Introduction
Clear cell carcinoma of the ovary (OCCC) is
recognized in the World Health Organization classification of
ovarian tumors as a distinct histological entity. Its clinical
behavior is distinctly different from other epithelial ovarian
cancers (1). OCCC accounts for
3.7–12.1% of epithelial ovarian cancers (2,3). We
found that response rates for platinum-based chemotherapy were
11.1% for OCCC and 72.5% for serous adenocarcinoma (SAC),
suggesting that OCCC resists conventional platinum-based
chemotherapy (4). A novel
therapeutic strategy is needed to improve the prognosis of patients
with OCCC.
PIK3CA is located at the 3q26.3 locus and
encodes the catalytic subunit of the phosphatidylinositol 3-kinase
(PI3K), p110α (5). In response to
an extracellular signal, the activated p110α phosphorylates PIP2 to
generate PIP3. The PIP3 recruits AKT to the plasma membrane, where
it is phosphorylated and activated by
phosphatidylinositol-dependent kinase 1 (PDK1) and PDK2. Activated
AKT can directly activate the mammalian target of rapamycin (mTOR)
by phosphorylation at Ser2448. mTOR is a serine/threonine kinase
that acts as an effector in the PI3K/Akt pathway. Aberrations of
the PI3K pathway are frequently present in many different types of
cancer. A number of studies have shown amplification or mutations
of the PIK3CA gene in ovarian cancers (6–8). AKT
and mTOR are also hyperactivated in ovarian cancer (9,10).
Additionally, a high frequency of activating mutations of
PIK3CA has been observed in OCCC (11).
NVP-BEZ235 is an imidazoquinoline derivative that
potently and reversibly inhibits class 1 PI3K and mTOR catalytic
activity by competing at its ATP-binding site (12). It has been demonstrated to reduce
tumor growth in several xenograft models and is currently in
clinical trials (12–14). The present study was conducted to
clarify the efficacy of NVP-BEZ235 treatment on OCCC.
Materials and methods
Cell lines and cell cultures
Eight human OCCC cell lines (OVISE, SMOV-2, KK,
TU-OC-1, OVTOKO, KOC-7c, RMG-I and OVMANA) and five OSAC cell lines
(KF, KOC-2s, TU-OS-3, TU-OS-4 and SHIN-3) were used. Cells were
obtained as follows: OVISE and OVTOKO from Dr Hiroshi Minaguchi
(Yokohama City University, Yokohama, Japan); SMOV-2 from Dr
Tomohiro Iida (St. Marianna University, Kawasaki, Japan); KK and KF
from Dr Yoshihiro Kikuchi (National Defense Medical College,
Tokorozawa, Japan); KOC-7c and KOC-2s from Dr Toru Sugiyama (Kurume
University, Kurume, Japan); RMG-I from Dr Shiro Nozawa (Keio
University, Tokyo, Japan); and SHIN-3 from Dr Yasuhiko Kiyozuka
(Nara Medical University, Kashihara, Japan). TU-OC-1, TU-OS-3, and
TU-OS-4 cells were established by our department (15,16).
All cell lines were maintained in Dulbecco’s modified Eagle’s
medium (DMEM)/F12 (Gibco, Grand Island, NY, USA) with 10% fetal
bovine serum (FBS) in a humidified atmosphere containing 5%
CO2 at 37°C.
Mutation screening
Screening for mutations was performed as previously
described (17). Genomic DNA was
purified from all cell lines using a DNeasy Tissue kit (Qiagen,
Valencia, CA, USA). PCR primers used to amplify the sequence of
interest (exons 9 and 20 of the PIK3CA gene, exons 2 and 3
of the KRAS gene) were the same as reported in the
literature (18,19). DNA was amplified in reactions of 30
sec at 94°C; 30 sec at 55°C; followed by 90 sec at 72°C for 30
cycles. Then, PCR products were subjected to sequencing using
BigDye Terminator v3.1 Cycle Sequencing kit and an Applied
Biosystems 3130xl Genetic Analyzer (Applied Biosystems Foster City,
CA, USA).
Reagents
NVP-BEZ235 and temsirolimus were purchased from LC
Laboratories (Woburn, MA, USA). Stock solutions were prepared in
dimethyl sulfoxide (DMSO) and stored at −20°C for the in
vitro experiments. The drugs were diluted in fresh medium
immediately before each experiment. In all the experiments, the
final DMSO concentration was <0.1%.
Dose-response studies
The cytotoxicities of NVP-BEZ235 and temsirolimus
were assessed by the WST-8 assay using Cell Counting Kit-8 (Dojindo
Laboratories, Tabaru, Japan) as previously described (17). Cells (2–4×103 cells/80
μl) were seeded into each well of a 96-well tissue culture plate,
cultured overnight, and then treated with 20 μl of NVP-BEZ235 or
temsirolimus solution at a final concentration of 0.001, 0.01, 0.1,
1 or 10 μM for 72 h. After that, 20 μl of Cell Counting Kit-8
solution was added to each well, and the plates were incubated for
another 1–2 h. Absorbance was measured at 450 nm with a microplate
reader (iMark Microplate Absorbance Reader). Cell viability was
calculated as the percentage of cells killed by the treatment. All
experiments were conducted in triplicate. Median inhibitory
concentrations were determined from these calculations.
Western blot analysis
Cells were washed twice with ice-cold PBS. Cell
pellets were then lysed in a buffer [50 mM Tris-HCl (pH 7.5), 150
mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA, 50 mM NaF, 2 mM
Na3VO4 and protease inhibitors (Complete
Protease Inhibitor Cocktail Tablets; Roche Diagnostics)] as
previously described (17). Protein
concentrations were measured against a standardized control using a
protein assay kit (Bio-Rad Laboratories). A total of 50 mg protein
was separated by electrophoresis on a 5–20% polyacrylamide gel and
transferred to a polyvinylidene difluoride membrane (Millipore).
The antibodies were as follows: rabbit anti-erbB3 antibody (C17)
(diluted 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
mouse anti-β-actin (AC-40) antibody (1:1,000; Sigma-Aldrich, St.
Louis, MO, USA); and anti-phospho-erbB3 (Tyr1289) (21D3) antibody
(1:1,000), rabbit anti-AKT antibody (1:1,000), rabbit
anti-phospho-AKT (Ser473) antibody (1:500), rabbit anti-mTOR
antibody (1:500), rabbit anti-phospho-mTOR (Ser2448) antibody
(1:500), rabbit anti-p70S6K antibody (1:500), rabbit
anti-phospho-p70S6K (Thr389) antibody (1:500), rabbit anti-4E-BP1
antibody (1:1,000) and rabbit anti-phospho-4E-BP1 (Thr37/49)
antibody (1:1,000) (all from Cell Signaling Technology (Danvers,
MA, USA). Signals were detected with secondary anti-mouse or
anti-rabbit immunoglobulin G antibody coupled with horseradish
peroxidase, using an Ez-Capture II chemiluminescent imaging system
(ATTO, Tokyo, Japan).
Cell cycle distribution analysis
Cell cycle distribution was analyzed by flow
cytometry. Briefly, cells were plated in a 6-well plate, cultured
overnight, and then treated with NVP-BEZ235 or left untreated for
48 or 72 h (final concentration of 10 or 100 nM). Floating and
adherent cells were fixed overnight in ice-cold 70% ethanol. The
cells were then resuspended in PBS containing propidium iodide (PI,
25 μg/ml) supplemented with 0.1% RNase A and incubated at 37°C for
30 min. DNA content was measured with a FACSCalibur flow cytometer
with CellQuest software (Becton-Dickinson, Franklin Lakes, NJ,
USA). Cell fit analysis determined the percentage of the cell count
in a specific phase of the cell cycle.
Annexin V staining
The Annexin V-FITC Apoptosis Detection kit
(BioVision, Mountain View, CA, USA) was used to assess apoptosis as
the externalization of phosphatidylserine residues, according to
the specifications of the manufacturer. Briefly, cells were
suspended in 500 ml of 1× binding buffer. The cells then were
stained with 5 ml Annexin V-FITC (fluorescein isothiocyanate) and 5
ml PI (50 mg/ml) for 5 min in the dark at room temperature.
Finally, the cells were analyzed with a flow cytometer
(FACSCalibur; Becton-Dickinson).
Ovarian cancer xenograft model
OVISE or TU-OC-1 cells in log-phase growth were
trypsinized, washed twice with PBS and centrifuged at 250 × g. For
subcutaneous tumor development, 4×106 viable cells (in
0.1 ml of PBS) were injected subcutaneously under aseptic
conditions into female athymic mice. Seven days after the
injection, we confirmed the development of measurable tumors, and
then treatment was initiated with NVP-BEZ235 at doses of 25 or 50
mg/kg/day, and continued for 3 weeks. Mice treated with vehicle
(10% 1-methyl-2-pyrrolidone-90% polyethylene glycol 300) were used
as the control group. All agents were administered by oral gavage.
Ten mice were used in each experimental group. The tumor volume was
measured with a caliper twice weekly. The body weight of mice was
also measured twice weekly.
Statistical analysis
Statistical analyses were performed with Prism
version 5 (GraphPad Software Inc., San Diego, CA, USA). Data are
presented as means ± 1 standard error. Means for all data were
compared by one-way analysis of variance with post hoc
testing or by unpaired t-test. A P-value of <0.05 was considered
to indicate a statistically significant result.
Results
Identification of PIK3CA and KRAS
mutations in OCCC and OSAC cell lines
We first screened the mutation status of
PIK3CA (exons 9 and 20) and KRAS (exons 2 and 3) in
the 8 OCCC and 5 OSAC cell lines. Four out of the 8 OCCC cell lines
showed a PIK3CA mutation while none of the 5 OSAC cell lines
showed the mutation (Table I). One
of the 5 OSAC cell lines showed a KRAS mutation (34G>A)
while none of the 8 OCCC cell lines showed this mutation.
 | Table ICharacteristics of the OCCC and OSAC
cell lines. |
Table I
Characteristics of the OCCC and OSAC
cell lines.
| | KRAS | PIK3CA | | |
|---|
| |
|
| | |
|---|
| Cell line | Original tumor | Exon 2 | Exon 3 | Exon 9 | Exon 20 | IC50 of
BEZ235 (nM) |
|---|
| OVISE | Clear cell
carcinoma | wt | wt | wt | wt | 44 |
| SMOV-2 | Clear cell
carcinoma | wt | wt | | 3141 A>A/T | 65 |
| KK | Clear cell
carcinoma | wt | wt | 1634 A>A/C | wt | 74 |
| TU-OC-1 | Clear cell
carcinoma | wt | wt | 1624 G>G/A | wt | 131 |
| OVTOKO | Clear cell
carcinoma | wt | wt | wt | wt | 534 |
| KOC-7c | Clear cell
carcinoma | wt | wt | wt | wt | 600 |
| OVMANA | Clear cell
carcinoma | wt | wt | 1634 A>T | wt | 641 |
| RMG-I | Clear cell
carcinoma | wt | wt | wt | wt | 777 |
| KF | Serous
adenocarcinoma | wt | wt | wt | wt | 779 |
| KOC-2s | Serous
adenocarcinoma | wt | wt | wt | wt | 989 |
| TU-OS-3 | Serous
adenocarcinoma | wt | wt | wt | wt | 1,004 |
| TU-OS-4 | Serous
adenocarcinoma | wt | wt | wt | wt | 3,951 |
| SHIN-3 | Serous
adenocarcinoma | 34 G>A | wt | wt | wt | 25,400 |
Sensitivity to NVP-BEZ235 or
temsirolimus
The IC50 values of NVP-BEZ235 in the OCCC
cell lines were lower than these values in the OSAC cell lines
(Table I). In the OCCC cell lines,
the IC50 of temsirolimus was higher than that of BEZ235
(Table II). Although the
PIK3CA mutation was more frequently noted in OCCC than OSAC,
the sensitivity of these cell lines to NVP-BEZ235 or temsirolimus
was not related to the mutation status.
 | Table IIIC50 of temsirolimus in
the OCCC cell lines. |
Table II
IC50 of temsirolimus in
the OCCC cell lines.
| IC50
(nM) |
|---|
|
|
|---|
| Cell line | BEZ235 | Temsirolimus |
|---|
| OVISE | 44 | 9,122 |
| SMOV-2 | 64 | 8,924 |
| KK | 74 | 5,929 |
| TU-OC-1 | 131 | 7,224 |
| OVTOKO | 534 | 12,776 |
| KOC-7c | 600 | 9,779 |
| OVMANA | 641 | 17,650 |
| RMG-I | 777 | 4,045 |
Expression levels of PI3K-Akt-mTOR
pathway molecules in the OCCC and OSAC cell lines
Comparison of the OCCC and OSAC cell lines showed
that pHER3 and pAkt expression was more frequent in OCCC than OSAC
(Fig. 1A). That is, 7 of the 8 OCCC
cell lines expressed pHER3 whereas 2 of the 5 OSAC cells lines
exhibited expression. Similarly, 6 of the 8 OCCC cell lines
expressed pAkt while 2 of the 5 OSAC cell lines did. The protein
expression levels were distributed widely, and did not relate to
the sensitivity to NVP-BEZ235 or temsirolimus.
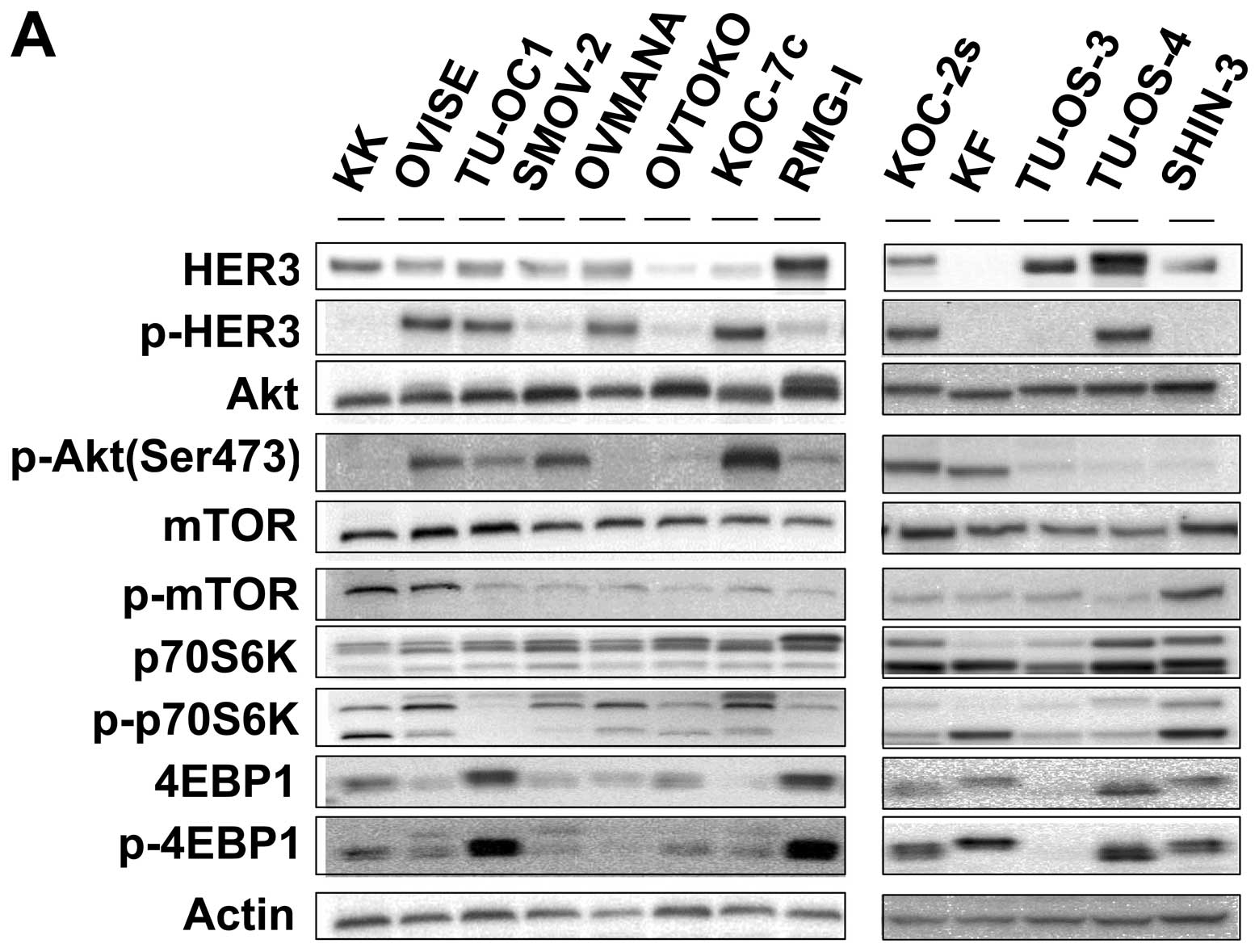 | Figure 1(A) Baseline expression of
PI3K-Akt-mTOR pathway molecules in the OCCC and OSAC cell lines.
Eight OCCC cell lines (KK, OVISE, TU-OC-1, SMOV-2, OVMANA, OVTOKO,
KOC-7c and RMG-I) and 5 OSAC cell lines (KOC-2s, KF, TU-OS-3,
TU-OS-4 and SHIN-3) were cultured in DMEM/F12 medium with 10% fetal
bovine serum in a humidified atmosphere containing 5%
CO2 at 37°C. Western blot analysis was performed to
detect the expression levels of HER3, p-HER3, Akt, p-Akt, mTOR,
p-mTOR, p70S6K, p-p70S6K, 4E-BP1 and p-4E-BP1. β-actin was used as
a loading control. Each experiment was repeated 3 times
independently. (B and C) NVP-BEZ235 suppressed pAkt expression in
OCCC cells. Two OCCC cell lines (OVISE and KK) were plated in
6-well plates. The protein samples were collected after treatment
with 10 and 100 nM NVP-BEZ235 or temsirolimus for 6 or 24 h.
Western blot analysis was performed to detect Akt, p-Akt, p70S6K,
p-p70S6K, 4E-BP1 and p-4E-BP1 expression. β-actin was used as a
loading control. |
When OVISE cells were treated with NVP-BEZ235,
expression levels of p-p70S6K and p4E-BP1 were suppressed in a
dose-dependent manner (Fig. 1B).
Treatment with temsirolimus incompletely suppressed p-p70S6K and
p4E-BP1 expression in the OVISE cells. Moreover, treatment with
NVP-BEZ235 suppressed pAKT expression, while treatment with
temsirolimus did not. Similar results were observed in the KK cells
(Fig. 1C).
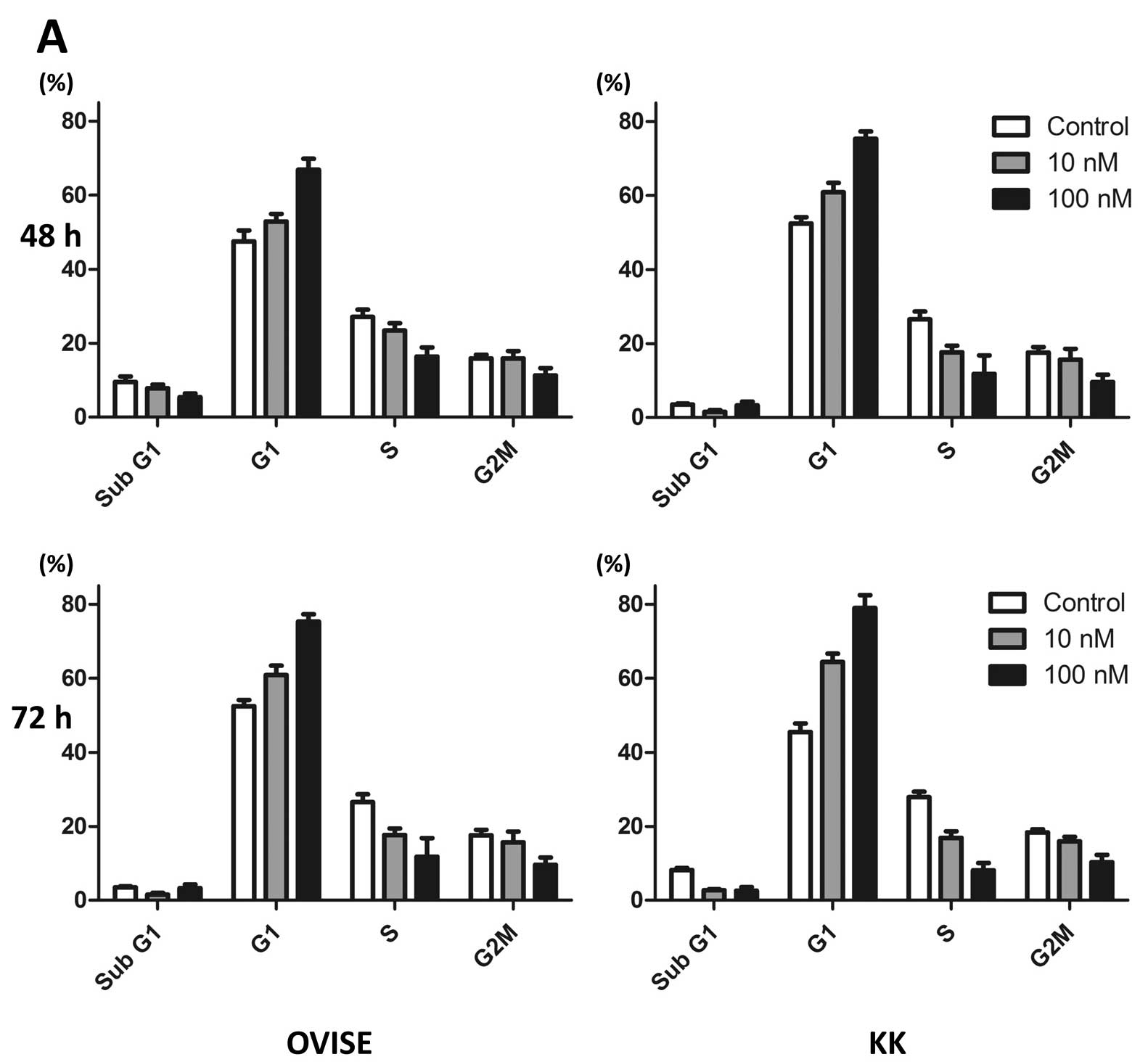
NVP-BEZ235 induces G1 phase
arrest and apoptosis in OCCC cells
OVISE cells were arrested at the G1
phase, but did not exhibit apoptosis (denoted by an increased
proportion of cells in sub-G1), after 72 h of treatment
with 10 and 100 nM NVP-BEZ235 (Fig.
2A). We observed similar results of G1 arrest in the
KK cells (Fig. 2A). Although the
same conditions as those in the cell cycle analysis did not induce
apoptosis, treatment of OVISE cells with 1 or 5 μM of NVP-BEZ235
for 96 h increased the number of Annexin V-positive and PI-negative
cells (Fig. 2B). Similar results
were observed in the KK cells (Fig.
2B).
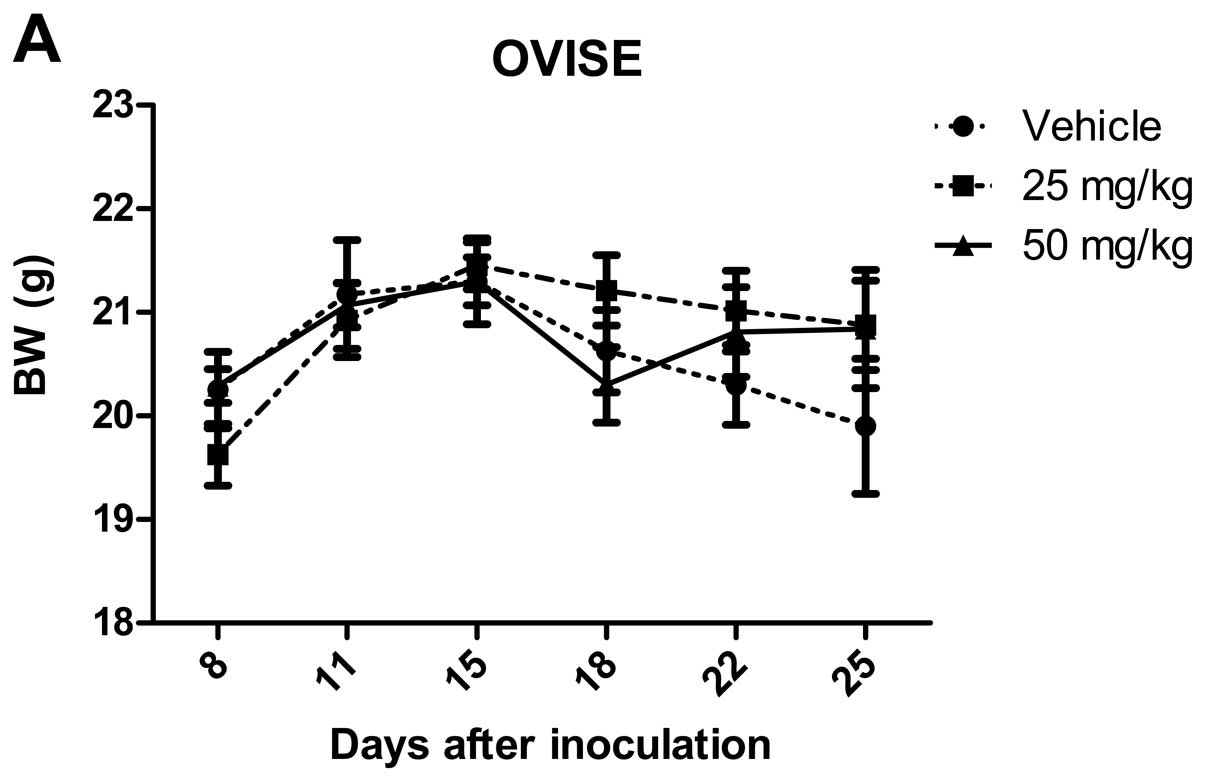
NVP-BEZ235 suppresses tumor growth in an
OCCC xenograft model
To assess short-term systemic toxicity of the agent,
we recorded body weight changes of mice in addition to visual
observation. After treatment, no mice had detectable changes in
body weight, implying that there was no severe toxicity (Fig. 3A). At doses of 25 or 50 mg/kg/day,
NVP-BEZ235 significantly inhibited subcutaneous tumor growth in
mice bearing OVISE cells (P<0.05 for 25 mg/kg/day, P<0.01 for
50 mg/kg/day) (Fig. 3B). TU-OC-1
tumor volume in the 50 mg/kg/day group was significantly lower than
that of the vehicle control although that in the 25 mg/kg/day group
was not (P<0.01 for 50 mg/kg/day) (Fig. 3C).
Discussion
Many authors have reported poorer prognoses for
patients with advanced stage OCCC (4,20,21).
Low survival rates in OCCC may, in part, reflect its lack of
sensitivity to platinum-based chemotherapy. There are no
antineoplastic agents definitely active and effective to treat
OCCC. Therefore, novel therapeutic strategies, including targeted
therapy, are needed to improve the prognosis of patients with
OCCC.
It is known that mutations of PIK3CA are
common molecular genetic alterations identified in OCCC (11). Expression of phospho-mTOR was found
to be more prominent in OCCC than in OSAC (22). mTOR exists in two distinct
complexes, mTOR complex 1 (mTORC1) and mTORC2. The downstream
targets of mTORC1 are p70 ribosomal S6 kinase 1 (p70S6K) and
eukaryotic translation initiation factor 4E-binding protein 1
(4E-BP1), both of which are crucial to the regulation of protein
synthesis.
In practice, inhibitors of mTORC1, such as
temsirolimus and everolimus, have been used for renal cell
carcinoma. A phase II study (GOG268) combining temsirolimus with
carboplatin and paclitaxel following temsirolimus consolidation as
first-line therapy is underway in patients with OCCC (23). However, the efficacy of mTORC1
blockade may be attenuated due to the loss of an mTORC1-dependent
negative feedback loop on PI3K signaling and the mTORC2-mediated
activation of Akt (24). p70S6K
inhibits insulin receptor substrate 1 (IRS-1) by phosphorylating
it, by inducing it to degrade and by altering its localization
(25,26). The inhibition of IRS by p70S6K
attenuates PI3K-AKT activation. Rapamycin (and its analogs
temsirolimus and everolimus) stops this negative feedback loop from
the p70S6K to the PI3K signaling pathway, resulting in activation
of proliferative and prosurvival effectors such as AKT.
NVP-BEZ235 is a dual pan-class I PI3K and an mTOR
kinase inhibitor that has the possible advantage of inhibiting
PI3K, mTORC1 and mTORC2. Therefore, it should turn off this pathway
completely and overcome feedback inhibition that is normally
observed with mTORC1 inhibitors (e.g. rapamycin analogs). It is
known that NVP-BEZ235 displays significant antitumor activities in
glioblastoma, lung, breast, renal cell and uterine endometrial
carcinomas (12,14,13,27).
In the present study, IC50 of
temsirolimus was markedly higher than NVP-BEZ235 in all OCCC cell
lines. In contrast, NVP-BEZ235 effectively suppressed proliferation
of OCCC cells. Additionally, treatment with temsirolimus increased
expression of pAKT while p-p70S6K and p4E-BP1 were suppressed.
Treatment with NVP-BEZ235 suppressed pAkt, p-p70S6K and p4E-BP1.
Accordingly, NVP-BEZ235 may be the more effective agent.
We found that NVP-BEZ235 suppressed tumor growth in
an OCCC xenograft model. A few authors have reported on the
antitumor activity of this compound in ovarian carcinoma. Montero
et al (28) showed that
NVP-BEZ235 effectively suppressed proliferation of 4 ovarian
carcinoma cell lines which were not derived from OCCC.
Santiskulvong et al (29),
investigated the in vivo effects of NVP-BEZ235 on an
immunocompetent transgenic murine ovarian endometrioid
adenocarcinoma model. They also examined in vitro activity
of the compound in 17 human ovarian carcinoma cell lines including
2 OCCC cell lines (ES-2 and OV207). Unfortunately, these studies
did not focus on OCCC. Recently, Rahman et al (30) investigated the frequency of
PIK3CA mutations in patients with OCCC and the relationship
between the mutations and clinicopathological or biological
variables. They also examined the in vitro sensitivity of 9
OCCC cell lines to LY294002, temsirolimus and NVP-BEZ235. No
relationship was observed between the mutation status and
sensitivity to these inhibitors. We also examined the mutation
status of PIK3CA and KRAS genes and baseline protein
expression levels of the PI3K/Akt/mTOR pathway molecules. Although
the PIK3CA mutation was more common in OCCC than in OSAC in
our series, there were no relationships between the mutation status
or protein expression levels and sensitivity to NVP-BEZ235. These
findings supported those of a previous report (30).
Our results revealed that NVP-BEZ235 effectively
suppressed not only p-p70S6K and p4E-BP1, but also pAKT expression
in OCCC cell lines and suppressed tumor growth in an OCCC xenograft
model. This is the first report to demonstrate the efficacy of
NVP-BEZ235 in OCCC.
We conclude that the PI3K-AKT-mTOR pathway is a
potential therapeutic target for OCCC and that NVP-BEZ235 warrants
investigation as a therapeutic agent.
Acknowledgements
We thank Dr Kazuyuki Kitatani of the Medical
Megabank Organization at Tohoku University for providing technical
advice and Dr Yuji Nakayama and Ms. Hiromi Miyauchi of the Division
of Functional Genomics, Research Center for Bioscience and
Technology at Tottori University for assisting with the cell cycle
analysis. The present study was supported by the Project for
Development of Innovative Research on Cancer Therapeutics, from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan and Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science, and Technology of Japan
(25462594 to T.O.).
Abbreviations:
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PDK1
|
phosphatidylinositol-dependent kinase
1
|
|
mTOR
|
mammalian target of rapamycin
|
|
OCCC
|
ovarian clear cell carcinoma
|
|
OSAC
|
ovarian serous adenocarcinoma
|
References
|
1
|
Scully RE: World Health Organization
classification and nomenclature of ovarian cancer. J Natl Cancer
Inst Monogr. 42:5–7. 1975.PubMed/NCBI
|
|
2
|
McGuire V, Jesser CA and Whittemore AS:
Survival among U.S. women with invasive epithelial ovarian cancer.
Gynecol Oncol. 84:399–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kennedy AW, Biscotti CV, Hart WR and
Webster KD: Ovarian clear cell adenocarcinoma. Gynecol Oncol.
32:342–349. 1989. View Article : Google Scholar
|
|
4
|
Sugiyama T, Kamura T, Kigawa J, et al:
Clinical characteristics of clear cell carcinoma of the ovary: a
distinct histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtney KD, Corcoran RB and Engelman JA:
The PI3K pathway as drug target in human cancer. J Clin Oncol.
28:1075–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakayama K, Nakayama N, Kurman RJ, et al:
Sequence mutations and amplification of PIK3CA and AKT2 genes in
purified ovarian serous neoplasms. Cancer Biol Ther. 5:779–785.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Huang J, Yang N, et al:
Integrative genomic analysis of phosphatidylinositol 3′-kinase
family identifies PIK3R3 as a potential therapeutic target
in epithelial ovarian cancer. Clin Cancer Res. 13:5314–5321.
2007.
|
|
8
|
Campbell IG, Russell SE, Choong DYH, et
al: Mutation of the PIK3CA gene in ovarian and breast
cancer. Clin Cancer Res. 64:7678–7681. 2004.
|
|
9
|
Altomare DA, Wang HQ, Skele KL, et al: AKT
and mTOR phosphorylation is frequently detected in ovarian cancer
and can be targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mabuchi S, Kawase C, Altomare DA, et al:
mTOR is a promising therapeutic target both in cisplatin-sensitive
and cisplatin-resistant clear cell carcinoma of the ovary. Clin
Cancer Res. 15:5404–5413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuo KT, Mao TL, Jones S, et al: Frequent
activating mutations of PIK3CA in ovarian clear cell
carcinoma. Am J Pathol. 174:1597–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maira SM, Stauffer F, Brueggen J, et al:
Identification and characterization of NVP-BEZ235, a new orally
available dual phosphatidylinositol 3-kinase/mammalian target of
rapamycin inhibitor with potent in vivo antitumor activity.
Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brachmann S, Hofmann I, Schnell C, et al:
Specific apoptosis induction by the dual PI3K/mTOR inhibitor
NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells.
Proc Natl Acad Sci. 106:22299–22304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho DC, Cohen MB, Panka DJ, et al: The
efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235
compared with rapamycin in renal cell carcinoma. Clin Cancer Res.
16:3628–3638. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itamochi H, Kato M, Nishimura M, et al:
Establishment and characterization of a novel ovarian serous
adenocarcinoma cell line, TU-OS-4, that overexpresses EGFR and
HER2. Hum Cell. 25:111–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Itamochi H, Kato M, Nishimura M, et al:
Establishment and characterization of a novel ovarian clear cell
carcinoma cell line, TU-OC-1, with a mutation in the PIK3CA
gene. Hum Cell. 26:121–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itamochi H, Oishi T, Shimada M, et al:
Inhibiting the mTOR pathway synergistically enhances cytotoxicity
in ovarian cancer cells induced by etoposide through upregulation
of c-Jun. Clin Cancer Res. 17:4742–4750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McIntyre AJ, Summersgill BM, Spendlove HE,
et al: Activating mutations and/or expression levels of tyrosine
kinase receptors GRB7, RAS, and BRAF in
testicular germ cell tumors. Neoplasia. 7:1047–1052. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li VSW, Wong CW, Chan TL, et al: Mutations
of PIK3CA in gastric adenocarcinoma. BMC Cancer. 5:292005.
View Article : Google Scholar
|
|
20
|
Rauh-Hein AJ, Winograd D, Growdon WB, et
al: Prognostic determinants in patients with uterine and ovarian
clear carcinoma. Gynecol Oncol. 125:376–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pectasides D, Fountzilas G, Aravantinos G,
et al: Advanced stage clear-cell epithelial ovarian cancer: the
Hellenic Cooperative Oncology Group experience. Gynecol Oncol.
102:285–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazawa M, Yasuda M, Fujita M, et al:
Therapeutic strategy targeting the mTOR-HIF-1α-VEGF pathway in
ovarian clear cell adenocarcinoma. Pathol Int. 59:19–27. 2009.
|
|
23
|
Itamochi H and Kigawa J: Clinical trials
and future potential of targeted therapy for ovarian cancer. Int J
Clin Oncol. 17:430–440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Efeyan A and Sabatini DM: mTOR and cancer:
many loops in one pathway. Curr Opin Cell Biol. 22:169–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harrington LS, Findlay GM, Gray A, et al:
The TSC1-2 tumor suppressor controls insulin-PI3K signaling via
regulation of IRS proteins. J Cell Biol. 166:213–223. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hartley D and Cooper GM: Role of mTOR in
the degradation of IRS-1: regulation of PP2A activity. J Cell
Biochem. 85:304–314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shoji K, Oda K, Kashiyama T, et al:
Genotype-dependent efficacy of a dual PI3K/mTOR inhibitor,
NVP-BEZ235, and an mTOR inhibitor, RAD001, in endometrial
carcinomas. PLoS One. 7:e374312012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montero JC, Chen X, Ocaria A, et al:
Predominance of mTORC1 over mTORC2 in the regulation of
proliferation of ovarian cancer cells: therapeutic implications.
Mol Cancer Ther. 11:1342–1352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santiskulvong C, Konecny GE, Fekete M, et
al: Dual targeting of phosphoinositide 3-kinase and mammalian
target of rapamycin using NVP-BEZ235 as a novel therapeutic
approach in human ovarian carcinoma. Clin Cancer Res. 17:2373–2384.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rahman M, Nakayama K, Rahman MT, et al:
Clinicopathologic and biological analysis of PIK3CA mutation
in ovarian clear cell carcinoma. Hum Pathol. 43:2197–2206.
2012.PubMed/NCBI
|