Introduction
Cholangiocarcinoma is one of the most intractable
malignancies in the human body. The disease was recently
highlighted in Japan, since 11 patients presented with intrahepatic
or extrahepatic cholangiocarcinoma among 62 workers who had been
employed in the offset color proof-printing room or in the front
room of a printing company for at least 1 year between 1991 and
2006. The incidence suggests that cholangiocarcinoma might be a new
occupation-related disease. Long-term exposure to chemicals, such
as 1,2-dichloropropane and/or possibly dichloromethane, were
attributable to the etiology (1).
The prognosis of the disease is unfavorable. The
5-year survival of newly diagnosed patients is less than 15%
(2). Surgical removal is the only
curable treatment for the disease. However, since the disease
initially lacks subjective symptoms and many patients are diagnosed
at the advanced stage, only less than a third of all patients are
indicated for radical resection (3). Furthermore, even when the affected
region is surgically removed, the disease frequently recurs and
thus the outcome is not satisfactory. Therefore, intensive
chemotherapy is mandatory as an adjunctive treatment of the
disease. However, the response rate of systemic chemotherapy is
only ~25% and it is critical to find new treatment strategies for
these tumors (3).
Gemcitabine (GEM) is the most potent
chemotherapeutic agent for cholangiocarcinoma (4) and from the results of the ABC-02
trial, its combination with cisplatin has become the standard
treatment for the disease (5).
Nevertheless, the median survival time is less than 12 months in
patients at an advanced stage. Studies regarding other approaches,
such as its combination with molecular targeting drugs (2) or methods increasing cellular
sensitivity to the drugs, are in progress (4).
In order to develop these therapies, cell biological
techniques have been utilized. The in vitro study method
with corresponding cell lines is particularly useful for evaluating
sensitivity to chemotherapeutic agents and testing for the
improvement of efficacy. Several cholangiocarcinoma cell lines have
been reported (6–10). However, the number and access to
these lines is limited and more importantly, detailed information
and their documented characteristics are insufficient.
Previously, we established a cholangiocarcinoma cell
line and designated it as TK. The cell line was derived from the
ascites of a 78-year-old female cholangiocarcinoma patient; it
produces carbohydrate antigen (CA)19-9, CA50 and carcinoembryonic
antigen (CEA) (11). The cell line
also morphologically forms a characteristic duct-like edifice
covered by structured microvilli when cultured three-dimensionally,
hence the cell line is expected to be usable as a model for in
vivo study of cholangiocarcinoma (12). The cell line is also implantable and
forms a tumor in the nude mouse, and therefore, can be used as a
xenograft animal model.
In the present study, the efficacy of GEM on the
cell line was investigated. To develop highly effective
chemotherapy, cytotoxicities to the drug and transcripts of enzymes
relating to GEM incorporation and excretion in the cell line were
compared to a pancreatic carcinoma cell line. Pancreatic carcinoma
is similarly treated by GEM. By means of a comparative
investigation, we attempted to determine whether or not the TK cell
line can be used for further studies.
Materials and methods
Cell lines
Establishment and the characteristics of the TK
human cholangiocarcinoma cell line were previously described
(11). The TK cell line was
cultured with RPMI-1640 (Gibco Life Technologies, Carlsbad, CA,
USA) supplemented with 15% fetal bovine serum (FBS), 2 mM glutamine
and 1 mM sodium pyruvate. The human pancreatic adenocarcinoma cell
line BXPC3 was obtained from the American Type Culture Collection
(Manassas, VA, USA). BXPC3 was grown in RPMI-1640 containing 10%
FBS, 2 mM glutamine and 1 mM sodium pyruvate. Both cell lines were
maintained as monolayer cultures at 37°C in a 5% CO2
atmosphere. Cells were harvested with 0.05% trypsin and 0.02% EDTA
in Dulbecco’s phosphate-buffered saline (PBS).
Cell growth
Growth of the cell line was assessed in 6-cm culture
dishes. The cells were seeded at the density of 1.4×104
cells per dish, and the cell growth was determined by counting the
cell number.
Drug
Gemcitabine (GEM; difluorodeoxycytidine, dFdC) was
purchased from Wako (Osaka, Japan). GEM was dissolved in saline,
sterilized by 0.22-μm filtration, and stored at −20°C until
use.
Cytotoxic assay
Cells were seeded in 96-well sterile plates
(Corning, Tokyo, Japan) at the density of 5×103 cells
per well. After 24 h, the cells were treated with stepwise
dilutions of GEM and incubated for 120 h. To assess the cell
viability, the cells were fixed in 5.4% glutaraldehyde for 15 min
at room temperature and stained with 50 μl of 0.05% methylene blue
for 15 min. The dye was eluted with 0.4 N HCl for 15 min, and the
absorbance was measured in a microplate reader (model 680 XR,
Bio-Rad, Tokyo, Japan) at 595 nm (13). Sensitivity to the agent was
evaluated by calculation of the 50% inhibitory concentration
(IC50) of the drug.
Cell cycle analysis
The cells were dispersed and attached to the bottom
of the culture flasks before use. Subsequently, the cells were
exposed to GEM at the dose of the IC50 values for the
respective cells. After 48 and 96 h, the cells were dispersed with
trypsin, washed with PBS and fixed with 75% ethanol. They were
stained with 50 μg/ml propidium iodide (PI) in PBS with 180 U of
RNase A for 30 min (14). The cell
cycle populations were determined by a flow cytometer (FACSCalibur;
Becton Dickinson Japan, Tokyo, Japan) and analyzed by ModFit Lt
software (Becton Dickinson).
Quantification of RNA transcripts
The cellular RNAs of TK and BXPC3 cells were
extracted by the acid guanidium-phenol-chloroform (AGPC) method
(RNAzol B; Tell Test, Friendswoods, TX, USA). The RNAs were treated
with RNase inhibitor and RNase-free recombinant DNase I (Takara
Bio, Otsu, Japan) for 20 min, and the resulting DNA-free RNAs were
reversely transcribed using Prime Script RT Master Mix (Takara
Bio). For quantification of the RNAs of deoxycytidine kinase (dCK),
cytidine deaminase (CDA) and deoxycytidine monophosphate (dCMP)
deaminase, semi-quantitative polymerase chain reactions were
performed at 95°C for 30 sec followed by 40 cycles at 95°C for 5
sec and then at 60°C for 31 sec (SYBR-Premix Ex Taq II; Takara
Bio). Primers used for the study are shown in Table I. Signals were detected using the
ABI 7300 real-time PCR system (Applied Biosystems, Life
Technologies, Carlsbad, CA, USA) Specificities of the reaction were
confirmed with melting curves. The expression level was compared by
relative quantification (ΔΔCt) (15).
 | Table IPrimers used for RT-PCR. |
Table I
Primers used for RT-PCR.
| Gene | Primers |
|---|
| dCK | F:
ggaagtggttcctgaacctgttg
R: ctctgcatctttgagcttgcc |
| CDA | F:
gaagcgtcctgcctgca
R: ctggaccgtcatgacaatatacg |
| GAPDH | F:
gaaggtgaaggtcggagtc
R: gaagatggtgatgggatttc |
Adenoviral infection
Constructs of the adenoviral vectors encoding dCK
(Ad-dCK) and green fluorescent protein (GFP) (Ad-GFP) were
described previously (13,16). These viral vectors were isolated
from single plaques, expanded in human embryonic kidney 293 cells
and purified by double Cs centrifugation (17). The TK and BXPC3 cells were infected
with the Ad-dCK or control Ad-GFP at several different
multiplicities of infection (MOIs).
Cell growth inhibition assay
After 24 h of adenoviral infection, the cells were
dispersed, seeded in 96-well plates at the density of
1.2×102 cells per well, and incubated for 24 h. Cells in
each group were exposed to GEM at concentrations of 0 or the dose
of the respective IC50 for 120 h and the cytotoxicity
was measured. The effect of dCK transduction on GEM treatment was
evaluated by inhibition divided by mock treatment.
Statistical analysis
Statistical analysis was performed by the two-sample
t-test.
Results
Cell growth of the TK cell line
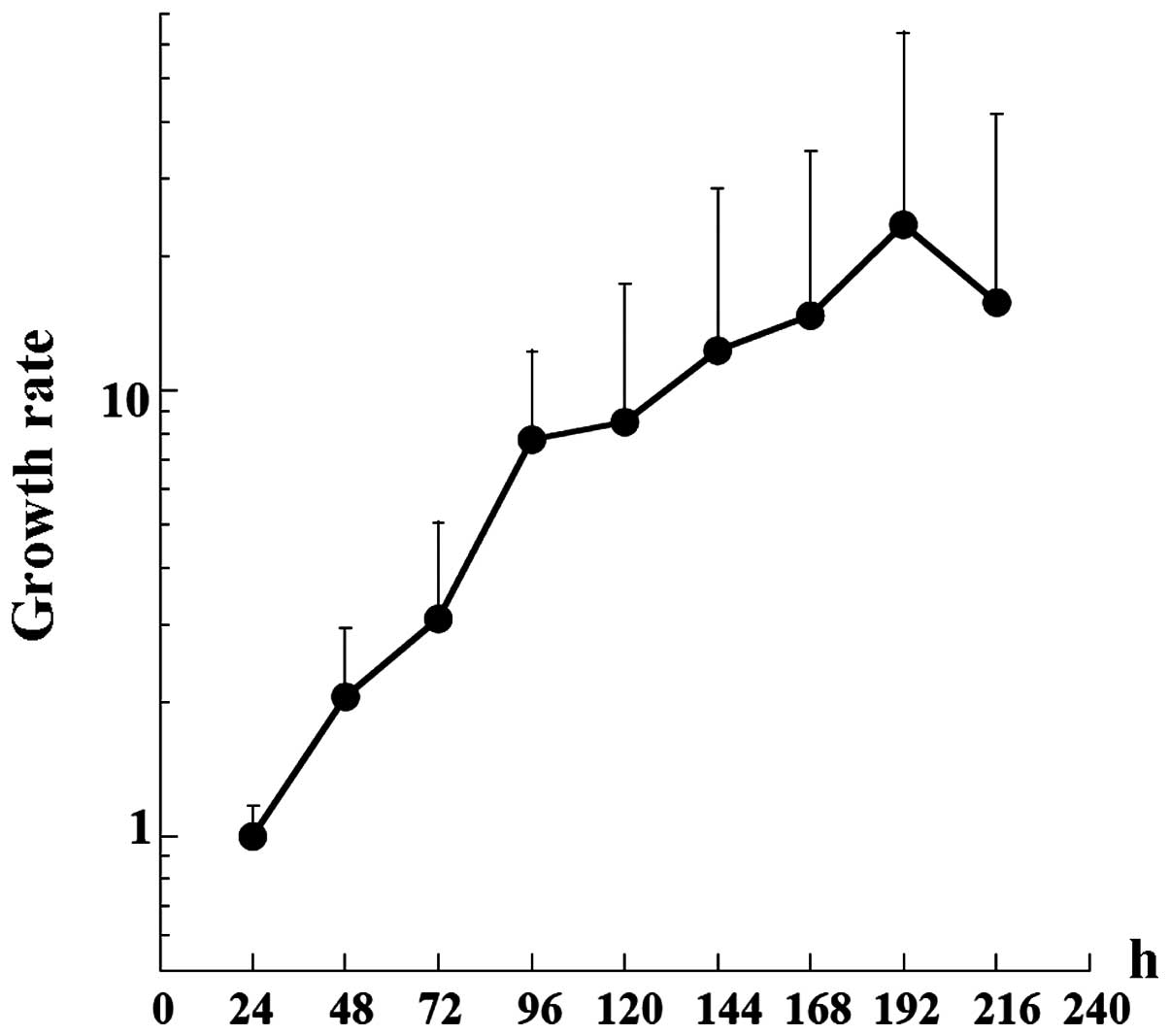
The manner of TK cell growth was examined first. The
growth curve of the TK cells is shown in Fig. 1. The doubling time of the cells was
36.9 h, which was slightly longer than that of the earlier passage
cells (29 h) (11). The saturation
density of the cells was 2.7×105/cm2.
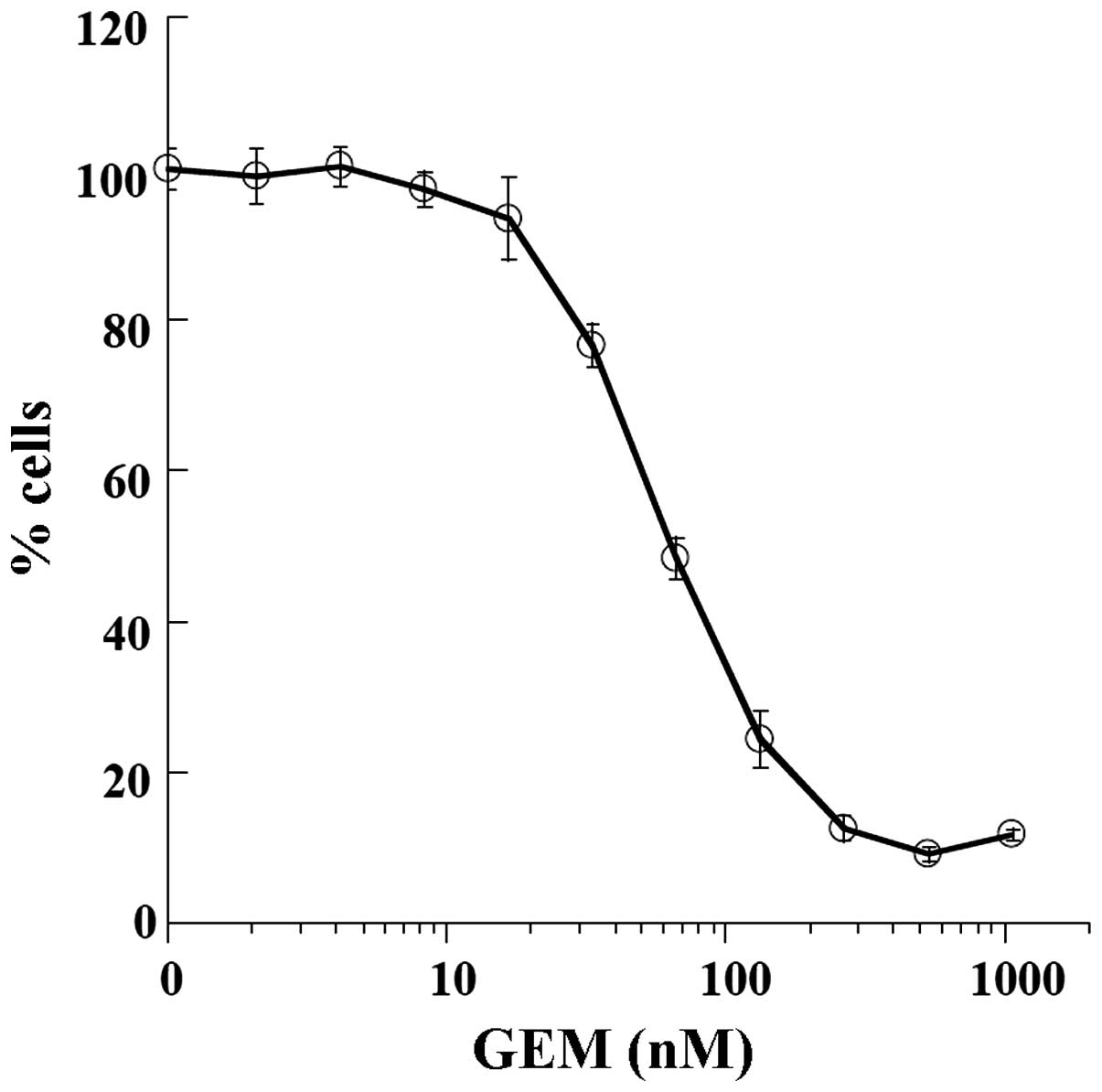
Sensitivity of the TK cell line to
GEM
Since GEM is a standard therapeutic agent,
sensitivity of the TK cells to the drug was determined (Fig. 2). The GEM IC50 value of
the TK cells was 66.22 nM and the value was comparable to that of
the pancreatic BXPC3 cells (47.10 nM).
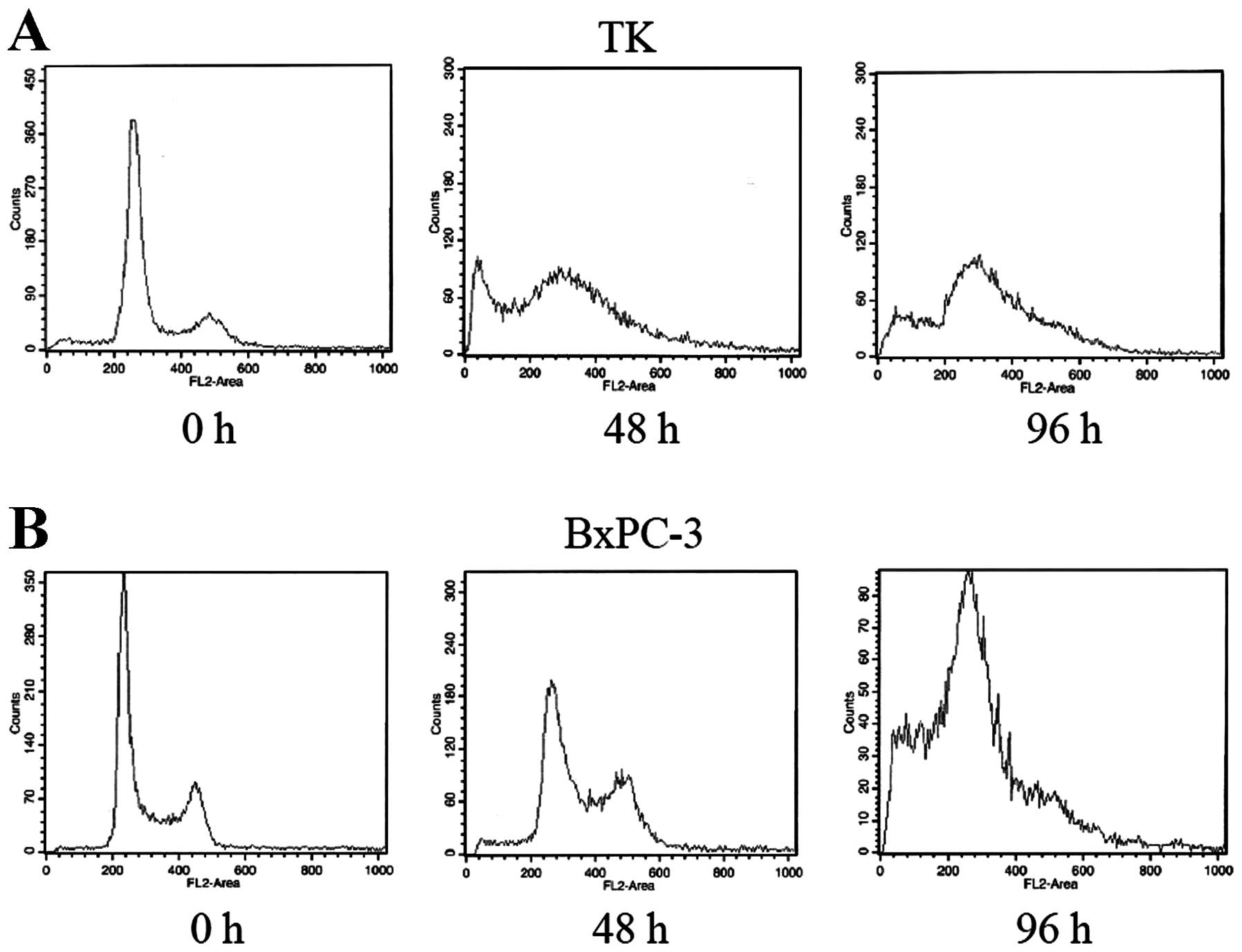
Cell cycle analysis of the TK cells
exposed to GEM
To analyze the mechanism of action on these cells,
cell cycle populations of both the TK and pancreatic BXPC cell
lines were analyzed (Fig. 3).
Forty-eight and 96 h after treatment with GEM, the cells were fixed
and the cell populations in each cell cycle were analyzed. At 48 h,
the S phase population was increased in both the TK and BXPC cell
lines. An apoptotic sub-G1 peak was observed only in the TK cells,
but this peak subsequently also appeared in the BXPC cells at 96 h
after treatment. The overall effects of GEM on the cell cycle of
both cell lines were similar.
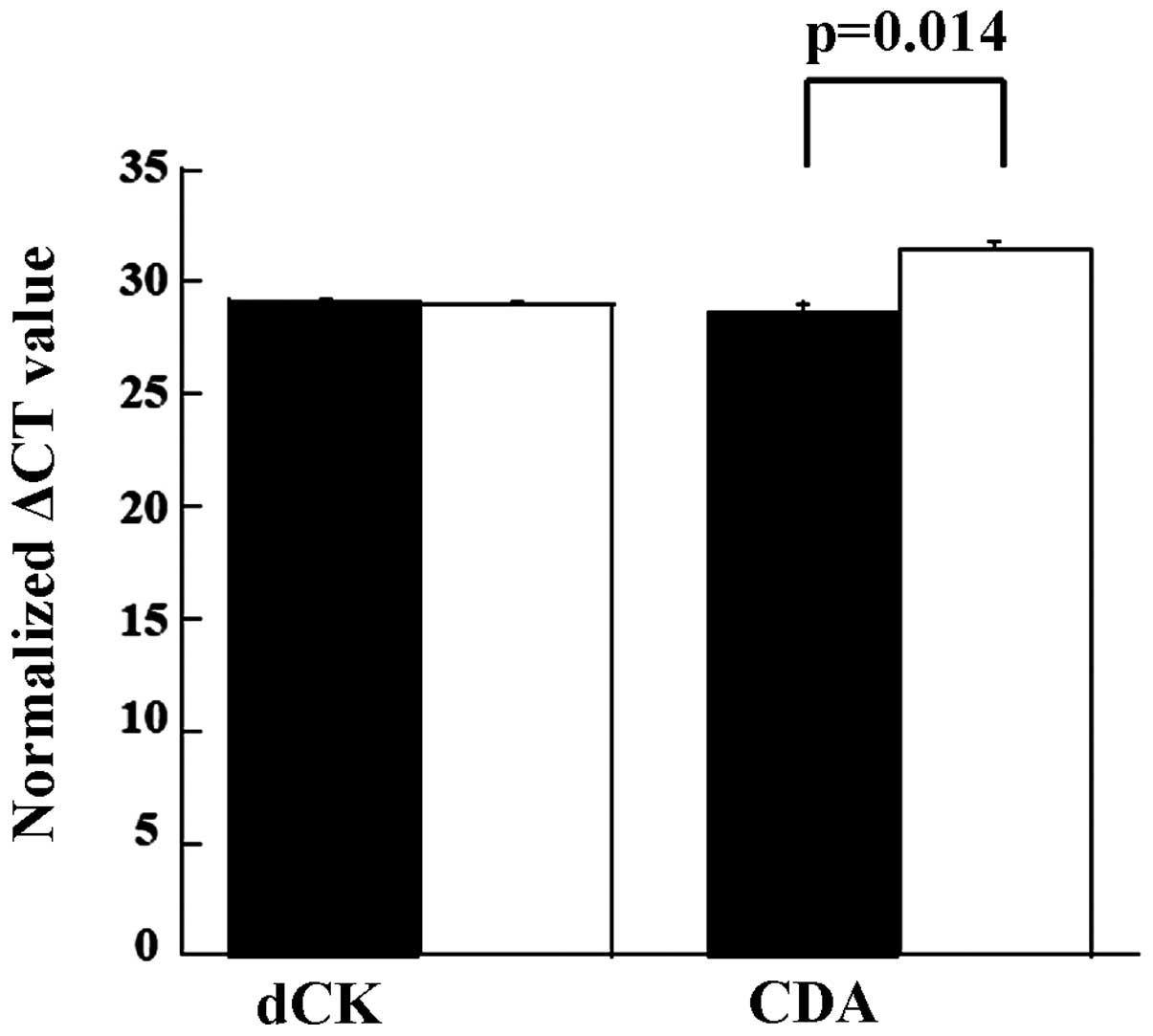
Quantification of transcripts of
deoxycytidine kinase (dCK) and cytidine deaminase (CDA)
GEM is a prodrug and confers toxicity only after it
is incorporated into DNA. When taken up by cells, the agent is
metabolized by cellular enzymes, among which the activities of
deoxycytidine kinase (dCK) and cytidine deaminase (CDA) are the
major contributors to chemosensitivity. The quantities of dCK and
CDA in the TK and BXPC3 cells were measured (Fig. 4). When compared, levels of the dCK
transcript were almost equal. However, the level of CDA in the TK
cell line was lower than that in the BXPC cell line (P<0.02)
(ΔΔCt TK, 28.6 vs. BXPC3, 31.4). Transcripts of dCMP deaminase,
which also inactivate dFdCMP, were almost equal in both cell lines
(ΔΔCt TK, 31.9 vs. BXPC3, 32.2).
Sensitization of the cell lines by forced
expression of the dCK enzyme
In the pancreatic tumor cells, an increase in dCK
improved the efficacy of GEM (18).
The effects of transduction of dCK on sensitivity to GEM were
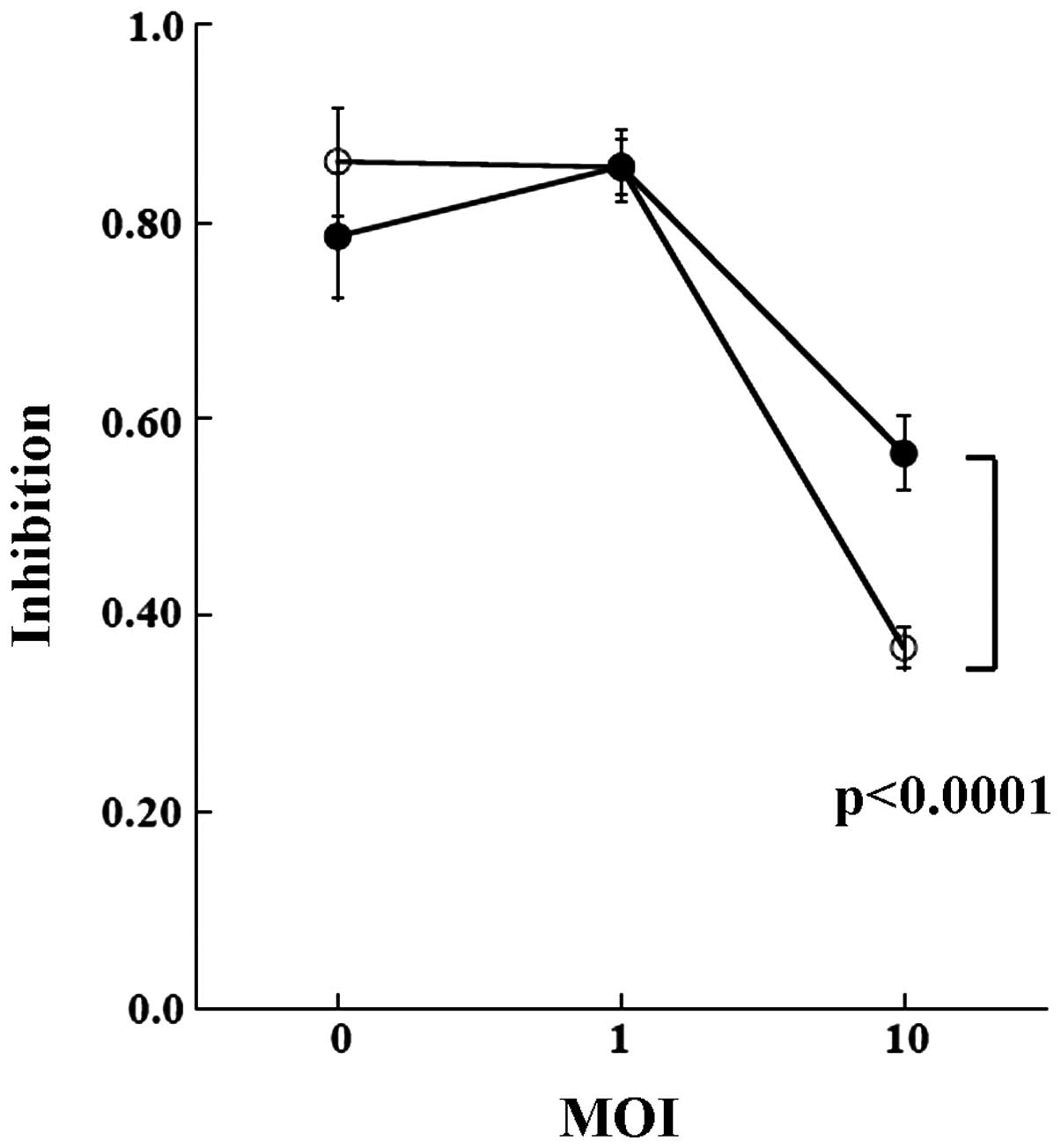
measured (Fig. 5). At viral
infection of MOI 0 and 1, overexpression of dCK did not alter the
sensitivity to the drug in either cell line. When MOI was increased
to 10, dCK conferred sensitivity to GEM. While both TK and BXPC3
cells were sensitized by the infection, the effect was marginally
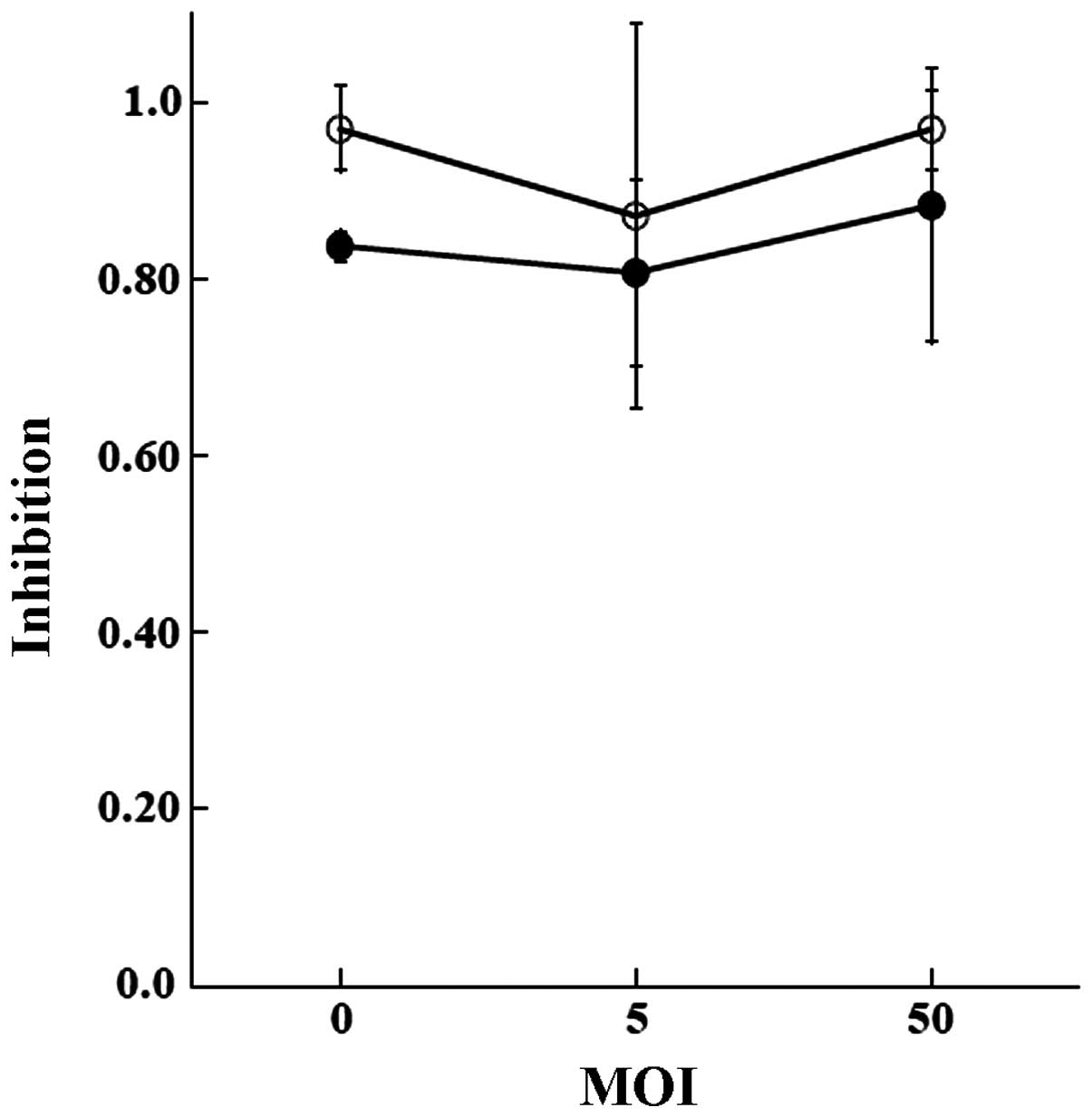
higher in the BXPC3 cells. Transduction of the control GFP did not
influence the sensitivities (Fig.
6).
Discussion
In the present study, the pharmacological response
to GEM of the cholangiocarcinoma TK cell line was evaluated. GEM is
a representative chemotherapeutic agent used to treat pancreatic
carcinoma. The drug is also used to treat cholangiocarcinoma as the
agent of first choice. Since GEM has a potent cytotoxic effect and
its mechanism of action is relatively well understood, methods to
increase its efficacy have been extensively studied. TK and BXPC3
cells were used to compare the effects of GEM on the cell cycle,
drug sensitivity, and levels of transcripts of key enzymes. In
addition, the effects of dCK transduction were addressed.
Cholangiocarcinoma is a refractory disease. More
than half of all patients are inoperable when diagnosed (19). Unresectable or metastatic lesions
are treated with chemotherapeutic agents (2). However, the effect has been limited
and in this situation, the development of more effective adjuvant
therapy is needed (3,4).
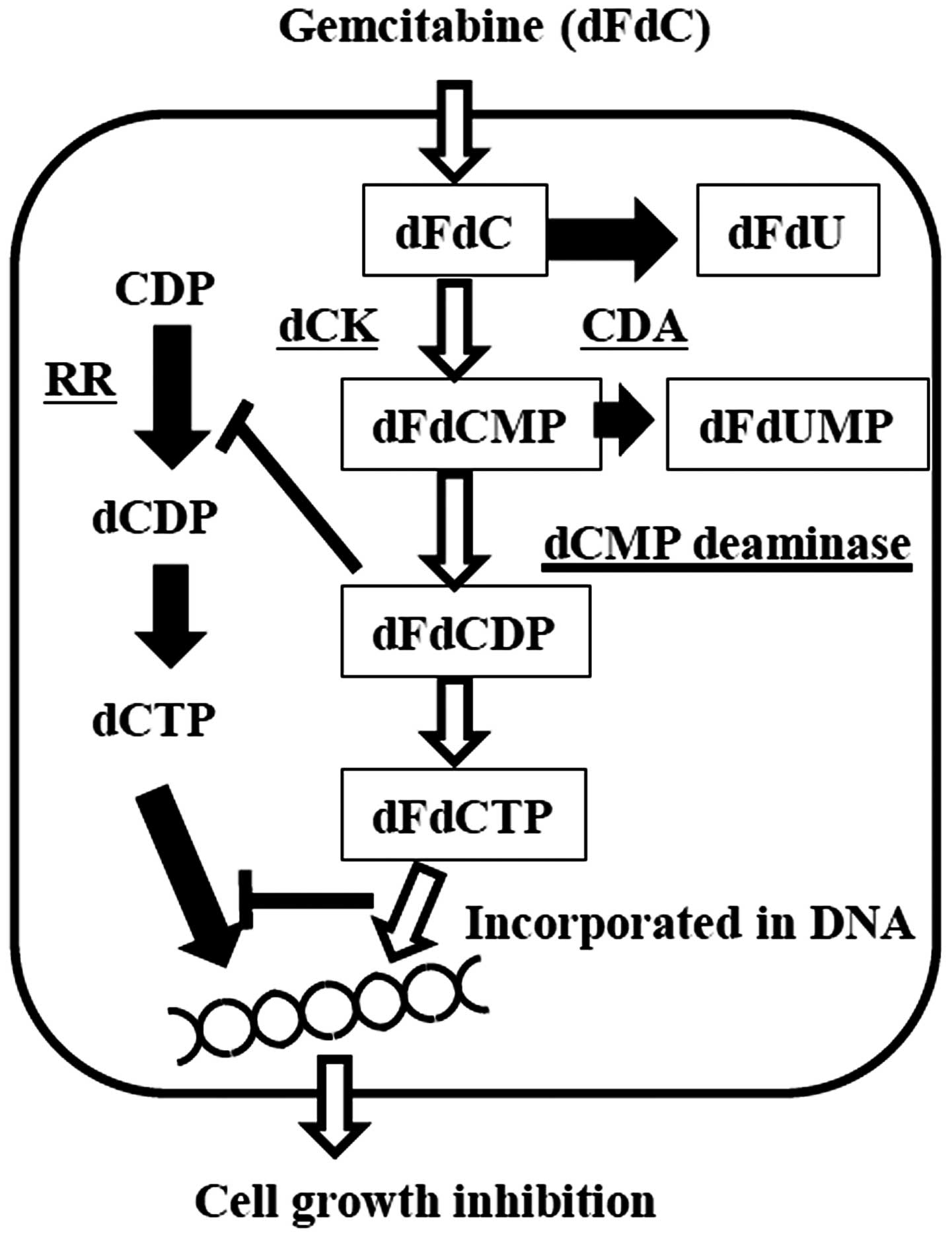
GEM (dFdC) is an analog of deoxycytidine and when
entering target cells, the drug is phosphorylated to gemcitabine
monophosphate (dFdCMP), gemcitabine diphosphate (dFdCDP), and then
gemcitabine triphosphate (dFdCTP) by corresponding enzymes. After
conversion, dFdCTP is incorporated into DNA by competing with
deoxycytidine triphosphate (dCTP). When integrated into DNA, it
terminates further chain elongation and causes apoptosis (4). In this sequence, phosphorylation of
GEM to dFdCMP by deoxycytidine kinase (dCK) is a rate-limiting
reaction. Furthermore, cytidine deaminase (CDA) abolishes the
cytotoxicity by deaminating GEM (Fig.
7). GEM and dFdCMP are deaminated by cytidine deaminase (CDA)
and dCMP deaminase, and become the inactive products,
2′,2′-difluorodeoxyuridine (dFdU) and 2′,2′-difluorodeoxyuridine
monophosphate (dFdUMP).
Multiple mechanisms potentiate the activity of GEM
both by increased formation of active dFdCDP and dFdCTP, and
decreased elimination of GEM. For example, dFdCDP itself inhibits
ribonucleotide reductase (RR) and depletes the deoxyribonucleotide
pool that is available for DNA synthesis and repair. A decreased
concentration of dCTP activates dCK, which accelerates the
phosphorylation of GEM. Furthermore, a decreased concentration of
intracellular dCTP inhibits dCMP deaminase and increases the
concentration of dFdCTP (20).
The incidence of cholangiocarcinoma differs in each
region and country, but overall, this disease accounts for only ~3%
of gastrointestinal system malignancies. The low incidence of the
disease, the diversity of the patients and tumor progression affect
clinical trial enrollments and retard the development of effective
treatments for cholangiocarcinoma (2).
Methods to increase the sensitivity to GEM have been
investigated using pancreatic carcinoma cell lines. Funamizu et
al demonstrated the effects of transduction of dCK in
activating GEM and tetrahydrouridine (THU), which inhibits the
action of CDA in causing GEM resistance (15,18).
Nakahira et al reported that inhibition of the
ribonucleotide reductase M1 subunit (RRM1) of a GEM-resistant cell
line by siRNA improved the effect of GEM (21).
Ohhashi et al demonstrated that inhibition of
dCK by siRNA decreased the sensitivity to GEM, and the inhibition
of RRM1 and RRM2 by siRNA increased the sensitivity to GEM
(22). Duxbury et al also
demonstrated that systemic administration of siRNA for RRM2
decreased the expression of RRM2 in an implanted tumor of
pancreatic adenocarcinoma and by combination with GEM treatment,
inhibited tumor growth, increased apoptotic cells and the
inhibition of metastasis using a nude mouse xenograft model
(23).
In contrast to pancreatic carcinoma, only a small
number of studies on cholangiocarcinoma have been reported and this
might be due to the limited number of suitable cell lines. Ohtaka
et al demonstrated that inhibition of RRM1 by siRNA
increased sensitivity to GEM and induced apoptosis in a gallbladder
carcinoma cell line (4). Faris and
Zhu investigated the combination of GEM and a molecular targeting
agent (2). Other studies aimed to
explore the factors for predicting the effect of gemcitabine-based
chemotherapies (3,5,24,25).
In the present study, we attempted to demonstrate
that the TK cell line might be useful for further studies.
Transcripts of dCK and CDA in the TK and pancreatic BXPC3 cell
lines were compared in the study and the level of dCK transcript
was comparable in both cell lines. The sensitivity of TK cells to
GEM was almost equal to that of the BXPC3 cell line. Similar to
pancreatic adenocarcinoma, patients are treated by GEM and the
sensitivity as well as the localization and blood supply of
cholangiocarcinoma would provide an adequate justification for
using GEM for the treatment of cholangiocarcinoma.
In pancreatic carcinoma, levels of dCK expression
determine the prognosis of patients (26) and an increase in dCK might directly
improve the sensitivity to GEM (18,27).
Our result demonstrated that the level of the dCK transcript in TK
cells was almost the same as that in the BXPC3 cells (Fig. 4) and adenoviral transduction of dCK
was able to increase the sensitivity to the drug (Fig. 5)
Alternatively, CDA abolishes the efficacy of GEM by
deamination of the agent. The level of transcripts of CDA was lower
in the TK cell line than in the BXPC cell line. Higher sensitivity
due to adenoviral transduction of dCK was expected in the TK cell
line. However, our result demonstrated that BXPC3 cells were more
sensitized by the procedure. The result itself might be
controversial, but many factors, such as RR subunit M1, RR subunit
M2, the RR subunit p53R2 gene, and the human equilibrative
nucleotide transporter 1 (hENT1) are also important for GEM
sensitivity (3–5,21,24,25,28).
In addition, human equilibrative nucleotide
transporter 2 (hENT2), human concentrative nucleotide transporter 1
(hCNT1), and human concentrative nucleotide transporter 3 (hCNT3)
are responsible for the uptake of GEM, and multidrug
resistance-associated protein 7 (MRP7) is related to excretion. All
of these factors are associated with the sensitivity of cells to
GEM. To understand the mechanisms of these actions, further study
is required using clinical samples and/or more cell lines (3).
The effects of GEM on cholangiocarcinoma have been
addressed using the TK cell line. Hence, this cell line may have a
role for further investigation of GEM sensitivity in relation to
cholangiocarcinoma chemotherapy.
Acknowledgements
The authors thank Mr. Chiaki Kuriyama, Ms. Nanami
Takatsuki, Airi Kugisaki, Keiko Tomaru and Mayumi Nomura of the
Jikei University School of Medicine for their expert technical
assistance.
References
|
1
|
Kumagai S, Kurumatani N, Arimoto A and
Ichihara G: Cholangiocarcinoma among offset colour proof-printing
workers exposed to 1,2-dichloropropane and/or dichloromethane.
Occup Environ Med. 70:508–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Faris JE and Zhu AX: Targeted therapy for
biliary tract cancers. J Hepatobiliary Pancreat Sci. 19:326–336.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sato J, Kimura T, Saito T, et al: Gene
expression analysis for predicting gemcitabine resistance in human
cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 18:700–711. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohtaka K, Kohya N, Sato K, Kitajima Y, Ide
T, Mitsuno M and Miyazaki K: Ribonucleotide reductase subunit M1 is
a possible chemoresistance marker to gemcitabine in biliary tract
carcinoma. Oncol Rep. 20:279–286. 2008.PubMed/NCBI
|
|
5
|
Borbath I, Verbrugghe L, Lai R, Gigot JF,
Humblet Y, Piessevaux H and Sempoux C: Human equilibrative
nucleoside transporter 1 (hENT1) expression is a potential
predictive tool for response to gemcitabine in patients with
advanced cholangiocarcinoma. Eur J Cancer. 48:990–996. 2012.
View Article : Google Scholar
|
|
6
|
Meng F, Henson R, Lang M, et al:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pignochino Y, Sarotto I, Peraldo-Neia C,
et al: Targeting EGFR/HER2 pathways enhances the antiproliferative
effect of gemcitabine in biliary tract and gallbladder carcinomas.
BMC Cancer. 10:6312010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saito S, Ghosh M, Morita K, Hirano T, Miwa
M and Todoroki T: The genetic differences between gallbladder and
bile duct cancer cell lines. Oncol Rep. 16:949–956. 2006.PubMed/NCBI
|
|
9
|
Selaru FM, Olaru AV, Kan T, et al:
MicroRNA-21 is overexpressed in human cholangiocarcinoma and
regulates programmed cell death 4 and tissue inhibitor of
metalloproteinase 3. Hepatology. 49:1595–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mott JL, Kobayashi S, Bronk SF and Gores
GJ: mir-29 regulates Mcl-1 protein expression and apoptosis.
Oncogene. 26:6133–6140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe M, Chigusa M, Takahashi H,
Nakamura J, Tanaka H and Ohno T: High level of CA19-9, CA50, and
CEA-producible human cholangiocarcinoma cell line changes in the
secretion ratios in vitro or in vivo. In Vitro Cell Dev Biol Anim.
36:104–109. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akiyoshi K, Kamada M, Akiyama N, et al:
Morphological study of cholangiocarcinoma cell line, TK with
three-dimensional cell culture. Mol Med Rep. 9:1359–1364.
2014.PubMed/NCBI
|
|
13
|
Manome Y, Wen PY, Dong Y, Tanaka T,
Mitchell BS, Kufe DW and Fine HA: Viral vector transduction of the
human deoxycytidine kinase cDNA sensitizes glioma cells to the
cytotoxic effects of cytosine arabinoside in vitro and in vivo. Nat
Med. 2:567–573. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamada M, Ikeda K, Fujioka K, et al:
Expression of mRNAs of urocortin and corticotropin-releasing factor
receptors in malignant glioma cell lines. Anticancer Res.
32:5299–5307. 2012.PubMed/NCBI
|
|
15
|
Funamizu N, Lacy CR, Fujita K, Furukawa K,
Misawa T, Yanaga K and Manome Y: Tetrahydrouridine inhibits cell
proliferation through cell cycle regulation regardless of cytidine
deaminase expression levels. PLoS One. 7:e374242012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki R, Kojima H, Moriyama H and Manome
Y: Utilization of caspase-14 promoter for selective transgene
expression in squamous layers of cholesteatoma in the middle ear. J
Intl Adv Otol. 8:21–29. 2012.
|
|
17
|
Manome Y, Wen PY, Chen L, et al: Gene
therapy for malignant gliomas using replication incompetent
retroviral and adenoviral vectors encoding the cytochrome P450 2B1
gene together with cyclophosphamide. Gene Ther. 3:513–520.
1996.
|
|
18
|
Funamizu N, Okamoto A, Kamata Y, et al: Is
the resistance of gemcitabine for pancreatic cancer settled only by
overexpression of deoxycytidine kinase? Oncol Rep. 23:471–475.
2010.PubMed/NCBI
|
|
19
|
de Marsh RW, Alonzo M, Bajaj S, et al:
Comprehensive review of the diagnosis and treatment of biliary
tract cancer 2012. Part I: diagnosis-clinical staging and
pathology. J Surg Oncol. 106:332–338. 2012.PubMed/NCBI
|
|
20
|
Yonemori K, Ueno H, Okusaka T, et al:
Severe drug toxicity associated with a single-nucleotide
polymorphism of the cytidine deaminase gene in a Japanese cancer
patient treated with gemcitabine plus cisplatin. Clin Cancer Res.
11:2620–2624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakahira S, Nakamori S, Tsujie M, et al:
Involvement of ribonucleotide reductase M1 subunit overexpression
in gemcitabine resistance of human pancreatic cancer. Int J Cancer.
120:1355–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohhashi S, Ohuchida K, Mizumoto K, et al:
Down-regulation of deoxycytidine kinase enhances acquired
resistance to gemcitabine in pancreatic cancer. Anticancer Res.
28:2205–2212. 2008.PubMed/NCBI
|
|
23
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: RNA interference targeting the M2 subunit of
ribonucleotide reductase enhances pancreatic adenocarcinoma
chemosensitivity to gemcitabine. Oncogene. 23:1539–1548. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kobayashi H, Murakami Y, Uemura K, Sudo T,
Hashimoto Y, Kondo N and Sueda T: Human equilibrative nucleoside
transporter 1 expression predicts survival of advanced
cholangiocarcinoma patients treated with gemcitabine-based adjuvant
chemotherapy after surgical resection. Ann Surg. 256:288–296. 2012.
View Article : Google Scholar
|
|
25
|
Murata A, Amano R, Yamada N, Kimura K,
Yashiro M, Nakata B and Hirakawa K: Prognostic predictive values of
gemcitabine sensitivity-related gene products for unresectable or
recurrent biliary tract cancer treated with gemcitabine alone.
World J Surg Oncol. 11:1172013. View Article : Google Scholar
|
|
26
|
Sebastiani V, Ricci F, Rubio-Viqueira B,
et al: Immuno-histochemical and genetic evaluation of deoxycytidine
kinase in pancreatic cancer: relationship to molecular mechanisms
of gemcitabine resistance and survival. Clin Cancer Res.
12:2492–2497. 2006. View Article : Google Scholar
|
|
27
|
Hapke D, Stegmann A and Mitchell B:
Retroviral transfer of deoxycytidine kinase into tumor cell lines
enhances nucleoside toxicity. Cancer Res. 56:2343–2347.
1996.PubMed/NCBI
|
|
28
|
Mori R, Ishikawa T, Ichikawa Y, et al:
Human equilibrative nucleoside transporter 1 is associated with the
chemosensitivity of gemcitabine in human pancreatic adenocarcinoma
and biliary tract carcinoma cells. Oncol Rep. 17:1201–1205.
2007.
|