GI: Anticoagulant
modulation of blood cells and platelet reactivity by garlic oil in
experimental diabetes mellitus. Biosci Rep. 26:1–6. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herman-Antosiewicz A, Kim YA, Kim SH, Xiao
D and Singh SV: Diallyl trisulfide-induced G2/M phase cell cycle
arrest in DU145 cells is associated with delayed nuclear
translocation of cyclin-dependent kinase 1. Pharm Res.
27:1072–1079. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herman-Antosiewicz A and Singh SV:
Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic
arrest in human prostate cancer cells. J Biol Chem.
280:28519–28528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Guo R, Li W, et al: A proteomic
investigation into a human gastric cancer cell line BGC823 treated
with diallyl trisulfide. Carcinogenesis. 27:1222–1231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Das A, Banik NL and Ray SK: Garlic
compounds generate reactive oxygen species leading to activation of
stress kinases and cysteine proteases for apoptosis in human
glioblastoma T98G and U87MG cells. Cancer. 110:1083–1095. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao D, Zeng Y, Hahm ER, Kim YA,
Ramalingam S and Singh SV: Diallyl trisulfide selectively causes
Bax- and Bak-mediated apoptosis in human lung cancer cells. Environ
Mol Mutagen. 50:201–212. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu XJ, Hu Y, Lamy E and Mersch-Sundermann
V: Apoptosis induction in human lung adenocarcinoma cells by
oil-soluble allyl sulfides: triggers, pathways, and modulators.
Environ Mol Mutagen. 50:266–275. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao D, Li M, Herman-Antosiewicz A, et al:
Diallyl trisulfide inhibits angiogenic features of human umbilical
vein endothelial cells by causing Akt inactivation and
down-regulation of VEGF and VEGF-R2. Nutr Cancer. 55:94–107. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shankar S, Chen Q, Ganapathy S, Singh KP
and Srivastava RK: Diallyl trisulfide increases the effectiveness
of TRAIL and inhibits prostate cancer growth in an orthotopic
model: molecular mechanisms. Mol Cancer Ther. 7:2328–2338. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia Q, Wang ZY, Li HQ, et al: Reversion of
p-glycoprotein-mediated multidrug resistance in human leukemic cell
line by diallyl trisulfide. Evid Based Complement Alternat Med.
2012:7198052012.PubMed/NCBI
|
|
25
|
Zhang YK, Zhang XH, Li JM, Sun de S, Yang
Q and Diao DM: A proteomic study on a human osteosarcoma cell line
Saos-2 treated with diallyl trisulfide. Anticancer Drugs.
20:702–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Colabufo NA, Contino M, Berardi F, et al:
A new generation of MDR modulating agents with dual activity: P-gp
inhibitor and iNOS inducer agents. Toxicol In Vitro. 25:222–230.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biedler JL and Riehm H: Cellular
resistance to actinomycin D in Chinese hamster cells in vitro:
cross-resistance, radioautographic, and cytogenetic studies. Cancer
Res. 30:1174–1184. 1970.PubMed/NCBI
|
|
29
|
Juliano RL and Ling V: A surface
glycoprotein modulating drug permeability in Chinese hamster ovary
cell mutants. Biochim Biophys Acta. 455:152–162. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niu J, Shi Y, Tan G, et al: DNA damage
induces NF-κB-dependent microRNA-21 up-regulation and promotes
breast cancer cell invasion. J Biol Chem. 287:21783–21795.
2012.
|
|
31
|
Mburu YK, Egloff AM, Walker WH, et al:
Chemokine receptor 7 (CCR7) gene expression is regulated by NF-κB
and activator protein 1 (AP1) in metastatic squamous cell carcinoma
of head and neck (SCCHN). J Biol Chem. 287:3581–3590. 2012.
|
|
32
|
Lin MT, Chang CC, Chen ST, et al: Cyr61
expression confers resistance to apoptosis in breast cancer MCF-7
cells by a mechanism of NF-κB-dependent XIAP up-regulation. J Biol
Chem. 279:24015–24023. 2004.PubMed/NCBI
|
|
33
|
Malewicz M, Zeller N, Yilmaz ZB and Weih
F: NF κB controls the balance between Fas and tumor necrosis factor
cell death pathways during T cell receptor-induced apoptosis via
the expression of its target gene A20. J Biol Chem.
278:32825–32833. 2003.
|
|
34
|
Bubici C, Papa S, Pham CG, Zazzeroni F and
Franzoso G: NF-κB and JNK: an intricate affair. Cell Cycle.
3:1524–1529. 2004.
|
|
35
|
Bourguignon LY, Xia W and Wong G:
Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates
beta-catenin signaling and NFκB-specific transcription activity
leading to MDR1 and Bcl-xL gene expression and chemoresistance in
breast tumor cells. J Biol Chem. 284:2657–2671. 2009.PubMed/NCBI
|
|
36
|
Zhou G and Kuo MT: NF-κB-mediated
induction of mdr1b expression by insulin in rat hepatoma cells. J
Biol Chem. 272:15174–15183. 1997.
|
|
37
|
Chelbi-alix MK, Bobe P, Benoit G, Canova A
and Pine R: Arsenic enhances the activation of Stat1 by interferon
gamma leading to synergistic expression of IRF-1. Oncogene.
22:9121–9130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JW, Tao S, Luo R, Zhang GS and Xu YX:
Molecular mechanism of reversing multi-drug resistance of K562/AO2
by puerarin. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 33:216–221.
2008.(In Chinese).
|
|
39
|
Kokura S, Yoshida N, Sakamoto N, et al:
The radical scavenger edaravone enhances the anti-tumor effects of
CPT-11 in murine colon cancer by increasing apoptosis via
inhibition of NF-κB. Cancer Lett. 229:223–233. 2005.PubMed/NCBI
|
|
40
|
Um JH, Kang CD, Lee BG, Kim DW, Chung BS
and Kim SH: Increased and correlated nuclear factor-kappa B and Ku
autoantigen activities are associated with development of multidrug
resistance. Oncogene. 20:6048–6056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin YG, Kunnumakkara AB, Nair A, et al:
Curcumin inhibits tumor growth and angiogenesis in ovarian
carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer
Res. 13:3423–3430. 2007.PubMed/NCBI
|
|
42
|
Tang QL, Xie XB, Wang J, et al: Glycogen
synthase kinase-3beta, NF-κB signaling, and tumorigenesis of human
osteosarcoma. J Natl Cancer Inst. 104:749–763. 2012.
|