Introduction
Apoptosis, or programmed cell death, is a physical
form of cell death that plays a central role in tissue homeostasis
and in the regulation of the cell suicide program. Apoptosis is
necessary to prevent cancer and its induction is a promising
approach for cancer gene therapy (1). Several proteins that control
programmed cell death in cancer cells have been identified
(1). Caspase-3 is one of the
members of the family of cysteine proteases which play a major role
in the transduction of apoptotic signals and the execution of
apoptosis in mammalian cells (2).
There has been considerable interest in caspase-3 as a potent tool
for cancer gene therapy, yet ectopic overexpression of the
wild-type caspase-3 precursor in cells does not induce apoptosis,
due to their inability to undergo autocatalytic activation. We
constructed constitutively active recombinant caspase-3 precursors
(rev-caspase-3) by making their small subunits preceding their
large subunits, as described by Srinivasula et al (3) and Song and Shen (4). Unlike their wild-type counterparts,
rev-caspase-3 is capable of autocatalytic processing in an in
vitro translation reaction, suggesting that they are
catalytically active. Our data demonstrated that overexpression of
rev-caspase-3 can significantly induce apoptotic cell death in both
tumor and normal cells in vitro and in vivo (4). This result is in accordance with that
of Yang et al (5).
To prevent the toxicity of apoptotic genes in normal
cells, targeted expression of therapeutic proteins within the tumor
mass is desired. Tumor-restricted gene expression through
transcriptional targeting is an attractive approach to reduce
toxicity and potentially increase the efficacy of cancer gene
therapy. Human telomerase reverse transcriptase (hTERT) is the
catalytic subunit of human telomerase and is highly active in
immortalized cell lines and in over 85% of human cancers, but is
inactive in most somatic cells (6,7). It
has been shown that the hTERT promoter (hTERTp) is useful for
targeted transgene expression in human ovarian cancer cells
(8). However, hTERTp is relatively
weak resulting in insufficient transgene expression levels. To
overcome this major limitation, we set out to generate an amplified
hTERTp for efficient transcriptional targeting of rev-caspase-3
gene expression. We constructed a two-step transcription
amplification (TSTA) system by using hTERTp (named the
‘hTERTp-TSTA’ system) to drive a chimeric transcription factor
consisting of the powerful herpes simplex virus VP16
transcriptional activation domain fused to the DNA-binding domain
of the yeast protein GAL4, which then binds to the GAL4-binding
sites upstream of a minimal promoter to activate rev-caspase-3 gene
expression. Our in vitro and in vivo data comparing
adenovirus-mediated rev-caspase-3 gene therapy utilizing the binary
promoter system vs. a cytomeglovirus promoter (CMVp) demonstrated
strong hTERT-restricted antitumor activity with significant
reduction in liver toxicity.
Materials and methods
Cell culture
Human ovarian adenocarcinoma cell lines AO, OVCAR3
and HO8910 were developed in our laboratory and maintained in
RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS) at 37°C with 5%
CO2 in air. Human umbilical vein epithelial cells
(HUVECs; CBI, USA) were maintained in growth medium of M200 (CBI,
USA) supplemented with 2% low serum growth supplement (LSGS; CBI,
USA). All of the cells were incubated at 37°C with 5%
CO2 in air.
Recombinant adenoviral vectors
AdHT-rev-casp3 and Ad-rev-casp3 are recombinant
adenoviruses expressing rev-caspase-3 driven by hTERTp and CMVp,
respectively, which were prepared using the methods described
previously (9). AdHTVP2G5-EGFP,
AdHT-EGFP and Ad-EGFP, which express enhanced green fluorescent
protein (EGFP) driven by the hTERTp-TSTA system, hTERTp and CMVp,
respectively, were successfully constructed in our laboratory
previously.
The vector of pHTVP2G5-rev-casp3, a bicistronic
adenoviral vector that expresses rev-caspase-3, driven by the
hTERTp-TSTA system via the GAL4 gene regulatory system was
constructed as follows. pBTdel-279 (kindly denoted by Dr Izumi,
National Institute of Environmental Health Science, USA) is a
vector that contains the core region of hTERTp from −279 to +5.
pBTdel-279 was cut by KpnI/HindIII, resulting in the
hTERTp segment. The resulting segment was then subcloned into the
KpnI/HindIII sites of pBluescript SK (+) vector
(Takara, Dalian, China), resulting in pBlue-hTERTp. The hTERTp
genome was synthesized with following primers of F and R, using
pBlue-hTERTp cDNA as a template: Primer F, 5′-TAA GGT CAC CGG ACC
CCC GGG TCC G-3′; and primer R, 5′-GCG TGC TAG CAC TTA GAT CGC AGA
TCT CG-3′. The PCR product was ligated with pMD18-T, resulting in
pMD18-hTERTp. PBCVP2G5-lucNSN (kindly provided by Professor M.
Carey, California University, USA) is a reporter vector containing
two components of the TSTA system. pMD18-hTERTp was cut by
BstEII and NheI, resulting in hTERTp. The resulting
segment was then ligated with BstEII- and
NheI-digested PBCVP2G5-lucNSN, resulting in
pHTVP2G5-luc+, which expresses luciferase driven by the
hTERTp-TSTA system. pHTVP2G5-luc+ was cut by
XbaI/SalI, resulting in the segment of pA (~268 bp).
pMD18-T-SS-LS, which is the pMD18-T vector (Takara) containing
rev-caspase-3, was constructed by our laboratory using a method
described previously (3). The
XbaI/SalI fragments of pA and pMD18-T-SS-LS were
ligated and then cut by SspI and SalI, resulting in
rev-caspase-3+pA. The NcoI-digested and blunted
pHTVP2G5-luc+ was cut by SalI and then ligated
with rev-caspase-3+ pA, resulting in pHTVP2G5-rev-casp3,
which expresses rev-caspase-3 driven by the hTERTp-TSTA system. The
insertion was confirmed by restriction enzyme analysis and DNA
sequencing analysis. The XbaINotI fragment of pDC316
(Vector Gene Technology, Beijing, China) and pHTVP2G5-rev-casp3
were blunted, cut by SalI and then ligated, resulting in
pDCHTVP2G5-rev-casp3, a recombinant adenoviral vector expressing
rev-caspase-3 driven by the hTERTp-TSTA system. Briefly, this
vector contains two expression cassettes: one for rev-caspase-3,
whose gene is driven by a synthetic, minimal promoter composed of
five sets of GAL4 binding sites and a TATA sequence (GT promoter),
the other for GAL4/VP16, a transactivator whose gene is driven by
hTERTp.
pDCHTVP2G5-rev-casp3 was cotransfected into 293
cells with pBHGloxdelE13cre plasmids (Vector Gene Technology) using
a lipid-based transfection method (Invitrogen, USA) to generate
AdHTVP2G5-rev-casp3. Each recombinant adenovirus was isolated from
a single positive plaque and passed through three rounds of plaque
purification and subsequently confirmed by PCR and restriction
enzyme analysis. All viruses were propagated in 293 cells, purified
by ultracentrifugation in a cesium chloride gradient and subjected
to dialysis. Viral titer was measured by a standard plaque assay
using 293 cells and by absorbance of the dissociated virus at
A260nm. Titers for subsequent experiments were
viral particles (v.p.) per milliliter determined by
A260nm. Viral preparations had a ratio of v.p. to
plaque-forming units (pfu) 100:1. The cells were infected by
adenoviral vectors at 37°C for 2 h.
EGFP expression assays
Cellular EGFP expression was quantitatively examined
by FACS analysis and visualized using fluorescence microscopy.
Cells were collected at 48 h after virus infection. Cells
(1×106) were illuminated at 488 nm and fluorescence was
detected in the FITC channel. Non-specific fluorescence was
detected using a 575/30-nm emission filter in the PI channel. EGFP
fluorescence is the mean fluorescence signal in EGFP+
cells in relative light units (RLUs) after subtraction of
background fluorescence. An inverted-system fluorescence microscope
(Olympus, Japan) was used for detection of EGFP expression in cell
monolayer.
Cell viability assays
Cells were seeded at a density of 1.7×104
cells per well on 96-well plates and after an overnight incubation
were treated with AdHTVP2G5-rev-casp3, AdHT-rev-casp3, Ad-rev-casp3
or AdHTVP2G5-EGFP at the indicated doses. Cell viability was
assessed using the Dojindo Cell Counting Kit-8 (Dojindo
Laboratories, Gaithersburg, MD, USA) according to the supplier’s
recommendations. Absorbance was read at 450 nm, and cell viability
was expressed as the percentage of viable cells relative to
untreated cells. All experiments were performed in triplicate and
at least three times independently. Ninety-six hours later, the
surviving cells were counted. Results are presented as a percentage
of cell survival compared with the cell counts of cultures treated
only with PBS.
Cell-cycle analysis
Treated cells were washed once with
phosphate-buffered saline (PBS), trypsinized and washed again in
PBS with 2% FBS and then fixed in ice-cold ethanol for at least 1 h
at −20°C. After washing with PBS, the cells were stained with
propidium iodide (30 μg/ml) and treated with RNase (0.6 mg/ml) in
PBS plus 0.5% (v/v) Tween-20 and 2% FBS. Stained cells were
analyzed on a FACSCalibur flow cytometer (BD Bioscience) using
CellQuest software, and the ModFit program (Verity Software House
Inc., Topsham, ME, USA) was used to analyze the cell cycle
profiles.
Real-time PCR
Total cellular RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). First-strand cDNA synthesis was
performed using reverse transcriptase (Invitrogen) by incubation at
25°C for 10 min, 37°C for 60 min and 95°C for 5 min. The sequences
of the primers used for real-time PCR were as follows: hTERT,
5′-CGG AAG AGT GTC TGG AGC AA-3′ (sense) and 5′-GGA TGA AGC GGA GTC
TGG A-3′ (antisense); active caspase-3, 5′-CCATGCTGA
AACAGTATGCCG-3′ (sense) and 5′-TTCCAGAGTCCA TTGATTCGCT-3′
(antisense); GAPDH, 5′-ACCACAGTCCA TGCCATCAC-3′ (sense) and
5′-TCCACCACCCTGTT GCTGTA-3 (antisense). Amplification was performed
in a 25-μl reaction containing 2 μl sample cDNA, 0.4× TaqMan
Universal PCR Master Mix (Applied Biosystems, USA), 120 nM of each
primer and 1 nM DNA probe. The PCR was run at 94°C for 2 min
followed by 40 cycles of 94°C for 5 sec, 62°C for 10 sec and 60°C
for 30 sec. Amplification was performed according to the
manufacturer’s specifications.
Immunoblotting studies
To detect expression of exogenous active caspase-3
and induction of apoptosis in AO cells and HUVECs, 1×106
cells were plated onto 6-cm dishes and infected with
AdHTVP2G5-rev-casp3 at a multiplicity of infection (MOI) of 70.
Cell lysates were prepared as previously described. Immunoblotting
procedure was carried out as previously described (10). AdHT-rev-casp3 and Ad-rev-casp3 at
the same doses were used as controls. The following antibodies were
used for immunoblotting: anti-PARP antibody and anti-caspase-3
antibody (Cell Signaling Technology, Danvers, Inc., MA, USA).
Xenograft studies
The study protocol was approved by the local
institution review boards at the affiliated institutions of the
authors. Female mice, 4–6 weeks old and weighing 16–20 g (Animal
Research Institution of Chinese Academy of Medical Sciences,
Beijing, China), were used for the experiment. All animals were
cared for according to the guidelines for the Care and Use of
Laboratory Animals and the Institutional Guidelines of the Chinese
Academy of Medical Sciences. For the subcutaneous tumor model,
1×107 AO cells in 0.2 ml PBS were inoculated
subcutaneously into the dorsal flank of nude mice. When the tumor
reached a volume of ~150 mm3, the mice received an
intratumoral injection of PBS, AdHTVP2G5-rev-casp3
[2.5×109 tissue culture infectious dose 50
(TCID50)/tumor], AdHT-rev-casp3 (2.5×109
TCID50/tumor), Ad-rev-casp3 (2.5×109
TCID50/tumor) or AdHTVP2G5-EGFP (2.5×109
TCID50/tumor). Three intratumoral injections were given
every 10 days, and 5 mice from each group were followed up once
every 3 days to measure tumor size by calipers. Tumor volumes were
calculated using the formula: a × b2 × 0.5, where a and
b represent the larger and smaller diameters, respectively. Mice
were sacrificed according to the institutional guidelines when the
tumor with PBS (negative control) treatment reached 2000 mm3 in
volume.
For the peritoneal tumor model, 1×107 AO
cells resuspended in 0.5 ml of PBS were injected intraperitoneally
into nude mice. Subsequently, mice were weighed once every two
days. Twenty-one days after inoculation, the mice received an
intraperitoneal injection of PBS, AdHTVP2G5-rev-casp3
(2.5×109 TCID50/tumor), AdHT-rev-casp3
(2.5×109 TCID50/tumor), Ad-rev-casp3
(2.5×109 TCID50/tumor) or AdHTVP2G5-EGFP
(2.5×109 TCID50/tumor). Three intraperitoneal
injections were given every 10 days. Three mice from each group
were sacrificed according to the institutional guidelines 72 h
after the first injection to detect the expression of EGFP or
active caspase-3 by fluorescence microscopy or RT-PCR, in the tumor
and liver tissues, respectively. Survival was defined as the study
endpoint. Histopathological changes in the liver, spleen,
intestine, lung, kidney, ovary, pancreas and heart were examined,
and serum contents of alanine transaminase (ALT) and aspartate
transaminase (AST) were determined as described previously
(12). Blood samples were collected
via the tail vein on day 1 and 14 to monitor liver damage and
specifically, the serum levels of AST and ALT in each group.
Statistical analysis
Statistical differences among the treatment groups
were assessed by ANOVA using SPSS16 software program. A value
P<0.05 was considered to indicate a statistically significant
result. Additionally, the survival data were summarized and plotted
using the Kaplan-Meier method, and survival curves were compared
using the log-rank test.
Results
Selective strong activity of the
hTERTp-TSTA system in hTERT-positive cells
We constructed recombinant adenoviral vector
AdHTVP2G5-EGFP which contains two expression cassettes and
expresses EGFP driven by the hTERTp-TSTA system. One cassette
expresses the EGFP gene driven by the GAL4/TATA (GT) promoter,
while the other expresses the GAL4/VP16 fusion gene driven by the
hTERTp. To assess the activity and selectivity of the hTERTp-TSTA
system in various cells, we infected hTERT-positive ovarian cancer
AO, OVCAR3 and HO8910 cells and hTERT-negative normal cell HUVECs
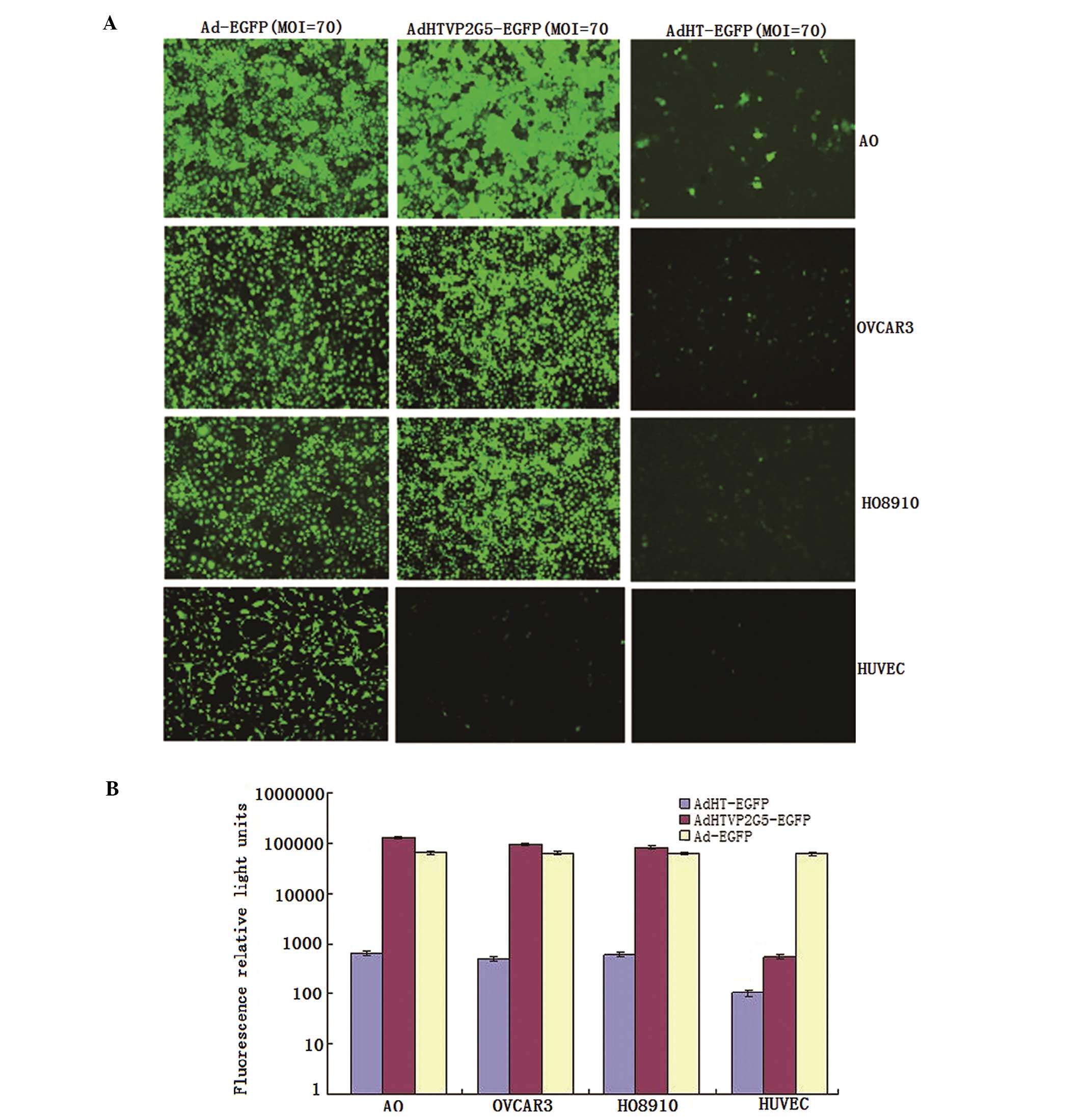
with AdHTVP2G5-EGFP, AdHT-EGFP or Ad-EGFP at an MOI of 70 for 2 h.
Cells were harvested 48 h after infection, and EGFP expression was
analyzed by fluorescence-activated cell sorting (FACS).
We found that the hTERTp-TSTA system showed the
strongest activity when compared with hTERTp and CMVp, although
these three promoter systems were all active in the hTERT-positive
cancer cells. The RLUs in the AO, OVCAR3 and HO8910 cells infected
with AdHTVP2G5-EGFP were 12,800, 95,210 and 83,500, which were
203-, 193- and 140-fold higher than the RLUs in the cells infected
by AdHT-EGFP and were as 2.0-, 1.5- and 1.3-fold in the cells
infected by Ad-EGFP, respectively. In contrast, AdHTVP2G5-EGFP
demonstrated a background level of EGFP expression (RLUs of 345) in
the hTERT-negative HUVECs, being ~180-fold lower than that of
Ad-EGFP (RLUs of 62,200) and showing no significant difference with
that of AdHT-EGFP (RLUs of 103) (Fig.
1A and B).
AdHTVP2G5-rev-casp3 markedly suppresses
the survival of AO cells with tumor selectivity in vitro
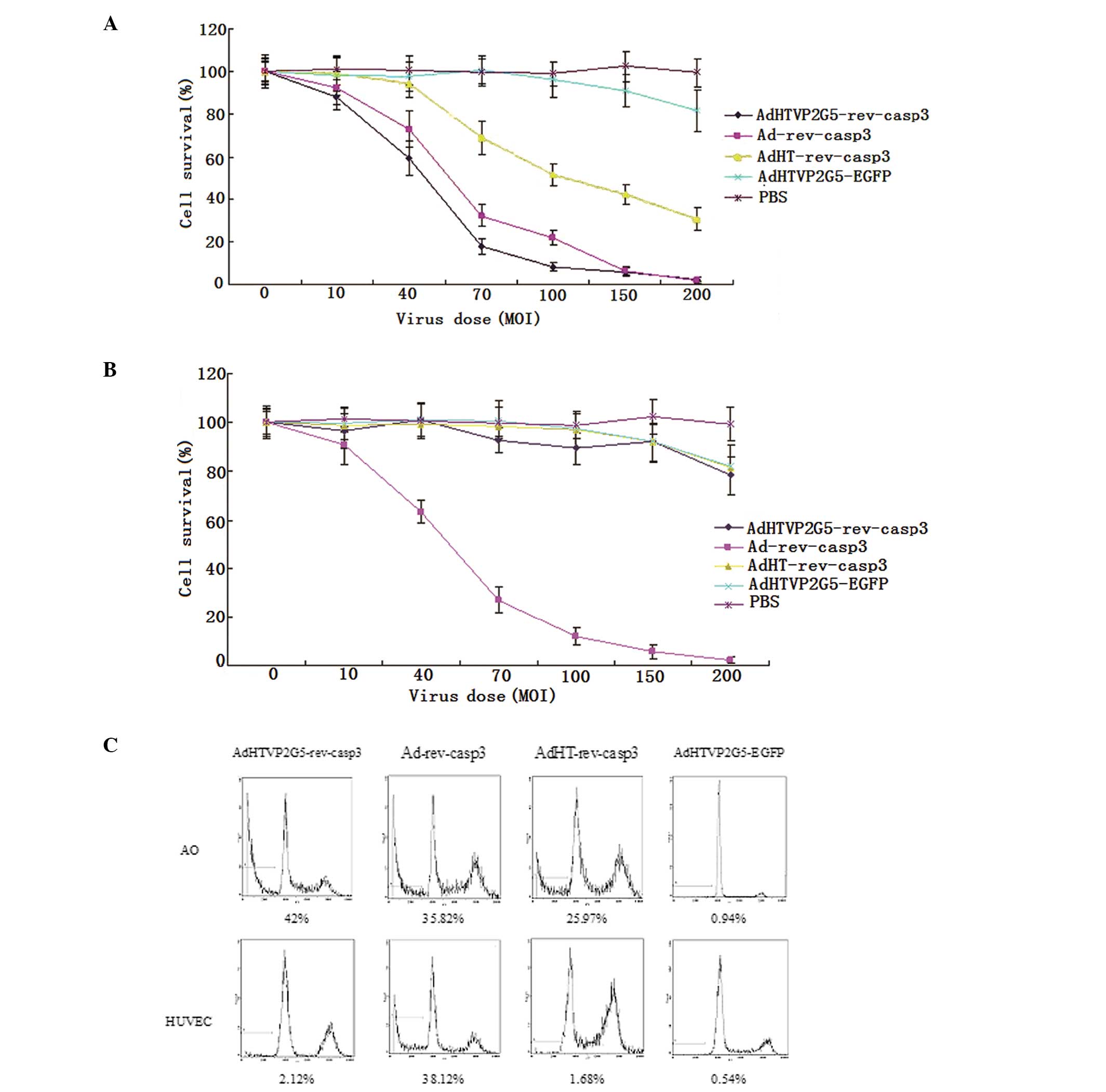
We infected AO cells with AdHTVP2G5-rev-casp3,
AdHT-rev-casp3 or Ad-rev-casp3 at an MOI of 5 to 200 and 96 h later
examined the viability of these cells. The viability assays using
the CCK-8 method showed that AdHTVP2G5-rev-casp3 significantly
suppressed the survival of AO cells in a dose-dependent manner with
a viability rate of 17.8±3.5% at an MOI of 70 and 8.1±1.9% at an
MOI of 100 (Fig. 2A), which was
markedly lower than that of AO cells treated with Ad-rev-casp3
(32.3±5.3% at an MOI of 70 and 21.8±3.4% at an MOI of 100) and
AdHT-rev-casp3 (69.2±7.8% at an MOI of 70 and 51.6±5.3% at an MOI
of 100), respectively. Flow cytometry additionally showed that the
sub-G1 fraction increased to 42, 35.82 and 25.97% in the AO cells
treated with AdHTVP2G5-rev-casp3, Ad-rev-casp3 and AdHT-rev-casp3,
respectively, at an MOI of 70, which was markedly higher than that
of AO cells treated with AdHTVP2G5-EGFP controls (0.94%,
P<0.05). We next examined the effect of AdHTVP2G5-rev-casp3,
AdHT-rev-casp3 or Ad-rev-casp3 on the proliferation of HUVECs. A
significant difference was detected in HUVEC viability following
three reconstructive adenoviruses, respectively.
AdHTVP2G5-rev-casp3 induced little HUVEC death with the cell
viability rate of 92.7±5.2 and 89.2±6.3% at an MOI of 70 and 100,
respectively, which showed no significant difference with that
induced by AdHT-rev-casp3 (98.5±6.9 and 96.9±7.7%, respectively).
In contrast, Ad-rev-casp3 induced significant cell death in the
HUVECs with a cell viability rate of 27.1±5.4 (MOI of 70) and
11.9±3.6% (MOI of 100), respectively, which was similar to that in
the AO cells (Fig. 2B). Flow
cytometry further showed that at an MOI of 70, AdHTVP2G5-rev-casp3
and AdHT-rev-casp3 did not induce significant apoptosis with an
apoptotic rate of only 2.1 and 1.7% in the HUVECs, respectively,
whereas Ad-rev-casp3 induced significant apoptosis with an
apoptotic rate of 38.1% in the HUVECs, similar to that in the AO
cells (Fig. 2C).
AdHTVP2G5-rev-casp3 causes significant
apoptotic activities in AO cells with significant tumor
selectivity
We further examined the levels of active caspase-3
in AO cells and HUVECs treated with various adenoviral vectors at
an MOI of 70, 48 h after viral infections by RT-PCR. We found that
the mRNA transcript levels of active caspase-3 in the AO cells
treated with AdHTVP2G5-rev-casp3 (9.44±1.36) was significantly
higher than that with Ad-rev-casp3 (8.19±1.72), AdHT-rev-casp3
(4.47±1.9) and AdHTVP2G5-EGFP (1.17±1.5) (P<0.05), respectively,
while no apparent active caspase-3 mRNA expression was found in the
HUVECs treated with AdHTVP2G5-rev-casp3 (1.95±1.28) and
AdHT-rev-casp3 (1.55±1.41) or AdHTVP2G5-EGFP control. By contrast,
Ad-rev-casp3 caused a significant increase in active caspase-3 mRNA
levels in both AO cells (8.66±1.8) and the HUVECs (7.93±2.1).
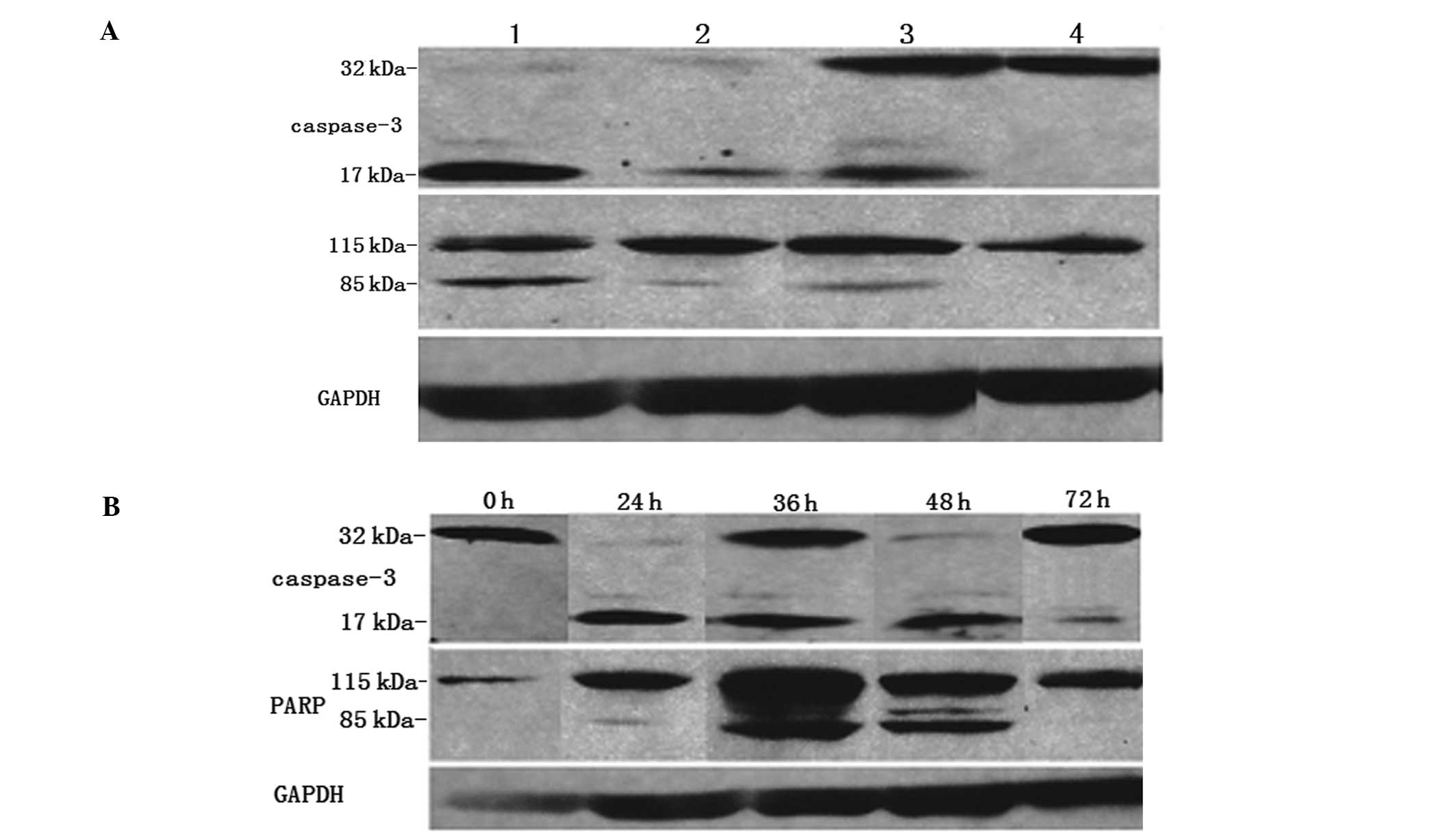
Our immunoblotting analysis further revealed that
the AdHTVP2G5-rev-casp3-, AdHT-rev-casp3- and Ad-rev-casp3-treated
AO cells expressed significant levels of p17 and p85 proteins.
Furthermore, the levels of p17 and p85 proteins in the
AdHTVP2G5-rev-casp3-treated cells were significantly higher than
levels in the Ad-rev-casp3-or AdHT-rev-casp3-treated cells 30 h
after the infections. In contrast, in HUVECs, only
Ad-rev-casp3-treated cells significantly expressed p17 and p85
proteins, while AdHTVP2G5-rev-casp3 and AdHT-rev-casp3 treatments
did not result in expression of p17 and p85 in the cells (Fig. 3A). These findings further confirmed
that AdHTVP2G5-rev-casp3 caused selective strong apoptotic
activities in the AO cells, not in the HUVECs.
p17 proteins became evident as early as 24 h after
AdHTVP2G5-rev-casp3 infection of AO cells and became stronger at
36–48 h after the infection. In addition, p85 proteins were
detected as early as 24 h and became evident at 36–48 h after the
infection. Protein expression of both p17 and p85 became weak at 72
h after infection (Fig. 3B).
hTERTp-TSTA system drives tumor-specific
EGFP and rev-caspase-3 gene expression in vivo
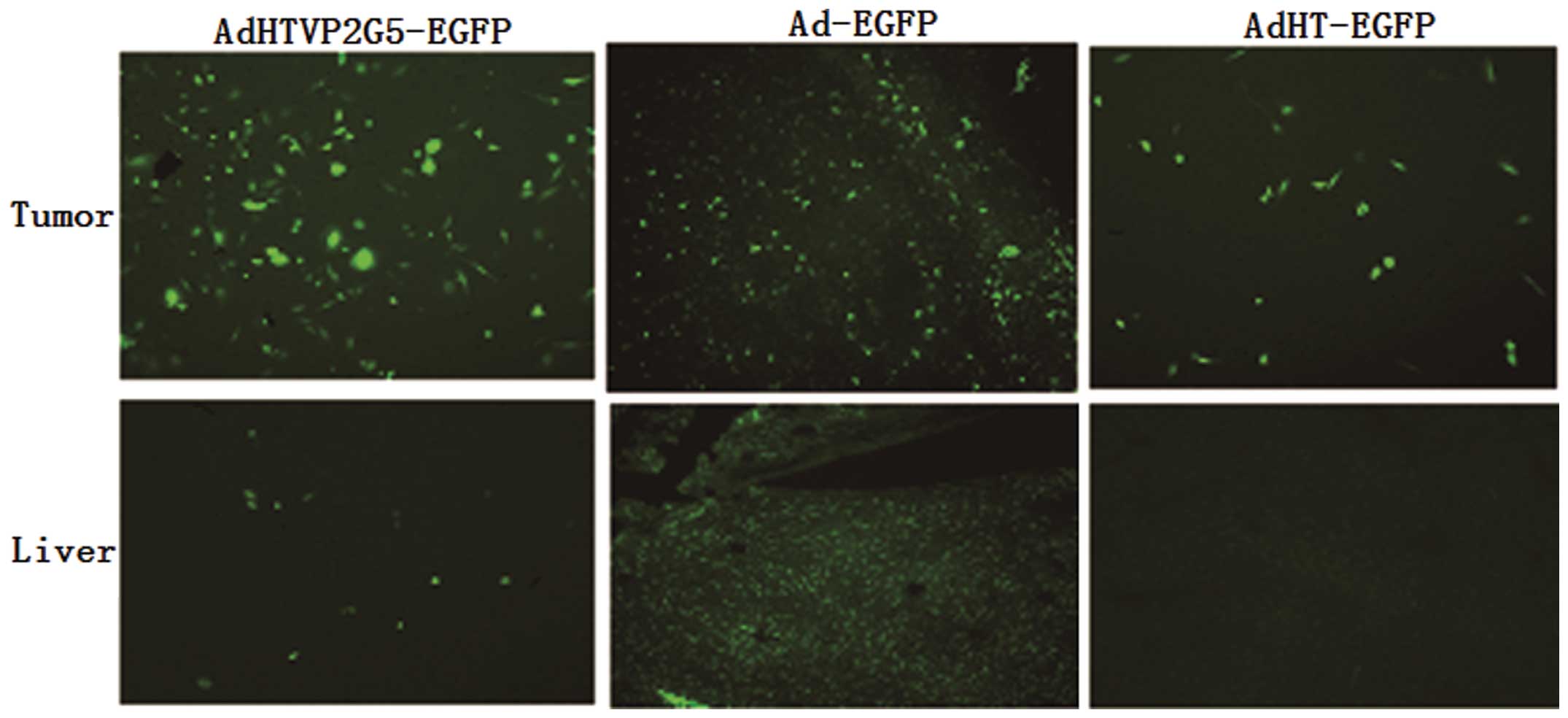
We gave mice bearing abdominal tumors derived from
AO cells an intraperitoneal injection of AdHTVP2G5-EGFP, AdHT-EGFP
or Ad-EGFP at a single dose of 2.5×109
TCID50/mouse. We sacrificed the mice 72 h after
injection and collected the tumor and liver tissues for
fluorescence detection to assess EGFP expression. We found that all
AdHTVP2G5-EGFP, AdHT-EGFP and Ad-EGFP caused EGFP expression in
tumor tissues. In comparison, only Ad-EGFP induced strong EGFP
expression in the liver, while AdHTVP2G5-EGFP and AdHT-EGFP induced
very weak EGFP expression (Fig. 4).
These results demonstrate that hTERT-TSTAp is highly active in
tumors, but quiescent in normal liver in vivo. We observed
similar selective expression of rev-caspase-3 by RT-PCR when we
sacrificed the mice 72 h after injection with AdHTVP2G5-rev-casp3,
AdHT-rev-casp3 or Ad-rev-casp3 and collected their tumor and liver
tissue for caspase-3 expression. AdHTVP2G5-rev-casp3 induced strong
caspase-3 expression in the tumors, while it did not induce
detectable transgene expression in the liver.
AdHTVP2G5-rev-casp3 markedly suppresses
tumor growth and extends the survival of tumor xenograft-bearing
mice
To evaluate the in vivo efficacy of
AdHTVP2G5-rev-casp3, we established a mouse xenograft model by
inoculating AO cells subcutaneously in nude mice and we treated the
mice with AdHTVP2G5-rev-casp3, AdHT-rev-casp3 or Ad-rev-casp3,
respectively. Fifty-three days after treatment, the tumor volume
was 406±61, 990±214 and 645±132 mm3 in mice bearing AO
xenografts treated with AdHTVP2G5-rev-casp3, AdHT-rev-casp3 and
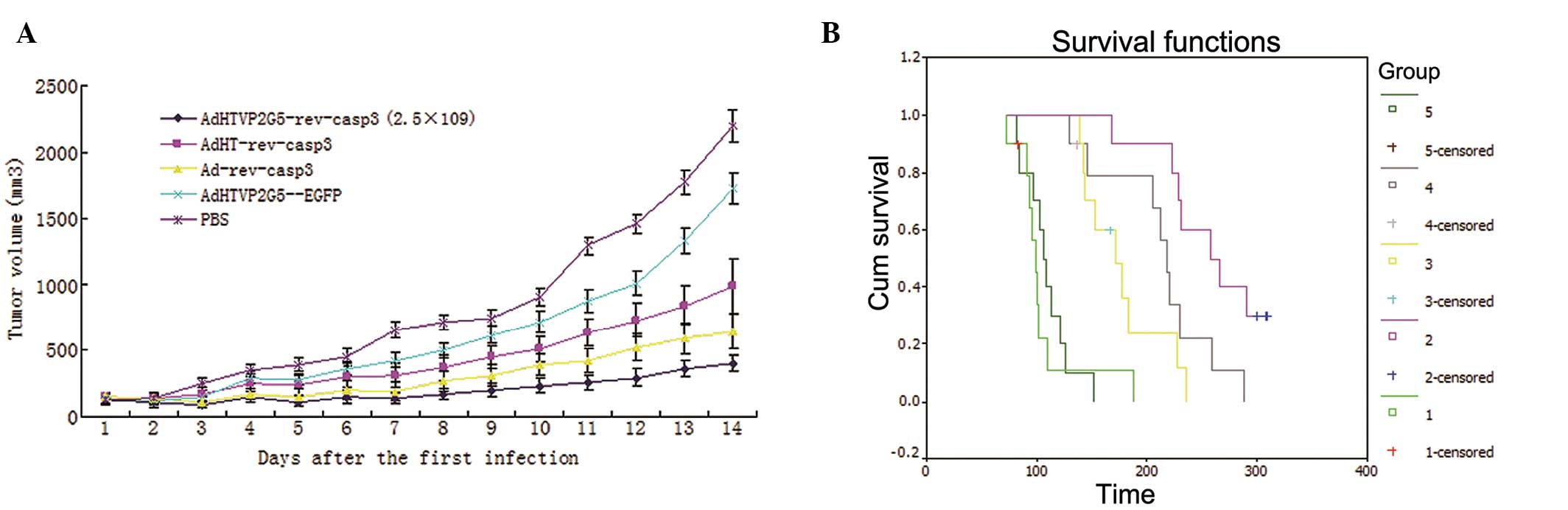
Ad-rev-casp3, respectively. As shown in Fig. 5A, the tumor growth suppression rates
of AdHTVP2G5-rev-casp3 and Ad-rev-casp3 were 81.52 and 70.64%,
respectively, significantly higher than that of AdHT-rev-casp3
(54.94%) or AdHTVP2G5-EGFP (21.35%) at the endpoint of the study
(Fig. 5A).
We next tested the effect of AdHTVP2G5-rev-casp3
in vivo in an abdominally spread tumor model of AO cells.
Twenty-one days after inoculation, the mice were randomized to
receive intraperitoneal injections of various adenoviral vectors or
PBS (n=5/group). Survival of mice in each group was recorded.
Kaplan-Meier analysis indicated that AdHTVP2G5-rev-casp3,
AdHT-rev-casp3 or Ad-rev-casp3 significantly improved the survival
of mice receiving an intraperitoneal inoculation of AO cells
compared with control mice treated with PBS (P<0.05) (Fig. 5B). The mean survival of mice treated
with AdHTVP2G5-rev-casp3 was 259±14 days with a median survival of
258±28 days. Furthermore, AdHTVP2G5-rev-casp3 treatments prolonged
survival significantly when compared with treatment using
AdHT-rev-casp3 (177±12 days, P<0.05) or Ad-rev-casp3 (213±16
days, P<0.05) (Table I).
 | Table IMean and median survival of mice
receiving the various adenoviral vectors (n=10). |
Table I
Mean and median survival of mice
receiving the various adenoviral vectors (n=10).
| Group | Mean (days) | N | Median (days) |
|---|
| PBS | 106±11 | 10 | 99±6 |
| AdHTVP2G5-EGFP | 109±7 | 10 | |
|
AdHTVP2G5-rev-casp3 | 259±14a | 10 | 258±28 |
| AdHT-rev-casp3 | 177±12b | 10 | 171±16 |
| Ad-rev-casp3 | 213±16c | 10 | 218±7 |
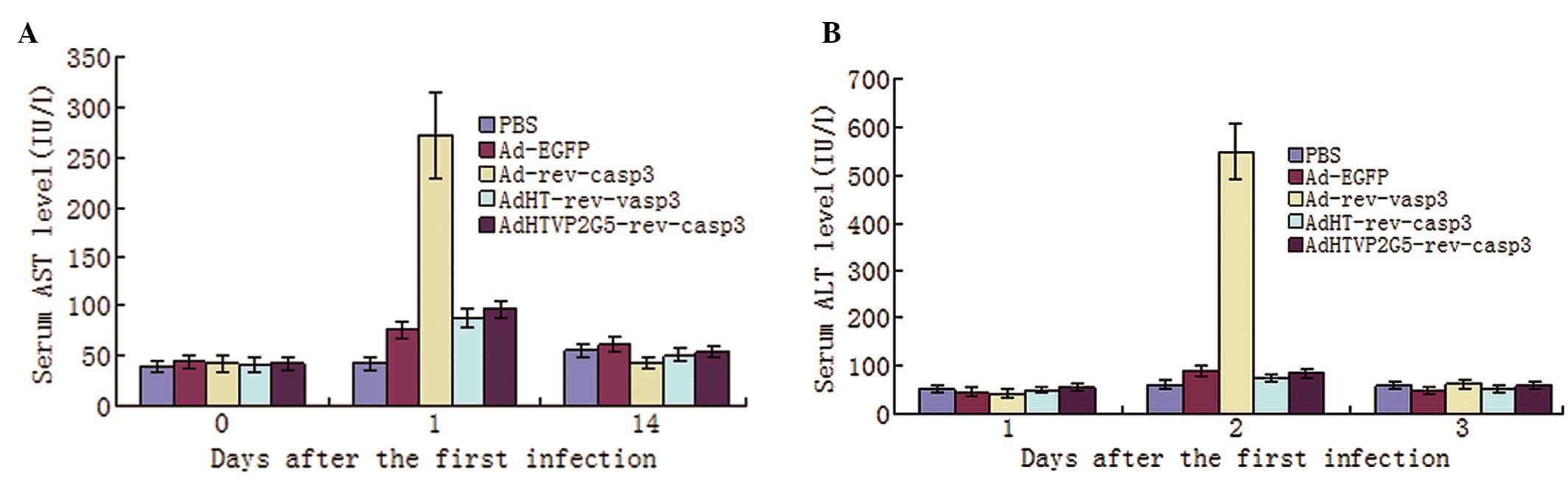
Toxicity after intraperitoneal vector
administration
We also examined the toxicity profiles of
intraperitoneal administration of AdHTVP2G5-rev-casp3,
AdHT-rev-casp3 or Ad-rev-casp3. We measured serum ALT and AST
levels and found that these levels were only slightly elevated in
the mice receiving AdHTVP2G5-rev-casp3 or AdHT-rev-casp3. The AST
and ALT levels were significantly lower in mice receiving
AdHTVP2G5-rev-casp3 or AdHT-rev-casp3 1 day after treatment than
that in mice receiving Ad-rev-casp3 (P<0.01). On day 14, no
significant difference in AST or ALT was noted among the groups
(Fig. 6A and B). We also examined
the histopathological changes in the liver, spleen, intestine,
lungs, kidneys, ovary and heart and we found no obvious lesions in
mice receiving AdHTVP2G5-rev-casp3 or AdHT-rev-casp3.
Discussion
It has been shown that rev-caspase-3 induces potent
apoptosis in human breast cancer, pancreatic cancer and colon
cancer cells (2,3–5). In
the present study, AdHTVP2G5-rev-casp3 suppressed AO cell growth
with a survival rate of <20%, at 96 h after its infection at an
MOI of 70–100, and the cells displayed typical apoptotic morphology
after treatments as detected by electron transmission microscopy.
This result demonstrated that the recombinant molecule,
rev-caspase-3, has potent activity for inducing apoptosis similar
to its wild-type counterparts. The apoptosis induced by
rev-caspase-3 was the most significant at 72 h after viral
infection. We examined the expression of active caspase-3 and the
induction of apoptosis at 24, 36, 48 and 72 h after rev-caspase-3
transfection. The results showed that active caspase-3 p17 and PARP
cleavage p85 proteins became evident as early as 24 h and were
strongly expressed at 36–48 h after the viral infection. Protein
expression of both p17 and p85 became weak at 72 h after infection,
probably due to increased cell death.
The optimal MOI was determined by infecting each
cell line with Ad-EGFP and assessing the expression of green
fluorescence protein under a fluorescence microscope. The optimal
MOI of recombinant adenoviruses expressing rev-caspase-3 was
70–100, at which most of the cells underwent apoptosis.
Alternatively, the non-cytotoxic MOI of the viral vectors used
(5–20) resulted in cessation of cell growth
with significant S-phase delay and retardation in S-phase
progression. No significant initial cell apoptosis occurred at this
MOI.
It is necessary to restrict the expression of
rev-caspase-3 to tumor cells, due to its potent efficacy of
inducing apoptosis. Utilization of the hTERT promoter that is
predominantly active in tumor cells would be an ideal system to
restrict rev-caspase-3 expression. However, in most cancer cells,
the hTERTp activity is >10-fold lower than that of CMVp
(11) and is too weak to achieve
sufficient transgene expression. It has been shown that transgene
expression from a tumor-specific promoter can be augmented by using
a TSTA system (12–14). In the present study, we employed a
TSTA system to elevate the activity of the hTERTp up to a 203-fold
range in human ovarian cancer cell lines. In this two-tiered
system, the hTERT regulatory region was employed to express the
potent synthetic transcription activator, GAL4-VP2, which in turn
powerfully activates a minimal TATA promoter to induce transgene
expression. In AdHTVP2G5-EGFP, the activator and reporter component
were inserted into the adenoviral vector in a divergently linked
head-to head configuration. In vitro infection assays of
hTERT-positive cells with AdHTVP2G5-EGFP confirmed the
hTERT-selective expression of this approach. Furthermore, the level
of EGFP expression by hTERTp-TSTA was 203-fold higher than that by
hTERTp alone and also compared favorably with CMVp, the strong
constitutively active viral promoter.
We then established the binary system expressing
rev-caspase-3 in a single recombinant adenovirus, named
AdHTVP2G5-rev-casp3. In vitro cell survival assays in
hTERT-positive and hTERT-negative cells confirmed the selectivity
of AdHTVP2G5-rev-casp3. Only hTERT-expressing cells were killed by
AdHTVP2G5-rev-casp3 as opposed to the non-specific killing of
Ad-rev-casp3. Moreover, the cell killing efficacy of
AdHTVP2G5-rev-casp3 was comparable to that of Ad-rev-casp3.
The selectivity of the hTERTp-TSTA system in
vivo was further confirmed by quantitative analysis of EGFP and
active caspae-3 expression. Fluorescence detection and RT-PCR
indicated transgene expression only in the area of hTERT-positive
tumors with significantly less spread to the adjacent liver tissues
than after administration of the universally expressed
Ad-rev-casp3. Moreover, AdHTVP2G5-rev-casp3 was more potent in
tumor killing than Ad-rev-casp3.
A transcriptionally targeted gene expression
approach could reduce the potential side effects of
adenovirus-mediated cytotoxic cancer gene therapy (15). After intratumoral injection of
adenovirus constitutively expressing luciferase or another reporter
gene, leakage of the vector into systemic circulation resulted in
transgene expression in the liver (16). From this finding liver toxicity can
be anticipated after intratumoral injection of Ad-rev-casp3. The
gene-expression targeting approach employed in this study will not
alter the in vivo liver distribution observed of Ad5
(17). This preferential adenovirus
transduction has contributed to liver toxicity (18,19)
due to the innate immune response to viral capsid proteins
(20) and cell-mediated immunity
against viral gene products (21,22).
Utilization of a specific promoter to drive transgene expression
was shown to reduce both the immune response against the adenovirus
and the associated liver toxicity; however, high viral doses
accompanied by severe liver toxicity were necessary to achieve
therapeutic effects (23). The
potent gene expression mediated by the TSTA system could
potentially reduce the amount of vector needed to transduce cancer
cells in vivo compared to nonamplified tissue-selective
vectors. Reducing the input dosage of Ad has been documented to
reduce liver toxicity (20–22,24).
Our data suggest that, at an viral dose of 2.5×109
TCID50, the AdHTVP2G5-rev-casp3 has the potential to
induce tumor killing with reduced liver toxicity. In the present
study, the same extent of vector delivery to the liver is expected
to occur when hTERTp-TSTA is employed to drive rev-caspase-3;
however, rev-caspase-3 expression in the liver is restricted by
hTERTp-TSTA and therefore rev-caspase-3-mediated liver toxicity is
reduced. The Ad/hTERTp-TSTA system exhibited a lower toxicity
profile than the Ad/CMVp system.
In summary, our results provide evidence that the
AdHTVP2G5-rev-casp3 is able to treat ovarian cancer with a high
degree of efficacy and selectivity. The use of a non-mammalian
transcriptional activator ensures that no other endogenous genes
are being activated. The TSTA system will undoubtedly profit from
the intense investigation of many tissue-specific promoters and may
ultimately emerge as a powerful tool for stringent gene expression
in a wide variety of tumors.
Acknowledgements
We thank Professor Izumi for the pBTdel-279 plasmid
and useful information, Professor M. Garey for the PBCVP2G5-lucNSN
plasmid and Professor K. Wang K help in preparing the adenoviral
vectors. This study was supported by NSFC (National Natural Science
Foundation of China) grant 30600746 (to Y.S.).
References
|
1
|
Jia LT, Chen SY and Yang AG: Cancer gene
therapy targeting cellular apoptosis machinery. Cancer Treat Rev.
38:868–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mazumder S, Plesca D and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 414:13–21.
2008.PubMed/NCBI
|
|
3
|
Srinivasula SM, Ahmad M, MacFarlane M, Luo
Z, Huang Z, Fernandes-Alnemri T and Alnemri ES: Generation of
constitutively active recombinant caspase-3 and -6 by rearrangement
of their subunits. J Bio Chem. 273:10107–10111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Y and Shen K: Construction of
autocatalytic caspase-3 and its effects of inducing apoptosis in
human ovarian carcinoma. Zhonghua Fu Chan Ke Za Zhi. 42:846–851.
2007.(In Chinese).
|
|
5
|
Yang L, Cao Z, Yan H and Wood WC:
Coexistence of high levels of apoptotic signaling and inhibitor of
apoptotic proteins in human tumor cells: implication for cancer
specific therapy. Cancer Res. 63:6815–6824. 2003.PubMed/NCBI
|
|
6
|
Fujiwara T, Shirakawa Y and Kagawa S:
Telomerase-specific oncolytic virotherapy for human
gastrointestinal cancer. Expert Rev Anticancer Ther. 11:525–532.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyo S, Takakura M, Fujiwara T and Inoue M:
Understanding and exploiting hTERT promoter regulation for
diagnosis and treatment of human cancers. Cancer Sci. 99:1528–1538.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong BH, Song Y, Ma DX, Qu X and Jiang S:
In vitro treatment of ovarian cancer cells with cytosine
deaminase-thymidine kinase fusion disuicide gene therapy system
driven by human telomerase reverse transcriptase gene promoter.
Zhonghua Fu Chan Ke Za Zhi. 39:390–395. 2004.(In Chinese).
|
|
9
|
Song Y, Xia Z, Shen K and Zhai X:
Autocatalytic caspase-3 driven by human telomerase reverse
transcriptase promoter suppresses human ovarian carcinoma growth in
vitro and in mice. Int J Gynecol Cancer. 23:642–649. 2013.
View Article : Google Scholar
|
|
10
|
Kagawa S, Gu J, Swisher SG, Ji L, Roth JA,
Lai D, Stephens LC and Fang B: Antitumor effect of
adenovirus-mediated Bax gene transfer on p53-sensitive and
p53-resistant cancer lines. Cancer Res. 60:1157–1161.
2000.PubMed/NCBI
|
|
11
|
Li JT, Bian K, Zhang AL, Kim DH, Ashley
WW, Nath R, McCutcheon I, Fang B and Murad F: Targeting different
types of human meningioma and glioma cells using a novel adenoviral
vector expressing GFP-TRAIL fusion protein from hTERT promoter.
Cancer Cell Int. 11:352011. View Article : Google Scholar
|
|
12
|
Hwang do W, Kang JH, Jeong JM, Chung JK,
Lee MC, Kim S and Lee DS: Noninvasive in vivo monitoring of
neuronal differentiation using reporter driven by a neuronal
promoter. Eur J Nucl Med Mol Imaging. 35:135–145. 2008.PubMed/NCBI
|
|
13
|
Watanabe M, Ueki H, Ochiai K, Huang P,
Kobayashi Y, Nasu Y, Sasaki K, Kaku H, Kashiwakura Y and Kumon H:
Advanced two-step transcriptional amplification as a novel method
for cancer-specific gene expression and imaging. Oncol Rep.
26:769–775. 2011.PubMed/NCBI
|
|
14
|
Chen IY, Gheysens O, Li Z, Rasooly JA,
Wang Q, Paulmurugan R, Rosenberg J, Rodriguez-Porcel M, Willmann
JK, Wang DS, Contag CH, Robbins RC, Wu JC and Gambhir SS:
Noninvasive imaging of hypoxia-inducible factor-1α gene therapy for
myocardial ischemia. Hum Gene Ther Methods. 24:279–288. 2013.
|
|
15
|
Wu L, Johnson M and Sato M:
Transcriptionally-targeted gene therapy to detect and treat cancer.
Trends Mol Med. 9:421–429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adams JY, Johnson M, Sato M, Berger F,
Gambhir SS, Carey M, Iruela-Arispe ML and Wu L: Visualization of
advanced human prostate cancer lesions in living mice by a targeted
gene transfer vector and optical imaging. Nat Med. 8:891–897.
2002.PubMed/NCBI
|
|
17
|
Alemany R and Curiel DT: CAR-binding
ablation does not change biodistribution and toxicity of adenoviral
vectors. Gene Ther. 8:1347–1353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tao N, Gao GP, Parr M, Johnston J, Baradet
T, Wilson JM, Barsoum J and Fawell SE: Sequestration of adenoviral
vector by Kuffer cells leads to a nonlinear dose response of
transduction in liver. Mol Ther. 3:28–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morral N, O’Neal WK, Rice K, Leland MM,
Piedra PA, Aguilar-Córdova E, Carey KD, Beaudet AL and Langston C:
Lethal toxicity, severe endothelial injury, and a threshold effect
with high doses of an adenoviral vector in baboons. Hum Gene Ther.
13:143–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Chirmule N, Gao GP, Qian R,
Croyle M, Joshi B, Tazelaar J and Wilson JM: Acute cytokine
response to systemic adenoviral vectors in mice is mediated by
dendric cells and macrophages. Mol Ther. 3:697–707. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Su Q and Wilson JM: Role of viral
antigens in destructive cellular immune responses to adenovirus
vector-transduced cells in mouse lungs. J Virol. 70:7209–7212.
1996.PubMed/NCBI
|
|
22
|
Jooss K, Yang Y, Fisher KJ and Wilson JM:
Transduction of dendritic cells by DNA viral vectors directs the
immune response to transgene products in muscle fibers. J Virol.
72:4212–4223. 1998.PubMed/NCBI
|
|
23
|
Brand K, Löser P, Arnold W, Bartels T and
Strauss M: Tumor cell-specific transgene expression prevents liver
toxicity of the adeno-HSV-tk/GCV approach. Gene Ther. 5:1363–1371.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pastore L, Morral N, Zhou H, Garcia R,
Parks RJ, Kochanek S, Graham FL, Lee B and Beaudet AL: Use of a
liver-specific promoter reduces immune response to the transgene in
adenoviral vectors. Hum Gene Ther. 10:1773–1781. 1999. View Article : Google Scholar : PubMed/NCBI
|