Introduction
Glycosylation is one of the most common
post-translational modifications. Many previous studies have
concluded that aberrant glycosylation of cell surface glycoproteins
is associated with the invasive and metastatic behavior of tumor
cells (1). These oligosaccharides
are synthesized by the sequential action of glycosyltransferases
localized in the endoplasmic reticulum (ER) and Golgi apparatus.
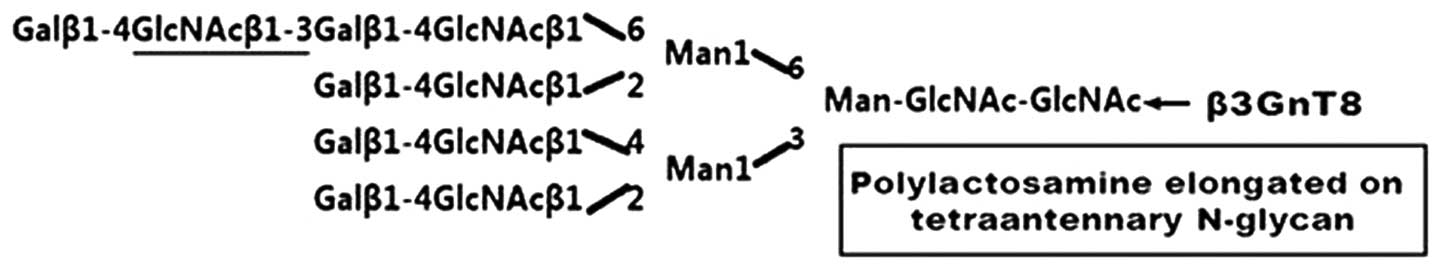
β-1,3-N-acetylglucosaminyltransferase-8 (β3GnT8), a member
of the β3GnT family, is involved in the biosynthesis of
polylactosamine chains on β1-6-branched N-glycans in vitro
(2–4). It transfers GlcNAc to the
tetraantennary non-reducing terminus of N-glycans to form a
polylactosamine structure in vitro (Fig. 1). Polylactosamine comprises repeated
(Galβ1-4GlcNAcβ1-3)n, and attaches to O-glycans, N-glycans or
glycolipids. Polylactosamine is often modified to carry important
carbohydrate structures such as Lewis-related antigens (5–7) and
the HNK-1 antigen (8), and has many
major roles in physiological functions.
Previous research in our laboratory confirmed the
ability of β3GnT8 to modulate matrix metalloproteinase-2 (MMP-2) in
AGS gastric cancer cells and elucidated the related mechanisms
(9). We found that siRNA-mediated
suppression of β3GnT8 directly reduced MMP-2 expression and
activity as assessed by RT-PCR, western blotting and gelatin
zymography. Moreover, a cell invasion assay using Matrigel-coated
Transwell inserts showed that the invasive ability was greatly
suppressed in β3GnT8 siRNA-transfected cells. Furthermore, cells
overexpressing the β3GnT8 gene (when transfected with the
pEGFP-C1-β3GnT8 plasmid) exhibited upregulated MMP-2 gene
expression, and the invasive ability of these cells was also
enhanced. Protein-protein interaction analysis using the STRING
database showed that β3GnT8 and MMP-2 may have a related signaling
pathway. Therefore, our results may reveal a new mechanism by which
β3GnT8 can regulate MMP-2 expression to affect tumor progression
(9). However, MMP-2 is not a
glycoprotein. Thus, we hypothesized that β3GnT8 affects cellular
signal transduction by altering the glycan structure of various
glycoproteins on the cell surface, which further influences
expression of MMP-2. This detailed signal transduction pathway is
yet unknown.
The molecule CD147 (basigin/EMMPRIN, extracellular
matrix metalloproteinase inducer) is a cell surface transmembrane
glycoprotein which is highly expressed in tumor cells (10–12).
CD147 consists of two immunoglobulin domains in the extracellular
region: a single transmembrane domain and a short cytoplasmic
domain containing 39 aa. The extracellular region of CD147 contains
three Asn glycosylation sites, and the N-glycosylation sites make
similar contributions to both high and low glycoforms of CD147
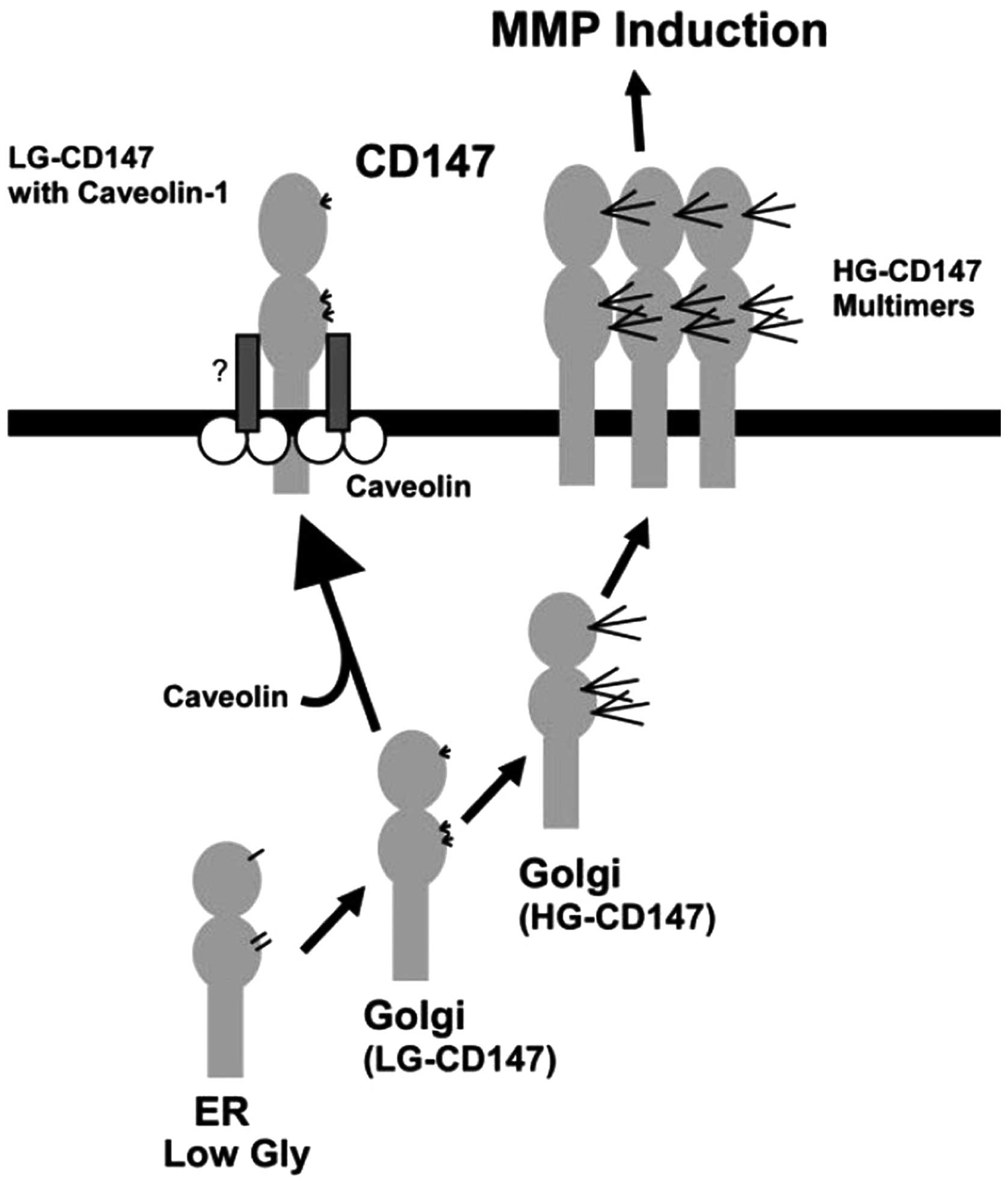
(HG-CD147 and LG-CD147). The different glycosylation pattern of the
native 28-kDa protein accounts for its variable molecular weight,
ranging between 44 and 66 kDa (10). HG-CD147 (~40–60 kDa) contains a
complex-type carbohydrate, and LG-CD147 (~32 kDa) contains the
mannose form. It is well known that the carbohydrate side chain is
then processed in the ER and Golgi network to produce a mature
glycoprotein that is exported through the secretory machinery to
the plasma membrane (13). After
CD147 enters the Golgi complex, it can mature via two possible
pathways (11,14,15).
Previous studies have confirmed that modulation of CD147 is
associated with the expression of MMPs in normal or tumor tissues
(16,17). This suggests that this
CD147-mediated MMP induction could be a common mechanism in
physiological or pathological situations. Many studies have
confirmed that CD147 induces MMP expression via Rac1-mediated
PI3K/Akt/IKK-dependent IκB-α degradation and NF-κB activation, and
by MKK7/JNK-dependent AP-1 activation (14,18,19).
It has also been confirmed that only the high glycosylated CD147,
except purified deglycosylated CD147 and LG-CD147, determines MMP
stimulatory activity (11,12,17,20)
(Fig. 2). Moreover, excess HG-CD147
glycosylation is attributed to β1-6-branched N-glycan to form
polylactosamine content.
β3GnT8 catalyzes and then extends a polylactosamine
chain specifically on β1-6-branched tetra-antennary N-glycans
(15,21). It has been cloned and characterized
by us and other groups (3,22). In the present study, we designed a
series of experiments to investigate whether β3GnT8 plays an
important role in CD147 signal transduction as an upstream
modulator of MMP production in tumor cells.
Materials and methods
Materials
The human glioma cell lines U251, LN229 and U87;
human breast adenocarcinoma cell lines MCF and M231 and human
gastric cancer cell line AGS were obtained from the American Type
Culture Collection (ATCC; Rockville, MD, USA). The human gastric
cancer cell line SGC-7901 was obtained from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Science.
Anti-human β3GnT8 polyclonal antibody was produced from rabbits in
our laboratory. Anti-CD147 polyclonal and anti-β-actin antibodies
were purchased from Santa Cruz Biotechnology. Anti-rabbit-HRP,
anti-goat-HRP and anti-mouse-HRP secondary antibodies were
purchased from Beyotime. Lycopersicon esculentum (tomato;
LEA) and phycoerythrin streptavidin were purchased from
Sigma-Aldrich. Other reagents were commercially available in
China.
Cell culture
The human glioma cell lines U251, LN229 and U87 were
cultured in Dulbecco’s modified Eagle’s medium (DMEM, high glucose;
Gibco-BRL) supplemented with 10% fetal bovine serum (FBS). The
human gastric cancer SGC7901 cell line and human breast
adenocarcinoma MCF and M231 cell lines were cultured in RPMI-1640
medium supplemented with 10% FBS. All cell lines were cultured in a
humidified atmosphere with 5% CO2 at 37°C.
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total-RNA was extracted from the various cancer cell
lines described above, using TRIzol (Gibco-BRL), according to the
manufacturer’s instructions. Complementary DNA (cDNA) was generated
from total-RNA using M-MLV reverse transcriptase (MBI, Fermentas,
Lithuania). The PCR conditions were as follows: initial denaturing
at 95°C for 5 min, 30 cycles of denaturing at 95°C for 30 sec,
annealing at 60°C for 45 sec, elongation at 72°C for 1 min, and
finally at 72°C for 10 min. The annealing temperature for β3GnT8
was 60°C; for MMP-2, 55°C and for β-actin, 53°C.
Specific primers (Invitrogen) used for the genes and
the expected product sizes were as follows:
5′-GGCCTGACCTAGACTCACTAGTG-3′ (sense) and
5′-CGCAGTGCGGTCTGCTGGCCAG-3′ (antisense) for β3GnT8 (518 bp);
5′-AACCCTCAGAGCCACCCCTA-3′ (sense) and 5′-GTGCATACAAAGCAAACTGC-3′
(antisense) for MMP-2 (286 bp); 5′-GAGCTACGAGCTGCCTGACG-3′ (sense)
and 5′-CCTAGAAGCATTTGCGGTGG-3′ (antisense) for β-actin (416 bp).
The PCR products were separated by electrophoresis on 10 g/l
agarose gel and visualized by ethidium bromide staining.
Lipofectamine-mediated cell
transfection
Cells (LN229, SGC-7901, U251) were seeded in a
6-well plate at a density of 60–70%. After 12 h, they were
transfected with pSilencircle-β3GnT8Scr and pSilencircle-β3GnT8Si
using Lipofectamine™ 2000 transfection reagent according to the
manufacturer’s protocol, followed by selection with G418 (500
μg/ml). Additionally, untransfected cells served as the control.
The stable cells were correspondingly named T8Scr and T8Si, and
untransfected cells were named NC.
Western blot analysis
Western blot analysis was conducted using standard
methods. Protein was extracted from the cell lysates using ice-cold
radioimmunoprecipitation assay (RIPA)lysis buffer (50 mM Tris pH
7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS,
sodium orthovanadate, sodium fluoride, EDTA and leupeptin)
supplemented with 1 mM PMSF. The protein concentration in the cell
lysates was determined using a protein assay kit (KeyGEN Biotech,
China). An equal amount of protein from each sample was mixed with
4X loading buffer (250 mM Tris-HCl, 40% glycerol, 5% SDS, 0.005%
bromophenol blue and 100 mM DTT) and denatured for 5 min at 100°C.
Total proteins were then separated by SDS/polyacrylamide gel
electrophoresis (10% acrylamide gel) and transferred onto
polyvinylidene fluoride (PVDF) membranes that had been pretreated
with methanol. The membranes were blocked for 1 h at room
temperature in PBS-T (PBS with 0.05% Tween-20™) containing 5% skim
milk. The proteins were analyzed using specific antibodies as
indicated. Blots were incubated overnight at 4°C with the primary
antibodies against β3GnT8 (1:400), CD147 (1:300) and β-actin
(1:1,000). After removal of the primary antibody, the blots were
incubated for 1 h at room temperature with goat anti-rabbit, donkey
anti-goat, rabbit anti-mouse IgG (1:1,000) horseradish peroxidase
(HRP)-conjugated secondary antibodies. For detection, enhanced
chemiluminescence was used according to the manufacturer’s
instructions (ECL Plus Detection System, Beyotime).
Flow cytometry
To detect the presence or absence of certain
carbohydrate determinants, the stable cells (LN229 and SGC-7901)
and NC, T8Scr and T8Si cells were stained with plant lectin and
analyzed by flow cytometry (FACScan; BD Biosciences). Approximately
5×105 cells were incubated for 2 h at room temperature
in 500 μl of assay buffer (10 mM HEPES, 0.15 M NaCl, 0.08%
NaN3, 0.1 mM CaCl2 and 1% BSA pH 7.5)
containing biotinylated lectin (Sigma-Aldrich) and LEA lectin (20
μg/ml). Lectin-stained cells were washed with TPBS (PBS + 0.05%
Tween-20) and incubated with streptavidin-R-phycoerythrin (0.4
μg/ml; Sigma-Aldrich) for 1 h at room temperature in 500 μl of
assay buffer (0.01 M phosphate-buffered saline pH 7.4, 1% BSA and
15 mM sodium azide). The cell suspensions were washed in TPBS in
300 μl PBS containing 1% BSA and analyzed for fluorescent intensity
by flow cytometry.
Immunofluorescent staining
Expression of certain carbohydrate determinants was
determined by fluorescence microscopy. The stable cells LN229, NC,
T8Scr and T8Si were fixed with 4% paraformaldehyde for 30 min at
room temperature, followed by permeabilization with 0.2% Triton
X-100 in PBS and blocking of non-specific binding with Carbo-Free™
blocking solution (Vector) for 30 min at room temperature.
Biotinylated LEA lectin (20 μg/ml) in assay buffer (10 mM HEPES,
0.15 M NaCl, 0.08% NaN3, 0.1 mM CaCl2, 1% BSA
pH 7.5) was applied to the LN229 cells and incubated for 2 h at
room temperature. After being washed with TPBS (PBS + 0.05%
Tween-20), the LN229 cells were incubated with
Streptavidin-R-phycoerythrin (0.4 μg/ml) for 1 h at room
temperature in assay buffer (0.01 M PBS pH 7.4, 1% BSA and 15 mM
sodium azide). After being washed with TPBS, images were obtained
using an inverted fluorescence microscope combined with a digital
camera.
Regulation of CD147 glycosylation and
MMP-2 expression by tunicamycin
Briefly, in vitro, the cells (SGC-7901 and
U251) were seeded in 6-well plate and pre-incubated overnight. The
cells were washed once with PBS and cultured for 24 h in fresh
culture media in the absence or presence of tunicamycin in a
dose-dependent manner (0, 2.5, 5 μg/ml). The cells were harvested,
and CD147 and MMP-2 expression was determined by western blotting
and RT-PCR analysis.
Analysis of protein-protein interaction
(PPI) networks of CD147 and MMPs using the STRING database
STRING is a database of known and predicted protein
interactions. The interactions include direct (physical) and
indirect (functional) associations. They are derived from four
sources: genomic context, high-throughput experiments, coexpression
(conserved) and previous knowledge. STRING quantitatively
integrates interaction data from these sources for a large number
of organisms, and transfers the information between these organisms
where applicable. The database currently covers 5,214,234 proteins
from 1,133 organisms. We set out to study the PPI network derived
from an analysis of the STRING database showing that CD147 and MMPs
have a related signaling pathway.
Statistical analyses
Statistical analysis was performed using SPSS 13.0
software®. Results are expressed as mean ± SD.
Statistical significance was evaluated for data from three
independent experiments using the Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
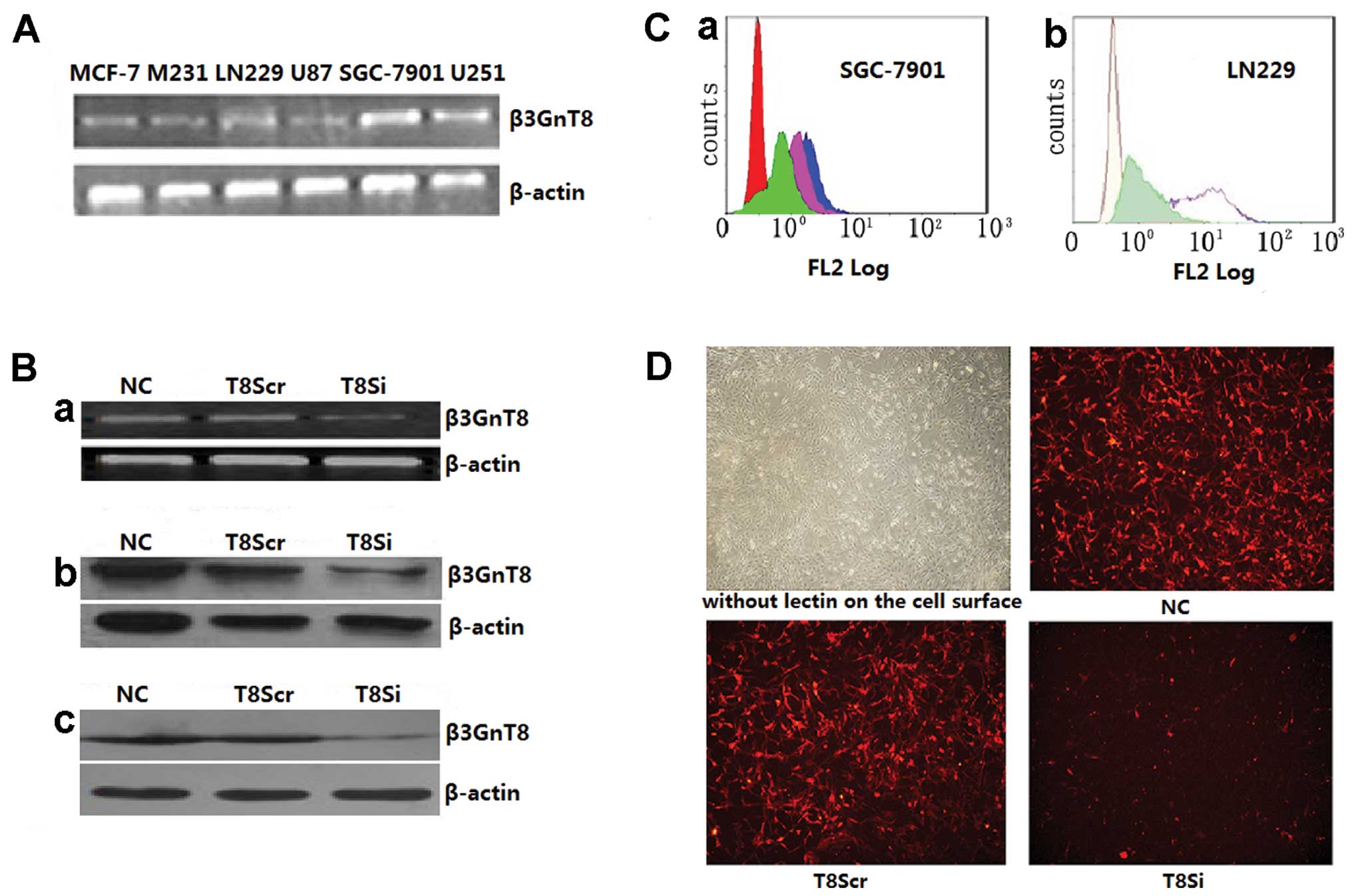
β3GnT8 mRNA expression in 6 cancer cell
lines (MCF-7, M231, LN229, U87, SGC-7901 and U251)
As shown in Fig. 3A,
the β3GnT8 mRNA expression level in the LN229, SGC-7901 and U251
cells was higher than that in the other cell lines. Hence, these
three cell lines with high β3GnT8 expression were chosen to study
the relationship between β3GnT8 and CD147 glycosylation.
Establishment of β3GnT8 downregulation in
SGC-7901 and LN229 cells
To investigate whether β3GnT8 plays an important
role in the CD147 signal transduction pathway as an upstream
modulator of MMP production in tumor cells, we constructed two
β3GnT8-knockdown cell lines derived from SGC-7901 and LN229 cells,
and measured the mRNA and protein expression levels by RT-PCR and
western blotting, respectively. As shown in Fig. 3B-a, the β3GnT8 transcripts were
decreased in the T8Si SGC-7901 cells when compared with its control
groups (P<0.05). Similar to the RT-PCR results, the β3GnT8
protein was markedly reduced in the T8Si SGC-7901 and T8Si LN229
cell lines (Fig. 3B-b and -c;
P<0.05). These results revealed that we successfully constructed
two β3GnT8-knockdown cell lines derived from SGC-7901 and
LN229.
Biosynthesis of polylactosamine following
β3GnT8 downregulation in the SGC-7901 and LN229 cells
To determine the enzymatic activity of β3GnT8 in
vivo, the two constructed β3GnT8-knockdown cell lines, SGC-7901
and LN229, were used in this experiment. Cell surface expression of
carbohydrate chains were detected by flow cytometric analysis and
immunofluorescent staining using LEA lectin, which can recognize
the polylactosamine chain of N-glycans. As shown in Fig. 3C, compared with the β3GnT8Scr
transfectants and NC, the β3GnT8Si transfectants of SGC-7901 and
LN229 exhibited reduced levels of LEA lectin (P<0.05).
Immunofluorescent staining was performed to observe the alteration
of carbohydrate chains in the LN229 cells. Similar to the results
of the flow cytometric analysis, the fluorescence intensity of the
T8Si cell groups was apparently weaker when compared with the Scr
and NC transfectants when incubated with LEA lectin against
polylactosamine (Fig. 3D,
P<0.05). These results indicate that β3GnT8 is involved in the
biosynthesis of polylactosamine chains on the β1-6-branched
N-glycans.
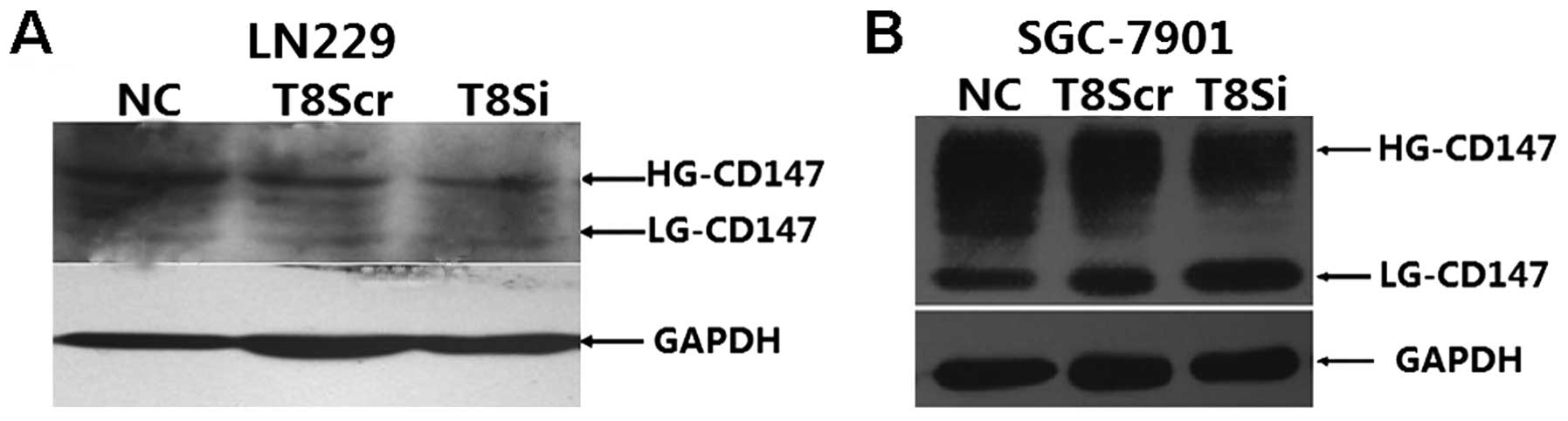
β3GnT8 regulates CD147 glycosylation
following β3GnT8 downregulation of the SGC-7901 and LN229
cells
To verify whether β3GnT8 regulates CD147
glycosylation, the two previously constructed SGC-7901 and LN229
cell lines with β3GnT8 downregulation were also tested in the
experiment. As shown in Fig. 4, the
T8Si SGC-7901 and T8Si LN229 cells exhibited a direct reduction in
the levels of HG-CD147 protein compared with the T8Scr
transfectants and NC groups (P<0.05).
Characterization of LG-CD147 and HG-CD147
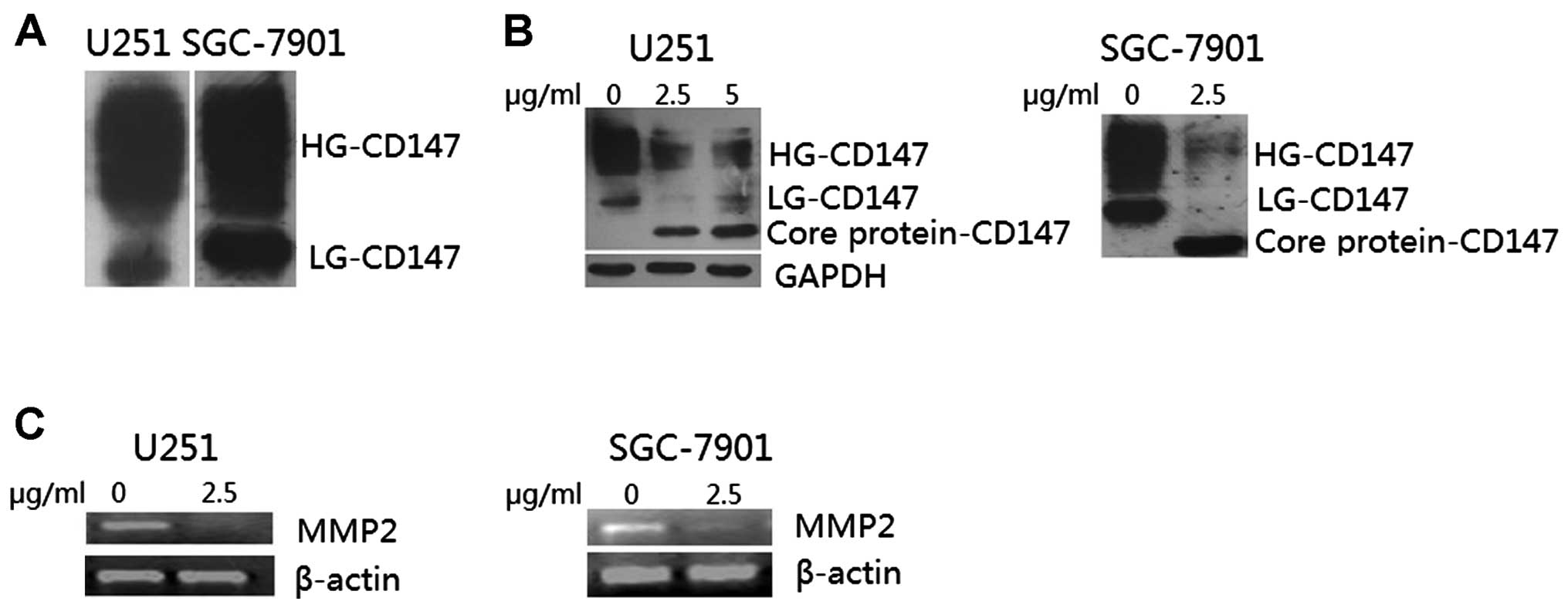
forms in the U251 and SGC-7901 cells
Western blot analysis revealed that a large amount
of CD147 was highly expressed in the U251 and SGC-7901 cells as
indicated by broad bands with apparent molecular weights ranging
from 32 to 65 kDa, which are characteristic of CD147 polypeptides
with extensive and heterogeneous glycosylation. Variability in
CD147 N-glycosylation was detected (Fig. 5A). Obviously, CD147 was present in
the U251 and SGC-7901 cells as a high glycosylated (HG) form
migrating at ~45–65 kDa and as a low glycosylated (LG) form
migrating at ~32–44 kDa, depending on the glycosylation of the core
protein (27 kDa).
Effect of tunicamycin on CD147
glycosylation and MMP-2 expression in U251 and SGC-7901 cells
Western blot analysis revealed that CD147 did have
two different forms: a high glycosylated (HG) form and a low
glycosylated (LG) form. U251 and SGC-7901 cells were treated with
various concentrations of tunicamycin, an inhibitor of N-linked
glycosylation of newly synthesized proteins, for 24 h. As shown in
Fig. 5B, following tunicamycin
treatment in U251 and SGC-7901 cells, a single new band of
molecular weight (27 kDa) consistent with the size of the core
protein was visualized by western blotting (P<0.05). These
results suggest that N-linked glycosylation of CD147 was highly
sensitive to the inhibition of tunicamycin.
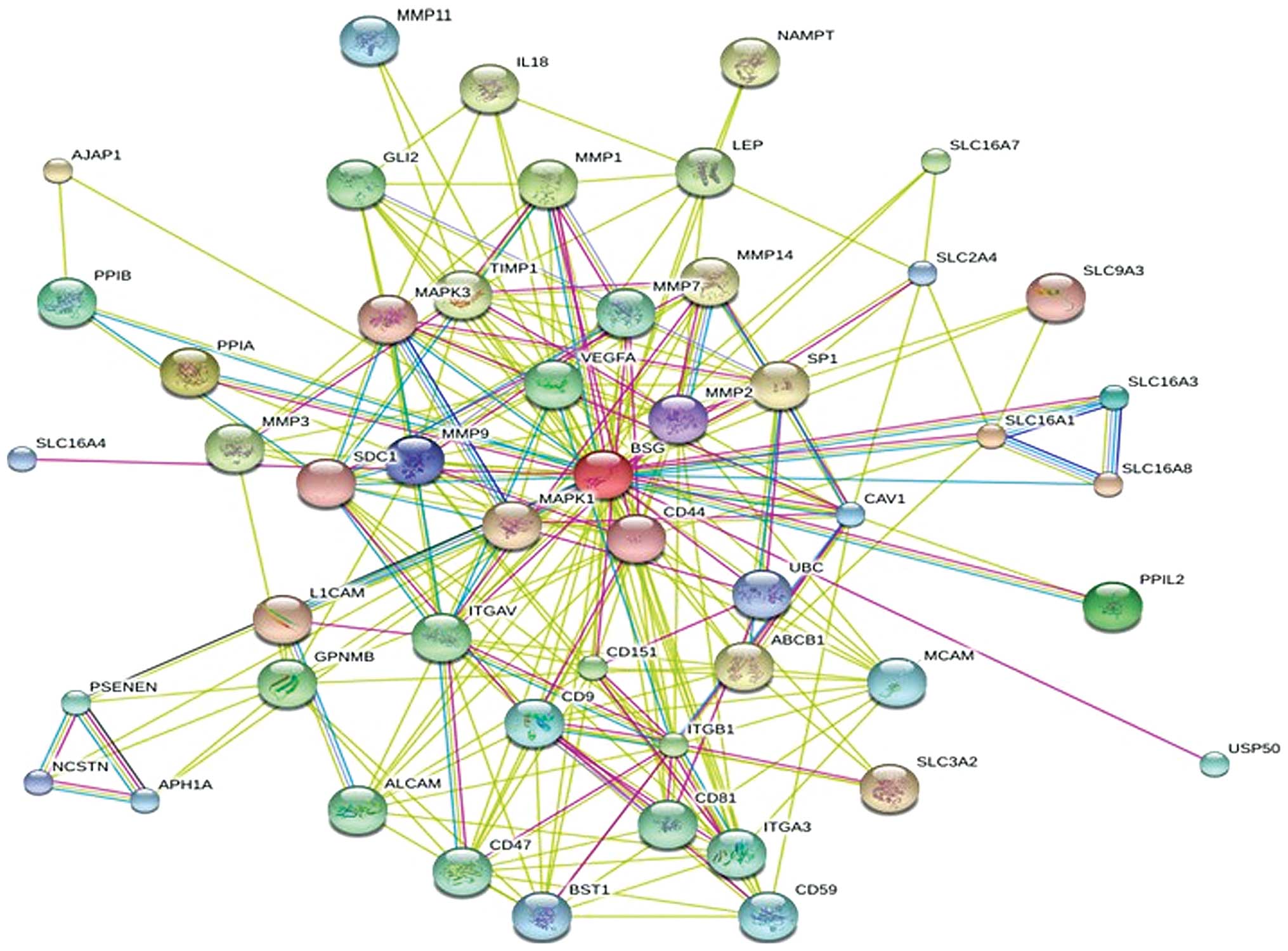
Analysis of protein-protein interaction
(PPI) networks of CD147 and MMPs
We studied the interacting neighbors of CD147 [also
called basigin (BSG)] using the STRING database. As shown in
Fig. 6, the analysis of
protein-protein interaction (PPI) networks of CD147 and MMPs was
derived from an analysis of the STRING database. The results
revealed that CD147 has a related signaling pathway with MMPs
including MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-11 and MMP-14.
Discussion
The complex carbohydrate chains of glycoproteins
(O-glycans and N-glycans), glycolipids and proteoglycans represent
secondary gene products formed through the reactions of numerous
glycosyltransferases. Aberrant carbohydration by the related
glycosyltransferases plays an important role in cell-cell and
cell-molecular recognition, such as receptor combination, signaling
pathway and molecular, which may lead to a variety of biological
alterations in cells (24).
The β3GnT familly contains 7 members (β3GnT2,
β3GnT3, β3GnT4, β3GnT5, β3GnT6, β3GnT7 and β3GnT8) which are able
to catalyze the initiation and elongation of polylactosamine
chains. However, they exhibit a different substrate specificity
dependent on the length of the polylactosamine chain (25,26).
All β3GnTs, namely β3GnT2, β3GnT3, β3GnT4, β3GnT5, β3GnT7 and
β3GnT8, except for β3GnT6, can transfer GlcNAc to Gal to synthesize
a polylactosamine chain. However, each differs in its preference
for acceptor molecules, i.e., core 1 O-glycan, glycolipids or
keratan sulfate (KS). Each enzyme may have distinct roles in
physiological processes. Notably, β3GnT8 was cloned and
characterized by various groups, including ours, as being involved
in the biosynthesis of polylactosamine chains on β1-6-branched
N-glycans in vitro (3,22). It
has been reported that β1-6-branched N-glycans containing
polylactosamine act on a variety of malignant phenotypes of tumor
cells, affecting cell proliferation (27) and metastatic potential (28–30).
Previous studies have demonstrated the ability of
β3GnT8 to modulate matrix metalloproteinase-2 (MMP-2) in AGS
gastric cancer cells and the possible mechanism involved (9,23).
However, the detailed signal transduction pathway is still unknown.
In the present study, we found that β3GnT8 plays an important role
in the CD147 signal transduction pathway as an upstream modulator
of MMP production in the tumor microenvironment. We found that
β3GnT8 is widely expressed in several different types of cancer
cell lines by RT-PCR analysis (Fig.
3A). In addition, Lipofectamine-mediated siRNA knockdown of
β3GnT8 was successfully conducted in the LN229 and SGC-7901 cell
lines for use in our further experiments (Fig. 3B). After β3GnT8 was downregulated in
LN229 and SGC-7901 cell lines, the levels of polylactosamine chains
were markedly reduced, when compared with the control groups by
flow cytometric analysis (Fig. 3C).
Similarly, immunofluorescent staining analysis demonstrated that
the cell surface polylactosamine chains of the β3GnT8Si
transfectants was visibly weaker when compared to the control
groups (Fig. 3D). These results
indicate that β3GnT8 is involved in the biosynthesis of
polylactosamine chains. Moreover, it has been reported that
glycoprotein CD147 has LG-CD147 and HG-CD147 forms (10,16).
Studies have confirmed that excess CD147 glycosylation is
attributable to β1-6-branches to form polylactosamine content
(11). Thus, it was hypothesized
that β3GnT8 is involved in the biosynthesis of polylactosamine
chains on the HG form of CD147. To demonstrate the validity of this
hypothesis, previously constructed β3GnT8 siRNA knockdown LN229 and
SGC-7901 cell lines were used. As evident from Fig. 4, the cells transfected with β3GnT8
siRNA exhibited a direct reduction in the levels of HG-CD147
protein when compared with levels in the control groups (Fig. 4A and B). In addition, a previous
study showed that N-glycosylation of CD147, particularly the
β1-6-branched N-glycans contributes to MMP-inducing activity in
tumor cells (20). Tumicamycin is
widely used as an inhibitor of N-linked glycosylation that blocks
the initial step in glycoprotein synthesis, thus blocking the
synthesis of all N-linked glycoproteins (31). Hence, various concentrations of
tunicamycin were used in this experiment to study its influence on
CD147 N-glycosylation and MMP-2 expression. As shown in Fig. 5A, LG-CD147 and HG-CD147 forms were
detected in the U251 and SGC-7901 cell lines by western blot
analysis. Following tumicamycin treatment, the levels of HG-CD147
and LG-CD147 decreased considerably, and a single new band of
molecular weight, consistent with the size of the core
protein-CD147, appeared, as compared with the control groups
(Fig. 5B). Meanwhile, the
expression of MMP-2 almost disappeared following tumicamycin
treatment, when compared with the level in the control groups
(Fig. 5C). This may indicate that
the LG-CD147 and HG-CD147 forms are present in cancer cells, and
further play a role in tumor progression, including promotion of
MMP-2 expression. However, once the glycosylation part of CD147
decreased or disappeared to the core protein-CD147, it lost the
function of promoting MMP-2 expression. Previous studies have shown
that inhibition of N-linked glycosylation reduces Akt
phosphorylation in U251 cells (13). Previously, we found that CD147
N-glycosylation regulates MMP expression through numerous possible
pathways; one is Akt-dependent IκB-α degradation (14). We speculated that N-glycosylation
levels of CD147 play an important role in MMP-2 expression through
Akt signal transduction. In general, from these results, we
demonstrated that the level of CD147 N-glycosylation mainly
contains β1-6-branched polylactosamine, which were catalyzed by
β3GnT8. In addition, the N-glycans of CD147 are important to
determine its MMP-2 stimulating expression.
In summary, the present study demonstrated that
β3GnT8 is involved in the biosynthesis of polylactosamine, which
regulates CD147 N-glycosylation and further influences MMP-2
expression in tumor cells. Therefore, we demonstrated that β3GnT8
may play an important role in the CD147 signal transduction pathway
as an upstream modulator of MMP-2 production in tumor cells. Thus,
this pathway may serve as a therapeutic target in preventing tumor
progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 31170772 ) and the Suzhou
Municipal Natural Science Foundation (SYS201208).
References
|
1
|
Hakomori S: Glycosylation defining cancer
malignancy: new wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narimatsu H: Human glycogene cloning:
focus on beta 3-glycosyltransferase and beta 4-glycosyltransferase
families. Curr Opin Struct Biol. 16:567–575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishida H, Togayachi A, Sakai T, et al: A
novel β1,3-N-acetylglucosaminyltransferase (β3Gn-T8), which
synthesizes poly-N-acetyllactosamine, is dramatically
upregulated in colon cancer. FEBS Lett. 579:71–78. 2005.
|
|
4
|
Mitsui Y, Yamada K, Hara S, Kinoshita M,
Hayakawa T and Kakehi K: Comparative studies on glycoproteins
expressing polylactosamine-type N-glycans in cancer cells. J Pharm
Biomed Anal. 70:718–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamze S, Harvey DJ, Chen YJ, Guile GR,
Dwek RA and Wing DR: Sialylated N-glycans in adult rat brain tissue
- a widespread distribution of disialylated antennae in complex and
hybrid structures. Eur J Biochem. 258:243–270. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishihara S, Iwasaki H, Kaneko M, Tawada
A, Ito M and Narimatsu H: α1,3-fucosyltransferase 9 (FUT9; Fuc-TIX)
preferentially fucosylates the distal GlcNAc residue of
polylactosamine chain while the other four α1,3FUT members
preferentially fucosylate the inner GlcNAc residue. FEBS Lett.
462:289–294. 1999.
|
|
7
|
Dennis JW, Granovsky M and Warren CE:
Glycoprotein glycosylation and cancer progression. Biochim Biophys
Acta. 1473:21–34. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S, Oka S, Inoue M, et al: Mice
deficient in nervous system-specific carbohydrate epitope HNK-1
exhibit impaired synaptic plasticity and spatial learning. J Biol
Chem. 277:27227–27231. 2002. View Article : Google Scholar
|
|
9
|
Shen L, Liu Z, Tu Y, Xu L, Sun X and Wu S:
Regulation of MMP-2 expression and activity by
β-1,3-N-acetylglucosaminyltransferase-8 in AGS gastric cancer
cells. Mol Biol Rep. 38:1541–1550. 2011.
|
|
10
|
Gabison EE, Hoang-Xuan T, Mauviel A and
Menashi S: EMMPRIN/CD147, an MMP modulator in cancer, development
and tissue repair. Biochimie. 87:361–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang W, Chang SB and Hemler ME: Links
between CD147 function, glycosylation, and caveolin-1. Mol Biol
Cell. 15:4043–4050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caudroy S, Polette M, Nawrocki-Raby B, et
al: EMMPRIN- mediated MMP regulation in tumor and endothelial
cells. Clin Exp Metastasis. 19:697–702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Contessa JN, Bhojani MS, Freeze HH,
Rehemtulla A and Lawrence TS: Inhibition of N-linked glycosylation
disrupts receptor tyrosine kinase signaling in tumor cells. Cancer
Res. 68:3803–3809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkatesan B, Valente AJ, Prabhu SD,
Shanmugam P, Delafontaine P and Chandrasekar B: EMMPRIN activates
multiple transcription factors in cardiomyocytes, and induces
interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB
andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 49:655–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto H, Swoger J, Greene S, et al:
β1,6-N-acetylglucosamine-bearing N-glycans in human
gliomas: implications for a role in regulating invasivity. Cancer
Res. 60:134–142. 2000.
|
|
16
|
Agrawal SM and Yong VW: The many faces of
EMMPRIN-roles in neuroinflammation. Biochim Biophys Acta.
1812:213–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fan J, Wang S, Yu S, He J, Zheng W and
Zhang J: N-acetylglucosaminyltransferase IVa regulates
metastatic potential of mouse hepatocarcinoma cells through
glycosylation of CD147. Glycoconj J. 29:323–334. 2012. View Article : Google Scholar
|
|
18
|
Yu XL, Jiang JL, Li L, Feng Q, Xu J and
Chen ZN: The glycosylation characteristic of hepatoma-associated
antigen HAb18G/CD147 in human hepatoma cells. Int J Biochem Cell
Biol. 38:1939–1945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J and Hemler ME: Regulation of MMP-1
and MMP-2 production through CD147/extracellular matrix
metalloproteinase inducer interactions. Cancer Res. 61:2276–2281.
2001.PubMed/NCBI
|
|
20
|
Huang W, Luo WJ, Zhu P, et al: Modulation
of CD147-induced matrix metalloproteinase activity: role of CD147
N-glycosylation. Biochem J. 449:437–448. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seko A and Yamashita K: Activation of
β1,3-N-acetylglucosaminyltransferase-2 (β3Gn-T2) by β3Gn-T8:
Possible involvement of β3Gn-T8 in increasing
poly-N-acetyllactosamine chains in differentiated HL-60 cells. J
Biol Chem. 283:33094–33100. 2008.
|
|
22
|
Huang C, Zhou J, Wu S, Shan Y, Teng S and
Yu L: Cloning and tissue distribution of the human B3GALT7 gene, a
member of the beta1,3-glycosyltransferase family. Glycoconj J.
21:267–273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saitoh O, Wang WC, Lotan R and Fukuda M:
Differential glycosylation and cell surface expression of lysosomal
membrane glycoproteins in sublines of a human colon cancer
exhibiting distinct metastatic potentials. J Biol Chem.
267:5700–5711. 1992.
|
|
24
|
Taniguchi N, Miyoshi E, Ko JH, Ikeda Y and
Ihara Y: Implication of N-acetylglucosaminyltransferases III and V
in cancer: gene regulation and signaling mechanism. Biochim Biophys
Acta. 1455:287–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Togayachi A, Akashima T, Ookubo R, et al:
Molecular cloning and characterization of
UDP-GlcNAc:lactosylceramide beta
1,3-N-acetylglucosaminyltransferase (beta 3Gn-T5), an essential
enzyme for the expression of HNK-1 and Lewis X epitopes on
glycolipids. J Biol Chem. 276:22032–22040. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shiraishi N, Natsume A, Togayachi A, et
al: Identification and characterization of three novel β
1,3-N-acetylglucosaminyltransferases structurally related to
the β1,3-galactosyltransferase family. J Biol Chem. 276:3498–3507.
2001.
|
|
27
|
Dennis JW, Laferte S and Vanderelst I:
Asparagine-linked oligosaccharides in malignant tumour growth.
Biochem Soc Trans. 17:29–31. 1989.PubMed/NCBI
|
|
28
|
Dennis JW and Laferte S: Oncodevelopmental
expression of -GlcNAc β1-6Man α 1-6Man β1-branched
asparagine-linked oligosaccharides in murine tissues and human
breast carcinomas. Cancer Res. 49:945–950. 1989.
|
|
29
|
Seberger PJ and Chaney WG: Control of
metastasis by Asn-linked, β-6-branched oligosaccharides in mouse
mammary cancer cells. Glycobiology. 9:235–241. 1999.
|
|
30
|
Granovsky M, Fata J, Pawling J, Muller WJ,
Khokha R and Dennis JW: Suppression of tumor growth and metastasis
in Mgat5-deficient mice. Nat Med. 6:306–312. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Del Grosso F, De Mariano M, Passoni L,
Luksch R, Tonini GP and Longo L: Inhibition of N-linked
glycosylation impairs ALK phosphorylation and disrupts pro-survival
signaling in neuroblastoma cell lines. BMC Cancer.
11:5252011.PubMed/NCBI
|