Introduction
Nasopharyngeal carcinoma (NPC), a malignant tumor
arising from the epithelium of the nasopharynx, has a unique
geographical and ethnic distribution. As one of the most common
cancers found among individuals of Chinese or Asian ancestry, it
poses one of the most serious health problems in southern China
where an annual incidence of more than 20 cases per 100,000 is
reported (1). A number of studies
indicate that the etiology of NPC is associated with multiple
factors, including genetics, Epstein-Barr virus and environment
(2). Patients with NPC tend to
present at a more advanced stage of disease as the primary
anatomical site of tumor growth is located in a silent area, and
NPC exhibits higher metastatic potential when compared with other
head and neck squamous cell carcinomas (3,4).
Despite being potentially curable at an early stage, more than 50%
of NPC patients present with advanced locoregional disease, which
results in a poor patient prognosis (5). Therefore, the screening and early
detection in a population at risk have been proposed to decrease
both the morbidity and the mortality associated with NPC.
T lymphoma invasion and metastasis 1 (Tiam1), a
specific guanine nucleotide exchange factor (GEF) for Rac1 and an
important member of the Rho GTPase family, was first identified as
a gene which might induce invasion and metastasis by proviral
tagging, in combination with in vitro selection for
invasiveness in T lymphoma cells (6). The role of Tiam1 in cellular
migration, invasion and metastasis may not be limited to T
lymphoma. It has been reported to be significant in promoting tumor
progression in a variety of cancers, such as breast, colorectal,
lung, liver cancer and Ras-induced skin tumors (7–11).
Recent study indicates that Tiam1 has different effects on various
types of cancers. Uhlenbrock et al (12) found that Tiam1 inhibited the
migration and invasion of metastatic melanoma via a novel adhesive
mechanism. However, little is known concerning the potential
prognostic value and molecular mechanisms of Tiam1 in NPC.
In the present study, we aimed to investigate the
endogenous expression of Tiam1 in primary NPC tissues and cell
lines and to identify the relationship between Tiam1 expression and
clinicopathological features, including the survival of patients
with NPC. Furthermore, we observed the effects of intentionally
regulated changes in Tiam1 expression levels on the in vivo
and in vitro functions of NPC cells. The present study aimed
to evaluate the prognostic value of Tiam1, in regards to patient
survival in NPC and to explore the essential role of Tiam1 in tumor
cell growth, migration, invasion and metastasis in NPC.
Materials and methods
Patients and tissue specimens
A total of 140 previously untreated patients with
NPC, who were histologically and clinically diagnosed at the
Nanfang Hospital, Southern Medical University, China, between 1998
and 2000, were enrolled in the present study. For the use of these
clinical materials for research purposes, prior patient consent and
approval from the Ethics Committee of the Southern Medical
University were obtained. The stage of cancer was defined according
to the 1992 Fuzhou NPC staging system of China. Clinical
information concerning the samples is described in detail in
Table I. The patients included 104
males and 36 females, ranging in age from 20 to 74 years (mean,
48.3 years). The median follow-up time for overall survival was
41.2 months for patients still alive at the time of analysis, and
ranged from 1.83 to 80.6 months. In addition, 30 patients with
chronic nasopharyngitis were used as the control.
 | Table ICorrelation between the
clinicopathological features and expression of Tiam1 protein. |
Table I
Correlation between the
clinicopathological features and expression of Tiam1 protein.
| | Tiam1 (%) | |
|---|
| |
| |
|---|
|
Characteristics | n | Negative | Positive | P-value |
|---|
| Gender |
| Male | 104 | 29 (27.88) | 75 (72.12) | 0.125 |
| Female | 36 | 15 (41.67) | 21 (58.33) | |
| Age (years) |
| ≤45 | 65 | 21 (32.31) | 44 (67.69) | 0.464 |
| >45 | 75 | 20 (26.67) | 55 (73.33) | |
| Pathological
classification |
| Type II | 131 | 36 (27.48) | 95 (72.52) | |
| Type III | 9 | 5 (55.56) | 4 (44.44) | 0.073 |
| T
classification |
|
T1–T2 | 83 | 28 (33.73) | 55 (66.27) | |
|
T3–T4 | 57 | 13 (22.81) | 44 (77.19) | 0.163 |
| N
classification |
| N0 | 91 | 34 (37.36) | 57 (62.64) | |
|
N1–N3 | 49 | 7 (14.29) | 42 (85.71) | 0.004 |
| Distant
metastasis |
| Yes | 15 | 1 (6.67) | 14 (93.33) | |
| No | 125 | 40 (32.00) | 85 (68.00) | 0.042 |
| Clinical stage |
| I–II | 60 | 23 (38.33) | 37 (61.67) | |
| III–IV | 80 | 18 (22.50) | 62 (77.50) | 0.042 |
Cell lines and animals
The human NPC cell lines, CNE1, CNE2, HONE1 and
C666–1, were obtained from the Cancer Institute, Southern Medical
University (Guangzhou, China). 5–8F (high tumorigenic and
metastatic ability) and 6–10B (tumorigenic, but lacking metastatic
ability) cells from colony lines of the NPC SUNE1 cell line
(13) were provided by the Cancer
Center of Sun Yet-Sen University (Guangzhou, China). These cell
lines were maintained in RPMI-1640 medium (Gibco, Grand Island, NY,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS) and 100 U/ml of penicillin/streptomycin at 37°C in a
humidified atmosphere of 5% CO2 in air. The 293T cells
were cultured in Dulbecco’s modified Eagle’s medium (Gibco). Female
nude mice (6- to 8-weeks old and 16–21 g in weight) were purchased
from the Central Laboratory of Animal Science, Southern Medical
University. All animals were housed under standard pathogen-free
conditions. All animal experiments were performed with approval
from the Institutional Animal Ethics Committees of the Southern
Medical University.
Immunohistochemistry (IHC)
IHC staining was performed to study altered protein
expression in 140 human NPC and 30 chronic nasopharyngitis tissues
using a Dako EnVision System (Dako, Carpinteria, CA, USA) following
the manufacturer’s recommended protocol. The procedures were
carried out with standard methods. Briefly, all paraffin sections,
4 μm in thickness, were baked for 1 h at 65°C. Sections were
deparaffinized with xylene and rehydrated with graded ethanol to
distilled water. Sections were submerged in EDTA antigenic
retrieval buffer (pH 8.0) and subjected to microwave treatment.
After being treated with 0.3% H2O2 for 15 min
to block the endogenous peroxidase, the sections were treated with
1% bovine serum albumin for 30 min to reduce non-specific binding,
and then rabbit polyclonal anti-Tiam1 antibody (1:200; Santa Cruz
Biotechnology) was incubated with the sections overnight at 4°C.
After washing, the sections were incubated with HRP at 4°C for 30
min. For color reactions, diaminobenzidine (DAB) was used. For
negative controls, the antibody was replaced by normal goat
serum.
Evaluation of staining
The immunohistochemically stained tissue sections
were reviewed and scored separately by two pathologists blinded to
the clinical parameters. For Tiam1 assessment, the entire tissue
section was scanned to assign the scores. The staining intensity
was scored as 0 (negative), 1 (weak), 2 (medium) and 3 (strong).
The extent of staining was scored as 1 (0%), 1 (1–25%), 2 (26–50%),
3 (51–75%) and 4 (76–100%) according to the percentages of the
positive staining areas in relation to the entire
carcinoma-involved area or the entire section for the normal
samples. The sum of the intensity and extent score was used as the
final staining score (0–7) for Tiam1. This relatively simple,
reproducible scoring method provides highly concordant results
between independent evaluators and has been used in previous
studies (14–16). Tumors having a final staining score
of 3 or higher were considered to be positive.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
According to the manufacturer’s instructions, total
RNA from the cell lines was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). DNase I-treated total RNA (~1 μg)
from each sample was used to synthesize cDNA using SuperScript II
Reverse Transcriptase (Invitrogen). The segment of the Tiam1 gene
was PCR amplified from cDNA samples of NPC cell lines. The
following primers were used for amplification of Tiam1 (GeneBank,
NM_003253): sense primer, 5′-AAGACGTACTCAGGCCATGTCC-3′ and
antisense primer, 5′-GACCCAAATGTCGCAGTCAG-3′.
Glyceraldehyde-3-phosphate dehydrogenase was amplified as an
internal control using sense primer, 5′-AATCCCATCACC ATCTTCCA-3′
and antisense primer, 5′-CCTGCTTCACCA CCTTCTTG-3′. The appropriate
size of the PCR products was confirmed by agarose gel
electrophoresis. 1-D Advanced software (Eastman-Kodak, Co.,
Stamford, CT, USA) was used to calculate the ratios of the
densitometry of the Tiam1 band to the GAPDH band, indicating the
relative Tiam1 expression levels.
Immunofluorescence staining
Cells were cultured on coverslips overnight and
fixed with 4% paraformaldehyde for 20 min and treated with 0.25%
Triton X-100 for 10 min. After blocking in 10% normal blocking
serum at room temperature for 10 min, slides were incubated with
the anti-Tiam1 antibody (Santa Cruz Biotechnologies) at 4°C
overnight and then washed with phosphate-buffered saline (PBS)
three times. Coverslips were then incubated with FITC-conjugated
anti-rabbit secondary antibodies (Invitrogen) for 30 min at room
temperature, followed by staining with
4′,6-diamidino-2-phenylindole (DAPI; Invitrogen).
Western blot analysis
Cells were washed twice with cold PBS and lysed on
ice in RIPA buffer (1× PBS, 1% NP-40, 0.1% SDS, 5 mM EDTA, 0.5%
sodium deoxycholate and 1 mM sodium orthovanadate) with protease
inhibitors. The protein concentration was determined by the
Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal
amounts of proteins were separated electrophoretically on 12%
SDS/polyacrylamide gels and transferred onto polyvinylidene
difluoride (PVDF) membranes (Amersham Pharmacia Biotech,
Piscataway, NJ, USA) and blocked in 5% non-fat dry milk in
Tris-buffered saline (TBST) (pH 7.5; 100 mM NaCl, 50 mM Tris and
0.1% Tween-20). Membranes were immunoblotted with the anti-Tiam1
polyclonal antibody and the anti-GAPDH antibody (Santa Cruz
Biotechnology) overnight at 4°C, followed by their respective
secondary antibodies conjugated to horseradish peroxidase (HRP).
The signals were detected by enhanced chemiluminescence (ECL;
Pierce, Rockford, IL, USA).
Plasmid preparation
For construction of the Tiam1-knockdown vectors in
mammalian cells, four human siRNAs (shRNA1, CTCAGAGCATCCACATTGA;
shRNA2, GGA GATGAGATTCTTGAGA; shRNA3, CGGAAATGGTAGA GTTTCA; and
shRNA4, GCAGCAAGAGTACAGAACA) that targeted Tiam1 were selected, and
a human scrambled siRNA sequence (5′-TTCTCCGAACGTGTCACGT-3′)
possessing limited homology to human genes served as a negative
control (17). The siRNAs were
synthesized and subcloned into the pGC-LV vector by double
digestion with AgeI and EcoRI to form VshRNA (pGC-LV
recombination vector) in accordance with the manufacturer’s
guidelines (Shanghai GeneChem, Co., Ltd., Shanghai, China). The
correct coding regions of all plasmids were confirmed by
sequencing. In addition, the overexpression vector of Tiam1,
Tiam1/C1199HA plasmids, was a gift from Professor J.G. Collard
(6).
Stable transfections regulate the
expression of the Tiam1 gene
Cells were seeded onto a 6-well plate 16 h before
transfection. Transfection assays were performed by Lipofectamine
2000 according to the manufacturer’s instructions (Invitrogen).
Tiam1/C1199HA vector and pcDNA3.1 vector were transfected into CNE2
cells, respectively. Lentiviruses containing the 4 Tiam1 shRNAs or
the negative control shRNA were produced by plasmid cotransfection
of 293T cells. The viral supernatants containing shRNA1, shRNA2,
shRNA3, shRNA4 and the negative control vector were added
respectively to the plates with CNE2 cells. The selections for
stable integrants were carried out by culturing cells with a
complete medium containing G418 (for overexpression) or blasticidin
(for knockdown). Tiam1 expression in the transfectants was verified
by both RT-PCR and western blot analysis.
In vitro cell growth assay
The cells were prepared at a concentration of
1×104 cells/ml, respectively. Aliquots (100 μl) were
dispensed into 96-well microtiter plates and cultured overnight.
The cells were cultured for 1 to 7 days. Each subsequent day, 20 μl
of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) (Sigma, St. Louis, MO, USA) was added to each well.
After being incubated for 4 h at 37°C, the supernatants were
carefully removed. One hundred and fifty microliters of dimethyl
sulfoxide (Sigma) was added to each well. Fifteen minutes later,
the absorbance value (OD) of each well was measured with a
microplate reader set at 570 nm. All experiments were performed in
triplicate.
Plate colony formation assay
Approximately 1×102 cells were added to
each well (3 cm in diameter) of a 6-well culture plate, and each
cell group contained three wells. After incubation at 37°C for 12
days, the cells were washed twice with PBS and stained with Giemsa
solution. The number of colonies containing ≥50 cells was counted
under a microscope. Plate colony formation efficiency = number of
colonies/number of cells inoculated × 100%.
Soft agar assay
Cells (1×104/ml, 20 μl) were seeded in
0.4% agar and incubated at 37°C for 14 days. Colonies that grew
>50 μm in diameter were counted. Colony formation efficiency =
number of colonies/number of cells inoculated × 100%. Each
experiment was carried out in triplicate.
In vitro invasion assay
Invasion assays were performed using a cell invasion
chamber (Chemicon, Temecula, CA, USA) according to the protocol of
the manufacturer. The cell invasion chamber contained a
polycarbonate membrane with an 8-μm pore size, over which a thin
layer of ECMatrix (Chemicon) was dried. The extracellular matrix
(ECM) layer occludes membrane pores, blocking non-invasive cells
from migrating. In brief, warm serum-free medium was added to the
top chamber of the cell invasion chamber to rehydrate the ECM layer
for 2 h at room temperature. Tumor cells in a serum-free medium
(300 μl containing 1×105 cells) were added to the top
chamber. The bottom chamber was prepared using 10% FBS as
chemoattactant. After incubation for 24 h, the non-invasive cells
were removed with a cotton swab. The cells that had migrated
through the membrane and had stuck to the lower surface of the
membrane were fixed with methanol and stained with hematoxylin. For
quantification, the cells were counted under a microscope in five
randomly selected fields (original magnification, ×200).
Cell adhesion assay
To investigate the ability of cell-matrix adhesion,
we analyzed the binding capacity of CNE2 cells to extracellular
matrix (ECM) by seeding cells on a fibronectin substrate. Cell
adhesion assay was performed as previously reported (18). Briefly, 96-well plates were firstly
coated with fibronectin (100 μl, 50 μg/ml; Sigma) for 2 h at 37°C.
Then, cells (2×104/well) were allowed to adhere to the
substrate for 2 h. After unattached cells were removed by gentle
washing with PBS, the adherent cells were rinsed, fixed with 4%
paraformaldehyde and quantified by 1% crystal violet staining. The
OD value was measured at 570 nm. Every group had 5 repeated
wells.
Wound healing assay
Cells were cultured under standard conditions, as
previously described, until 70–80% confluency. Cells were grown to
~90% confluency in 6-well plates. Scratches of 1 mm were
constructed with the tip of a micropipette, followed by addition of
fresh serum-containing medium. Phase-contrast microscopic images
were captured 0–30 h after the scratches were made in order to
observe their closure. Migration was quantified by counting the
number of cells that had moved across the starting line.
In vivo metastasis assays
The cells were first harvested by trypsinization,
washed thrice with cold serum-free medium, and resuspended with
serum-free medium. Six-week-old female nude mice were injected
respectively with the differently treated cells, including the
Tiam1-overexpressing cells, Tiam1 shRNA cells and the corresponding
control cells, via the lateral tail vein (n=9 per group). The mice
were sacrificed 9 weeks later when cachexia appeared in some of the
animals. All mice were dissected and/or observed with GFP imaging
system. The organs were collected for histological analysis of
metastases. The number of metastatic nodules per organ was counted
under a dissecting microscope.
Statistical analysis
All statistical analyses were performed by SPSS 13.0
for Windows (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test
was used to analyze the relationship between Tiam1 expression and
clinicopathological characteristics. Survival curves were plotted
by the Kaplan-Meier method and compared by the log-rank test. The
significance of various variables for survival was analyzed by the
Cox proportional hazards model in the multivariate analysis.
Differences between groups were analyzed by a one-way analysis of
variance (ANOVA) or a Student’s t-test; multiple comparison of the
mean was performed by variance analysis; bivariate relevant
information was analyzed using correlation coefficient. P<0.05
in all cases was considered to indicate a statistically significant
result.
Results
Tiam1 is overexpressed in NPC
tissues
To investigate the correlation of Tiam1 expression
with human NPC, we first analyzed Tiam1 protein expression in 140
paraffin-embedded, archival NPC tissues by immunohistochemical
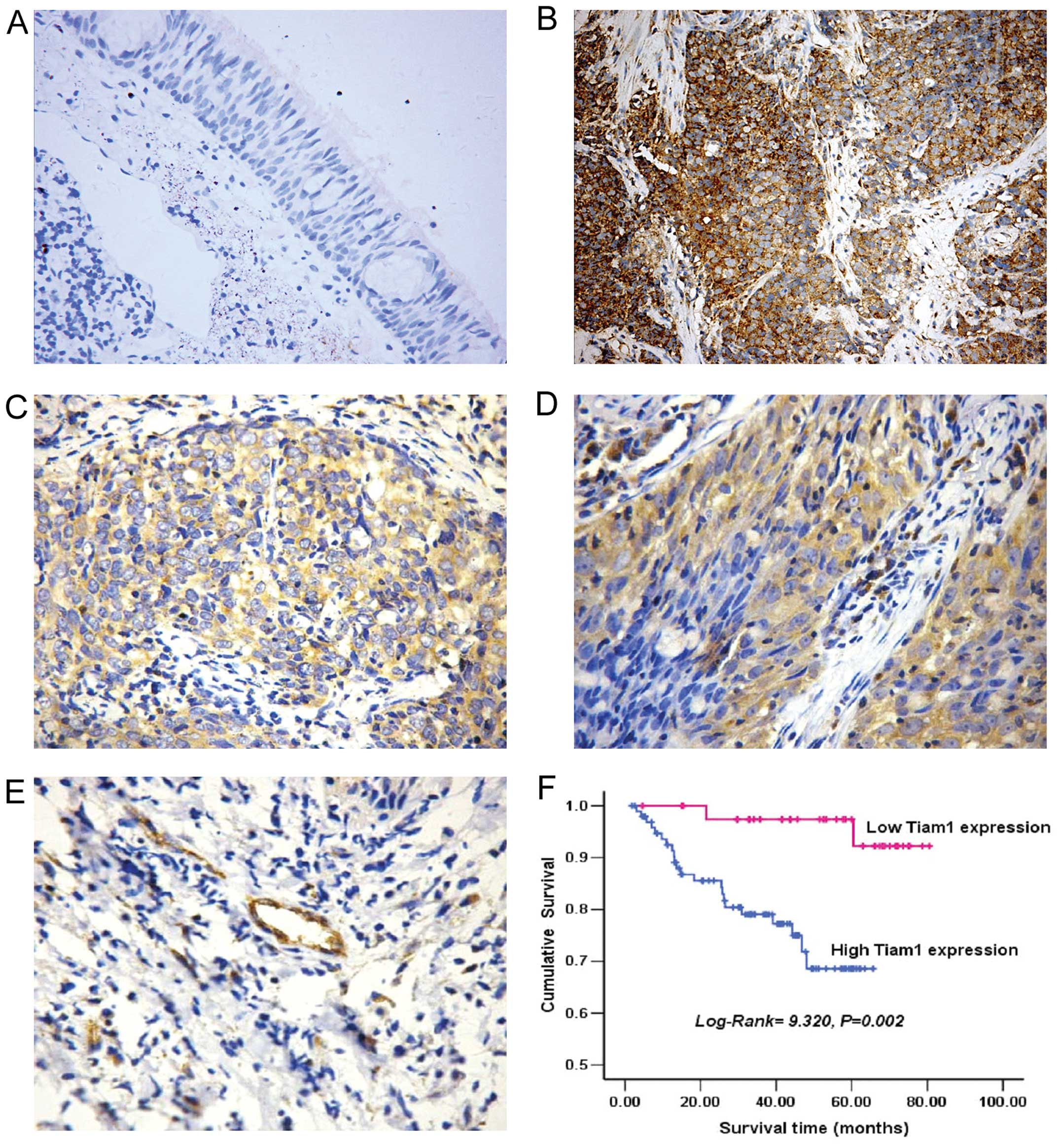
staining. As shown in Fig. 1, Tiam1
staining was mainly localized to the cytoplasm and the cytoplasmic
membrane of carcinoma (Fig. 1B–D)
and endothelial cells (Fig. 1E).
Overexpression of Tiam1 protein was detected in 96 of 140 (68.6%)
NPC tissues, compared with 14 of 30 (46.67%) normal nasopharynx
tissues. A significant difference in overexpression was found for
tumor and normal tissue samples (P=0.023).
Increased expression of Tiam1 correlates
with progression of NPC
The association between Tiam1 expression and
clinicopathological characteristics of tumors was examined. As
summarized in Table I, there was no
significant correlation between Tiam1 expression and gender, age,
pathological classification or T classification (P>0.05).
However, Tiam1 expression was positively correlated with N
classification (P=0.004), distant metastasis (P=0.042) and clinical
stage (P=0.042).
Tiam1 expression is inversely correlated
with prognosis in NPC patients
To investigate the prognostic value of Tiam1 for
NPC, we evaluated the association between Tiam1 expression and
survival duration using Kaplan-Meier analysis with the log-rank
test. Tiam1 expression in NPC was significantly correlated with
overall survival (P=0.002) (Fig.
1F). The log-rank test showed that the survival time was
significantly different between groups with high and low expression
of Tiam1, indicating that the high level of Tiam1 correlated with
shorter survival time.
To determine whether the expression of Tiam1 is an
independent prognostic factor of outcomes, multivariate survival
analysis, which included gender, age, T classification, N
classification, clinical stage and Tiam1 expression, was carried
out. Results showed that the expression of Tiam1 protein had a
significant correlation with prognosis and was an independent
prognostic factor of outcomes of NPC (Table II).
 | Table IISummary of overall survival analyses
by univariate and multivariate COX regression analysis. |
Table II
Summary of overall survival analyses
by univariate and multivariate COX regression analysis.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Parameters | P-value | HR | 95% CI | P-value | HR | 95% CI |
|---|
| Age (years) |
| >45 vs.
≤45 | 0.463 | 1.364 | 0.596–3.124 | | | |
| Gender |
| Male vs.
female | 0.104 | 0.367 | 0.109–1.230 | | | |
| Histologic
classification |
| Type II vs. type
III | 0.185 | 0.441 | 0.131–1.479 | | | |
| T
classification |
|
T1–T2 vs.
T3–T4 | 0.025 | 2.582 | 1.129–5.903 | 0.666 | 0.809 | 0.309–2.117 |
| N
classification |
| N0 vs.
N1–N3 | 0.094 | 2.016 | 0.888–4.577 | | | |
| M
classification |
| M0 vs.
M1 | 0.000 | 5.728 | 2.250–14.585 | 0.013 | 3.395 | 1.295–8.897 |
| Clinical stage |
| I–II vs.
III–IV | 0.003 | 6.391 | 1.906–21.433 | 0.022 | 5.149 | 1269–20.890 |
| Tiam1
expression |
| Negative vs.
positive | 0.008 | 7.417 | 1.689–32.564 | 0.031 | 5.029 | 1.158–21.845 |
Analysis of the expression of Tiam1 in
NPC cell lines by RT-PCR and immunofluorescence staining
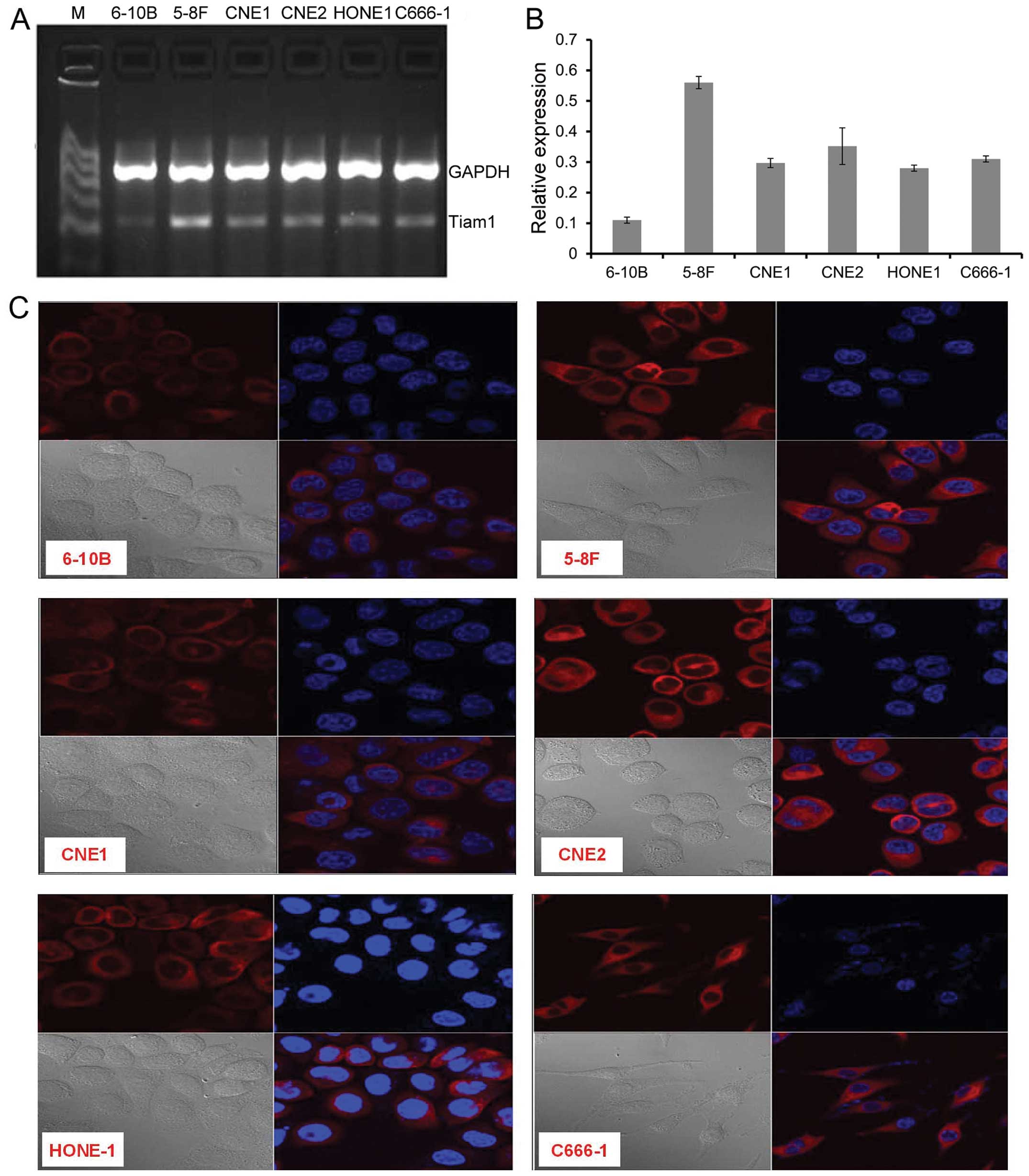
To investigate the levels of expression and
subcellular location of Tiam1 in NPC cell lines, RT-PCR and
immunofluorescence staining were performed. Tiam1 was highly
expressed in 5–8F cells, lowly expressed in 6–10B, and at a
moderate level in the other 4 NPC cell lines (Fig. 2A and B). Tiam1 protein was mainly
located in the perinuclear cytoplasm and cytoplasmic membrane of
the NPC cells (Fig. 2C). The
expression intensity of Tiam1 protein shown by immunofluorescence
staining in NPC cells was consistent with the results of
RT-PCR.
Alteration of Tiam1 expression affects
proliferation and colony formation of NPC cells in vitro
To further determine whether Tiam1 affects the
biological behaviors of NPC cells, we generated two cell clones
with stably overexpressed Tiam1 or with stably knocked down
endogenous Tiam1. CNE2 cells were stably transfected with
C1199/Tiam1 plasmids or infected with viral supernatants containing
shRNA, and then were transformed into CNE2/Tiam1+ and
CNE2/Tiam1down cells, respectively.
We first examined the effect of increased Tiam1
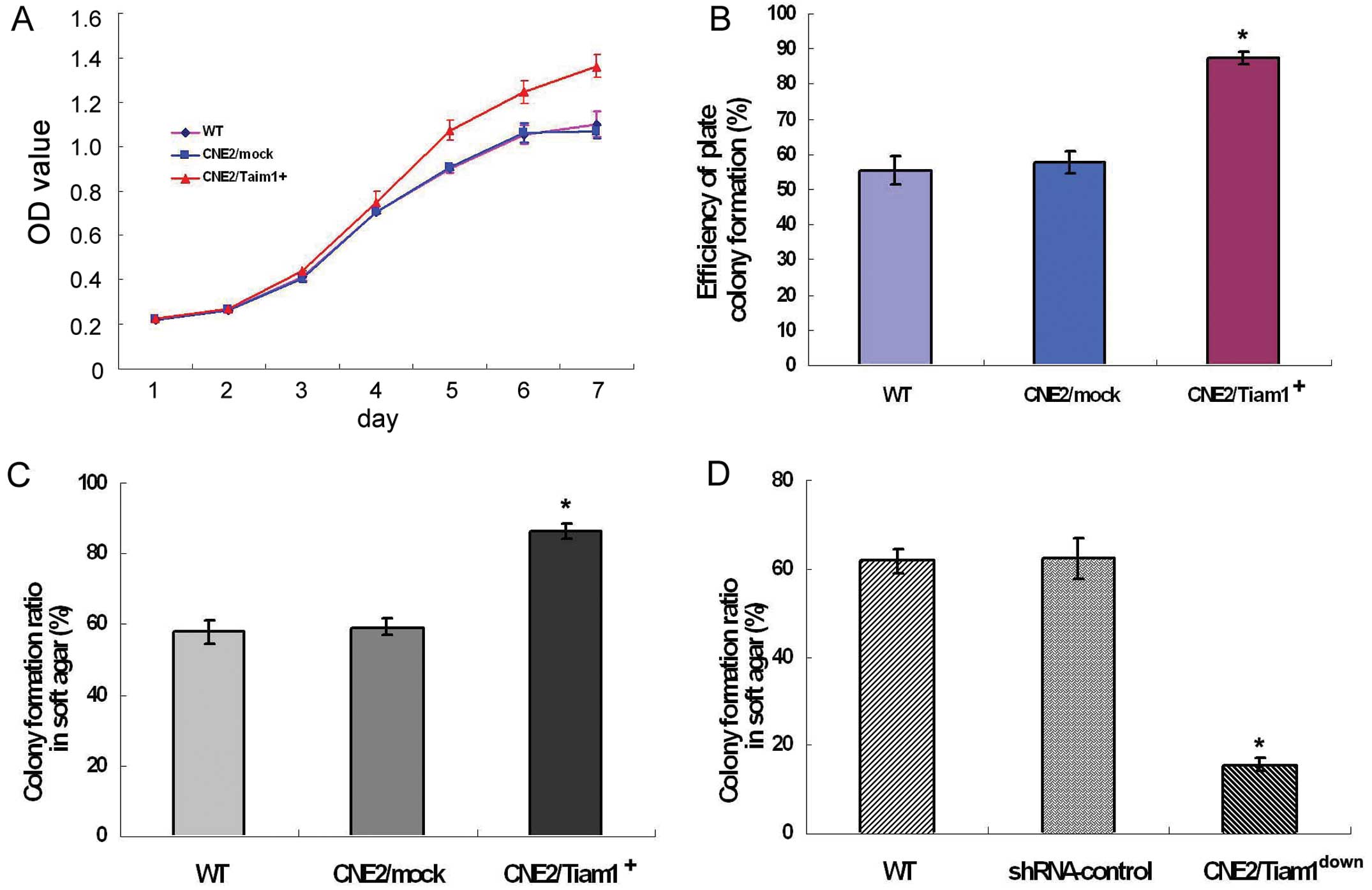
expression on NPC cell growth in vitro. Using MTT assay, we
found that parental wild-type CNE2 (WT) cells had a similar growth
rate as CNE2/mock cells over a 7-day period, while starting from
day 3 the growth rate of CNE2/Tiam1+ cells was
significantly faster than that of the former two cell lines
(P<0.05) (Fig. 3A). Notably,
plate colony formation assay showed that CNE2/Tiam1+
cells had a stronger ability to form colonies than CNE2 or mock
cells (Fig. 3B). The consistent
results also appeared in the soft agar assay. The colony formation
rates of CNE2/Tiam1+, CNE2/mock and WT cells were
86.44±1.98, 59.22±2.40 and 57.94±3.50%, respectively.
CNE2/Tiam1+ cells had a higher efficiency to form
colonies than the other two groups, suggesting that overexpression
of Tiam1 promoted the anchorage-independent growth ability of CNE2
cells (Fig. 3C). In contrast,
knockdown of endogenous Tiam1 markedly reduced the colony formation
ratio of CNE2 cells. The colony formation ratio of
CNE2/Tiam1down cells was reduced by ~80%, as compared
with that of WT and shRNA-control cells (P<0.001) (Fig. 3D). The above results indicate that
increased Tiam1 expression promotes the proliferation and colony
formation of NPC cells.
Alteration of Tiam1 expression affects
the adhesion, invasion and migration of NPC cells in vitro
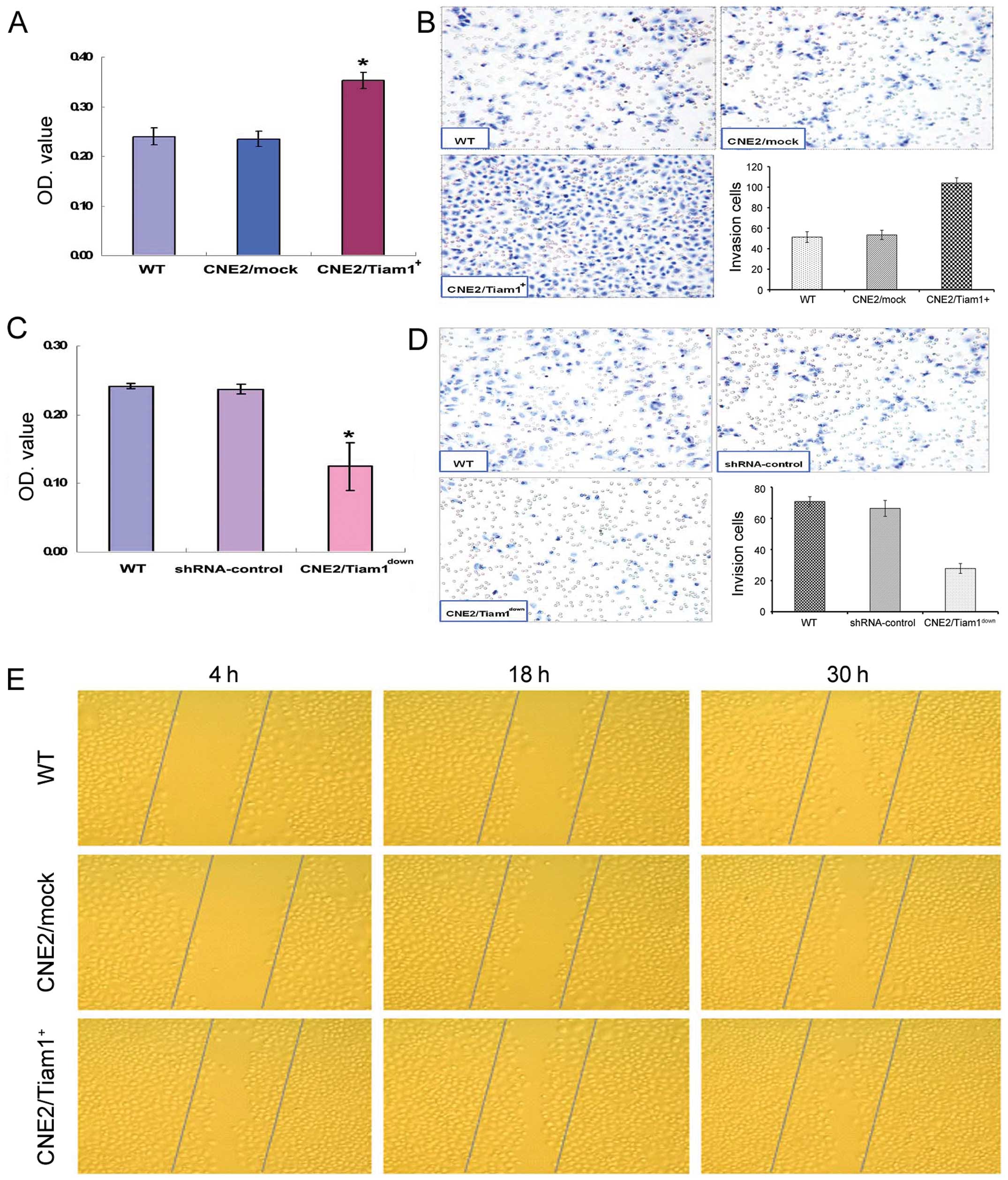
We next focused on determining the effects of Tiam1
on cell adhesion, invasion and migration activity in vitro.
Effects of Tiam1 overexpression or knockdown on adhesion and
invasion capacities of NPC cells were assayed using an adhesion
assay and ECMatrix invasion assay. The adhesion assay revealed that
the adhesive ability to fibronectin of CNE2/Tiam1+ cells
increased by nearly 50% as compared with the WT and mock cells
(Fig. 4A). ECMatrix invasion assay
revealed that the number of CNE2/Tiam1+, mock and WT
cells that penetrated through the matrix was 104.00±5.12,
53.44±4.56 and 51.33±5.22, respectively. Compared with the mock and
WT cells, CNE2/Tiam1+ cells exhibited markedly greater
penetration potency (P<0.05) (Fig.
4B). The opposite results were observed in the experimental
groups with disruption of endogenous Tiam1 expression. The
Tiam1-knockdown cells exhibited only a nearly 0.5-fold adhesion
ability when compared with the WT or shRNA-control cells
(P<0.05) (Fig. 4C). ECMatrix
invasion assay demonstrated that Tiam1 shRNA cells displayed a
significant decrease in invasive ablity, as compared with that of
the WT and shRNA-control cells (P<0.05) (Fig. 4D). The results imply that the
abilities of adhesion and invasion of NPC cells are correlated with
the alteration of Tiam1 expression. The upregulation or
downregulation of Tiam1 expression enhances or attenuates the
adhesion and invasion of NPC cells.
The effect of Tiam1 overexpression on the migratory
ability of CNE2 cells was determined using a scratch-wound healing
assay (Fig. 4E). After 4, 18 and 30
h of wounding, the number of migrated cells in the wound space was
counted in 3 randomly selected fields. The experiments were
repeated in triplicates. The number of WT, CNE2/mock and
CNE2/Tiam1+ cells that migrated to the wound area from
the edge 4, 18 and 30 h after the scratching was as follows: 4 h,
27.67±6.03/image, 28.00±5.00/image and 48.00±13.00/image; 18 h,
47.00±4.58/image, 45.67±5.67/image and 96.33±13.50/image; 30 h,
78.00±4.58/image, 86.33±10.07/image and 141.00±15.14/image,
respectively. The number of migratory cells was significantly
increased in the CNE2/Tiam1+ group as compared with that
of the WT and mock groups (P<0.05), demonstrating that the
migratory ability of NPC cells was positively correlated with
increased Tiam1 expression.
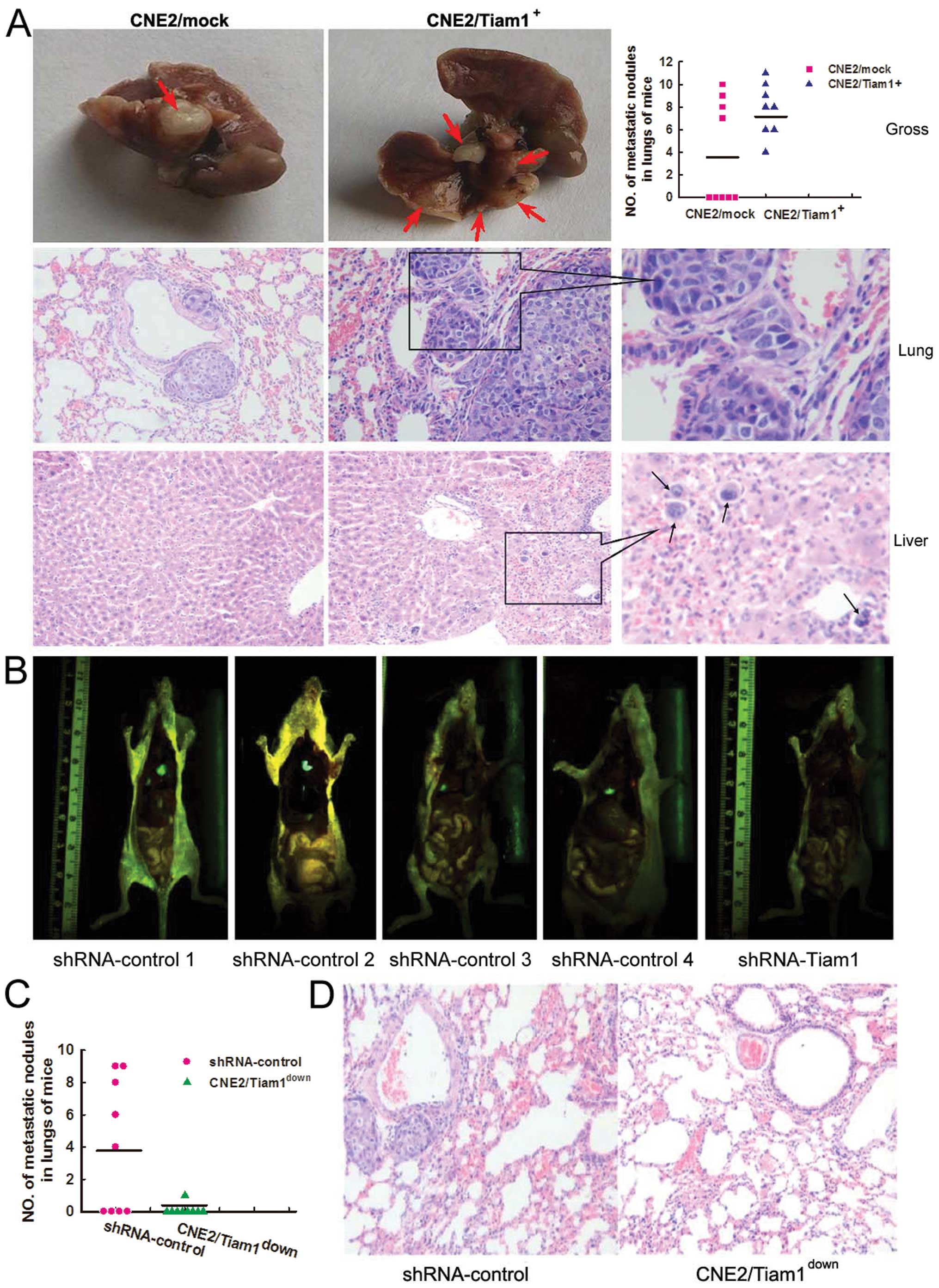
Alteration of Tiam1 expression affects
NPC metastasis in vivo
In addition to examining the biological functions of
Tiam1 in vitro, we also assessed the in vivo function
of Tiam1 by establishing an animal metastasis model by
intravenously injecting CNE2/Tiam1+,
CNE2/Tiam1down and corresponding control cells into nude
mice, respectively. In the mice injected with the
CNE2/Tiam1+ cells, one died due to abscesses in multiple
organs 15 days after injection. Two months later when cachexia
occurred in the CNE2/Tiam1+ group, all of the remaining
mice were sacrificed. Autopsy revealed that nude mice in the
CNE2/Tiam1+ group had many more metastatic nodules in
lungs than those in the CNE2/mock group, indicating extravasation
and tumor growth in the lungs (Fig.
5A). In the CNE2 mock group, 4 of 9 (44%) mice developed
metastasis in the lungs, while all 8 (100%) mice in the
CNE2/Tiam1+ group displayed marked numbers of metastatic
nodules in the lungs (Fig. 5A). The
results were confirmed by histopathological staining (Fig. 5A). Furthermore, scattered NPC cells
were found in the liver in one of the CNE2/Tiam1+ group
mice by microscopy (Fig. 5A). No
detectable tumors were found in other organs in mice in either the
CNE2/Tiam1+ or CNE2/mock group. In the groups of mice
injected with CNE2/Tiam1down or shRNA-control CNE2
cells, 5 of 9 mice in the shRNA-control group developed
collectively 36 metastatic nodules in the lungs, indicating
extravasation of tumor growth. In contrast, the 9 mice injected
with Tiam1 shRNA cells developed only one metastatic nodule in the
lungs as determined using a GFP imaging system (Fig. 5B and C). No metastatic nodules were
found in the other organs. The gross findings were further
confirmed by histological observation (Fig. 5D). These data showed that Tiam1
promotes the metastasis of NPC cells in vivo, which was
validated both by overexpression and knockdown experiments.
Discussion
NPC is a rare malignancy in most regions of the
world, while it is highly prevalent in southern Asia where the
disease occurs at an approximately 100-fold higher incidence
compared with other populations not at risk. NPC is one of the most
confusing, commonly misdiagnosed and poorly understood disease.
Significant achievements in the basic sciences have led to a
greater knowledge of the underlying molecular genetics of NPC,
which holds promise in attempts to tailor patient prognostication
and for future treatment strategies. An enhanced ability to predict
patient survival will allow for better selection of patients most
likely to benefit from systemic therapies and for more accurate
comparison of clinical trials. Tiam1, an important member of the
Rho GTPase family, was identified to play an important role during
the invasion and metastasis of many types of cancer cells (7–11,19–21).
Engers et al (22) found
that significantly increased Tiam1 expression levels were observed
in both prostate cancer and almost all preneoplastic high-grade
prostatic intraepithelium neoplasia lesions, indicating that
increased Tiam1 expression may occur early in prostate carcinoma
development. Huang et al (21) and Liu et al (23) clarified the possible role of the
Tiam1 gene in the proliferation, invasion and metastasis of
colorectal cancer (CRC) and hepatocellular carcinoma (HCC),
respectively, and established Tiam1 as a new target for early
metastatic diagnostic markers. Mo et al (19) and Zhang et al (20 both
reported that that overexpression of Tiam1 was correlated with
invasion and metastasis of NPC; however, little is known about the
potential prognostic value and molecular mechanisms of Tiam1 in
NPC.
In the present study, we assessed Tiam1 expression
in NPC using an immunochemical staining approach and explored the
clinical prognostic value of Tiam1 by using complete long-term
follow-up data of a large cohort of patients with NPC. We found
that ~68.6% (96 of 140 cases) of NPC tissues overexpressed Tiam1
protein. The positive rate of Tiam1 protein was higher in NPC than
that in normal nasopharyngeal tissues. We further confirmed that
Tiam1 was overexpressed in NPC tissues. Furthermore, we also found
that Tiam1 overexpression was significantly associated with T, M
classification and clinical stage of NPC patients. In addition, the
expression of Tiam1 was detected in all of the 6 NPC cell lines. As
indicated by RT-PCR and immunofluorescence staining, the highly
metastatic cell line, 5–8F, exhibited the highest staining of
Tiam1, while the non-metastatic 6–10B cell line showed the lowest
expression of Tiam1. Our finding, in agreement with many other
observation of overexpression of Tiam1 in a variety of cancer
tissues, including NPC, confirmed the close association between
Tiam1 expression and progression and metastasis of tumors.
Our analysis further indicated that Tiam1 protein
expression was inversely correlated with overall survival of NPC
patients. The higher the expression of Tiam1, the shorter was the
survival time for patients with NPC. By univariate analysis of Cox
proportional-hazards model, Tiam1 expression, T classification, M
classification and clinical stage were found to be associated with
an increased risk of death from NPC. In the multivariate analyses,
a high expression of Tiam1 protein was a significant predictor of
poor prognosis for NPC patients. These results suggest the clinical
significance of Tiam1 as a biomarker for NPC prognosis.
The expression of Tiam1 has been widely studied in
various human cancers. Most previous reports indicate that
upregulation of Tiam1 is associated with metastasis and serves as
an unfavorable prognostic factor in cancers (8,11,22–26).
However, contradicting results were also observed in various
studies. Engers et al (27)
found that two invasive cell lines either failed to express Tiam1
or exhibited very low expression levels of Tiam1, but expression of
Tiam1 was markedly higher in the intermediate or low invasive
potential cell lines in renal cell carcinona. Uhlenbrock et
al (12) found that Tiam1
inhibited migration and invasion of metastatic melanoma via a novel
adhesive mechanism. In gastric carcinoma, increased expression of
Tiam1 was found in half of the examined gastric carcinomas, tending
to be associated with favorable prognosis (28). All of these data suggest a complex
role of Tiam1 in different types of human cancers. Thus, it is
important to elucidate the precise roles and molecular mechanisms
of Tiam1 in NPC tumorigenesis and development. To understand the
biological function of Tiam1, we employed two different approaches
to alter the expression level of endogenous Tiam1 in NPC cells,
that is, by the exogenous overexpression and the knockdown of
expression of Tiam1. CNE2 cells exhibited a moderate expression
level of endogenous Tiam1 among all the 6 NPC cell lines examined,
and thus, represent an ideal model for the present study.
In the present study, we determined that
overexpression of Tiam1 enhanced the abilities of proliferation and
colony formation of CNE2 cells. On the other hand, the present
study demonstrated that an efficient knockdown of the Tiam1 gene
strongly inhibited in vitro cell growth and colony formation
efficiency. The soft agar colony formation experiment is well known
for determining non-anchored capacity for growth of a single cell,
reflecting the growth characteristics of tumor cells in
vivo. The present study showed that the colony formation
efficiency was enhanced in Tiam1-overexpressing NPC cells while
reduced in Tiam1-silencing NPC cells. This indicates that Tiam1 may
be a positive regulator of tumor growth and a significant modulator
of tumor development in NPC.
Since Tiam1 is known as a metastasis-associated gene
in other types of cancer, the effects of Tiam1
overexpression/knockdown on the metastatic potential of NPC cells
were also assessed in the present study. The migratory and invasive
ability, an important aspect of epithelial cells, depends
predominantly on cell migration and cell-substrate adhesion. The
cellular ability of attaching to extracellular matrix (ECM)
components contributes to the invasion and metastasis of tumor
cells, for heterogeneous adhesion can be mediated by interactions
between tumor cells and the host or matrix adhesion molecules. We
observed that knockdown of Tiam1 expression in NPC cells reduced
their ability to adhere to fibronectin in our adhesion assay and to
invade ECMatrix-coated membranes in our invasion chamber assay.
This is opposite to our observation that overexpression of Tiam1 in
NPC cells improves their abilities to adhere to fibronectin and to
invade ECMatrix-coated membrane. Our experiments also demonstrated
that overexpression of Tiam1 enhanced the ability of cell migration
by scratch wound assay in confluent monolayer NPC cells, as was
observed by Minard et al (29) in CRC. These results suggest that
Tiam1 is required for the invasive phenotypes of NPC cells in
vitro. To prove that Tiam1 plays an important role in promoting
NPC cell metastasis in vivo as it did in vitro, we
also constructed experimental metastasis models. The experimental
results clearly demonstrated that increased expression of Tiam1
enhanced the ability of NPC cells to metastasize to and grow in the
lungs and liver of mice, while reduction of Tiam1 expression
abrogated such ability of NPC cells. The positive relation of Tiam1
expression to the metastatic potential of NPC cells provides
another piece of evidence for the crucial role of Tiam1 in NPC
progression. These results strongly suggest that Tiam1
overexpression is associated with the malignant phenotype of
NPC.
In conclusion, this is the first relative
comprehensive study showing the functional mechanisms of Tiam1 in
NPC, highlighting the clinical significance of Tiam1 in NPC. The
present study revealed that the level of expression of Tiam1 was
highly increased in NPC tissues, and Tiam1 overexpression was
inversely correlated with survival and directly correlated with
malignant status of patients with NPC. Tiam1 could be used as a
valuable molecular marker for NPC and an indicator for tumor
progression and invasion. Moreover, we identified the roles of
Tiam1 in promoting cell proliferation, adhesion, invasion and
migration in vitro and in vivo. These observations
could provide new insight into understanding the molecular
mechanisms involved in NPC progression and prognosis, and may
result in the development of novel therapeutic strategies for NPC.
However, additional research is needed to investigate the
mechanisms and pathways of NPC pathogenesis mediated by Tiam1.
Acknowledgements
The present study was supported by the National
Natural Science Fund of China (grant nos. 30800414, 30801380,
30770977, 30670967, 30670968, 30500242, 81071735 and 81000953) and
the Nature Science Fund of Guangdong Province of China (nos.
5200512, 2010B031500012 and 10451051501004710).
References
|
1
|
Her C: Nasopharyngeal cancer and the
Southeast Asian patient. Am Fam Physician. 63:1776–1782.
2001.PubMed/NCBI
|
|
2
|
Lo KW and Huang DP: Genetic and epigenetic
changes in nasopharyngeal carcinoma. Semin Cancer Biol. 12:451–462.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cvitkovic E, Bachouchi M, Boussen H, et
al: Leukemoid reaction, bone marrow invasion, fever of unknown
origin, and metastatic pattern in the natural history of advanced
undifferentiated carcinoma of nasopharyngeal type: a review of 255
consecutive cases. J Clin Oncol. 11:2434–2442. 1993.
|
|
4
|
Chan AS, To KF, Lo KW, et al: High
frequency of chromosome 3p deletion in histologically normal
nasopharyngeal epithelia from southern Chinese. Cancer Res.
60:5365–5370. 2000.PubMed/NCBI
|
|
5
|
Loong HH, Ma BB and Chan AT: Update on the
management and therapeutic monitoring of advanced nasopharyngeal
cancer. Hematol Oncol Clin North Am. 22:1267–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Habets GG, Scholtes EH, Zuydgeest D, et
al: Identification of an invasion-inducing gene, Tiam-1, that
encodes a protein with homology to GDP-GTP exchangers for Rho-like
proteins. Cell. 77:537–549. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeng YM, Chang CC, Hu FC, et al:
RNA-binding protein insulin-like growth factor II mRNA-binding
protein 3 expression promotes tumor invasion and predicts early
recurrence and poor prognosis in hepatocellular carcinoma.
Hepatology. 48:1118–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou M, Tan L, Wang X and Zhu YS: Antisense
Tiam1 down-regulates the invasiveness of 95D cells in
vitro. Acta Biochim Biophys Sin (Shanghai). 36:537–540.
2004.
|
|
9
|
Minard ME, Kim LS, Price JE and Gallick
GE: The role of the guanine nucleotide exchange factor Tiam1 in
cellular migration, invasion, adhesion and tumor progression.
Breast Cancer Res Treat. 84:21–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minard ME, Herynk MH, Collard JG and
Gallick GE: The guanine nucleotide exchange factor Tiam1 increases
colon carcinoma growth at metastatic sites in an orthotopic nude
mouse model. Oncogene. 24:2568–2573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding Y, Chen B, Wang S, et al:
Overexpression of Tiam1 in hepatocellular carcinomas predicts poor
prognosis of HCC patients. Int J Cancer. 124:653–658. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uhlenbrock K, Eberth A, Herbrand U, et al:
The RacGEF Tiam1 inhibits migration and invasion of metastatic
melanoma via a novel adhesive mechanism. J Cell Sci. 117:4863–4871.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song LB, Yan J, Jian SW, et al: Molecular
mechanisms of tumorgenesis and metastasis in nasopharyngeal
carcinoma cell sublines. Ai Zheng. 21:158–162. 2002.(In
Chinese).
|
|
14
|
Masunaga R, Kohno H, Dhar DK, et al:
Cyclooxygenase-2 expression correlates with tumor
neovascularization and prognosis in human colorectal carcinoma
patients. Clin Cancer Res. 6:4064–4068. 2000.PubMed/NCBI
|
|
15
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: a
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang S, Zhou J, Wang XY, et al:
Down-regulated expression of SATB2 is associated with metastasis
and poor prognosis in colorectal cancer. J Pathol. 219:114–122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez M, Aladowicz E, Lanfrancone L
and Goding CR: Tbx3 represses E-cadherin expression and enhances
melanoma invasiveness. Cancer Res. 68:7872–7881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herve MA, Buteau-Lozano H, Vassy R, et al:
Overexpression of vascular endothelial growth factor 189 in breast
cancer cells leads to delayed tumor uptake with dilated
intratumoral vessels. Am J Pathol. 172:167–178. 2008. View Article : Google Scholar
|
|
19
|
Mo L, Wang H, Huang G, Zhao H and Kuang G:
Correlation between expression of the Tiam1 gene and the invasion
and metastasis in nasopharyngeal carcinoma. Lin Chuang Er Bi Yan
Hou Ke Za Zhi. 19:785–787. 2005.(In Chinese).
|
|
20
|
Zhang XM, Ding Y, Chen JZ, et al:
Overexpression of Tiam1 gene and its relationship with invasive and
metastatic ability of nasopharyngeal carcinoma. Zhonghua Bing Li
Xue Za Zhi. 38:268–272. 2009.(In Chinese).
|
|
21
|
Huang J, Ye X, Guan J, et al: Tiam1 is
associated with hepatocellular carcinoma metastasis. Int J Cancer.
132:90–100. 2013. View Article : Google Scholar
|
|
22
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Zhang Q, Zhang Y, Wang S and Ding
Y: Lentivirus-mediated silencing of Tiam1 gene influences multiple
functions of a human colorectal cancer cell line. Neoplasia.
8:917–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehta SA, Christopherson KW,
Bhat-Nakshatri P, et al: Negative regulation of chemokine receptor
CXCR4 by tumor suppressor p53 in breast cancer cells: implications
of p53 mutation or isoform expression on breast cancer cell
invasion. Oncogene. 26:3329–3337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao L, Liu Y, Sun X, He M and Ding Y:
Overexpression of T lymphoma invasion and metastasis 1 predict
renal cell carcinoma metastasis and overall patient survival. J
Cancer Res Clin Oncol. 137:393–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Adams HC III, Chen R, Liu Z and Whitehead
IP: Regulation of breast cancer cell motility by T-cell lymphoma
invasion and metastasis-inducing protein. Breast Cancer Res.
12:R692010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Engers R, Zwaka TP, Gohr L, Weber A,
Gerharz CD and Gabbert HE: Tiam1 mutations in human renal-cell
carcinomas. Int J Cancer. 88:369–376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walch A, Seidl S, Hermannstadter C, et al:
Combined analysis of Rac1, IQGAP1, Tiam1 and E-cadherin expression
in gastric cancer. Mod Pathol. 21:544–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minard ME, Ellis LM and Gallick GE: Tiam1
regulates cell adhesion, migration and apoptosis in colon tumor
cells. Clin Exp Metastasis. 23:301–313. 2006. View Article : Google Scholar : PubMed/NCBI
|