Introduction
The incidence rates of glioma and hepatic carcinoma
increase annually in China and invasion suppression is key to
clinical management (1–2). Invasion is a complex process,
involving altered expression of adhesion molecules, proteinase,
cytokines and transcriptional factors in tumor cells (3). Therefore, studies on the potential
suppressor genes involved in glioma and hepatic carcinoma are
required to inhibit invasion and improve cancer treatment
efficacy.
Some studies have shown that tumor cells possess
higher levels of ether lipids compared with normal cells through
the alkyl or alkenyl chain. Certain ether lipids, such as,
lysophosphatidic acid-ether (LPAe) or platelet-activating
factor-ether (PAFe), have shown certain oncogenic properties. The
alkylglycerone phosphate synthase (AGPS) is a critical enzyme for
ether lipid synthesis, overexpressing in tumor cells and increasing
tumor cell growth and metastasis. Some studies support the fact
that AGPS is a pro-metastasis gene, promoting the invasion and
metastasis of several types of human cancer (4).
In the present study, we sought to elucidate the
effects and potential molecular mechanism of AGPS in suppressing
glioma and hepatic carcinoma cell invasion in vitro. A
series of glioma and hepatic carcinoma U87 and HepG2 cell lines
with knockdown of AGPS was established, and the effects of AGPS on
invasion and expression of relevant genes and signal pathways were
investigated in vitro.
Materials and methods
Cell lines and cell culture
Human glioma and hepatic carcinoma cell lines U87
and HepG2 were obtained from the American Type Culture Collection.
Cells were grown and maintained in DMEM medium (Life Technologies)
supplemented with 10% fetal bovine serum (Life Technologies) at
37°C with 5% CO2.
Cell transfection and establishment of
stable clones
Cells (3×105) were plated into a 6-well
plate. AGPS shRNA plasmid and empty vector plasmid (Santa Cruz)
were transfected into the cells using the Transfection Reagent
(Biomiga) according to the manufacturer’s instructions. Stable
clones were selected with 300 μg/ml of G418 (Life Technologies).
Resistant cell clones were harvested and analyzed using western
blotting. Cells transfected with shRNA plasmid were designated as
the AGPS-knockdown group (shRNA group). Cells transfected with
empty vectors were used as the negative groups. The parent cells
were used as the parent group.
Extraction and quantization of LPA, LPAe
and PGE2
Cancer cells were starved in serum-free media for 4
h to minimize the contribution of serum-derived metabolites.
Approximately 1×106 cells were washed twice in PBS and
harvested by scraping and centrifugation. Cell pellets were flash
frozen at −80°C. Nonpolar lipid metabolites were extracted and
analyzed by LC-MS/MS. Briefly, cell lysis was extracted by 4 ml of
mixture of 2 (chloroform): 1 (methanol): 1 PBS with C12:0
dodecylglycerol (10 nmol) and pentadecanoic acid (10 nmol) as
internal standards. Organic and aqueous layers were separated by
centrifugation. The aqueous layer was acidified by adding 0.1%
formic acid and reextracted by chloroform. The organic layer was
dried by N2 and dissolved in 120 μl chloroform and a 10 μl aliquot
was analyzed by Agilent 6430 LC-MS/MS.
Cells (4×105/ml), were plated in 96-well
plates and cultured overnight. The next day, cells were treated
with 0.1 ml arachidonic acid (final concentration 15 μM) for 1 h
before collecting the culture medium. PGE2 levels in the medium
were determined by PGE2 enzyme immunoassay kits (Cayman
Chemical).
Cell adhesion assay
96-well plates were coated with 25 μl (0.2 μg/μl)
Matrigel overnight at 37°C and blocked with 50 μl serum-free medium
with 2% BSA for 1 h at 37°C. Cells (4×104) in 100 μl
serum free medium with 0.1% BSA were added to the well for 1 h at
37°C with 5% CO2. Cells were washed with PBS and were
determined by MTS assay.
Cell invasion assay
The cell invasion activity was assessed by 24-well
Transwell chambers (Costar). The inserts contain an 8 μm pore size
polycarbonate membrane and 25 μl (0.2 μg/μl) FN (Sigma) was added
on the lower surface of the membrane, over which was a thin layer
of 50 μl (0.2 μg/μl) Matrigel (Sigma). Cells were suspended to a
final concentration of 2×106 cell/ml in serum free
medium with 0.1% BSA. Cell suspensions (100 μl) were added to the
upper compartment and 600 μl serum free medium with 0.1% BSA was
added to the lower compartment. Transwell chambers were incubated
for 24 h at 37°C with 5% CO2. Invasive cells on the
lower surface of the membrane were stained by H&E and counted
by photographing the membrane in five microscopic fields.
Flow cytometry assay
Cells (3×105) were plated into a 6-well
plate at 37°C for 24 h, collected, and fixed by 70% cold ethanol at
4°C overnight. Then, ethanol was removed and the 300 μl PI (50
μg/ml) with RNase A were added, incubated at 37°C in the dark for
30 min. Cell cycle was measured by flow cytometry at 488 nm.
Western blot analysis
Cells were washed with cold PBS and the cell pellets
were lysed using the lysis buffer (Beyotime Institute of
Biotechnology) for 45 min on ice. Lysates were centrifuged (15,000
× g, 20 min, 4°C), and the supernatants were analyzed to determine
the protein concentration. Three-fold concentrated SDS sample
buffer was added into the cell lysates and boiled for 5 min. Total
cell lysates (50 μg) and the protein molecular weight standard were
electrophoresed on a 12% SDS-polyacrylamide gel concurrently, and
then the proteins were transferred electrophoretically to
nitrocellulose membranes. Blots were blocked for 1 h at room
temperature using 5% milk and were incubated with monoclonal
antibody (E-cadherin, 1:800; CD44, 1:800; MMP-2, 1:800; MMP-9,
1:800; cyclin D1, 1:800; Santa Cruz) and mouse monoclonal β-actin
(Sigma, 1:5,000) which was used for protein loading analyses
respectively at 4°C overnight. Then, the membranes were washed and
incubated with HRP substrate for 1 h at room temperature. The
results were visualized using the Immobilon Western HRP Substrate
(Millipore).
Real-time PCR assay
The real-time PCR reagents were purchased from
Takara and the primers were synthesized by AuGCT Biological
Technology Co., Ltd (Beijing, China). SYBR-Green was used to
quantify the mRNA level. The PCR reaction was performed under the
following conditions: 10 sec at 95°C, and 40 cycles at 95°C for 5
sec and 65°C for 34 sec on the ABI Prism 7500 Sequence Detector
System. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used
for normalization. The primers for the detected genes are listed in
Table I.
 | Table IReal-time PCR primer sequences. |
Table I
Real-time PCR primer sequences.
| Primers | Sequence (5′-3′) |
|---|
| CD44 | F:
TTGATGGACCAATTACCATAACTATTG
R: CGTTCTGTATTCTCCTTTCTGGACAT |
| E-cadherin | F:
AGAACGCATTGCCACATACACTC
R: CATTCTGATCGGTTACCGTGATC |
| MMP-2 | F:
ACTGCGGTTTTCTCGAATCCA
R: GGTATCCATCGCCATGCTCC |
| MMP-9 | F:
CGGCTTGCCCTGGTGCAGT
R: CGTCCCGGGTGTAGAGTCTCTCG |
| cyclin D1 | F:
TCTAAGATGAAGGAGACCATC
R: GCGGTAGTAGGACAGGA |
| GAPDH | F:
GAAGGTGAAGGTCGGAGTC
R: GAAGATGGTGATGGGATTTC |
Luciferase reporter assay
Cells (1×105) were plated in a 24-well
plate and then transfected using the Transfection Reagent (Biomiga)
following the manufacturer’s instructions. Cells were
co-transfected with promoter-luciferase plasmids (Twist, AP-1 or
Snail) and pRL-SV40 (Promega) as an internal control for 24 h. The
luciferase reporter assay was performed using the Dual-luciferase
Reporter Assay System (Promega) on Berthold TriStar LB 941
(Berthold Technologies, Bad Wildbad, Germany).
Statistical analysis
The data are presented as mean ± standard deviation
(SD). Variance analysis between groups was performed using one-way
ANOVA. All data in the study were evaluated with Statistical
Package for the Social Sciences software version 10.0. (SPSS Inc.).
Differences were considered significant at values of P<0.05.
Results
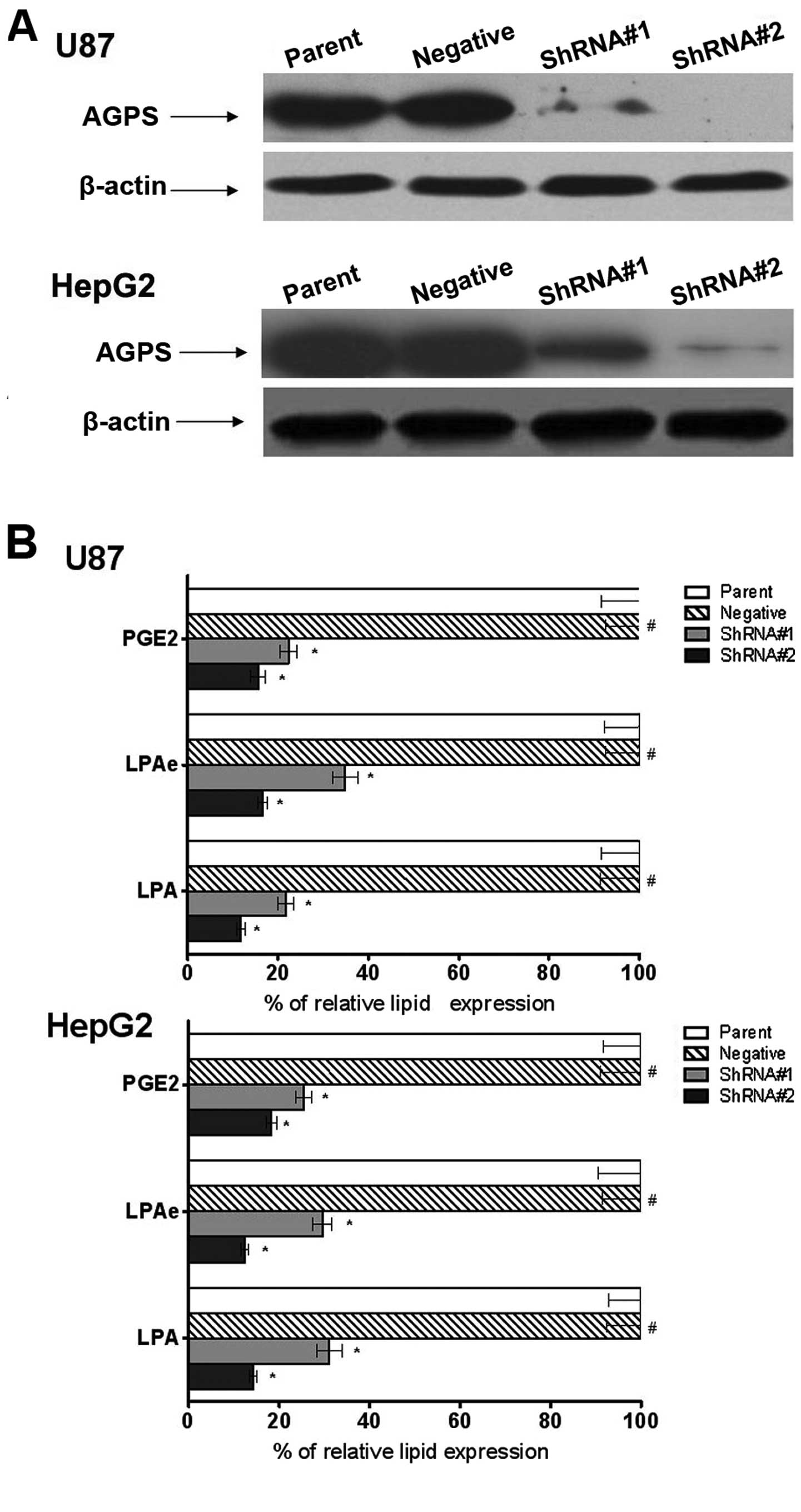
Expression of AGPS on establishment and
cellular content of LPA, LPAe and PGE2 of stable glioma and hepatic
carcinoma cell clones
To explore the role of AGPS in OVCA cells, the human
glioma U87 and hepatic carcinoma HepG2 cell lines were used to
reconstitute the expression of AGPS via the stable knockdown of
AGPS. As shown in Fig. 1A, the AGPS
expression level was downregulated in the AGPS-knockdown group
compared to the parent and negative groups.
LPA, LPAe and PGE2 are lipids that have been found
to be involved in AGPS-mediated cancer pathogenicity. As shown in
Fig. 1B, all LPA, LPAe and PGE2
were downregulated in the AGPS-knockdown group compared to the
parent and negative groups, demonstrating that in the two cell
lines, silencing of AGPS resulted in reduction of LPA, LPAe and
PGE2 (Fig. 1B).
Effect of AGPS on adhesion, invasion and
cell cycle of glioma and hepatic carcinoma cells
We hypothesized that AGPS played a role in
regulating glioma and hepatic carcinoma cell adhesion and invasion
ability. To test this hypothesis, we investigated the effect of
AGPS-knockdown in adhesion and invasion assays.
As shown in Fig. 2A,
cells of AGPS-knockdown groups show an increased adhesion compared
to parent and negative groups cells, suggesting that AGPS was
acting as a regulative gene on U87 and HepG2 cell adhesion. We
assessed the modulation effect of AGPS on the ability of epithelial
tumor cells to invade a reconstituted extracellular matrix (ECM) by
cell invasion assay. When cells of AGPS-knockdown groups were grown
on Matrigel, a significant increase in the number of invasive cells
was observed compared to the parent and negative groups after 24 h
(Fig. 2B). The results suggest that
AGPS expression is inversely associated with the invasion ability
of glioma and hepatic carcinoma cells in vitro. Cell cycle
is also an important factor in tumor metastasis; we found that
AGPS-knockdown arrested the cell cycle in the G0/G1 phase (Fig. 2C). Furthermore, we found that there
was a decreased expression of cyclin D1 in U87 and HepG2 cells
(Fig. 3). Therefore, we considered
that cell cycle arrest may be one of the mechanisms through which
AGPS regulates tumor invasive potential.
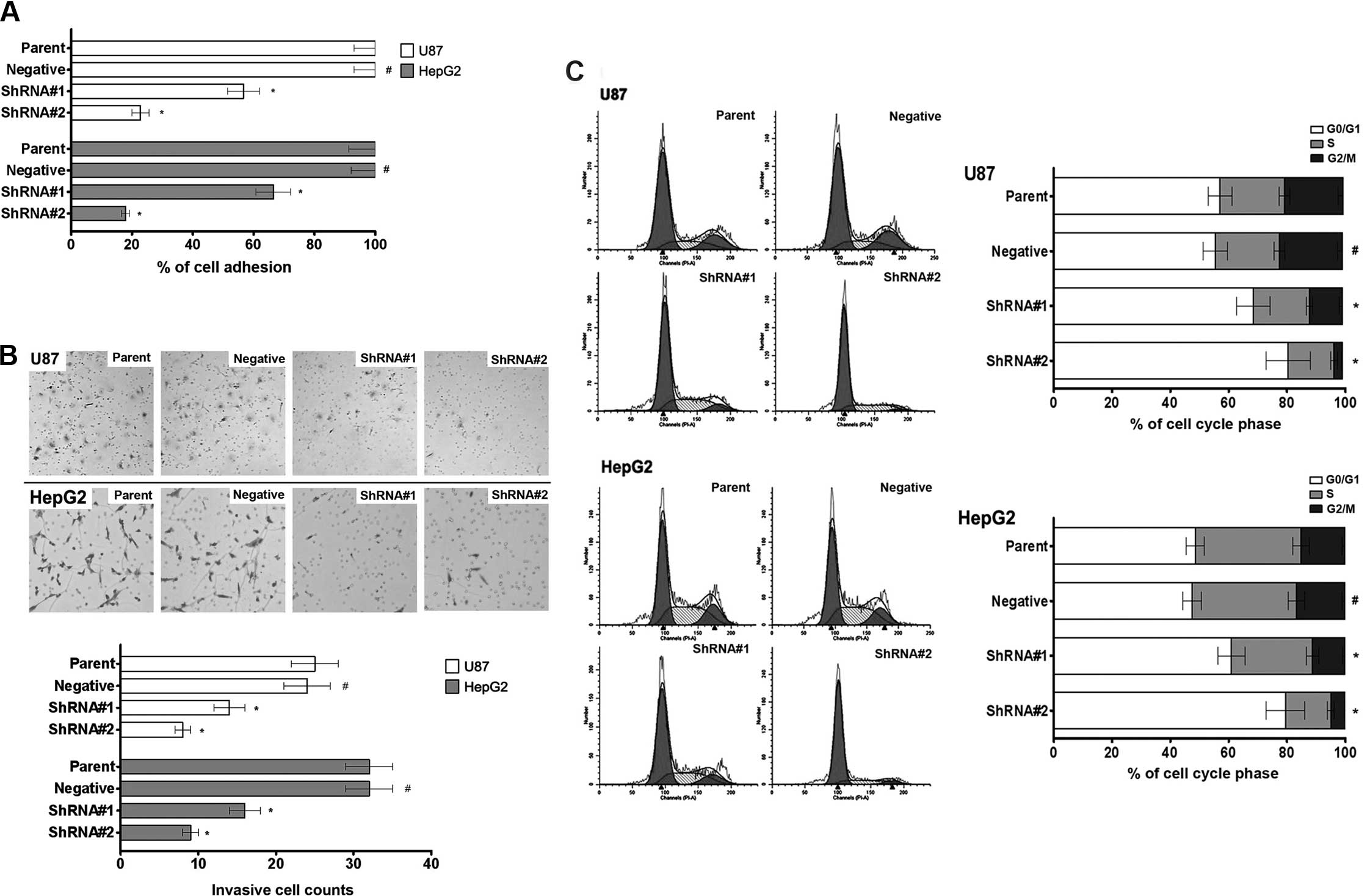 | Figure 2Effect of AGPS on adhesion, invasion
and cell cycle of glioma and hepatic carcinoma cells. (A) MTS assay
revealed a decreased adhesion ability in AGPS-knockdown human
glioma U87 and hepatic carcinoma HepG2 cell lines (shRNA#1 and
shRNA#1) compared with parent and negative groups. Bars, mean ± SD.
#P>0.05 compared to the control group,
*P<0.05 compared to the control group, n=10. (B) The
invasiveness assay revealed a decreased invasiveness ability in
AGPS-knockdown human glioma U87 and hepatic carcinoma HepG2 cell
lines (shRNA#1 and shRNA#1) compared with parent and negative
groups. Bars, mean ± SD. #P>0.05 compared to the
control group, *P<0.05 compared to the control group,
n=3. (C) The flow cytometry assay revealed a G0/G1 phase arrest in
AGPS-knockdown human glioma U87 and hepatic carcinoma HepG2 cell
lines (shRNA#1 and shRNA#1) compared with parent and negative
groups. Bars, mean ± SD. #P>0.05 compared to the
control group, *P<0.05 compared to the control group,
n=3. |
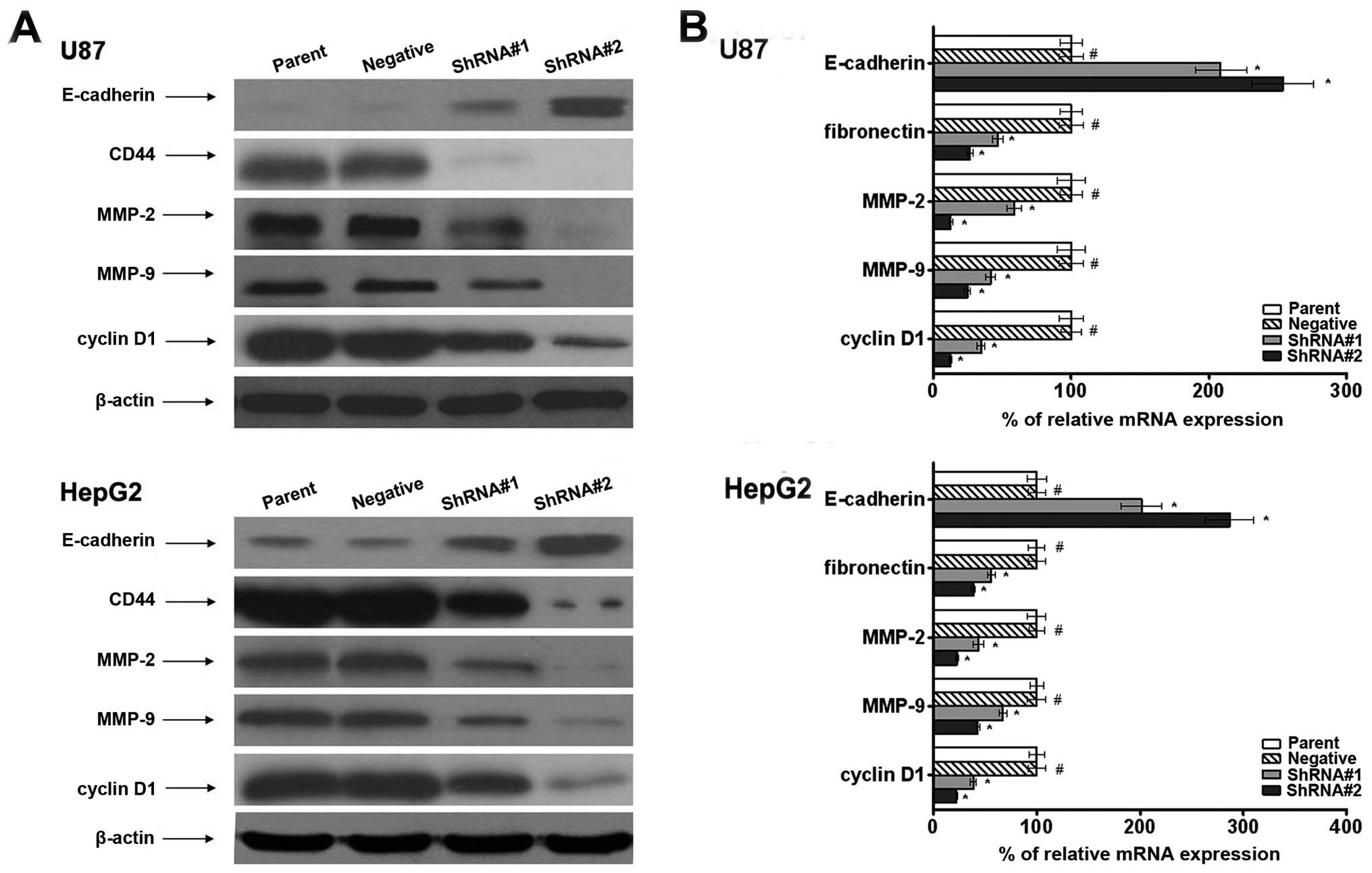
Effect of AGPS on expression of
invasion-related genes in vitro
To investigate the relationships between AGPS and
expression of invasion-related genes, we examined the expression
level of adhesion molecules such as E-cadherin and CD44 in cells.
We found that AGPS knockdown promoted the mRNA and protein
expression of CD44. In addition, the results demonstrated that the
expression of E-cadherin was suppressed in the parent and negative
groups but was increased in the AGPS-knockdown group (Fig. 3).
To further investigate the molecular mechanism, we
also observed that AGPS had significantly regulated expression
levels for MMP-2/9. MMP-2/9 play important roles in cancer
invasion. The AGPS-knockdown group exhibited significant
downregulation of MMP-2/9 at the mRNA and protein levels compared
to the parent and negative groups, as shown in Fig. 3. This suggested a regulatory effect
of AGPS on the expression of MMP in glioma and hepatic carcinoma
cells.
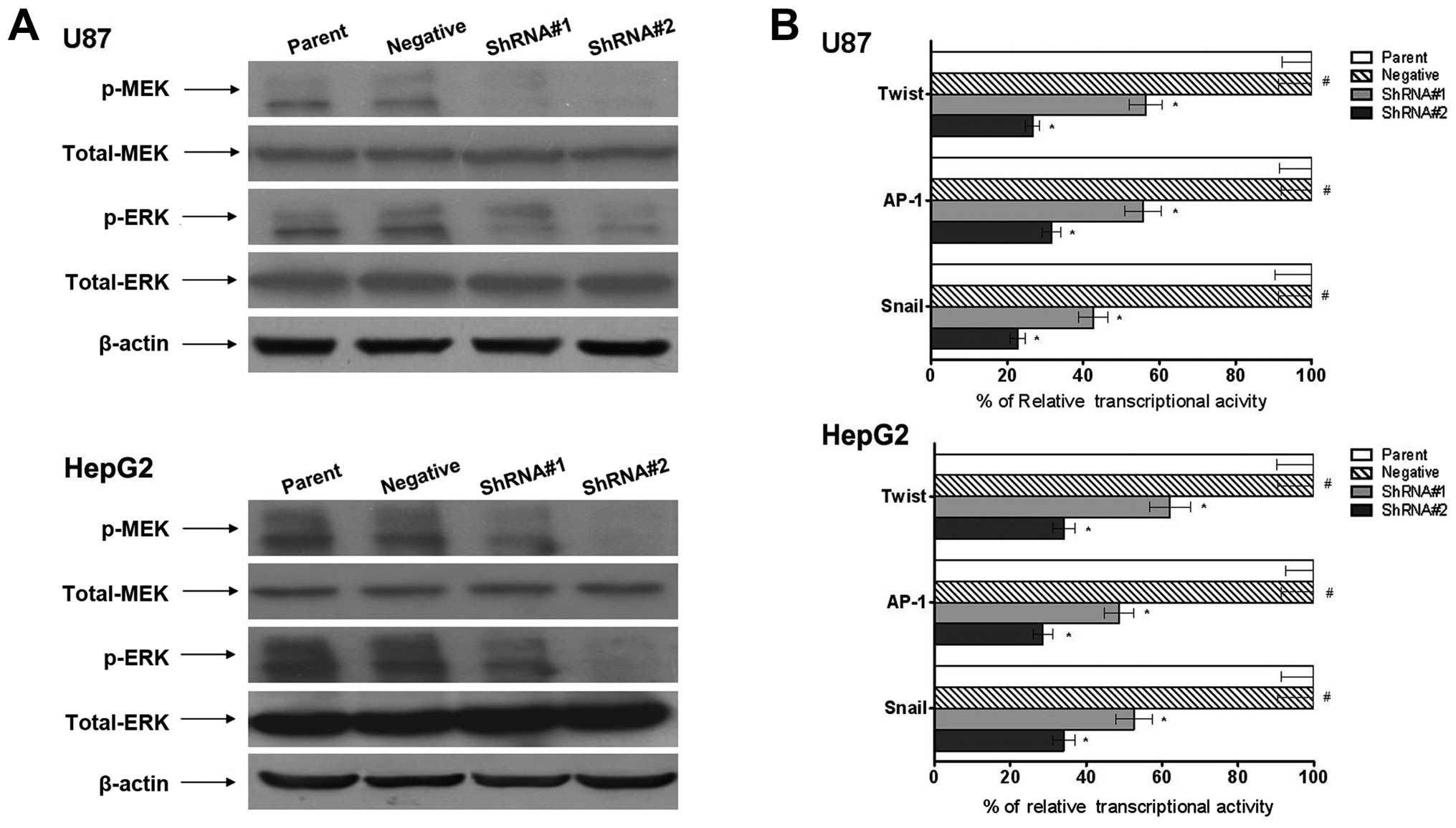
Effect of AGPS on the MAPK signaling
pathway in glioma and hepatic carcinoma cells
The MAPK signaling pathway is an important cell
signaling pathway, involved in the regulation of many fundamental
cellular processes. As shown in Fig.
4A, AGPS-knockdown reduced phosphorylation of both MEK and ERK
compared with the parent and negative groups; meanwhile, total MEK
and ERK levels remained unchanged.
Twist, AP-1 and Snail are considered to play
important roles in tumor development. In this study, using the
luciferase reporter assay, we investigated the effects of AGPS on
the transcriptional activity of Twist, AP-1 and Snail. As shown in
Fig. 4B, compared to the parent and
negative groups, AGPS-knockdown inhibited the transcriptional
activity of Twist, Snail and AP-1.
Discussion
Tumor-related mortality is primarily due to the
invasion progression of tumor cells and increasing evidence
supports the important role of invasion in cancer progression
(5,6). Thus, to improve the clinical outcome,
it is essential to understand the mechanism of AGPS-knockdown
inhibition in this complex processes.
The research and development of invasion-related
genes is one of the most active fields in cancer research. The
metabolic enzyme alkylglycerone phosphate synthase (AGPS) is a
critical step in the synthesis of ether lipids and is upregulated
across multiple types of tumor. We examined the effect of
AGPS-knockdown on the potential of invasion of these cells, and
investigated the regulation mechanism and roles of the
AGPS-regulated invasion in glioma and hepatic carcinoma cells.
Invasion is a program of the development of tumor
cells by the altered expression of cellular lipids and genes and
increased invasion was potentially reported as the key step towards
cancer aggravation (7). We found
that AGPS-knockdown could suppress expression of cellular lipids
such as LPA, LPAe and PGE2, adhesion and invasion of glioma U87 and
hepatic carcinoma HepG2 cells. To further understand the mechanism,
we investigated a series of key factors in the process of invasion.
Cell cycle is a key step of tumor invasion, the results showed that
AGPS-knockdown could arrest cell cycle in the G0/G1 phase and
downregulated the expression of cyclin D1 in tumor cells. CD44 has
numerous functions involved in tumor adhesion, growth,
differentiation and resistance to apoptosis-inducing
chemotherapeutic agents. Suppression of adhesion molecule
expression such as E-cadherin and increased cell mobility were
reported to be the key steps toward cancer metastasis. MMP-2/9
degraded the components of the basement membrane and are strongly
implicated in the invasion of many types of tumor (8–12). Our
study clearly demonstrated that AGPS-knockdown improved the
expression of E-cadherin, and downregulated the expression of CD44
in tumor cells. We further demonstrated the regulatory role of AGPS
in invasion through regulating MMP-2/9 gene expression. These
results strongly suggest that AGPS plays an important role in
invasion through the modulation of invasion-related genes.
The MAPK signaling pathway is involved in the
regulation of many fundamental cellular processes, including
proliferation, differentiation, survival and cell death, as well as
controlling the expression of a number of pro-metastasis genes.
Twist, AP-1, and Snail are the transcription factors required for
the upregulation of a large number of genes encoding proteins
important for invasion in cancer (13–16).
We found that AGPS-knockdown reduced activity of the MAPK signaling
pathway and transcription activity of Twist, AP-1, and Snail, and
we considered that it may be a key mechanism for AGPS to regulate
the invasion of tumor cells.
In summary, our study showed that AGPS is an
invasion promoter of glioma and hepatic carcinoma. The decreased
AGPS expression was correlated with decreased invasion potential by
the modulation of the MAPK signaling pathway and activities of
transcriptional factors. Therefore, we consider AGPS to be a
potential molecular target for cancer invasion.
Acknowledgements
This study was supported by The Fund of the Health
Bureau of Tianjin (Grant No. 2013KY17) and Tianjin Research Program
of Application Foundation and Advanced Technology (Grant No.
14JCQNJC12000).
References
|
1
|
Zhang H, Nie W, Zhang X, Zhang G, Li Z, Wu
H, Shi Q, Chen Y, Ding Z, Zhou X and Yu R: NEDD4-1 regulates
migration and invasion of glioma cells through CNrasGEF
ubiquitination in vitro. PLoS One. 12:e827892013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Shi X, Zhang J, Zhang X and Martin
RC: Hepatic protection and anticancer activity of curcuma: A
potential chemopreventive strategy against hepatocellular
carcinoma. Int J Oncol. 2:505–513. 2014.PubMed/NCBI
|
|
3
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(-like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 2:131–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benjamin DI, Cozzo A, Ji X, Roberts LS,
Louie SM, Mulvihill MM, Luo K and Nomura DK: Ether lipid generating
enzyme AGPS alters the balance of structural and signaling lipids
to fuel cancer pathogenicity. Proc Natl Acad Sci USA.
37:14912–14917. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Zhao Y, Ju Q, Chen L, Li F, Zhou G,
Xie P, Li G and Li Y: Molecular clone and functional study of a
novel hepatoma associated gene. Int J Oncol. 3:1105–1112.
2013.PubMed/NCBI
|
|
6
|
Zhu Y, Yang P, Wang Q, Hu J, Xue J, Li G,
Zhang G, Li X, Li W, Zhou C, Zhao M and Wang D: The effect of CXCR4
silencing on epithelial-mesenchymal transition related genes in
glioma U87 cells. Anat Rec (Hoboken). 12:1850–1856. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Wang J, Gou WF, Xiu YL, Zheng HC,
Zong ZH, Takano Y and Zhao Y: The involvement of RhoA and Wnt-5a in
the tumorigenesis and progression of ovarian epithelial carcinoma.
Int J Mol Sci. 12:24187–24199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Xiao W, Chen W, Luo L, Ye S and
Liu Y: The epigenetic modifier trichostatin A, a histone
deacetylase inhibitor, suppresses proliferation and
epithelial-mesenchymal transition of lens epithelial cells. Cell
Death Dis. 4:e8842013. View Article : Google Scholar
|
|
9
|
Koay MH, Crook M and Stewart CJ: Cyclin
D1, E-cadherin and beta-catenin expression in FIGO Stage IA
cervical squamous carcinoma: diagnostic value and evidence for
epithelial-mesenchymal transition. Histopathology. 6:1125–1133.
2012. View Article : Google Scholar
|
|
10
|
La Monica S, Caffarra C, Saccani F,
Galvani E, Galetti M, Fumarola C, Bonelli M, Cavazzoni A, Cretella
D, Sirangelo R, Gatti R, Tiseo M, Ardizzoni A, Giovannetti E,
Petronini PG and Alfieri RR: Gefitinib inhibits invasive phenotype
and epithelial-mesenchymal transition in drug-resistant NSCLC cells
with MET amplification. PLoS One. 10:e786562013.PubMed/NCBI
|
|
11
|
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang
S, Zou X and Peng X: Wild-type p53 suppresses the
epithelial-mesenchymal transition and stemness in PC-3 prostate
cancer cells by modulating miR-145. Int J Oncol. 4:1473–1481.
2013.PubMed/NCBI
|
|
12
|
Hsieh YS, Chu SC, Hsu LS, Chen KS, Lai MT,
Yeh CH and Chen PN: Rubus idaeus L. reverses
epithelial-to-mesenchymal transition and suppresses cell invasion
and protease activities by targeting ERK1/2 and FAK pathways in
human lung cancer cells. Food Chem Toxicol. 62:908–918. 2013.
View Article : Google Scholar
|
|
13
|
Li NY, Weber CE, Wai PY, Cuevas BD, Zhang
J, Kuo PC and Mi Z: An MAPK-dependent pathway induces
epithelial-mesenchymal transition via Twist activation in human
breast cancer cell lines. Surgery. 2:404–410. 2013.PubMed/NCBI
|
|
14
|
Ferraro A, Mourtzoukou D, Kosmidou V,
Avlonitis S, Kontogeorgos G, Zografos G and Pintzas A: EZH2 is
regulated by ERK/AKT and targets integrin alpha2 gene to control
epithelial-mesenchymal transition and anoikis in colon cancer
cells. Int J Biochem Cell Biol. 2:243–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pakala SB, Singh K, Reddy SD, Ohshiro K,
Li DQ, Mishra L and Kumar R: TGF-β1 signaling targets
metastasis-associated protein 1, a new effector in epithelial
cells. Oncogene. 19:2230–2241. 2011.
|
|
16
|
Tsubaki M, Komai M, Fujimoto S, Itoh T,
Imano M, Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K,
Fujiwara D, Mukai J, Sakaguchi K, Satou T and Nishida S: Activation
of NF-κB by the RANKL/RANK system up-regulates snail and twist
expressions and induces epithelial-to-mesenchymal transition in
mammary tumor cell lines. J Exp Clin Cancer Res. 1:622013.
|