Introduction
Reactive oxygen species (ROS) are produced in the
cells during aerobic metabolism. At first, molecular oxygen can be
reduced to form superoxide anion (O2•−) by
the activity of NADPH oxidase (NOX) (1,2), a
multicomponent enzyme, or by xanthine oxidase, lipoxygenase and
mitochondrial oxidases (2).
Subsequently, superoxide, a highly ROS (hROS), is converted by
superoxide dismutase (SOD) (3,4) to
hydrogen peroxide (H2O2), a more stable
species, which can be reduced to water either by catalase or by
glutathione peroxidase (2).
An excess of ROS (O2•− and
H2O2) is commonly referred to as oxidative
stress. This condition, which is responsible for cell damage, can
be induced by many diseases and a number of toxic agents (5).
Also, nitric oxide (NO) is implicated in
pathological mechanisms. One of these mechanisms involves NO in a
reaction with superoxide to form peroxynitrite (6) that is a potent oxidant. Peroxynitrite
leads to lipid peroxidation and nitration of tyrosine residues in
proteins with their inactivation (7).
Parthenolide, a sesquiterpene lactone found in
Tanacetum parthenium, known for its anti-inflammatory
activity, is currently considered an effective antitumour agent
(8,9). It has been shown that this drug exerts
its effect by inducing generation of ROS (10) and inhibiting the nuclear factor κB
(NF-κB) (11).
We have previously shown that parthenolide rapidly
stimulates ROS generation in human osteosarcoma MG-63 and melanoma
SK-MEL-28 cells (12) as well as in
breast cancer MDA-MB-231 cells (13). Furthermore, it has been reported
that parthenolide induces the activity of NOX in prostate cancer
PC3 cells (14). Recently, we
demonstrated that the drug stimulates NOX activity with
O2•− production in MDA-MB-231 cells as well
(13,15), which could be responsible for the
toxic effects caused by the drug. These effects consist in the
depletion of thiol groups and glutathione, NF-κB inhibition, c-Jun
N-terminal kinase (JNK) activation, dissipation of mitochondrial
membrane potential (Δψm) and necrotic effects (12,13).
The aim of the present report was to study the
production of different oxygen species induced by parthenolide,
such as H2O2, O2•−, and
peroxynitrite, in order to demonstrate the relationships between
these compounds and the role exerted by parthenolide in the
induction of toxicity in MDA-MB-231 cells.
Materials and methods
Chemicals and reagents
Parthenolide was supplied by Sigma-Aldrich (Milan,
Italy). Stock solution of parthenolide was prepared in dimethyl
sulfoxide (DMSO) and diluted to a final concentration in the
culture medium. Final concentration of DMSO employed as vehicle
never exceeded 0.04% and had no discernible effects on MDA-MB-231
cells in comparison with the control. Unless otherwise indicated,
all reagents were purchased from Sigma-Aldrich.
Cell cultures
Human breast carcinoma MDA-MB-231 cells were
provided by Istituto Scientifico Tumori (Genoa, Italy). The cell
line was grown as monolayer in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% (v/v) heat inactivated fetal calf
serum (FCS), 2 mM glutamine and 1% non-essential amino acids, at
37°C in a humidified atmosphere containing 5% CO2. After
plating on 96- or 6-well plates, cells were allowed to adhere
overnight and were then treated with chemicals or vehicle only.
Analysis of cell viability and cell
necrosis
For these studies, the cells (8×103) were
seeded into 96-well plates. Twenty-four hours after seeding, cells
were treated with drugs for the time indicated in the Results. Cell
viability was determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay as previously reported (16). MTT is reduced to purple formazan in
the mitochondria of living cells. The absorbance of the formazan
was measured at 570 nm, with 630 nm as a reference wavelength using
an automatic ELISA plate reader (Opsys MR; Dynex Technologies,
Chantilly, VA, USA). After treatment with the drugs, cell survival
was estimated as a percentage of the value of the vehicle-treated
control.
To determine membrane damage, the cells were stained
with propidium iodide (PI), as previously reported (13). In particular, cells
(8×103/well) were incubated in culture medium and
treated for various times with parthenolide, and PI (2.0 μg/ml
medium) was then added and the incubation was protracted for an
additional 15 min. At the end, cell morphology was visualized by a
Leica DMR microscope equipped with a DC300F camera (Leica
Microsystems, Wetzlar, Germany) using an appropriate filter to
examine PI (rhodamine filter with excitation wavelength of 596 nm
and emission wavelength of 620 nm). All the images were acquired by
Leica Q-Fluoro Software. Cells with red fluorescence were counted
and normalized to the total number of cells/field to calculate the
percentage of PI-positive cells. Five fields/condition were
analysed.
Flow cytometric analysis
MDA-MB-231 cells (105/well) were
incubated with parthenolide and other effectors for various times.
At the end, the cells were trypsinized, collected, washed with PBS
and incubated with fluorescent probes.
DCFH-DA analysis
Generation of intracellular radicals was measured
using 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
(DCFH-DA; Molecular Probe, Life Technologies, Eugene, OR, USA), a
fluorochrome that passively diffuses into the cells. After cleavage
of acetate groups by intracellular esterases, a fluorescent adduct
(DCF) is produced by oxidation. To analyse DCF signal, the cells
were incubated with 50 μM DCFH-DA in Hank’s balanced salt solution
(HBSS) for 30 min at 37°C in darkness.
DHE analysis
O2•− production was assessed
by dihydroethidium (DHE) staining. The fluorochrome DHE is oxidized
by superoxide to fluorescent ethidium that intercalates with
nuclear DNA, staining the nucleus with a bright red fluorescence.
After treatment with parthenolide, the cells were incubated with 20
μM DHE in PBS for 15 min at 37°C in darkness.
MitoSox Red analysis
To detect mitochondrial production of
O2•−, MitoSox Red fluorochrome was used
(Molecular Probe, Life Technologies). MitoSox Red is a
mitochondria-targeted form of DHE that is relatively specific for
O2•− and undergoes oxidation to form a
DNA-binding red fluorescent product (17). After treatment, the cells were
incubated with 5 μM MitoSox Red in HBSS buffer for 10 min at 37°C
in the dark.
HPF analysis
Hydroxyphenyl fluorescein (HPF; Enzo Life Sciences
Inc., Farmingdale, NY, USA) was employed to detect hROS). HPF is a
cell permeable minimally fluorescent dye, which reacts with hROS
(·OH, ONOO- and -OCl) and is converted to fluorescein, which
exhibits strong, dose-dependent fluorescence (18). In this case, the cells after the
treatment were incubated with 10 μM HPF in HBSS for 1 h at 37°C in
the dark. Then, cells were collected and resuspended in 500 μl
HBSS.
All analyses were performed using a Beckman Coulter
Epics XL flow cytometer (Beckman Coulter, Brea, CA, USA) with
appropriate filters. For DCF and HPF signals, FITC filter was used
with excitation wavelength of 480 nm and emission wavelength of 525
nm. For DHE and MitoSox Red, rhodamine filter was used with
excitation wavelength of 518 nm and emission wavelength of 605 nm.
All the acquired data were analysed by EXPO32 software.
Western blot analysis
For these studies, after treatment, cells were
washed in PBS, lysed for 30 min at 4°C in ice-cold lysis buffer (1%
NP-40, 0.1% SDS and 0.5% sodium deoxycholate in PBS), containing
protease inhibitor cocktail and sonicated three times for 10 sec.
Then, protein concentration was determined by Lowry assay (19). Equal amounts of protein samples (50
μg/lane) were subjected to SDS-polyacrylamide gel electrophoresis,
then blotted to a nitrocellulose membrane.
All western blot analyses were performed using
specific antibodies obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Detection was developed using secondary antibodies
conjugated with alkaline phosphatase.
Protein bands were visualized using
5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium
(Promega Italia, Milan, Italy) and their intensity was quantified
by densitometric analysis using SMX Image software (Bio-Rad
Laboratories Srl, Segrate, Italy).
Statistical analysis
Results are presented as means ± standard error.
Data were analysed using the Student’s t-test.
Results
Effects induced by parthenolide on the
ROS
In order to establish the effect exerted by
parthenolide on the generation of radical species, we analysed the
changes induced by parthenolide treatment on four different
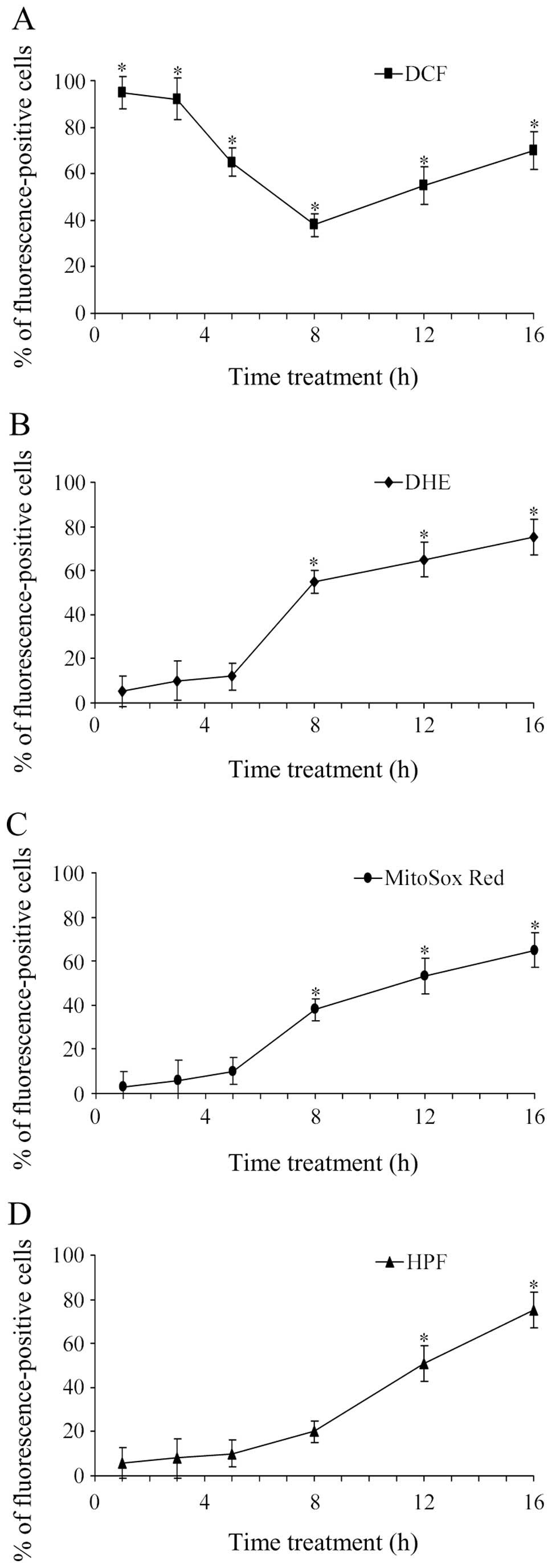
fluorescent signals, DCF, DHE, MitoSox Red and HPF (Fig. 1).
DCFH-DA was used as a fluorescent probe for ROS.
Although this probe is widely used for H2O2,
it lacks specificity among ROS and reacts strongly with hROS, such
as hydroxyl radicals and peroxynitrite (18).
The time course of the parthenolide effect exerted
on DCF signal is reported in Fig.
1A. It was observed that in the first hours of treatment (1–3
h) with 25 μM parthenolide most cells (>90%) were positive to
DCF signal. After 3 h, this percentage declined progressively with
the time of treatment so that at 8 h only 40% of cells were
positive to DCF. After 8 h, DCF signal increased again until 16 h
of treatment, when DCF-positive cells reached 70% of the total
cells.
The fluorochrome DHE was used to assess superoxide
radical production. The time course of the effect exerted by
parthenolide on DHE signal is shown in Fig. 1B. We observed that the percentage of
DHE-positive cells was very modest in the first 5 h of treatment,
then progressively increased in the second phase of treatment (8–16
h), reaching a level near 75% at 16 h.
MitoSox Red was used to ascertain superoxide
production at the mitochondrial level. As shown in Fig. 1C, the percentage of cells positive
to MitoSox Red progressively increased from 8 to 16 h of
incubation. Results reported for MitoSox Red are similar to those
ascertained for DHE signal. This finding suggests that
O2•− assayed in the second phase of treatment
with parthenolide was primarily produced at the mitochondrial
level.
HPF is a fluorescent probe used to detect
selectively hROS, such as hydroxyl radical and peroxynitrite, but
not H2O2, NO and superoxide (18). As shown in Fig. 1D, positivity to HPF signal was
particularly observed in the second phase of treatment (8–16 h),
reaching the maximum at 16 h when 75% of cells were positive to
this signal. This result demonstrated that parthenolide in the
second phase of treatment stimulated the production of hydroxyl
radical and peroxynitrite.
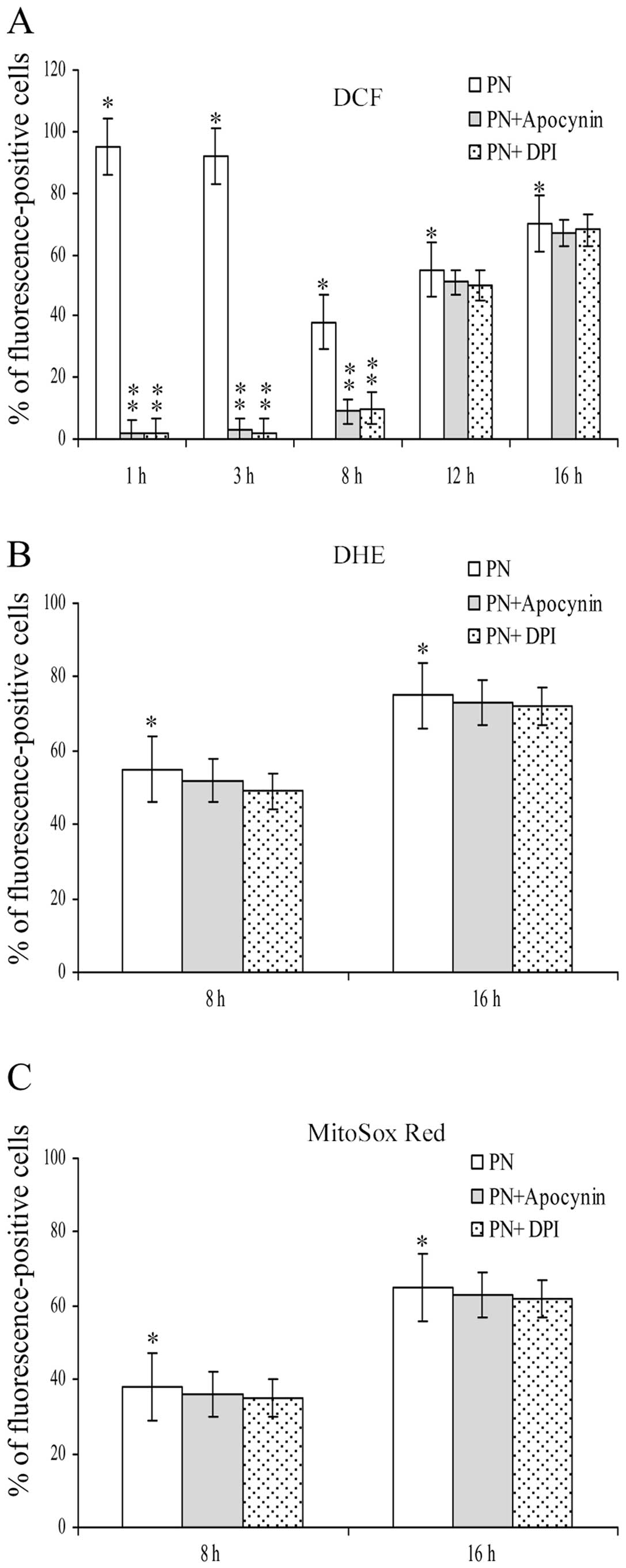
Influence of apocynin and DPI on the
effects exerted by parthenolide on ROS generation
Fig. 2 shows the
influence exerted by apocynin and DPI, two effective inhibitors of
NOX, on the effects induced by parthenolide on ROS generation. It
is of note that both inhibitors completely suppressed the effects
on DCF signal found at 1 and 3 h of treatment with parthenolide. In
addition, the inhibitors also markedly diminished the effect
exerted on DCF signal at 8 h of treatment, whereas their effect was
very modest, at 12 and 16 h of treatment (Fig. 2A).
Fig. 2B and C show
that apocynin and DPI did not modify the effect induced by
parthenolide on both DHE and MitoSox Red assays at 8 and 16 h.
These results demonstrate that O2•− detected
by these fluorochromes was not produced by NOX activity.
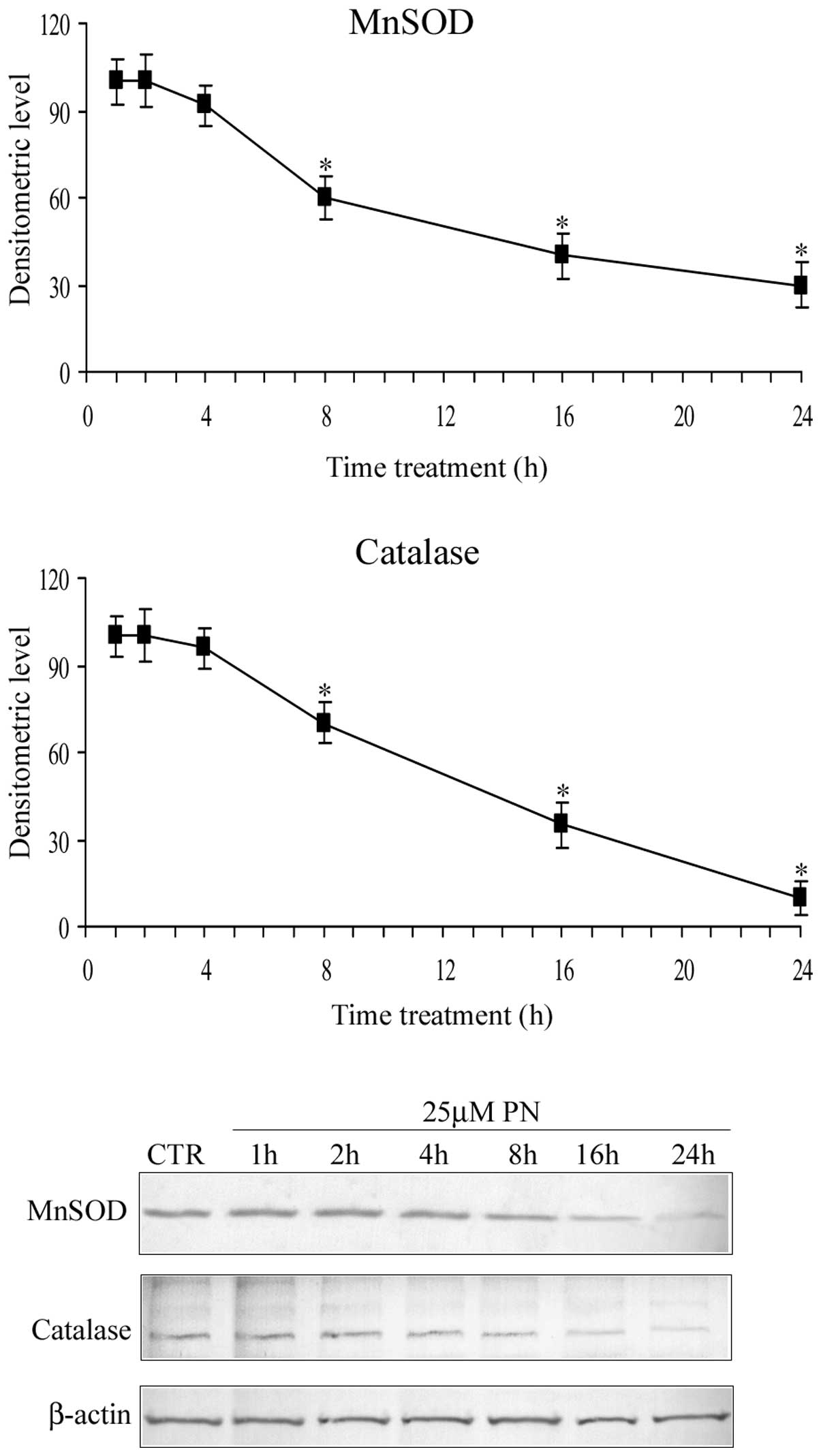
Time course of the parthenolide effect on
manganese superoxide dismutase (MnSOD) and catalase levels
Fig. 3 shows the
time course of the parthenolide effect on the level of MnSOD (SOD2)
and catalase. The results demonstrated that in the first hours (0–4
h) of treatment, parthenolide did not modify the level of MnSOD and
catalase, whereas after 4 h, the level of the two proteins
progressively diminished and at 16 h it was reduced to ~40% of the
control value. These results are in accordance with the previous
observations of Sun et al (14).
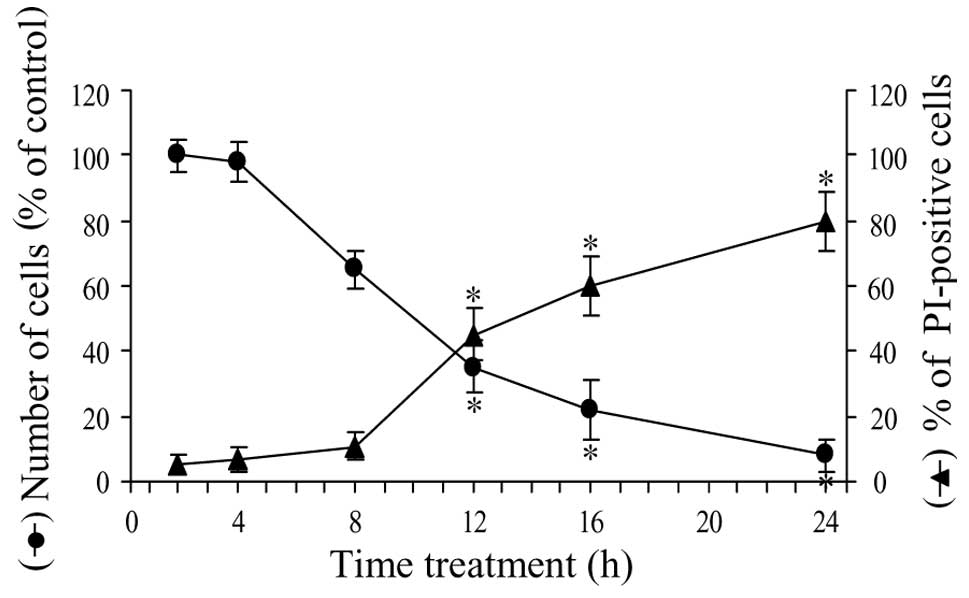
Time course of the parthenolide effect on
cell viability
Fig. 4 shows the
time course of the effect of parthenolide on cell viability. In
particular, in the first 4 h of treatment, viability was reduced by
only 4%, then the effect increased and at 8 h viability decreased
to 65% of control. In the second phase of treatment, viability
rapidly diminished and at 16 h was equal to 22% and at 24 h to only
8%.
To ascertain the effect of parthenolide on the
production of necrotic cells, we employed PI, a cell-impermeable
nuclear dye, which stains the nuclei of cells that have lost plasma
membrane integrity and are considered necrotic. As shown in
Fig. 4, the number of PI-positive
cells increased slowly in the first phase of treatment (10% at 8
h). Between 8 and 20 h, the increment was more rapid, reaching a
level of 60% at 16 h and 80% at 24 h of treatment.
Discussion
In previous studies (13,15),
we showed that parthenolide induces generation of ROS in MDA-MB-231
cells. The aim of the present study was to individuate the ROS
produced under parthenolide treatment to specify the modalities of
their production and the role exerted in the induction of cell
death.
In order to clarify these aspects, at first we
studied the effects exerted by 25 μM parthenolide during 24 h of
treatment on the viability of MDA-MB-231 cells. It was shown that
in the first 4 h of treatment, the effect was very modest, while a
marked reduction of the cell number was observed in the second
phase of incubation (8–16 h).
Flow cytometric analysis of cellular ROS was
performed in treated cells using fluorescent probes. DCFH-DA
detects not only H2O2, but also hROS, such as
hydroxyl radical and peroxynitrite, while DHE detects primarily
O2•−.
It was observed that most of the cells were positive
to DCF signal already at 1–3 h of treatment. Since this effect was
suppressed by the addition of either apocynin or DPI, two effective
inhibitors of NOX activity, we suggest that parthenolide, in line
with previous findings (13,15),
stimulated NOX activity, inducing production of
O2•−, which was rapidly dismutated by SOD1 to
produce H2O2. Also, at 8 h, apocynin and DPI
exerted a marked inhibitory effect on DCF signal. Therefore, we
conclude that in the first phase of treatment, the principal effect
of parthenolide consists in the activation of NOX, followed by
involvement of SOD1 with production of H2O2.
This conclusion is also supported by the consideration that in this
phase hROS were not produced, as indicated by the results obtained
with HPF analysis (Fig. 1D).
DHE analysis showed that parthenolide stimulated
this signal only in the second phase of treatment.
O2•−, assayed by DHE analysis, was not
produced by NOX activity since the signal was not diminished by the
addition of either apocynin or DPI. Therefore, it seems likely that
O2•− arises from mitochondrial activity, as
suggested by positivity observed using MitoSox Red fluorochrome. It
is possible that H2O2 can also be produced in
this second phase of treatment. However, as DCF signal at 12 and 16
h was not inhibited by apocynin and DPI, we exclude that in this
phase generation of H2O2 can be a consequence
of activation of NOX, unlike the effect observed in the first hours
of treatment.
Our results clearly demonstrate that parthenolide
induced, in this second phase, production of hROS (hydroxyl radical
and peroxynitrite), as suggested by the results of HPF analysis. In
this phase, as previously ascertained by us (13), parthenolide caused mitochondrial
dysfunction with a marked fall in Δψm. Mitochondrial damage could
be responsible for the production of either
O2•− and hROS. In particular, peroxynitrite
could be produced by a specific reaction between
O2•− and NO (6) and could contribute to positivity found
with both DCF and HPF signals in the second phase of incubation.
The marked decrement in the level of both MnSOD and catalase, found
in this phase of treatment, can contribute to determine the rise of
O2•− and hROS. Finally, it is interesting to
note that peroxynitrite and hydroxyl radical can in turn induce
mitochondrial damage and further production of ROS (6).
A comparison between the time course of the
parthenolide effect on cell viability and the production of ROS
leads to the following conclusions: i) in a first phase of
treatment (0–8 h), and in particular in the first 4 h, the effect
on cell viability was very modest, while most of the cells were
positive to DCF signal owing to the production of
H2O2; and ii) in the second phase (8–16 h), a
strong effect on cell viability was observed, just when
mitochondrial damage leads to the production of
O2•− and hROS. These results suggest that
O2•− and hROS may play a central role in the
induction of cell death by parthenolide treatment.
Acknowledgements
The present study was supported by grants from the
Italian Ministry of Education, University and Research (MIUR)
ex-60%, 2010; the European Regional Development Fund, European
Territorial Cooperation 2007–2013, CCI 2007 CB 163 PO 037, OP
Italia-Malta 2007–2013. Dr D. Carlisi is a recipient of a grant by
the Italian Ministry of Education, University and Research (MIUR).
Drs R. Martinez and G. Buttitta are PhD students of Doctorate in
Biomedicine and Clinical Neuroscience supported by the Italian
Ministry of Education, University and Research (MIUR).
Abbreviations:
|
DCF
|
dichlorofluorescein
|
|
DCFH-DA
|
5-(and-6)-
carboxy-2′,7′-dichlorodihydrofluorescein diacetate
|
|
DHE
|
dihydroethidium
|
|
DMSO
|
dimethyl sulfoxide
|
|
DPI
|
diphenyleneiodonium
|
|
Δψm
|
mitochondrial membrane potential
|
|
H2O2
|
hydrogen peroxide
|
|
HPF
|
hydroxyphenyl fluorescein
|
|
hROS
|
highly reactive oxygen species
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MnSOD
|
manganese superoxide dismutase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
NF-κB
|
nuclear factor κB
|
|
NO
|
nitric oxide
|
|
NOX
|
NADPH oxidase
|
|
O2•−
|
superoxide anion
|
|
PI
|
propidium iodide
|
|
PN
|
parthenolide
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Nauseef WM: Detection of superoxide anion
and hydrogen peroxide production by cellular NADPH oxidases.
Biochim Biophys Acta. 1840:757–767. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paletta-Silva R, Rocco-Machado N and
Meyer-Fernandes JR: NADPH oxidase biology and the regulation of
tyrosine kinase receptor signaling and cancer drug cytotoxicity.
Int J Mol Sci. 14:3683–3704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fukai T and Fukai MU: Superoxide
dismutases: role in redox signaling, vascular function, and
diseases. Antioxid Redox Signal. 15:1583–1606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Landis GN and Tower J: Superoxide
dismutase evolution and life span regulation. Mech Ageing Dev.
126:365–337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartosz G: Reactive oxygen species:
destroyers or messengers? Biochem Pharmacol. 77:1303–1315. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zunino SJ, Ducore JM and Storms DH:
Parthenolide induces significant apoptosis and production of
reactive oxygen species in high-risk pre-B leukemia cells. Cancer
Lett. 254:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghantous A, Sinjab A, Herceg Z and
Darwiche N: Parthenolide: from plant shoots to cancer roots. Drug
Discov Today. 18:894–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreuger MR, Grootjans S, Biavatti MW,
Vandenabeele P and D’Herde K: Sesquiterpene lactones as drugs with
multiple targets in cancer treatment: focus on parthenolide.
Anticancer Drugs. 23:883–896. 2012.PubMed/NCBI
|
|
10
|
Wang W, Adachi M, Kawamura R, et al:
Parthenolide-induced apoptosis in multiple myeloma cells involves
reactive oxygen species generation and cell sensitivity depends on
catalase activity. Apoptosis. 11:2225–2235. 2006. View Article : Google Scholar
|
|
11
|
Nakabayashi H and Shimizu K: Involvement
of Akt/NF-κB pathway in antitumor effects of parthenolide on
glioblastoma cells in vitro and in vivo. BMC Cancer.
12:4532012.
|
|
12
|
D’Anneo A, Carlisi D, Lauricella M,
Emanuele S, Di Fiore R, Vento R and Tesoriere G: Parthenolide
induces caspase-independent and AIF-mediated cell death in human
osteosarcoma and melanoma cells. J Cell Physiol. 228:952–967.
2013.PubMed/NCBI
|
|
13
|
D’Anneo A, Carlisi D, Lauricella M,
Martinez R, Emanuele S, Vento R and Tesoriere G: Parthenolide
generates reactive oxygen species and autophagy in MDA-MB-231
cells. A soluble parthenolide analogue inhibits tumour growth and
metastasis in a xenograft model of breast cancer. Cell Death Dis.
4:e8912013.
|
|
14
|
Sun Y, St Clair DK, Xu Y, Crooks PA and St
Clair WH: A NADPH oxidase-dependent redox signaling pathway
mediates the selective radiosensitization effect of parthenolide in
prostate cancer cells. Cancer Res. 70:2880–2890. 2010. View Article : Google Scholar
|
|
15
|
D’Anneo A, Carlisi D, Emanuele S, Buttitta
G, Vento R, Tesoriere G and Lauricella M: Parthenolide induces
superoxide anion production by stimulating EGF receptor in
MDA-MB-231 breast cancer cells. Int J Oncol. 43:1895–1900.
2013.
|
|
16
|
Carlisi D, D’Anneo A, Angileri L,
Lauricella M, Emanuele S, Vento R and Tesoriere G: Parthenolide
sensitizes hepatocellular carcinoma cells to TRAIL by inducing the
expression of death receptors through inhibition of STAT3
activation. J Cell Physiol. 226:1632–1641. 2011. View Article : Google Scholar
|
|
17
|
Han Z, Varadharaj S, Giedt RJ, Zweier JL,
Szeto HH and Alevriadou BR: Mitochondria-derived reactive oxygen
species mediate heme oxygenase-1 expression in sheared endothelial
cells. J Pharmacol Exp Ther. 329:94–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Setsukinai K, Urano Y, Kakinuma K, Majima
H and Nagano T: Development of novel fluorescence probes that can
reliably detect reactive oxygen species and distinguish specific
species. J Biol Chem. 278:3170–3175. 2003. View Article : Google Scholar
|
|
19
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|