Introduction
Pancreatic carcinoma is one of the most aggressive
cancers. Early metastasis to regional lymph nodes and finally
hematogenous spread to distant organs are the leading causes of the
low 5-year survival rate (1,2).
Previous studies have shown that various distinct proteins are
involved in the different steps of cancer progression. In
pancreatic carcinoma, CD133, CD44 and TF are distinct proteins
involved in invasion and metastasis (3–5).
CD133, as an important marker of cancer stem cells
(CSCs), has been used in the identification of CSCs from several
solid tumors (6–12). A CD44+/CD133+
CSC population has been identified that exhibits extensive
proliferation, self-renewal, differentiation and invasion (6,13).
Recent studies have shown that CD133+ circulating tumor
cells (CTCs) are believed to be directly involved in the metastatic
process of colon cancer (14). In
pancreatic carcinoma, CD133 has been shown to be associated with
lymph node metastasis (3).
CD44, a transmembrane glycoprotein, has multiple
variant isoforms (CD44v2-10) (15,16).
CD44 standard (CD44s) can be found in most tissues in the adult
organism, including the hematopoietic system, whereas the variant
isoforms are expressed in specific epithelial tissues and cancers
(17,18). Recent studies have indicated that
CD44v6, a metastatic marker, is unregulated in aggressive
pancreatic cancer CSC subpopulations, and blockage of CD44v6
suppresses the metastasis of pancreatic carcinoma cells (19,20).
There is ample evidence to show that a CD44 isoform, and
specifically CD44v6 as an HA binding protein, or a co-receptor for
c-Met and VEGFR-2 is crucial for the establishment of primary
tumors as well as for metastasis (21). Interaction of CD44 with P-selectin
facilitates tumor cell survive in circulation via binding to
platelets, thereby making CD44+ prone to be involved in
distant metastases (22–24).
Human tissue factor (TF) is the cell surface
receptor of factor VII (FVII), which is responsible for triggering
blood coagulation (25,26). The expression of TF in tumors can
alter the tumor microenvironment, thus facilitating the survival
and metastasis of CSCs (27–30). A
strong correlation between TF expression and hepatic metastasis,
but not lymph node metastasis, has been recognized in colorectal
carcinoma patients (31). In
pancreatic carcinoma, TF expression occurs preferentially at the
invasive front of the tumor and is correlated with angiogenesis,
lymph node and liver metastases and a poor prognosis (5,32).
Indeed, recent studies have shown that certain types
of cancer cells expressing markers of CSCs (CD133) also exhibit
elevated expression of TF or CD44 (28,30,33,34).
However, the role of these three molecules in tumor metastasis is
unclear. To determine whether tri-expression of these three
molecules is associated with metastasis and prognosis in pancreatic
carcinoma, we analyzed the expression profiles of these three
molecules in pancreatic carcinoma tissues by immunohistochemistry
and further evaluated the relationship of their expression profiles
with metastasis and prognosis of pancreatic carcinoma.
Materials and methods
Patients and specimens
A total of 109 patients (71 male and 38 female) with
a median age 58 years (range 36–86 years) underwent surgery at the
Department of Hepatobiliary Surgery Institute, Southwest Hospital,
Third Military Medical University, China, for pancreatic carcinoma
from January 2007 to June 2010. All the patients underwent curative
resection by pancreaticoduodenectomy and pylorus-preserving
pancreaticoduodenectomy with lymph node dissection. None of the
patients had received neoadjuvant or adjuvant radio/chemotherapy.
Formalin-fixed paraffin-embedded samples were obtained for
immunohistochemical analysis. The number of patients with pT1, pT2,
pT3, and pT4 tumors was 19 (17.4%), 33 (30.3%), 53 (48.6%), and 4
(3.7%), respectively. Resected primary tumors and lymph nodes were
histologically examined by hematoxylin and eosin staining using the
TNM (tumor-node-metastasis) classification system. Histologically,
all of the tumors were invasive ductal adenocarcinomas (9
well-differentiated, 73 moderately differentiated and 27 poorly
differentiated). Lymph node metastasis and vascular invasion were
observed in 44 (40.4%) and 37 tumors (33.9%), respectively. All
patients were assessed by radiography, ultrasonography and computed
tomography every 3 months after discharge. New lesions detected by
imaging were considered indicative of relapse. The median follow-up
period was 13 months (range 3–46 months). During this period, 14
patients experienced recurrence of liver disease.
This study was approved by the Ethics Committee of
the Southwest Hospital, and all patients provided written informed
consent.
Immunohistochemistry
Specimens were fixed in formalin, embedded in
paraffin and cut into 3-mm sections. Sections were deparaffinized
in xylene, rehydrated in a graded series of ethanol solutions and
incubated in 3.0% hydrogen peroxide in methanol for 30 min to block
endogenous peroxidase action. Slides were heated at 120°C in an
autoclave in 10 mM sodium citrate (pH 6.0) for 130 sec and cooled
to room temperature. After blocking with 10% goat serum for 30 min,
the sections were incubated overnight at 4°C with primary
antibodies for CD133 (rabbit polyclonal; Bioss; bs-0209R, dilution
1:100), CD44v6 (mouse monoclonal; Invitrogen; 33-6700, dilution
1:50) and TF (rabbit polyclonal, Boster; BA1714, dilution 1:100).
Negative controls were obtained by omitting the primary antibody.
The sections were incubated with peroxidase-conjugated
anti-mouse/rabbit immunoglobulins (Dako EnVisionTM
System; K5007) for 60 min at 37°C. The peroxidase reaction was
developed with 3,3′-diaminobenzidine as the chromogen and
counterstained with hematoxylin.
Criteria for assessing
immunohistochemical results
All the immunostained sections were evaluated
independently by two investigators without knowledge of the
clinical or pathological backgrounds of the patients. Ten random
fields were selected, and expression was evaluated in 1,000 tumor
cells (100 cells per field) with an image analyzer (MetaMorph
Imaging System version 6.0). The immunohistochemical grade was
quantified according to the proportion of stained cells. Specimens
were defined as having positive expression if there were tumor
cells distinctly stained by anti-CD133, CD44v6 or TF antibody. The
staining intensity of CD133 was scored as 3+ (>25%), 2+ (5–25%),
1+ (<5%) or 0 (0%) respectively, according to the percentage of
positively stained cells (Fig. 1A, D
and G) (3,35). Similarly, the staining intensity of
CD44v6 was scored as 3+ (>50%), 2+ (10–50%), 1+ (<10%) or 0
(0%), respectively (Fig. 1B, E and
H) (36). The staining
intensity of TF was scored as 3+ (>66%), 2+ (33–66%), 1+
(<33%) or 0 (0%), respectively (Fig.
1C, F and I) (5). For
statistical analysis, as well as to reduce intraobserver
variability, the immunohistochemical scores were further grouped
into two categories: low (grade 0 or 1+) or high (grade 2+ or
3+).
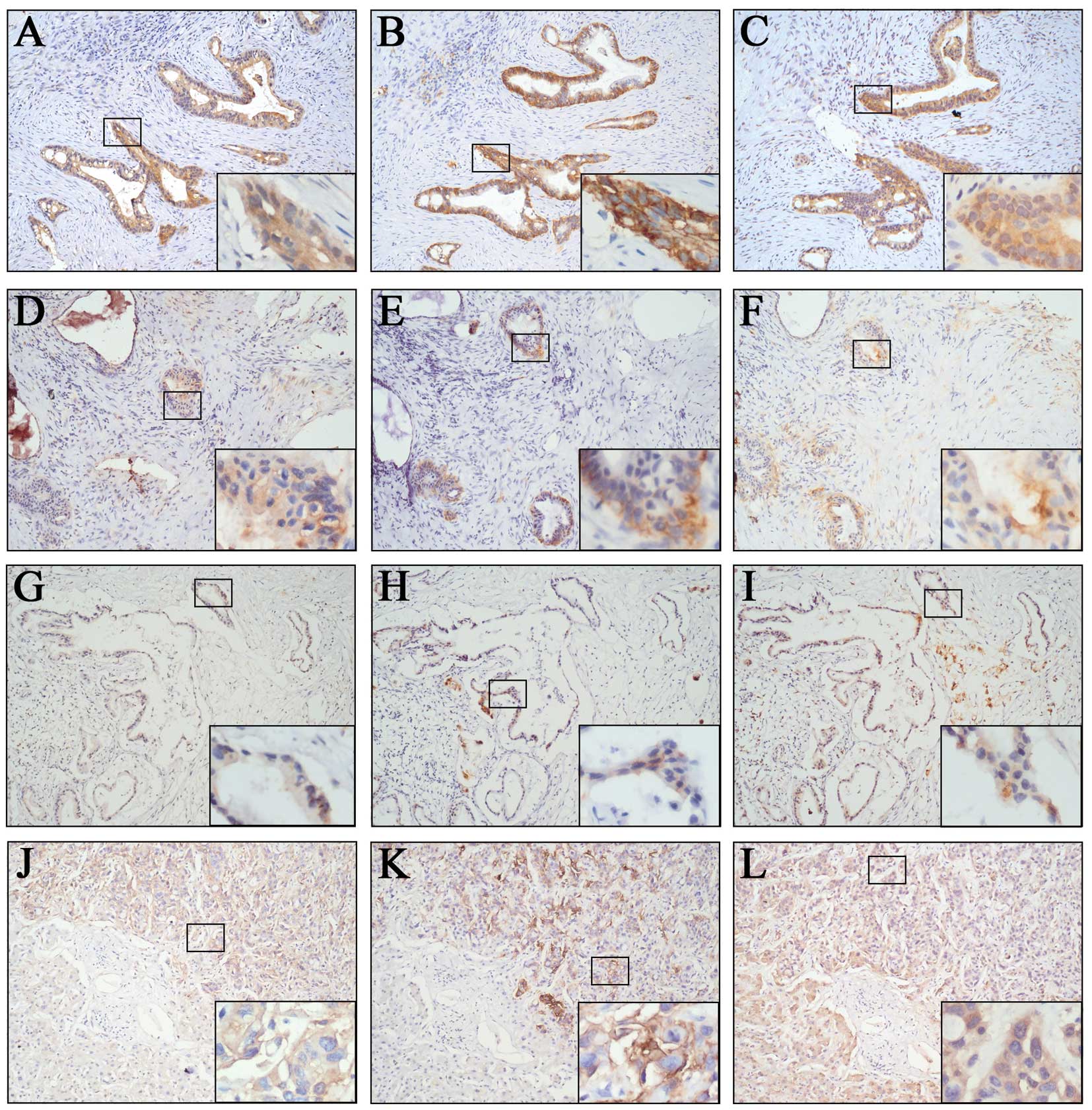 | Figure 1Immunohistochemical staining of
CD133, CD44v6 and TF in pancreatic carcinoma. (A–I) Samples from
primary pancreatic carcinoma. (J–L) Liver samples from metastatic
pancreatic carcinoma. (A, D and G) are CD133-positive (3+),
CD133-positive (2+) and CD133-positive (1+), respectively. (B, E
and H) are CD44v6-positive (3+), CD44v6-positive (2+) and
CD44v6-positive (1+), respectively. (C, F and I) are TF-positive
(3+), TF-positive (2+) and TF-positive (1+), respectively. (J, K
and L) are CD133-positive (3+), CD44v6-positive (3+) and
TF-positive (3+), respectively. Original magnification, ×100; ×400
magnification is shown in the bottom right box. |
Statistical analyses
Group differences were statistically analyzed using
the χ2 test. The Kaplan-Meier method was used to analyze
survival and the log-rank test was used to estimate differences in
survival. Prognostic factors were examined using univariate and
multivariate analyses (Cox proportional hazards regression model).
During the Cox regression, Backward LR method was applied, and
values of variables not in the equation were picked from step one.
P-values <0.05 were considered statistically significant.
Statistical analysis was performed using IBM SPSS Statistics 19.
All the statistical analyses were completed under the guidance of
experienced experts in the Statistics Department.
Results
Overexpression of CD133, CD44v6 and TF in
pancreatic carcinoma
It has been shown that CD133, CD44v6 and TF play
important roles in the process of tumor metastasis. To analyze the
expression patterns of CD133, CD44v6 and TF in pancreatic
carcinoma, we applied immunohistochemistry in 109 pancreatic
carcinoma and 8 normal pancreatic samples. As shown in Table I and Fig. 2, compared with the normal pancreatic
tissues, the expression of CD133 in the pancreatic carcinoma was
low in 44 samples and high in 65 samples. Similarly, the expression
of CD44v6 was low in 59 samples and high in 50 samples.
Additionally, the expression of TF was low in 42 samples and high
in 67 samples. Furthermore, 46/109 pancreatic carcinoma samples
showed tri-expression of CD133, CD44v6 and TF and an additional
16/109 samples showed bi-expression of CD133 and TF but were CD44v6
low. Of the 46 tri-expression samples, 11 samples (23.9%) had
hepatic metastases. However, of the 16 bi-expression samples, only
1 sample (7.1%) had hepatic metastasis (data not shown). These
results suggest that the expression of CD133, CD44v6 and TF is
increased in pancreatic carcinoma and tri-expression of these three
molecules may be required for distant metastasis of pancreatic
carcinoma.
 | Table IExpression of CD133, CD44v6, TF and
their co-expression in the pancreatic carcinoma samples
(n=109). |
Table I
Expression of CD133, CD44v6, TF and
their co-expression in the pancreatic carcinoma samples
(n=109).
| Variables | Expression
level |
|---|
|
|
|---|
| Low | High |
|---|
| CD133 | 44 | 65 |
| CD44v6 | 59 | 50 |
| TF | 42 | 67 |
| CD133 + CD44v6 | | 0 |
| CD133 + TF | | 16 |
| CD44v6 + TF | | 1 |
| CD133 + CD44v6 +
TF | | 46 |
Expression levels of CD133, CD44v6 and TF
are correlated with vascular invasion and lymph node and liver
metastases
To investigate whether CD133, CD44v6 and TF are
involved in the metastasis of pancreatic carcinoma, we further
analyzed the relationship between the clinical characteristics of
the pancreatic carcinoma patients and the expression levels of
CD133, CD44v6 and TF. As shown in Table II, overexpression of CD133 was
correlated with vascular invasion (P=0.004, χ2=8.177),
lymph node metastasis (P=0.002, χ2=9.538), hepatic
metastasis (P=0.033, χ2=4.539) and TNM stage (P=0.002,
χ2=19.132). Overexpression of CD44v6 was correlated with
vascular invasion (P=0.041, χ2=4.165), lymph node
metastasis (P=0.002, χ2=9.378), hepatic metastasis
(P=0.003, χ2=8.728) and TNM stage (P=0.004,
χ2=17.479). Overexpression of TF was correlated with
vascular invasion (P=0.000, χ2=14.803), lymph node
metastasis (P=0.001, χ2=10.181), hepatic metastasis
(P=0.046, χ2=3.987) and TNM stage (P=0.001,
χ2=20.563). As shown in Table III, co-expression of CD133 and TF
was correlated with vascular invasion (P=0.001,
χ2=10.555), lymph node metastasis (P=0.000,
χ2=12.510) and hepatic metastasis (P=0.020,
χ2=5.445). The tri-expression of CD133, CD44v6 and TF
was correlated with vascular invasion (P=0.027,
χ2=4.865), lymph node metastasis (P=0.000,
χ2=13.898), hepatic metastasis (P=0.003,
χ2=8.711) and TNM stage (P=0.000, χ2=28.584),
but showed greater differences in metastases to the lymph nodes and
the liver. Notably, further analysis found that tri-expression and
bi-expression had similar rates of lymph node metastasis and
vascular invasion. Yet, in the 46 tri-expression samples, 11
samples (23.9%) had hepatic metastases. In contrast, in the 14
bi-expression samples, only 1 sample (7.1%) had hepatic metastasis.
These results indicate that the expression levels of CD133, CD44v6
and TF are correlated with vascular invasion, lymph node metastasis
and hepatic metastasis in pancreatic carcinoma and the
co-expression of these three molecules in pancreatic carcinoma may
imply a poorer prognosis.
 | Table IIClinicopathological parameters and
immunohistochemical labeling of CD44v6, CD133 and TF (n=109). |
Table II
Clinicopathological parameters and
immunohistochemical labeling of CD44v6, CD133 and TF (n=109).
| CD133 | CD44v6 | TF |
|---|
|
|
|
|
|---|
| Variables | High
n (%) | Low
n (%) | P-value
χ2-value | High
n (%) | Low
n (%) | P-value
χ2-value | High
n (%) | Low
n (%) | P-value
χ2-value |
|---|
| Gender | | | 0.583 | | | 0.527 | | | 0.498 |
| Female | 24 (36.9) | 14 (31.8) | 0.301 | 19 (38.0) | 19 (32.2) | 0.400 | 25 (37.3) | 13 (31.0) | 0.460 |
| Male | 41 (63.1) | 30 (68.2) | | 31 (62.0) | 40 (67.8) | | 42 (62.7) | 29 (69.0) | |
| Age, years | | | 0.962 | | | 0.743 | | | 0.526 |
| <65 | 47 (72.3) | 32 (72.7) | 0.002 | 37 (74.0) | 42 (71.2) | 0.107 | 50 (74.6) | 29 (69.0) | 0.403 |
| ≥65 | 18 (27.7) | 12 (27.3) | | 13 (26.0) | 17 (28.8) | | 17 (25.4) | 13 (31.0) | |
| Tumor location | | | 0.083 | | | 0.237 | | | 0.112 |
| Head | 53 (81.5) | 41 (93.2) | 2.997 | 41 (82.0) | 53 (89.8) | 1.398 | 55 (82.1) | 39 (92.9) | 2.522 |
| Body/Tail | 12 (18.5) | 3 (6.8) | | 9 (18.0) | 6 (10.2) | | 12 (17.9) | 3 (7.1) | |
| Tumor size, cm | | | 0.908 | | | 0.805 | | | 0.702 |
| ≤2 | 20 (30.8) | 14 (31.8) | 0.013 | 15 (30.0) | 19 (32.2) | 0.061 | 20 (29.9) | 14 (33.3) | 0.146 |
| >2 | 45 (69.2) | 30 (68.2) | | 35 (70.0) | 40 (67.8) | | 47 (70.1) | 28 (66.7) | |
| Lymph node
status | | | 0.002 | | | 0.002 | | | 0.001 |
| Negative | 31 (47.7) | 34 (77.3) | 9.538 | 22 (44.0) | 43 (72.9) | 9.378 | 32 (47.8) | 33 (78.6) | 10.181 |
| Positive | 34 (52.3) | 10 (22.7) | | 28 (56.0) | 16 (27.1) | | 35 (52.2) | 9 (21.4) | |
| Vascular
invasion | | | 0.004 | | | 0.041 | | | 0.000 |
| Negative | 36 (55.4) | 36 (81.8) | 8.177 | 28 (56.0) | 44 (74.6) | 4.165 | 35 (52.2) | 37 (88.1) | 14.803 |
| Positive | 29 (44.6) | 8 (18.2) | | 22 (44.0) | 15 (25.4) | | 32 (47.8) | 5 (11.9) | |
| Neural
invasion | | | 0.261 | | | 0.302 | | | 0.792 |
| Negative | 41 (63.1) | 23 (52.3) | 1.264 | 32 (64.0) | 32 (54.2) | 1.064 | 40 (59.7) | 24 (57.1) | 0.070 |
| Positive | 24 (36.9) | 21 (47.7) | | 18 (36.0) | 27 (45.8) | | 27 (40.3) | 18 (42.9) | |
| Duodenal
invasion | | | 0.588 | | | 0.28 | | | 0.881 |
| Negative | 52 (80.0) | 37 (84.1) | 0.293 | 43 (86.0) | 46 (78.0) | 1.166 | 55 (82.1) | 34 (81.0) | 0.022 |
| Positive | 13 (20.0) | 7 (15.9) | | 7 (14.0) | 13 (22.0) | | 12 (17.9) | 8 (19.0) | |
| Hepatic
metastases | | | 0.033 | | | 0.003 | | | 0.046 |
| Negative | 53 (81.5) | 42 (95.5) | 4.539 | 39 (76.5) | 56 (96.0) | 8.728 | 55 (82.1) | 40 (95.2) | 3.987 |
| Positive | 12 (18.5) | 2 (4.5) | | 12 (23.5) | 2 (3.4) | | 12 (17.9) | 2 (4.8) | |
|
Differentiation | | | 0.168 | | | 0.025 | | | 0.298 |
| Poor | 20 (30.8) | 7 (15.9) | 3.566 | 18 (36.0) | 9 (15.3) | 7.400 | 20 (29.9) | 7 (16.7) | 2.421 |
| Moderate | 41 (63.1) | 32 (72.7) | | 30 (60.0) | 43 (72.9) | | 42 (62.7) | 31 (73.8) | |
| Well | 4 (6.2) | 5 (11.4) | | 2 (4.0) | 7 (11.9) | | 5 (7.5) | 4 (9.5) | |
| T-factor
(UICC) | | | 0.388 | | | 0.671 | | | 0.306 |
| T1 | 11 (16.9) | 8 (18.2) | 3.023 | 9 (18.0) | 10 (16.9) | 1.549 | 12 (17.9) | 7 (16.7) | 3.614 |
| T2 | 16 (24.6) | 17 (38.6) | | 14 (28.0) | 19 (32.2) | | 16 (23.9) | 17 (40.5) | |
| T3 | 35 (53.8) | 18 (40.9) | | 24 (48.0) | 29 (49.2) | | 36 (53.7) | 17 (40.5) | |
| T4 | 3 (4.6) | 1 (2.3) | | 3 (6.0) | 1 (1.7) | | 3 (4.5) | 1 (2.4) | |
| Stage (UICC) | | | 0.002 | | | 0.004 | | | 0.001 |
| 1A | 3 (4.6) | 7 (15.9) | 19.132 | 2 (4.0) | 8 (13.6) | 17.479 | 3 (4.5) | 7 (16.7) | 20.563 |
| 1B | 7 (10.8) | 14 (31.8) | | 6 (12.0) | 15 (25.4) | | 7 (10.4) | 14 (33.3) | |
| 2A | 14 (21.5) | 12 (27.3) | | 8 (16.0) | 18 (30.5) | | 15 (22.4) | 11 (26.2) | |
| 2B | 27 (41.5) | 8 (18.2) | | 21 (42.0) | 14 (23.7) | | 28 (41.8) | 7 (16.7) | |
| 3 | 1 (1.5) | 1 (2.3) | | 1 (2.0) | 1 (1.7) | | 1 (1.5) | 1 (2.4) | |
| 4 | 13 (20.0) | 2 (4.5) | | 12 (24.0) | 3 (5.1) | | 13 (19.4) | 2 (4.8) | |
 | Table IIIClinicopathological parameters and
immunohistochemical labeling of bi-expression of CD133 + TF and
tri-expression of CD133, CD44v6 and TF (n=109). |
Table III
Clinicopathological parameters and
immunohistochemical labeling of bi-expression of CD133 + TF and
tri-expression of CD133, CD44v6 and TF (n=109).
| CD133 + TF | CD44v6 + CD133 +
TF |
|---|
|
|
|
|---|
| Variables | High
n (%) | Low
n (%) | P-value
χ2-value | High
n (%) | Low
n (%) | P-value
χ2-value |
|---|
| Gender | | | 0.574 | | | 0.228 |
| Female | 23 (37.1) | 15 (31.9) | 0.316 | 19 (41.3) | 19 (30.2) | 1.454 |
| Male | 39 (62.9) | 32 (68.1) | | 27 (58.7) | 44 (69.8) | |
| Age, years | | | 0.645 | | | 0.774 |
| <65 | 46 (74.2) | 33 (70.2) | 0.212 | 34 (73.9) | 45 (71.4) | 0.082 |
| ≥65 | 16 (25.8) | 14 (29.8) | | 12 (26.1) | 18 (28.6) | |
| Tumor location | | | 0.166 | | | 0.133 |
| Head | 51 (82.3) | 43 (91.5) | 1.920 | 37 (80.4) | 57 (90.5) | 2.259 |
| Body/Tail | 11 (17.7) | 4 (8.5) | | 9 (19.6) | 6 (9.5) | |
| Tumor size, cm | | | 0.576 | | | 0.572 |
| ≤2 | 18 (29.0) | 16 (34.0) | 0.313 | 13 (28.3) | 21 (33.3) | 0.319 |
| >2 | 44 (71.0) | 31 (66.0) | | 33 (71.7) | 42 (66.7) | |
| Lymphatic
invasion | | | 0.000 | | | 0.000 |
| Negative | 28 (45.2) | 37 (78.7) | 12.510 | 18 (39.1) | 47 (74.6) | 13.898 |
| Positive | 34 (54.8) | 10 (21.3) | | 28 (60.9) | 16 (25.4) | |
| Vascular
invasion | | | 0.001 | | | 0.027 |
| Negative | 33 (53.2) | 39 (83.0) | 10.555 | 25 (54.3) | 47 (74.6) | 4.865 |
| Positive | 29 (46.8) | 8 (17.0) | | 21 (45.7) | 16 (25.4) | |
| Neural
invasion | | | 0.308 | | | 0.433 |
| Negative | 39 (62.9) | 25 (53.2) | 1.040 | 29 (63.0) | 35 (55.6) | 0.615 |
| Positive | 23 (37.1) | 22 (46.8) | | 17 (37.0) | 28 (44.4) | |
| Duodenal
invasion | | | 0.755 | | | 0.470 |
| Negative | 50 (80.6) | 39 (83.0) | 0.097 | 39 (84.8) | 50 (79.4) | 0.521 |
| Positive | 12 (19.4) | 8 (17.0) | | 7 (15.2) | 13 (20.6) | |
| Hepatic
metastases | | | 0.020 | | | 0.003 |
| Negative | 50 (80.6) | 45 (95.7) | 5.445 | 35 (76.1) | 60 (95.2) | 8.711 |
| Positive | 12 (19.4) | 2 (4.3) | | 11 (23.9) | 3 (4.8) | |
|
Differentiation | | | 0.105 | | | 0.022 |
| Poor | 20 (32.3) | 7 (14.9) | 4.515 | 17 (37.0) | 10 (15.9) | 7.672 |
| Moderate | 38 (61.3) | 35 (74.5) | | 25 (54.3) | 46 (73.0) | |
| Well | 4 (6.5) | 5 (10.6) | | 2 (4.3) | 7 (11.1) | |
| T-factor
(UICC) | | | 0.522 | | | 0.381 |
| T1 | 10 (16.1) | 9 (19.1) | 2.250 | 9 (19.6) | 10 (15.9) | 3.067 |
| T2 | 16 (25.8) | 17 (36.2) | | 11 (23.9) | 22 (34.9) | |
| T3 | 33 (53.2) | 20 (42.6) | | 23 (50.0) | 30 (47.6) | |
| T4 | 3 (4.8) | 1 (2.1) | | 3 (6.5) | 1 (1.6) | |
| Stage (UICC) | | | 0.000 | | | 0.000 |
| 1A | 2 (3.2) | 8 (17.0) | 22.836 | 2 (4.3) | 8 (12.7) | 28.584 |
| 1B | 7 (11.3) | 14 (29.8) | | 3 (6.5) | 18 (28.6) | |
| 2A | 12 (19.4) | 14 (29.8) | | 7 (15.2) | 19 (30.2) | |
| 2B | 27 (43.5) | 8 (17.0) | | 27 (58.7) | 14 (22.2) | |
| 3 | 1 (1.6) | 1 (2.1) | | 1 (2.2) | 1 (1.6) | |
| 4 | 13 (21.0) | 2 (4.3) | | 12 (26.1) | 3 (4.8) | |
Individual expression or tri-expression
of the three molecules indicates a poor prognosis in pancreatic
carcinoma patients
We further analyzed whether CD133, CD44v6 and TF
expression levels affect the 1-year survival rate, median survival
time and overall survival of pancreatic carcinoma patients as
analyzed using Kaplan-Meier survival analyses. As shown in Table IV, patients with overexpression of
CD133 had lower 1-year, 2-year and 3-year survival rates and a
shorter median survival time than patients with low expression of
CD133 (67.6 vs. 41.9%, 25.5 vs. 12.7%, 11.3 vs. 3.2% and 14 vs. 11
months, respectively). Patients with overexpression of two or three
molecules had even lower survival rates and shorter median survival
times.
 | Table IVMedian survival and 1-, 2- and 3-year
survival rates and CD133, CD44v6, TF and their co-expression. |
Table IV
Median survival and 1-, 2- and 3-year
survival rates and CD133, CD44v6, TF and their co-expression.
| CD133 | CD44v6 | TF | CD133 + TF | CD44v6 + CD133 +
TF |
|---|
|
|
|
|
|
|
|---|
| Variables | Low | High | Low | High | Low | High | Low | High | Low | High |
|---|
| Median survival
time (months) | 14 | 11 | 13 | 9 | 13 | 11 | 14 | 11 | 14 | 9 |
| 1-year survival
rate (%) | 67.6 | 41.9 | 55.6 | 31.0 | 56.8 | 35.8 | 59.0 | 33.2 | 58.1 | 25.3 |
| 2-year survival
rate (%) | 25.5 | 12.7 | 24.6 | 10.3 | 25.5 | 12.6 | 26.8 | 11.1 | 25.0 | 8.4 |
| 3-year survival
rate (%) | 11.3 | 3.2 | 8.9 | 3.9 | 14.2 | 0.0 | 8.0 | 0.0 | 10.4 | 0.0 |
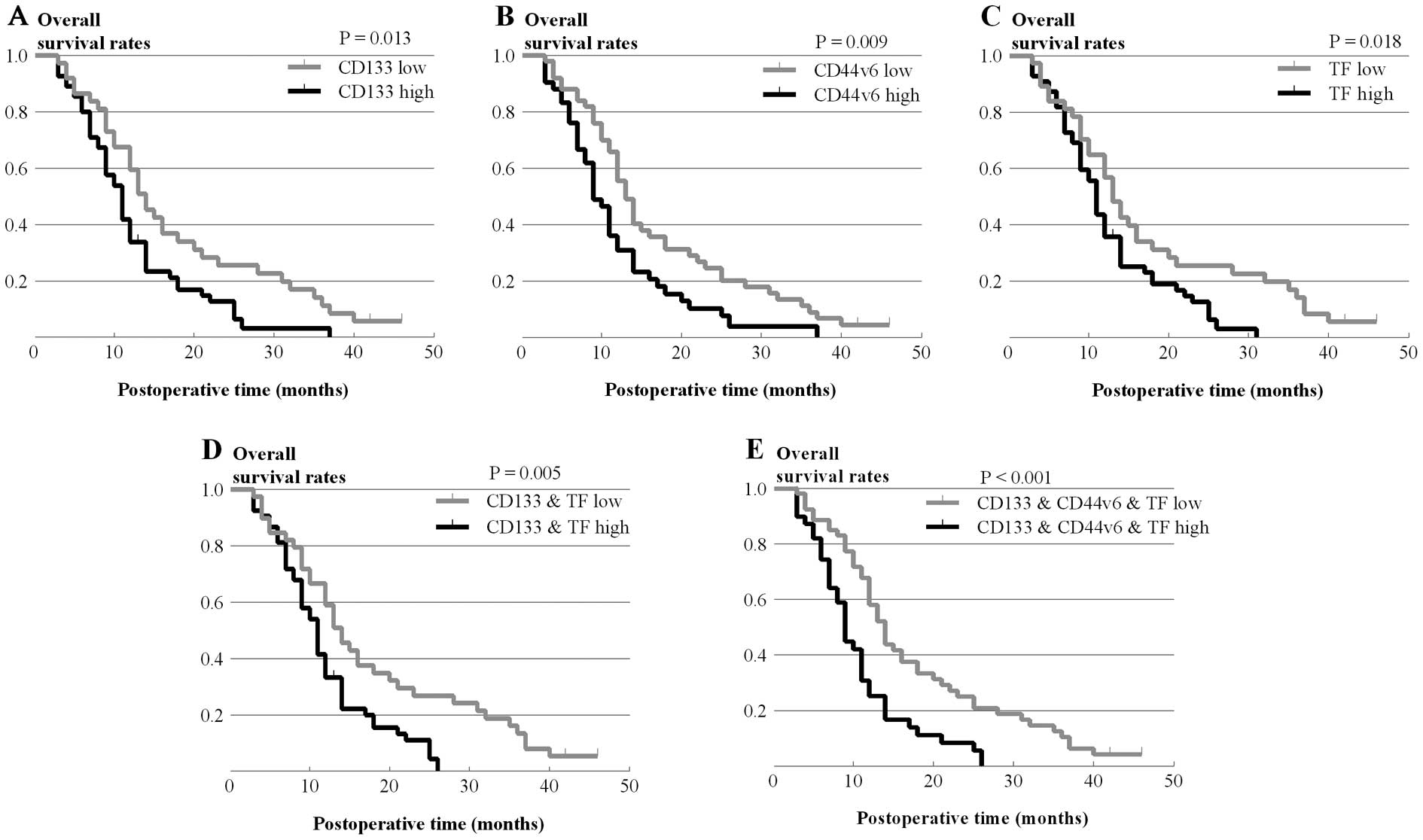
Kaplan-Meier survival curves showed that the
individual overexpression of CD133, CD44v6 or TF significantly
decreased overall survival (P=0.013, χ2=6.217; P=0.009,
χ2=6.756; P=0.018, χ2=5.622, respectively)
(Fig. 3A–C). Additionally,
co-expression of CD133 and TF was also associated with lower
overall survival (P=0.005, χ2=7.964) (Fig. 3D). Furthermore, patients with
overexpression of all three molecules had the lowest overall
survival (P=0.001, χ2=12.021) (Fig. 3E). These data suggest that
individual expression or co-expression of these three molecules
indicate a poorer prognosis in pancreatic carcinoma patients.
Co-expression of CD133, CD44v6 and TF is
an independent predictor of survival in pancreatic carcinoma
patients
To further identify the independent risk factors of
survival, we applied univariate and multivariate analyses to
investigate the survival rate for pancreatic adenocarcinoma. In the
univariate analysis, we found that age, gender and differentiation
were not risk factors of survival. However, T-factor (pT), lymph
node metastasis (pN), vascular invasion (pV), TNM stage and
individual expression or co-expression of CD133, CD44v6 and TF were
all risk factors for survival (Table
V). Multivariate analysis showed that lymph node metastasis
(pN) and TNM stage were independent predictors of survival rate
(P=0.046 and P=0.020, respectively). Yet, the individual expression
levels of CD133, CD44v6 and TF were not independent predictors.
Importantly, the co-expression of CD133, CD44v6 and TF was also
found to be an independent predictor of survival (P=0.018)
(Table V). Therefore, although
CD133, CD44v6 and TF all had an effect on the survival rate, only
co-expression of CD133, CD44v6 and TF was found to be an
independent predictor of survival in pancreatic carcinoma
patients.
 | Table VUnivariate and multivariate Cox
regression of prognostic factors for overall survival in pancreatic
adenocarcinoma. |
Table V
Univariate and multivariate Cox
regression of prognostic factors for overall survival in pancreatic
adenocarcinoma.
| Univariate P | Multivariate P |
|---|
|
|
|
|---|
| Independent
factors | P-value | HR (95% CI) | P-value |
|---|
| Age (years
<65/≥65) | 0.281 | | |
| Gender
(female/male) | 0.944 | | |
| Tumor size
(<2/≥2 cm) | 0.860 | | |
| pN
(negative/positive) | 0.000 | 1.642
(1.009–2.674) | 0.046 |
| Pv
(negative/positive) | 0.024 | 1.354
(0.646–2.835) | 0.422 |
| Hepatic metastases
(negative/positive) | 0.028 | 1.190
(0.430–3.293) | 0.737 |
| Differentiation
(poor/moderate/well) | 0.672 | | |
| pT (T1,2/T3,4) | 0.017 | 0.984
(0.471–2.058) | 0.966 |
| pStage
(I,II/III,IV) | 0.000 | 1.652
(1.081–2.524) | 0.020 |
| CD44v6 expression
(low/high) | 0.009 | 0.968
(0.278–3.372) | 0.960 |
| CD133 expression
(low/high) | 0.013 | 1.008
(0.227–4.486) | 0.992 |
| TF expression
(low/high) | 0.018 | 0.726
(0.165–3.194) | 0.672 |
| CD133 + TF
expression (low/high) | 0.005 | 1.305
(0.149–11.435) | 0.810 |
| CD44v6 + CD133 + TF
expression (low/high) | 0.000 | 1.774
(1.102–2.854) | 0.018 |
Discussion
We first characterized the expression patterns of
three tumor metastasis-related molecules, CD44v6, CD133 and TF, in
109 pancreatic carcinoma samples using immunohistochemistry. In
this study, we applied antibodies that specifically reacted with
CD44-v5, v6 and v7/v8, respectively. We found that, except for
CD44v6, the other CD44 variants were all negative in the pancreatic
carcinoma samples (data not shown). Therefore, among the different
splice variants of CD44, CD44v6 is most likely a reliable marker in
pancreatic carcinoma (20). Our
study showed that the individual expression levels of CD44v6, CD133
and TF were increased in pancreatic carcinoma compared with normal
pancreas. These results are consistent with previous studies that
have shown that CD44v6, CD133 and TF are increased in pancreatic
carcinoma and are associated with metastasis (3,32,37).
CD133, CD44v6 and TF have been shown to play a very
important role in the process of metastasis (3,37,38).
CD133 and CD44v6 are markers of CSCs (11,39,40).
Previous studies have shoen that a subset of CSCs with
CD133+/CD44+ has stronger invasive and
metastatic capabilities (33,41).
TF is unregulated in many cancer cells and impacts tumor
progression by altering the tumor microenvironment (27,28,42).
Various recent reports have shown that either cancer cells with
increased expression of TF or cancer cells with the CSC marker
CD133 are important in cancer progression (43,44).
However, the relationship between tri-expression of
CD133+/CD44+/TF+ and tumor
progression are still unknown. In the present study, we found that
tri-expression of CD133, CD44v6 and TF may be involved in the
metastasis of pancreatic carcinoma. First, 46/109 pancreatic
carcinoma samples showed tri-expression of CD133, CD44v6 and TF and
an additional 16/109 samples showed bi-expression of CD133 and TF
but were CD44v6 low. Of the 46 tri-expression samples, 11 (23.9%)
presented with hepatic metastasis. However, of the 14 bi-expression
samples, only 1 (7.1%) presented with hepatic metastasis,
suggesting that tri-expression of these three molecules may be
associated with distant metastasis of pancreatic carcinoma. Indeed,
we found strong tri-expression of these three molecules in liver
samples from metastasis of pancreatic carcinoma (Fig. 1J–L). Furthermore, the tri-expression
of these three molecules showed stronger association with tumor
metastasis compared with each molecule individually. These findings
may suggest that TF probably alters the tumor microenvironment and
facilitates survival and metastasis of pancreatic carcinoma
CD133+/CD44v6+ CSCs. Therefore,
tri-expression of these three molecules may imply a poor prognosis
for pancreatic carcinoma patients.
It has been shown that many invasion-related
molecules are implicated in the prognosis of pancreatic carcinoma.
In our study, univariate analysis showed that T-factor (pT), lymph
node metastasis (pN), vascular invasion (pV), TNM stage, individual
CD133, CD44v6 and TF expression levels, bi-expression and
tri-expression were all significantly correlated with patient
survival. In this study, although bi-expression, tri-expression and
individual CD133, CD44v6 and TF expression showed similar patient
survival curves, bi-expression and tri-expression were associated
with reduced survival times in comparison with individual CD133,
CD44v6 and TF expression. The ‘heat map’ of expression indicated
that these three markers were frequently co-expressed and that
bi-expression or even uni-expression were uncommon, suggesting that
tri-expression of these three markers may be more important in the
prognosis of pancreatic carcinoma. Consistent with the above
results, multivariate analysis also showed that tri-expression was
an independent prognostic factor. Therefore, tri-expression of
CD133, CD44v6 and TF has significant predictive value for overall
survival and a poor prognosis. Therefore, we believe that
tri-expression is important in predicting prognosis in pancreatic
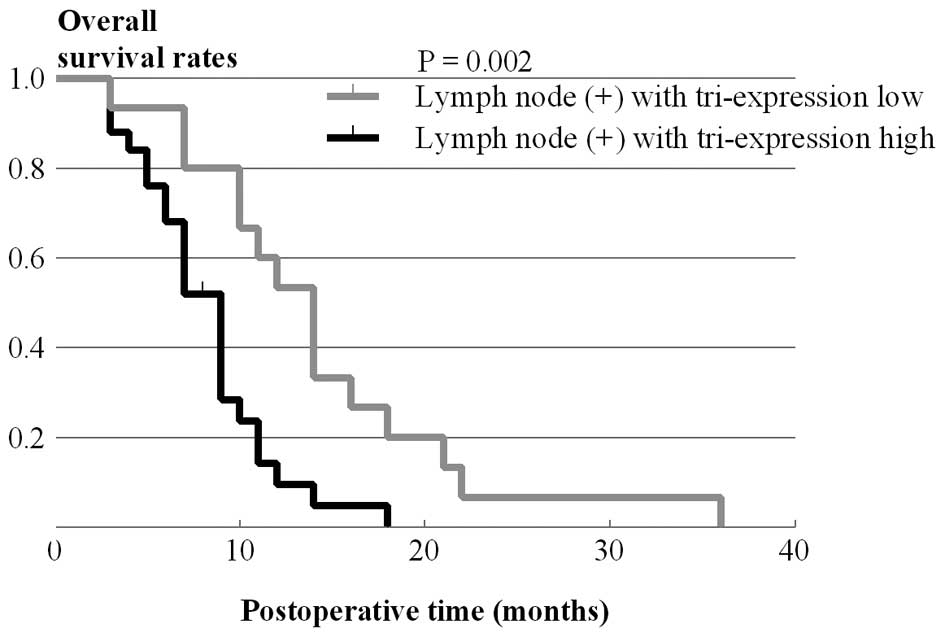
carcinoma patients. In fact, among patients with lymph node
metastasis, patients with tri-expression tumors had a markedly
poorer prognosis (P=0.002) (Fig.
4). Thus, our findings suggest that tri-expression of these
three molecules correlates with the aggressiveness of the
pancreatic carcinoma.
Taken together, we demonstrated that three tumor
metastasis-related molecules, CD133, CD44v6 and TF were
overexpressed in pancreatic carcinoma and were associated with
tumor metastasis and we initially detected the internal
relationships among these three molecules in pancreatic carcinoma
tissues. Tri-expression of these three molecules may be a useful
predictor for pancreatic carcinoma prognosis. This novel finding
may provide insight into a novel metastatic mechanism for
pancreatic carcinoma.
Acknowledgements
This study was supported by the Chongqing Natural
Science Fund Project (no. 2012JB1032), the Project of the National
Natural Science Fund (nos. 81272363, 30872497), the National 863
Project of China (no. 2012AA02A201), the National Basic Research
Program of China (973 Program) (no. 2010CB529400), and the National
Science and Technology Major Project of China (no.
2008ZX10002-026). We thank Dr Yazhou Wu and Dr Lin Liu of the
Statistics Department, Military Preventive Medicine, Third Military
Medical University.
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, et al:
Recent progress in pancreatic cancer. CA Cancer J Clin. 63:318–348.
2013. View Article : Google Scholar
|
|
2
|
Maeda S, Shinchi H, Kurahara H, et al:
Clinical significance of midkine expression in pancreatic head
carcinoma. Br J Cancer. 97:405–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maeda S, Shinchi H, Kurahara H, et al:
CD133 expression is correlated with lymph node metastasis and
vascular endothelial growth factor-C expression in pancreatic
cancer. Br J Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong SP, Wen J, Bang S, Park S and Song
SY: CD44-positive cells are responsible for gemcitabine resistance
in pancreatic cancer cells. Int J Cancer. 125:2323–2331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khorana AA, Ahrendt SA, Ryan CK, et al:
Tissue factor expression, angiogenesis, and thrombosis in
pancreatic cancer. Clin Cancer Res. 13:2870–2875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrandina G, Bonanno G, Pierelli L, et
al: Expression of CD133-1 and CD133-2 in ovarian cancer. Int J
Gynecol Cancer. 18:506–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Florek M, Haase M, Marzesco AM, et al:
Prominin-1/CD133, a neural and hematopoietic stem cell marker, is
expressed in adult human differentiated cells and certain types of
kidney cancer. Cell Tissue Res. 319:15–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ieta K, Tanaka F, Haraguchi N, et al:
Biological and genetic characteristics of tumor-initiating cells in
colon cancer. Ann Surg Oncol. 15:638–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma S, Chan KW, Hu L, et al: Identification
and characterization of tumorigenic liver cancer stem/progenitor
cells. Gastroenterology. 132:2542–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
12
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haraguchi N, Ohkuma M, Sakashita H, et al:
CD133+CD44+ population efficiently enriches
colon cancer initiating cells. Ann Surg Oncol. 15:2927–2933.
2008.
|
|
14
|
Pilati P, Mocellin S, Bertazza L, et al:
Prognostic value of putative circulating cancer stem cells in
patients undergoing hepatic resection for colorectal liver
metastasis. Ann Surg Oncol. 19:402–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Screaton GR, Bell MV, Jackson DG, Cornelis
FB, Gerth U and Bell JI: Genomic structure of DNA encoding the
lymphocyte homing receptor CD44 reveals at least 12 alternatively
spliced exons. Proc Natl Acad Sci USA. 89:12160–12164. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Screaton GR, Bell MV, Bell JI and Jackson
DG: The identification of a new alternative exon with highly
restricted tissue expression in transcripts encoding the mouse
Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons
between mouse, human, and rat. J Biol Chem. 268:12235–12238.
1993.
|
|
17
|
Rall CJ and Rustgi AK: CD44 isoform
expression in primary and metastatic pancreatic adenocarcinoma.
Cancer Res. 55:1831–1835. 1995.PubMed/NCBI
|
|
18
|
Bhatavdekar JM, Patel DD, Chikhlikar PR,
et al: Overexpression of CD44: a useful independent predictor of
prognosis in patients with colorectal carcinomas. Ann Surg Oncol.
5:495–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seiter S, Arch R, Reber S, et al:
Prevention of tumor metastasis formation by anti-variant CD44. J
Exp Med. 177:443–455. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gunthert U, Hofmann M, Rudy W, et al: A
new variant of glycoprotein CD44 confers metastatic potential to
rat carcinoma cells. Cell. 65:13–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tremmel M, Matzke A, Albrecht I, et al: A
CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and
angiogenesis. Blood. 114:5236–5244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alves CS, Yakovlev S, Medved L and
Konstantopoulos K: Biomolecular characterization of
CD44-fibrin(ogen) binding: distinct molecular requirements mediate
binding of standard and variant isoforms of CD44 to immobilized
fibrin(ogen). J Biol Chem. 284:1177–1189. 2009. View Article : Google Scholar
|
|
23
|
Alves CS, Burdick MM, Thomas SN, Pawar P
and Konstantopoulos K: The dual role of CD44 as a functional
P-selectin ligand and fibrin receptor in colon carcinoma cell
adhesion. Am J Physiol Cell Physiol. 294:C907–C916. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanley WD, Napier SL, Burdick MM, Schnaar
RL, Sackstein R and Konstantopoulos K: Variant isoforms of CD44 are
P- and L-selectin ligands on colon carcinoma cells. FASEB J.
20:337–339. 2006.PubMed/NCBI
|
|
25
|
Nemerson Y: Tissue factor and hemostasis.
Blood. 71:1–8. 1988.PubMed/NCBI
|
|
26
|
Carson SD and Brozna JP: The role of
tissue factor in the production of thrombin. Blood Coagul
Fibrinolysis. 4:281–292. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milsom CC, Yu JL, Mackman N, et al: Tissue
factor regulation by epidermal growth factor receptor and
epithelial-to-mesenchymal transitions: effect on tumor initiation
and angiogenesis. Cancer Res. 68:10068–10076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milsom C, Magnus N, Meehan B, Al-Nedawi K,
Garnier D and Rak J: Tissue factor and cancer stem cells: is there
a linkage? Arterioscler Thromb Vasc Biol. 29:2005–2014. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milsom C, Yu J, May L, et al: The role of
tumor-and host-related tissue factor pools in oncogene-driven tumor
progression. Thromb Res. 120(Suppl 2): S82–S91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Milsom C, Anderson GM, Weitz JI and Rak J:
Elevated tissue factor procoagulant activity in CD133-positive
cancer cells. J Thromb Haemost. 5:2550–2552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seto S, Onodera H, Kaido T, et al: Tissue
factor expression in human colorectal carcinoma: correlation with
hepatic metastasis and impact on prognosis. Cancer. 88:295–301.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nitori N, Ino Y, Nakanishi Y, et al:
Prognostic significance of tissue factor in pancreatic ductal
adenocarcinoma. Clin Cancer Res. 11:2531–2539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliveira LR, Castilho-Fernandes A,
Oliveira-Costa JP, Soares FA, Zucoloto S and Ribeiro-Silva A:
CD44+/CD133+immunophenotype and matrix
metalloproteinase-9 influences on prognosis of early stage oral
squamous cell carcinoma patients. Head Neck. Nov 1–2013.(Epub ahead
of print).
|
|
34
|
Rentala S, Chintala R, Guda M, Chintala M,
Komarraju AL and Mangamoori LN: Atorvastatin inhibited
Rho-associated kinase 1 (ROCK1) and focal adhesion kinase (FAK)
mediated adhesion and differentiation of CD133CD44 prostate cancer
stem cells. Biochem Biophys Res Commun. 441:586–592. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Q, Li JG, Zheng XY, Jin F and Dong HT:
Expression of CD133, PAX2, ESA, and GPR30 in invasive ductal breast
carcinomas. Chin Med J. 122:2763–2769. 2009.PubMed/NCBI
|
|
36
|
Lee SM, Lee KE, Chang HJ, et al:
Prognostic significance of CD44s expression in biliary tract
cancers. Ann Surg Oncol. 15:1155–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gansauge F, Gansauge S, Zobywalski A, et
al: Differential expression of CD44 splice variants in human
pancreatic adenocarcinoma and in normal pancreas. Cancer Res.
55:5499–5503. 1995.PubMed/NCBI
|
|
38
|
Saigusa S, Tanaka K, Toiyama Y, et al:
Correlation of CD133, OCT4, and SOX2 in rectal cancer and their
association with distant recurrence after chemoradiotherapy. Ann
Surg Oncol. 16:3488–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miki J, Furusato B, Li H, et al:
Identification of putative stem cell markers, CD133 and CXCR4, in
hTERT-immortalized primary nonmalignant and malignant tumor-derived
human prostate epithelial cell lines and in prostate cancer
specimens. Cancer Res. 67:3153–3161. 2007. View Article : Google Scholar
|
|
40
|
Hermann PC, Huber SL, Herrler T, et al:
Distinct populations of cancer stem cells determine tumor growth
and metastatic activity in human pancreatic cancer. Cell Stem Cell.
1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun Y, Han J, Lu Y, Yang X and Fan M:
Biological characteristics of a cell subpopulation in tongue
squamous cell carcinoma. Oral Dis. 18:169–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Jiang P, Capkova K, et al: Tissue
factor-activated coagulation cascade in the tumor microenvironment
is critical for tumor progression and an effective target for
therapy. Cancer Res. 71:6492–6502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rong Y, Post DE, Pieper RO, Durden DL, Van
Meir EG and Brat DJ: PTEN and hypoxia regulate tissue factor
expression and plasma coagulation by glioblastoma. Cancer Res.
65:1406–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fernandez PM and Rickles FR: Tissue factor
and angiogenesis in cancer. Curr Opin Hematol. 9:401–406. 2002.
View Article : Google Scholar : PubMed/NCBI
|