Introduction
Colorectal cancer (CRC) is the third most common
cancer in men and the second in women worldwide and accounts for
almost 8% of all cancer-related deaths (1). In Asian countries including China,
Singapore, South Korea and Japan, CRC has displayed a rapidly
rising trend (2). A 2- to 4-fold
upsurge in the incidence of CRC has been encountered over the past
few decades (3). In order to abate
disease mortality, timely detection of CRC by population screening
has been a pivotal strategy. However, existing screening tools such
as fecal occult blood test (FOBT) or colonoscopy either lack
sensitivity and specificity (4) or
are too invasive and costly. Fecal DNA (5) and mRNA testing (6) are emerging non-invasive techniques,
but their wide acceptance awaits further optimization and
validation with respect to the clinical diagnostic value. These
limitations have spawned efforts to develop an alternative
fecal-based method that is accurate yet easily administered as a
population screening tool for CRC.
As prevailing studies focused on DNA and mRNA
aberrations as potential fecal markers, the role of
post-transcriptional changes remains underexplored. MicroRNAs
(miRNAs) are 19–24 nucleotide non-coding RNAs which negatively
regulate gene expression post-transcriptionally by suppressing
translation and facilitating degradation of target mRNAs (7). Due to their ability to modulate the
expression of oncogenes and tumor suppressors directly, miRNAs have
increasingly been implicated in tumorigenesis (8–11). A
growing number of clinical studies have congruently shown
deregulated miRNA expression in surgically excised colorectal
tumor, for instance an upregulation of oncogenic miR-135b that
inhibits the tumor suppressor adenomatous polyposis coli (APC)
gene, compared to matched normal mucosae (12–20).
Owing to its direct contact with the colorectum, feces represent a
valuable resource of this unique miRNA signature for the detection
of CRC. It was hence hypothesized that fecal miRNAs could serve as
candidate markers for the non-invasive screening of CRC.
While targeted profiling of selected fecal miRNAs
have been explored (21–26), a holistic understanding of the
global fecal miRNA profile in CRC patients is lacking, particularly
in Asian patients. Non-targeted fecal miRNA profiling enables the
discovery of novel diagnostic markers but has hitherto been
conducted predominantly in Caucasian CRC patients (27,28)
who may possess different genetic predispositions (3,29) and
hence dissimilar fecal miRNA signature from their Asian
counterparts. A global profiling study is, therefore, warranted in
the Asian context. Furthermore, it remains to be determined if the
global miRNA profile characterizing the feces of CRC patients
reflects, or differs from, that within the colorectal tumor. In
addition, gut bleeding is a clinically prevalent phenomenon of CRC.
With the significant presence of miRNAs in the plasma, platelets
and blood cells (30,31), the potential impact of blood in
stool on the fecal miRNA profile needs to be addressed. To date,
this pertinent factor that influences the interpretation of fecal
miRNA data in disease biomarker profiling remains unclear.
In the present study, the global human fecal miRNA
profiles were characterized in CRC patients and healthy subjects in
Singapore to elucidate fecal markers for non-invasive detection of
the disease. Independently, tissue miRNA profiles were also
characterized and compared between cancer and matched normal
mucosae resected from the same cohort of CRC patients. The effect
of blood in stool on the levels of fecal miRNA markers was
investigated for the first time.
Materials and methods
Clinical population and samples
CRC patients and healthy subjects were recruited at
the Outpatient Specialist Clinic at the Singapore General Hospital
from September 2011 to February 2013. All subjects were Han
Chinese. The inclusion criterion for CRC patients was the diagnosis
of sporadic CRC where surgical tumor resection had not been
performed. Exclusion criterion was administration of neoadjuvant
chemotherapy or radiotherapy. Randomly selected healthy subjects
were eligible for the present study if they had undergone a
colonoscopy within the past 5 months to confirm the absence of CRC
or pre-cancerous polyps. Exclusion criteria comprised a history of
CRC, a history of polyps within the last 3 years, the presence of
inherited CRC syndromes (such as, Lynch syndrome and familial
adenomatous polyposis) and a family history of hereditary CRC.
Subjects in both groups did not have co-existent inflammatory bowel
disease. The present study was endorsed by the Institutional Review
Board at the Singapore General Hospital (2010/042/B) and all
subjects provided written informed consent prior to participation
in the study.
Fecal samples were collected domestically by CRC
patients before surgical resection and by healthy subjects within 1
month of enrolment in the study. Detailed instructions for sample
collection were provided to all subjects. Briefly, a representative
aliquot of feces was collected in a sealed plastic container. Care
was taken to prevent contact with urine and toilet water. The
samples were transported to the hospital on the same day, or were
otherwise kept at 4°C and transferred to the hospital within 2
days. All samples were stored at −80°C immediately upon receipt at
the hospital.
Surgical tissues resected from the same cohort of
CRC patients were snap-frozen in liquid nitrogen, microdissected
and stored at −80°C until analysis. Careful microdissection of the
excised tissue ensured that at least 90% of the tumor specimen
consisted of cancer cells. Matched normal mucosa samples were
obtained from excised tissue at least 5 cm away from the edges of
the tumor. Routine histological evaluation of the tumor specimens
was also conducted by a blinded pathologist to determine the stage
and grade of the cancer.
Study design
The study was implemented in 2 phases, namely phase
I, biomarker screening, and phase II, biomarker confirmation. In
phase I, biomarker screening, global profiling by microarray was
performed on the fecal miRNAs extracted from 8 CRC (stage B–C)
patients and 8 healthy subjects. Concurrently, the platform was
employed to compare the miRNA profiles of tumors (n=8) and paired
normal mucosa (n=8) surgically excised from the same cohort of CRC
patients. The differential miRNA expression patterns established
from the fecal and tissue analyses were compared.
In phase II, biomarker confirmation, selected fecal
markers identified from phase I were confirmed by real-time PCR. In
phase IIa, the validation of the real-time PCR approach to
biomarker confirmation was performed using miR-135b, an established
fecal marker for CRC (22,27,28),
on a randomly chosen subset of 9 CRC patients and 19 healthy
subjects. In phase IIb, the CRC-related dysregulation of selected
fecal miRNAs shown in phase I was confirmed on an extended cohort
of CRC patients (n=17) and healthy subjects (n=28). The robustness
of these markers in discriminating CRC subjects from healthy
subjects was evaluated.
Materials
MirVana™ miRNA isolation kit was purchased from
Ambion. TaqMan® MicroRNA Assays, Taqman®
microRNA RT kit and Taqman® Universal PCR Master Mix
(2X) with no AmpErase UNG were products of Applied Biosystems.
QIAshredder was supplied by Qiagen. Nuclease-free water was
obtained from Invitrogen.
Total RNA extraction from feces and
colorectal tissue
Total RNA (including miRNAs) was isolated from ~50
mg of frozen feces (sampled from 3 different points of the fecal
mass) or 20 mg of fresh frozen tissue using the mirVana™ miRNA
isolation kit. Briefly, the samples were homogenized by plastic
pestle on ice in 600 μl of lysis buffer. The homogenate was passed
through QIAshredder and centrifuged at 18,000 × g for 2 min.
Subsequent steps were performed in accordance with the
manufacturer’s instructions. Total RNA was then quantified using
NanoDrop (Thermo Scientific). Quality of the RNA was determined
using Agilent bioanalyzer 2100 (Agilent Technologies).
Microarray-based profiling (phase I)
Labeling and hydridization
Microarray analysis was performed on Agilent Human
miRNA 8×60K format v16 (based on Sanger miRbase version 16.0) by
Genomax Technologies. Each array contained probes interrogating
1,347 miRNAs. In the analysis, miRNAs were labeled with Agilent
miRNA Complete Labelling and Hyb kit. Briefly, total RNA (100 ng)
was phosphorylated with Calf Intestinal Alkaline Phosphatase before
ligating with cyanine3-pCp by T4 RNA Ligase. Labeled RNA was dried
completely using vacuum centrifugation and reconstituted in 17 μl
of nuclease-free water and hybridized onto Agilent SurePrint G3
Human miRNA 8×60K microarray for 20 h at 55°C. After hybridization,
the microarray slide was washed before scanning on the Agilent High
Resolution Microarray Scanner (C-model). Raw signal data were
extracted with Agilent Feature Extraction Software (version
10.7.1.1).
Data pre-processing
Data was pre-processed by logarithmic transformation
and normalization to the 90th percentile. Only miRNAs present in at
least 75% of samples in any 1 out of 2 conditions, at raw signal
intensities >20, were analyzed.
Selection of endogenous controls for
real-time PCR
To the processed data from the fecal miRNA
microarray, the following cut-offs were applied: i) small
coefficient of variation (CV) of <25% across samples, ii)
minimal fold-change in the range of 0.8–1.25 between groups, and 3)
raw signal intensity >100. Selection of appropriate endogenous
control was carried out based on the commercial availability of
Taqman® real-time PCR assays and the absence of
publications on their dysregulation in CRC. Finally, candidate
endogenous controls were verified on real-time PCR. GeNorm
Algorithm was utilized to determine M value, a measure of
expression stability (32).
Suitable endogenous controls have M values <1.5.
Real-time PCR-based profiling (phase
II)
Primers for reverse transcription (RT) and real-time
PCR were provided by TaqMan® MicroRNA assays. The assays
target miR-223 (assay ID: 002295), miR-451 (assay ID: 001141),
miR-135b (assay ID: 002261), miR-1202 (assay ID: 002858), miR-4257
(assay ID: 244369) and miR-3937 (assay ID: 462743). Total RNA (10
ng) was reverse-transcribed using Taqman® microRNA RT
kit, according to the manufacturer’s instructions. The PCR reaction
mix contained 1.33 μl of cDNA, 1 μl of primers, 10 μl of
Taqman® Universal PCR Master Mix (2X) with no AmpErase
UNG and 7.67 μl of nuclease-free water. The thermal cycling
procedure encompassed pre-cycling heat activation at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing/extension at 60°C for 60 sec, in a CFX96 real-time PCR
system (Bio-Rad). Reactions were performed in triplicates. Data
were obtained as average Ct values, and normalized against
endogenous controls as ΔCt.
Validation of real-time PCR method for
clinical profiling
The amplification efficiencies of 3 target miRNAs
(miR-223, miR-451 and miR-135b) and 2 endogenous controls (miR-1202
and miR-4257) were examined. In the PCR reaction, primers were
added to five different cDNA concentrations that were serially
diluted. The generated Ct values were then plotted against the
logarithm of cDNA concentrations. Amplification efficiency was
derived from the slope of the log-linear portion of the calibration
curve (efficiency = 10−1/slope −1). No-template-control
(NTC) and no-reverse-transcription (NRT) control were included.
Effect of blood on the fecal miRNA
levels
Feces (Bristol stool scale type 4) were collected
from a healthy subject who was tested negative by FOBT. Blood was
spiked into feces to simulate different clinical levels of blood in
stool, namely 0.1 mg Hb/g stool (occult), 1 mg Hb/g stool (occult),
10 mg Hb/g stool and 100 mg Hb/g stool (gross). Unspiked neat feces
and blood served as controls. The experiment was performed in
triplicates. RNA was isolated, reverse-transcribed and analyzed by
real-time PCR as detailed above. The normalized levels of miR-451,
miR-223 and miR-135b were expressed as a ratio with respect to neat
feces.
Statistical analysis
In microarray-based profiling, data pre-processing,
filtering, univariate t-test and fold-change analysis were
performed using GeneSpring GX 11.5. Specifically, independent
samples t-test was used to compare miRNAs from fecal samples of CRC
patients and healthy subjects, while paired samples t-test was used
to compare miRNAs from tumor tissue and matched normal mucosae.
Multivariate partial least-squares discriminant analysis (PLS-DA)
was performed using SIMCA-P version 11.0 software (Umetrics). The
validity of the PLS-DA model was ascertained by response
permutation testing (100 repetitions). A list of differential
miRNAs was identified based on variable importance for projection
(VIP) score of >1.2 in the PLS-DA coupled to P-value of <0.05
in the t-tests. Heat map was generated using R (www.r-project.org).
In real-time PCR-based profiling, univariate
analysis of relative levels of miRNAs was accomplished using the
Relative Expression Software Tool (REST© 2009) (Qiagen).
Statistical significance was established at P<0.05. Multivariate
logistic regression was performed using MedCalc version 12.7 using
ΔCt values. To evaluate the robustness of the markers in
classifying CRC patients and healthy individuals, ROC analyses were
performed using the predicted Y-values from the regression model.
Corresponding areas under the ROC curve (AUC) were determined.
Results
Patient characteristics
A total of 45 participants including 17 CRC patients
and 28 healthy subjects were recruited. The clinical
characteristics of the participants are summarized in Table I. Notably, there was an uneven
distribution of age (P<0.005, independent samples t-test) and
gender (P<0.01, χ2 test) between the groups. These
factors were hence adjusted in subsequent analysis. Among the CRC
patients, the cancer stages ranged from Dukes’ stages B to D. Most
tumors were moderately differentiated.
 | Table IClinical characteristics of CRC
patients and healthy subjects. |
Table I
Clinical characteristics of CRC
patients and healthy subjects.
|
Characteristics | CRC patients
(n=17) | Healthy subjects
(n=28) |
|---|
| Age (years) |
| Mean | 63.7 | 55.1 |
| Range | 46–80 | 36–79 |
| Gender, n (%) |
| Male | 13 (76.5) | 10 (35.7) |
| Female | 4 (23.5) | 18 (64.3) |
| Tumor site, n
(%) |
| Ascending
colon | 1 (5.9) | |
| Sigmoid | 4 (23.5) | |
| Rectosigmoid | 3 (17.6) | |
| Rectum | 9 (52.9) | |
| Dukes’ stage, n
(%) |
| B | 8 (47.1) | |
| C | 7 (41.2) | |
| D | 2 (11.8) | |
| Grade |
| Well
differentiated | 2 (11.8) | |
| Moderately
differentiated | 14 (82.4) | |
| Poorly
differentiated | 1 (5.9) | |
Elucidation of CRC-related miRNA profiles
in feces and tissue (phase I)
In the microarray-based fecal miRNA expression
analysis, 277 miRNAs were detected after the exclusion of low or
non-uniform signals. Univariate tests uncovered 17 human fecal
miRNA markers characterizing CRC (P<0.05), although multivariate
analysis did not differentiate CRC patients from healthy subjects.
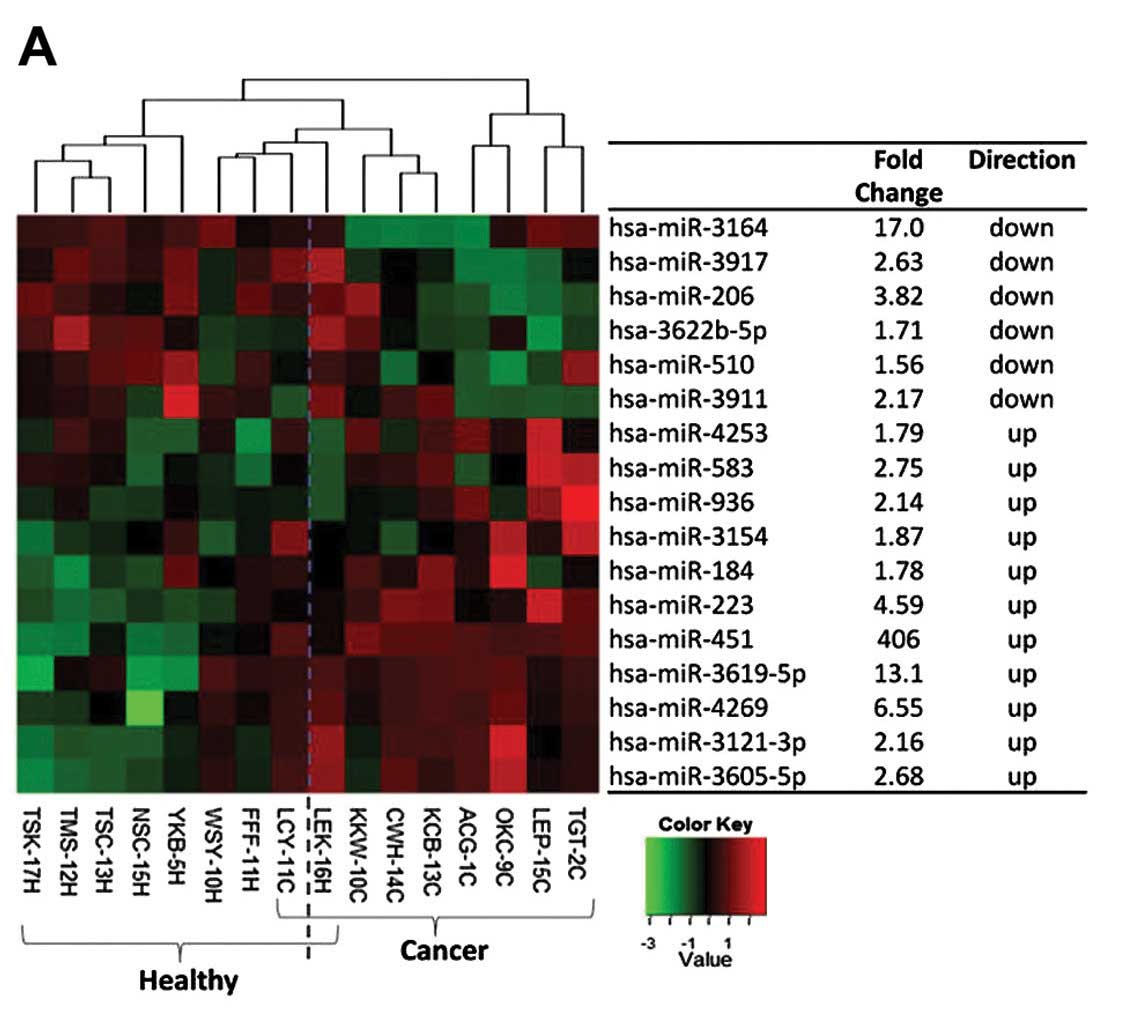
Fig. 1A shows the heat map of these
17 fecal miRNAs and their associated fold-changes relative to
healthy subjects. Among the fecal miRNAs, miR-451 was the most
prominently dysregulated.
In the global tissue miRNA profiling, 287 miRNAs
were detected. Tumor miRNA signature was clearly distinct from
matched normal mucosa as shown in the PLS-DA scores plot (3 latent
variables, R2X=0.619, R2Y=0.992,
Q2=0.883; Fig. 1B).
Based on PLS-DA, 79 discriminating human miRNAs were uncovered
(VIP>1.2, P<0.05) and summarized as a heat map in Fig. 1C.
Candidate endogenous controls for
real-time PCR
In addition to uncovering differentially expressed
miRNAs, a further capability of microarray analysis was to identify
consistently expressed fecal miRNAs that may serve as normalizers
on subsequent real-time PCR analysis, given the lack of reports on
this subject. Seventeen stably expressed candidate human miRNAs
with suitable signal intensity, CV across samples and fold-change
between groups were shortlisted. Three miRNAs, miR-3937, miR-4257
and miR-1202, were further selected based on the commercial
availability of Taqman® real-time PCR assays and the
absence of published dysregulation in CRC. While miR-3937 showed a
Ct value >40, miR-1202 and miR-4257 were detected and confirmed
as suitable endogenous controls (M value <1.5) by real-time PCR.
Fecal miR-1202 and miR-4257 were hence employed as endogenous
controls in subsequent real-time PCR analyses.
Validation of real-time PCR method for
clinical profiling
Real-time PCR provides a quantitative tool to
support clinical profiling of specific miRNA markers identified
from the earlier global profiling. Firstly, reaction efficiencies
of all amplifications were determined to be between 84.8 and
105.7%, ascertaining minimal presence of PCR inhibitors.
R2 coefficients of the calibration curves were >0.9.
Corresponding NRT controls verified negligible amplification of
genomic DNA.
miR-135b, an established fecal marker for CRC
(22,27,28),
was used as a probe for validating the real-time PCR approach to
biomarker confirmation. In accordance with previous reports, fecal
miR-135b was significantly upregulated in CRC patients compared
with healthy subjects. A mean fold-change of 7.25 (P<0.05) was
observed.
Biomarker confirmation of miR-223 and
miR-451 using real-time PCR (phase II)
After validating the real-time PCR approach, the
CRC-related dysregulation of fecal miR-223 and miR-451 was
confirmed in the complete cohort of 17 CRC patients and 28 healthy
subjects. From a univariate analysis, the levels of fecal miR-223
and miR-451 were appreciably higher in CRC patients compared to
healthy subjects [17.5- and 102-fold higher for miR-223
(P<0.001) and miR-451 (P<0.001), respectively]. Logistic
regression analyses with age, gender and fecal miRNAs as
independent variables further revealed both fecal miR-223 and
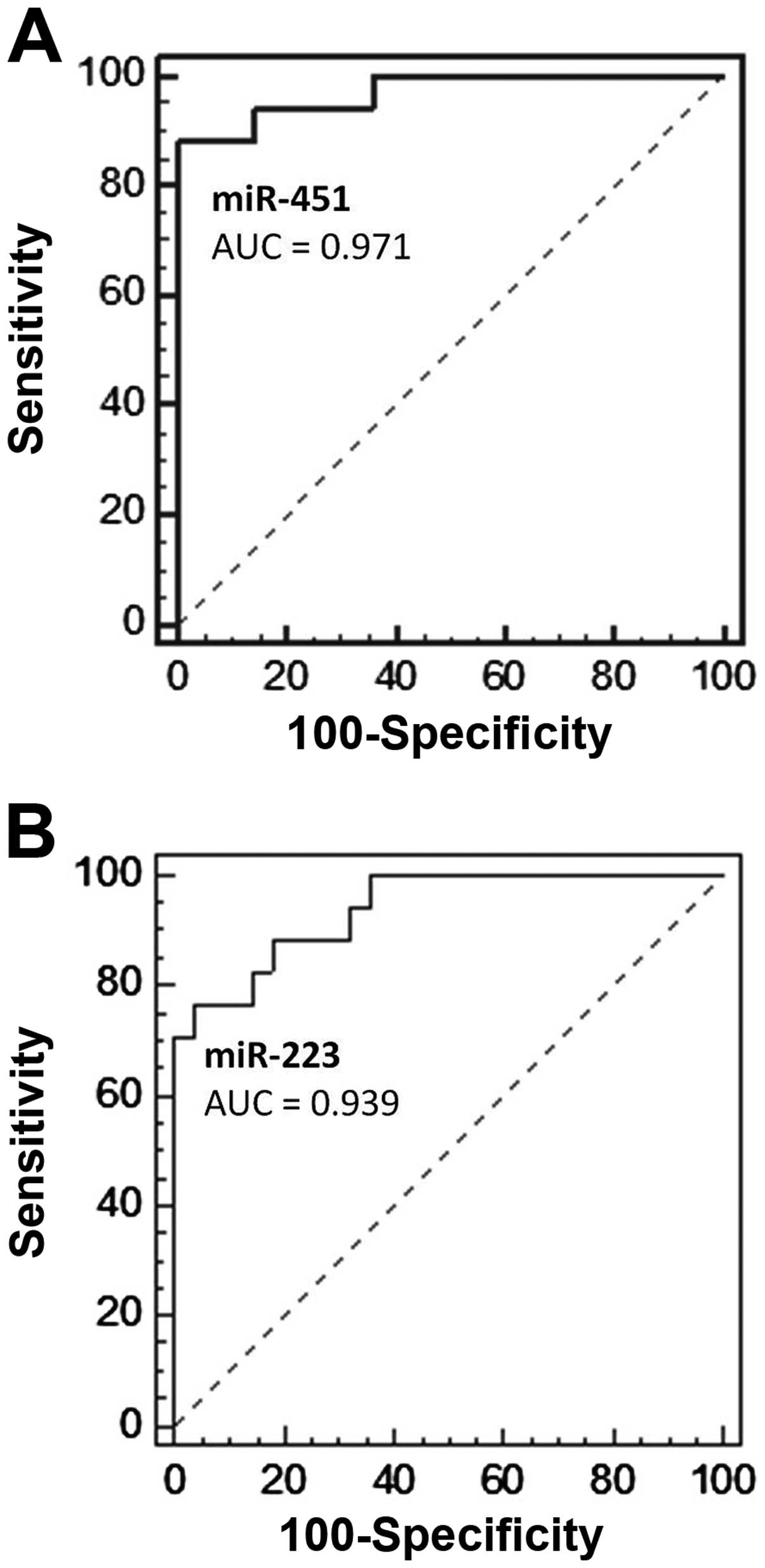
miR-451 as potential diagnostic markers (P<0.01). Receiver
operator characteristic (ROC) analyses of the regression models
showed areas under the curves (AUCs) of 0.939 (95% CI, 0.825–0.988)
and 0.971 (95% CI, 0.871–0.998), respectively (Fig. 2). Based on ROC, fecal miR-223
produced a sensitivity of 76.5% and specificity of 96.4%, while
fecal miR-451 yielded a sensitivity of 88.2% and specificity of
100.0%, in detecting CRC.
Effect of blood in stool on the fecal
levels of miRNA
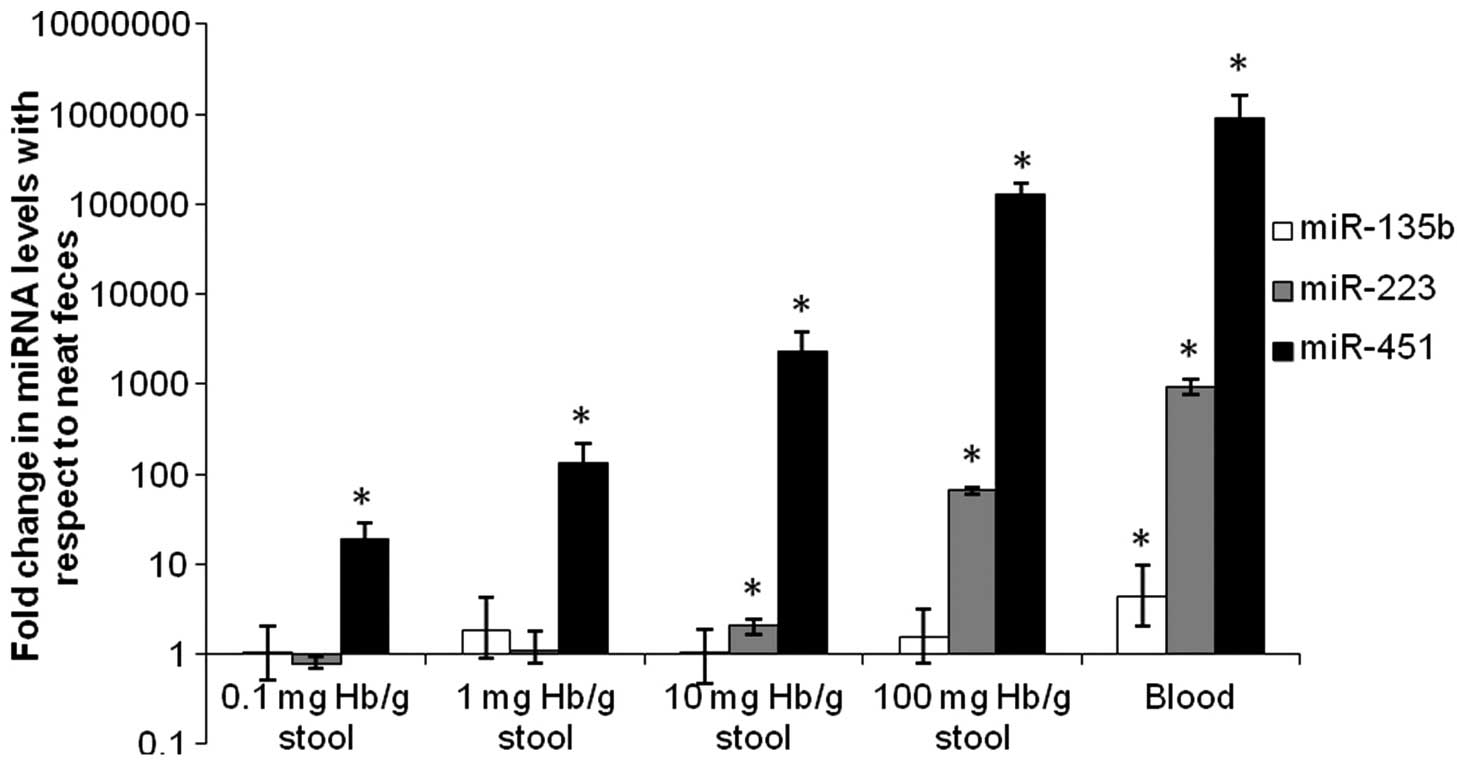
miR-451, miR-223 and miR-135b were present in human
blood (Fig. 3), with miR-451 and
miR-223 being particularly abundant. Fecal miR-451 level was
increased significantly (P<0.05) by the presence of blood at
concentrations as low as 0.1 mg Hb/g stool. Fecal miR-223 level was
unaffected by occult levels of blood at concentrations up to 1 mg
Hb/g stool, but was progressively increased (P<0.05) at 10 mg
Hb/g stool and beyond. On the contrary, fecal miR-135b remained
unchanged at varying levels of blood in stool.
Discussion
The search for a reliable, non-invasive fecal-based
screening tool has been an ongoing endeavour in the management of
CRC. In the present study, we adopted a non-targeted
microarray-based approach as a means to uncover novel fecal miRNA
markers for detecting CRC in the Asian population.
Unique fecal and tissue miRNA markers
characterizing CRC
The choice of a microarray designed based on the
newer Sanger miRBase version 16.0 conferred a broad analytical
space for the comprehensive evaluation of fecal and tissue miRNAs.
In fecal miRNA profiling, multivariate analysis did not aid in
differentiating CRC patients from healthy subjects. The dilution of
the disease-related signature was not unexpected considering the
plausible inter-individual variations in miRNA expression and the
complex nature of the fecal biomatrice. Nevertheless, a univariate
analysis unravelled 17 fecal miRNA markers, most of which were
previously unreported in the context of CRC. On the other hand,
higher expression of fecal miR-223 in CRC patients concurred with
Caucasian data (27,28). Taken together, the findings from our
global survey of miRNAs confirmed and complemented existing
knowledge on fecal miRNA markers of CRC.
Similar to fecal miRNA profiling, both
well-characterized and novel markers were elucidated from the
global study of miRNAs in paired tumor and normal mucosa. Some
differentially expressed miRNAs corresponded to oncomirs
well-defined in the pathogenesis of CRC. For example, the miR-17-92
cluster suppresses thrombospondin-1 (Tsp-1) and connective tissue
growth factor (CTGF); miR-145 inhibits Myc; and miR-135b targets
APC. Tsp-1, CTGF, Myc and APC are regulators along the Wnt
signaling pathway known to be altered in CRC (12–20,33).
On the other hand, we also uncovered oncomirs not known to
correlate with colorectal carcinogenesis, including miR-99a and
miR-100 that regulate mTOR in various types of cancer (34–36),
as well as newer miRNAs whose functions remain unclear (such as
miR-3648).
Notably, unlike a prior Caucasian report (28), these global miRNA aberrations within
the tumor were not represented within the fecal miRNA profiles of
CRC patients in our study. Osborn and Ahlquist (5) previously defined fecal markers from
three sources: exfoliated, secreted and leaked markers. Exfoliated
markers are derived from the shedding of colonocytes at the gut
luminal surface; secreted markers are emanated from the epithelial
cells lining the colonic lumen; and leaked markers are products of
disturbed blood or lymph vessels following tumor growth. Our
microarray data herein demonstrated that the unique fecal miRNA
profile characterizing CRC in Asian patients was possibly
associated with processes beyond the direct exfoliation of tumor
cells in feces. These complex mechanisms remained unclear, although
possible speculations were shedding of normal colonocytes that
harbored altered miRNA profiles and the presence of leaked markers
from blood in stool of CRC patients. In particular, the former
postulation was supported by a recent report that alluded to
changes in the miRNA milieu of normal mucosae given its interaction
with the adjacent tumor (37).
Notably, our observation posed considerable possibilities for
future research into the origin of fecal miRNA markers and the
multiple features of CRC.
Fecal miR-223 and miR-451 confirmed as
clinical markers of CRC
Following phase I screening, biomarker confirmation
(phase II) was performed on fecal miR-451, a novel and the most
differentially expressed fecal marker identified in the microarray
analysis. Fecal miR-223 was also included to confirm the observed
upregulation that had mirrored Caucasian findings (27,28).
From a univariate analysis, fecal miR-223 and miR-451 were
noticeably higher in CRC patients compared with healthy subjects,
corroborating findings from phase I. Multivariate and ROC analyses
further ratified the potential of fecal miR-223 and miR-451 as
biomarkers for non-invasive detection of CRC. In future studies,
non-CRC subjects with other diseases of the lower gastrointestinal
tract, for instance inflammatory bowel disease, could be included
to assess the specificity of these fecal markers for CRC.
Recent literature provides plausible targets for
these miRNAs but a divided opinion of their influence on
oncological phenotypes. For miR-223, it was shown to target tumor
suppressor erythrocyte membrane protein band 4.1-like 3 (EPB41L3)
in gastric cancer, hence promoting invasion and metastasis
(38). Conversely, it suppressed
artemin, a tumor promoter, in esophageal carcinoma cells (39) and inhibited cell proliferation
through regulation of Forkhead box O-1 (FOXO1) expression in
HCT-116 colorectal cancer cell line (40). Hence, miR-223 appeared to exert
different influence depending on the cancer type. miR-451 regulated
LKB1 signaling via direct targeting of calcium-binding protein 39
(CAB39) in glioma cells (41,42).
Its overexpression under high glucose conditions permitted
unrestrained mTOR activity and thereby promoted cell growth
(41,42). However, in an esophageal cancer cell
line, high levels of miR-451 downregulated BCL-2, AKT and pAKT and
increased apoptosis (43).
Similarly, in acute lymphoblastic leukemia, miR-451 suppressed Myc
expression (44) while in a DLD-1
colorectal cancer cell line, miR-451 reduced cell proliferation
through modulation of macrophage migration inhibitory factor (MIF)
expression (45). Therefore, the
exact pathological role of miR-451 requires further investigation
and contextualization by appropriately considering the influence of
their tumor-specific microenvironment. For now, their clinical
value mainly rests on their differential expression in patients’
fecal material to serve as diagnostics for disease
stratification.
Interpretation of fecal miRNA changes
considering the influence of blood in stool
As gut bleeding is a clinically prevalent phenomenon
of CRC, miRNAs of blood-origin become a pertinent factor in the
mechanistic interpretation of fecal miRNA alterations in CRC
patients. To the best of our knowledge, this is the first study to
investigate the influence of blood in stool on fecal miRNA
analysis. Our in vitro data clearly established that blood
in the stool affected the levels of three fecal markers, miR-451,
miR-223 and miR-135b, to a varying extent. Substantial amounts of
miR-451 and miR-223 in the blood were consistent with their
reported abundance in erythrocytes and myeloid cells, respectively
(30). These notable findings
impacted the interpretation of our clinical findings, as discussed
below.
Fecal miR-451 level was significantly enriched by
the presence of both occult and gross levels of blood. Considering
the propensity of gut bleeding in CRC, the data indicated that the
observed upregulation of fecal miR-451 in CRC patients may be
attributed predominantly to blood in their stool. In other words,
it pointed towards the potential role of blood-borne miRNAs as
sensitive fecal occult blood markers, or leaked markers, of CRC. On
this basis, future studies may be extended to correlating fecal
miR-451 with clinical fecal blood levels and comparing its
diagnostic sensitivity with existing FOBTs.
Conversely, fecal miR-223 level was unaffected by
occult levels of blood up to 1 mg Hb/g stool. Based on these data,
coupled with the likelihood of gross gut bleeding in some CRC
patients, it was postulated that blood in stool accounted partially
for the clinical upregulation of fecal miR-223. A separate analysis
was conducted on samples documented to contain <1 mg Hb/g stool
of blood (8 CRC patients and 10 healthy subjects). This pilot
investigation reinforced the pronounced elevation of miR-223 in the
feces of CRC patients notwithstanding the absence of blood beyond 1
mg Hb/g stool (mean fold-change, 14.6; P<0.001). Despite the
small cohort size, it underscored the presence of alternative
contributors to the upregulation of fecal miR-223 in CRC. Having
said that, as we did not observe dysregulation of miR-223 at the
tumor level (Table II) in phase I,
fecal miR-223 was unlikely a tumor-derived exfoliated marker.
Collectively, these findings may stimulate future investigations
into additional mechanisms for fecal miR-223 perturbation, for
instance the shedding of adjacent normal colonocytes and other
non-parenchymal cells.
 | Table IIFold change in tumor tissue (n=8)
relative to matched normal mucosae (n=8) from phase I microarray
analysis. |
Table II
Fold change in tumor tissue (n=8)
relative to matched normal mucosae (n=8) from phase I microarray
analysis.
| miRNA | Average
fold-changea | P-valueb |
|---|
| miR-451 | 1.26 | 0.708 |
| miR-223 | 1.05 | 0.272 |
| miR-135b | 11.8 | 0.004 |
In contrast to fecal miR-451 and miR-223, fecal
miR-135b level was impervious to varying amounts of blood,
suggesting that blood in stool did not mediate the upregulation of
fecal miR-135b in CRC patients; rather, fecal miR-135b was likely a
tumor-derived exfoliated marker, as supported by the parallel
upregulation at the tumor level (Table
II).
In light of the varying degree of influence exerted
by the presence of blood in stool on different fecal miRNA markers,
we propose that similar experiments be incorporated in the future
design of clinical fecal miRNA profiling studies. This approach may
also be considered in advancing existing studies where miRNAs
highly expressed in blood (including miR-92a in erythrocytes)
(30) have been reported as fecal
markers of CRC (22,24,27,28).
From these efforts, a more comprehensive insight into the
alterations of potential fecal miRNA markers could be gleaned.
In conclusion, the present study highlighted the
utility of a holistic miRNA screening approach in elucidating
potential diagnostic markers of CRC in the Asian population. Fecal
miR-223 and miR-451 were further confirmed as biomarkers that may
facilitate the non-invasive screening of CRC. It also illustrated
the importance of delineating the influence of blood in stool and
integrating these findings during the interpretation of clinical
fecal miRNA data.
Acknowledgements
The authors are grateful to Ms. Jiamin Koh, Dr Ming
Hian Kam, Ms. Pauline Ngiik Hung Wong and the Department of
Colorectal Surgery at Singapore General Hospital for their support
in subject recruitment, as well as Ms. Elya, Ms. Michelle Shu Mei
Lo, Ms. Wei Lin Goh and Ms. Grace Yu Hui Wong for their technical
assistance and retrieval of the clinicopathological data. This
project was funded by the Singapore Ministry of Education’s (MOE)
Academic Research Grants R -148-000-133-112 (HKH) and
R-148-000-135-112 (ECYC). LCP was supported by the NUS President
Graduate Fellowship.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v2.0, Cancer Incidence and
Mortality Worldwide: IARC CancerBase No. 10 (Internet).
International Agency for Research on Cancer; Lyon: 2010, http://globocan.iarc.fr.
access date: 11/06/2013
|
|
2
|
Moghimi-Dehkordi B and Safaee A: An
overview of colorectal cancer survival rates and prognosis in Asia.
World J Gastrointest Oncol. 4:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung JJ, Lau JY, Goh KL and Leung WK:
Increasing incidence of colorectal cancer in Asia: implications for
screening. Lancet Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ransohoff DF and Sandler RS: Clinical
practice. Screening for colorectal cancer. N Engl J Med. 346:40–44.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osborn NK and Ahlquist DA: Stool screening
for colorectal cancer: molecular approaches. Gastroenterology.
128:192–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takai T, Kanaoka S, Yoshida K, et al:
Fecal cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays
as a marker for colorectal cancer screening. Cancer Epidemiol
Biomarkers Prev. 18:1888–1893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
12
|
Motoyama K, Inoue H, Takatsuno Y, et al:
Over- and under-expressed microRNAs in human colorectal cancer. Int
J Oncol. 34:1069–1075. 2009.PubMed/NCBI
|
|
13
|
Bandres E, Cubedo E, Agirre X, et al:
Identification by Real-time PCR of 13 mature microRNAs
differentially expressed in colorectal cancer and non-tumoral
tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CJ, Zhou ZG, Wang L, et al:
Clinicopathological significance of microRNA-31, -143 and -145
expression in colorectal cancer. Dis Markers. 26:27–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Song Y, Wang Z, et al: Altered
expression of miR-148a and miR-152 in gastrointestinal cancers and
its clinical significance. J Gastrointest Surg. 14:1170–1179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slaby O, Svoboda M, Fabian P, et al:
Altered expression of miR-21, miR-31, miR-143 and miR-145 is
related to clinicopathologic features of colorectal cancer.
Oncology. 72:397–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamfjord J, Stangeland AM, Hughes T, et
al: Differential expression of miRNAs in colorectal cancer:
comparison of paired tumor tissue and adjacent normal mucosa using
high-throughput sequencing. PLoS One. 7:e341502012. View Article : Google Scholar
|
|
18
|
Faltejskova P, Svoboda M, Srutova K, et
al: Identification and functional screening of microRNAs highly
deregulated in colorectal cancer. J Cell Mol Med. 16:2655–2666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu XM, Qian JC, Deng ZL, et al: Expression
of miR-21, miR-31, miR-96 and miR-135b is correlated with the
clinical parameters of colorectal cancer. Oncol Lett. 4:339–345.
2012.PubMed/NCBI
|
|
20
|
Mazeh H, Mizrahi I, Ilyayev N, et al: The
diagnostic and prognostic role of microRNA in colorectal cancer - a
comprehensive review. J Cancer. 4:281–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed FE, Jeffries CD, Vos PW, et al:
Diagnostic microRNA markers for screening sporadic human colon
cancer and active ulcerative colitis in stool and tissue. Cancer
Genomics Proteomics. 6:281–295. 2009.
|
|
22
|
Koga Y, Yasunaga M, Takahashi A, et al:
MicroRNA expression profiling of exfoliated colonocytes isolated
from feces for colorectal cancer screening. Cancer Prev Res
(Phila). 3:1435–1442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Link A, Balaguer F, Shen Y, et al: Fecal
microRNAs as novel biomarkers for colon cancer screening. Cancer
Epidemiol Biomarkers Prev. 19:1766–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu CW, Ng SS, Dong YJ, et al: Detection of
miR-92a and miR-21 in stool samples as potential screening
biomarkers for colorectal cancer and polyps. Gut. 61:739–745.
2011.PubMed/NCBI
|
|
25
|
Yamazaki N, Koga Y, Yamamoto S, et al:
Application of the fecal microRNA test to the residuum from the
fecal occult blood test. Jpn J Clin Oncol. 43:726–733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JM, Zhao RH, Li ST, et al:
Down-regulation of fecal miR-143 and miR-145 as potential markers
for colorectal cancer. Saudi Med J. 33:24–29. 2012.PubMed/NCBI
|
|
27
|
Kalimutho M, Del Vecchio Blanco G, Di
Cecilia S, et al: Differential expression of miR-144* as a novel
fecal-based diagnostic marker for colorectal cancer. J
Gastroenterol. 46:1391–1402. 2011.
|
|
28
|
Ahmed FE, Ahmed NC, Vos PW, et al:
Diagnostic microRNA markers to screen for sporadic human colon
cancer in stool: I. Proof of principle. Cancer Genomics Proteomics.
10:93–113. 2013.PubMed/NCBI
|
|
29
|
Mao X, Yu Y, Boyd LK, et al: Distinct
genomic alterations in prostate cancers in Chinese and Western
populations suggest alternative pathways of prostate
carcinogenesis. Cancer Res. 70:5207–5212. 2010. View Article : Google Scholar
|
|
30
|
Pritchard CC, Kroh E, Wood B, et al: Blood
cell origin of circulating microRNAs: a cautionary note for cancer
biomarker studies. Cancer Prev Res (Phila). 5:492–497. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kirschner MB, Kao SC, Edelman JJ, et al:
Haemolysis during sample preparation alters microRNA content of
plasma. PLoS One. 6:e241452011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vandesompele J, De Preter K, Pattyn F, et
al: Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome
Biol. 3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Slaby O, Svoboda M, Michalek J and Vyzula
R: MicroRNAs in colorectal cancer: translation of molecular biology
into clinical application. Mol Cancer. 8:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun J, Chen Z, Tan X, et al:
MicroRNA-99a/100 promotes apoptosis by targeting mTOR in human
esophageal squamous cell carcinoma. Med Oncol. 30:4112013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torres A, Torres K, Pesci A, et al:
Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma
coexists with increased expression of mTOR kinase in endometrioid
endometrial carcinoma. BMC Cancer. 12:3692012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Z, Jin Y, Yu D, et al:
Down-regulation of the microRNA-99 family members in head and neck
squamous cell carcinoma. Oral Oncol. 48:686–691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang ZM, Yang J, Shen XY, et al: MicroRNA
expression profile in non-cancerous colonic tissue associated with
lymph node metastasis of colon cancer. J Dig Dis. 10:188–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Zhang Y, Zhang H, et al: miRNA-223
promotes gastric cancer invasion and metastasis by targeting tumor
suppressor EPB41L3. Mol Cancer Res. 824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li S, Li Z, Guo F, et al: miR-223
regulates migration and invasion by targeting Artemin in human
esophageal carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu L, Li H, Jia CY, et al: MicroRNA-223
regulates FOXO1 expression and cell proliferation. FEBS Lett.
586:1038–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Godlewski J, Bronisz A, Nowicki MO,
Chiocca EA and Lawler S: microRNA-451: a conditional switch
controlling glioma cell proliferation and migration. Cell Cycle.
9:2742–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Godlewski J, Nowicki MO, Bronisz A, et al:
MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to
metabolic stress in glioma cells. Mol Cell. 37:620–632. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang T, Zang WQ, Li M, Wang N, Zheng YL
and Zhao GQ: Effect of miR-451 on the biological behavior of the
esophageal carcinoma cell line EC9706. Digest Dis Sci. 58:706–714.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Molinari F, Felicioni L, Buscarino M, et
al: Increased detection sensitivity for KRAS mutations
enhances the prediction of anti-EGFR monoclonal antibody resistance
in metastatic colorectal cancer. Clin Cancer Res. 17:4901–4914.
2011.PubMed/NCBI
|
|
45
|
Bandres E, Bitarte N, Arias F, et al:
microRNA-451 regulates macrophage migration inhibitory factor
production and proliferation of gastrointestinal cancer cells. Clin
Cancer Res. 15:2281–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|