Introduction
MicroRNAs (miRNAs) are a recently discovered class
of small non-coding RNAs that regulate gene expression (1). Mature miRNAs are the results of
sequential processing of primary transcripts (pri-miRNAs) mediated
by two RNase III enzymes, Drosha and Dicer (2). Mature 18–24 mer long miRNAs negatively
regulate protein expression of specific mRNAs by either
translational inhibition or mRNA degradation (3). miRNAs are differentially expressed in
various human cancers. A number of previous reports have provided a
considerable amount of support for a critical role of many miRNAs
in cancer (4–6). Strong correlations between miRNA
expression profiles and specific cancer lineages have been observed
(for reviews,7,8), and that may be due to their fundamental
importance in regulatory processes involved in establishing and
maintaining the tumor phenotype. Further support for their role in
tumorigenesis may come from genetic evidence. Notably, Calin et
al showed that miRNAs are often encoded in fragile sites in the
genome, where their expression can be altered by events such as
genomic amplification, loss of heterozygosity, viral integration or
genomic rearrangement (9). Another
study also found that miRNAs display a high frequency of genomic
alterations in human cancers (10).
Array profiling studies have shown strong correlations between the
expression of specific miRNAs and the tumor phenotype (11–14).
Further evidence for an oncogenic role of certain miRNAs came from
transgenic mouse studies showing that overexpression of the miR-19
cluster or miR-155 results in increased frequency of tumor
formation (15,16).
miR-155, which is overexpressed in breast, lung and
colon cancer (17,18), was also previously shown to be
involved in numerous cellular processes including proliferation,
differentiation, apoptosis and metabolism. However, since it is
strongly upregulated in normal pancreatic tissue, when an all
tumors vs. all normal comparison is performed, miR-155 appears as
downregulated in solid tumors, albeit with a borderline
significance (19). In the present
study, we determined the expression of mir-155 in gastric cancer
and its functional role in gastric cancer cells (SGC-7901). We used
fluorescence-based quantitative real-time PCR as well as precursor
molecules to investigate the expression level of miR-155 in human
gastric carcinoma and normal tissue. Due to the considerable
evidence that miRNAs are involved in cancer (20–22),
it is key to elucidate their roles in solid tumors and to further
elucidate common miRNA-driven pathways; we also looked for
downstream targets of miR-155 in SGC-7901 gastric cancer cells in
order to ascertain the relationship between mir-155 and the
pathogenesis of gastric cancer.
Materials and methods
Patients and specimens
All human tissue samples were obtained from surgical
specimens of 52 patients with gastric carcinoma from 2006 to 2007
at the Second Affiliated Hospital, Harbin Medical University,
China. All tissues, including gastric carcinoma and corresponding
adjacent normal tissue, were divided into two parts and preserved
in liquid nitrogen for 30 min after removing from the body. All of
the samples were obtained with patient’s informed consent and were
histologically confirmed.
Cell culture
SGC-7901 (human gastric cancer cell line) and HEK293
(human embryonic kidney cell line) were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) (both from
Invitrogen) plus 0.5% penicillin-streptomycin at 37°C in a 5%
CO2 incubator.
Quantitative reverse
transcription-PCR
Real-time quantitative PCR was performed using
standard protocols on an Applied Biosystem 7500 HT Sequence
Detection System. All reagents and primers (miRNAs155, 5s) were
purchased from Ambion. Briefly, 5 μl of a 1/100 dilution of cDNA in
water was added into 12.5 μl of the 2× SYBR-Green PCR master mix
(Ambion), with 800 nmol/l of each primer in a total volume of 25
μl. The reactions were amplified for 15 sec at 95°C, and 1 min at
60°C for 40 cycles. All reactions were run in triplicate and
included no template and no reverse transcription controls for each
gene. The cycle number at which the reaction crossed an arbitrarily
placed threshold (CT) was determined for each gene, and the
relative amount of each miRNA to 5sRNA was calculated using the
equation 2−ΔCT, where ΔCT = (CTmiRNA − CT5s) (23).
Oncogene target predictions
The recent Sanger predictions (April 2008) were used
to identify the putative miRNA targets. They included essentially
the 3′-untranslated region (3′-UTR) targets reported by Lewis et
al (24) with a few changes
arising from updated gene boundary definitions from the April 2005
UCSC Genome Browser mapping of RefSeq mRNAs to the hg17 human
genome assembly. Among the putative targets, we specified known
cancer genes (tumor suppressors and oncogenes) as identified in the
Cancer Gene Census at http://www.pubmedcentral.nih.gov/redirect3.cs, or
reported by OMIM at http://www.pubmedcentral.nih.gov/redirect3 (19). We obtained the network of miR-155
regulation by RG Bioconductor.
Target in vitro assays
For luciferase reporter experiments, the 3′-UTR
segments (Invitrogen) of myc predicted to interact with miR-155
were annealed synthetic from myc cDNA (ENST00000377970) and ligated
into the pMIR-REPORT™ vector (Ambion), using the SpeI and
HindIII sites immediately downstream from the stop codon of
luciferase. The HEK293 cell line was grown in 10% FBS in RPMI-1640
medium, supplemented with 1× non-essential amino acid and 1 mmol
sodium pyruvate at 37°C in a humidified atmosphere of 5%
CO2. The cells were cotransfected in 12-well plates by
using siPORT NeoFx (Ambion), according to the manufacturer’s
protocol, with 0.4 μg of the firefly luciferase report vector and
galactosidase activity was used for normalizing the transfection
efficiency and protein input. For each well, 10 or 50 nM miRNA
oligonucleotides or scrambled oligonucleotides (both from Ambion)
were used. Firefly and Renilla luciferase activities were
measured consecutively by using Dual-Luciferase assays (Promega) 24
h after transfection.
Transfection of miR-155
Transfection of SGC-7901 cells with miR155 mimic or
the negative control oligonucleotide (both from Ambion) by siPORT
NeoFX (Ambion) was performed according to the manufacturer’s
protocol. SGC-7901 cells were seeded in 6-well plates at a
concentration of 1×105/well. The effects caused by the
introduction miR-155 mimic into the cells were assayed at 48 h
after the transfection by western blot analyses for the protein of
myc.
Cell adhesion assays
Fibronectin (Sigma, Beijing, China) was dissolved in
phosphate-buffered saline (PBS) to the 2 μg/50 μl solution, then
coated to each well of 96-well plates with the solution for 100 μl,
dried in horizontal laminar flow clean workbench overnight. The
plates were washed with PBS and blocked with RPMI-1640 medium (50
μl/well) for 1 h before use. SGC-7901 cells were cultured in the
presence of miR-155 mimics or miR-control (0, 30, 50, 80 or 100 nM)
for 24 h and then resuspended in serum free medium. The cell
concentration was adjusted to 6×108 cells/l. The
fibronectin coated wells were seeded with 100 μl of this cell
suspension and incubated for 2 h at 37°C. The medium was discarded
and the wells were washed with 100 μl of pre-warmed PBS to wash out
the unattached cells. DMSO (100 μl) was added to each well after 4
h incubation by 100 μl MTT (1 mg/ml). The OD values were measured
by a microplate reader as described above.
EdU proliferation assay and cell
viability
Proliferation was determined in vitro using
the EdU DNA proliferation in vitro detection kit (RiboBio,
China), according to the manufacturer’s instructions. Proliferating
SGC-7901 cells after transfection by miR-155 mimics were incubated
with 50 μm EdU for 2 h, then fixed and permeated by PBS
(respectively containing 4% polyoxymethylene, 0.5% Triton X-100),
and cell nuclei were stained with Apollo and Hoechst staining
solution at an advisable concentration for 30 min, observed by
confocal laser scanning microscope (CLSM) and the result was
analyzed by IPP6.0 software (Image-Pro Plus).
SGC-7901 cells were harvested at 24 h after
transfection with miR-155 mimics (30, 50, 80 and 100 nm). The cell
viability was obtained by CASY measurement (CASY-1; Schärfe System,
Reutlingen, Germany), according to the manufacturer’s
instructions.
Western blotting
Antibody samples, myc and β-actin, (purchased from
Santa Cruz) were lysed in buffer (1% NP-40, 0.1 M Tris, pH 8.0,
0.15 MNaCl, 5 mM EDTA and 1 mM phenylmethylsulfonyl fluoride). The
protein pellets were resuspended in the buffer, followed by pH
adjustment. The protein samples were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Proteins were transferred to nitrocellulose membrane with a semidry
electrophoresis transfer apparatus (Bio-Rad) and probed with
antibodies according to the manufacturer’s instructions.
Statistical analysis
All data are reported as means ± SEM. When
comparisons were made between two different groups, statistical
analysis was performed by the Student’s t-test and ANOVA. P<0.05
was considered to indicate a statistically significant difference.
All analyses were performed with the SPSS 19.0 statistics software
(SPSS Inc., Chicago, IL, USA).
Results
Expression level of miR-155 is
downregulated in SGC-7901 cells and gastric cancer samples
In order to confirm whether the level of miR-155 was
reduced in human gastric cancer, we examined the expression of
mature miR-155 and 5s in the samples from 52 patients with gastric
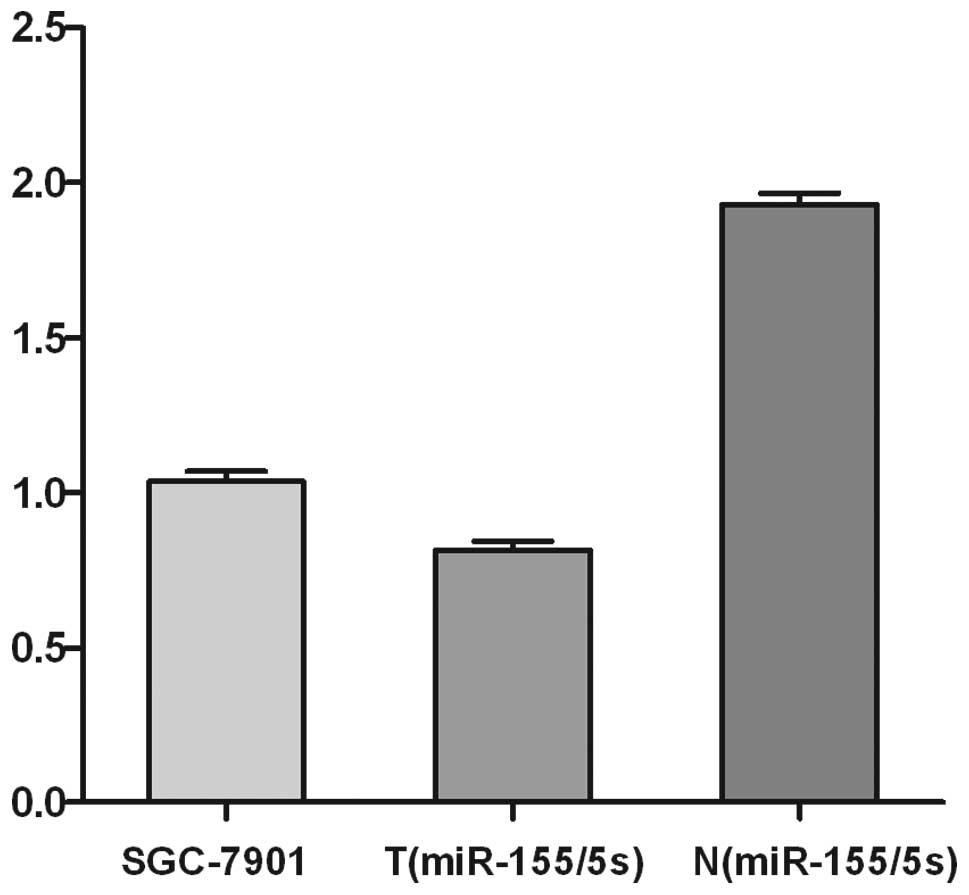
cancer as well as in SGC-7901 cells. As shown in Fig. 1, the expression level of miR-155
miRNA in tumors was significantly lower (downregulation rate
>2-fold) in SGC-7901 cells and 32 patients tested. However, in
the other 20 patients, no significant difference in miR-155
expression was observed between tumors and normal tissues, and the
up- or downregulation rates were within 2-fold.
Overexpression of miR-155 reduces
SGC-7901 cell proliferation and viability
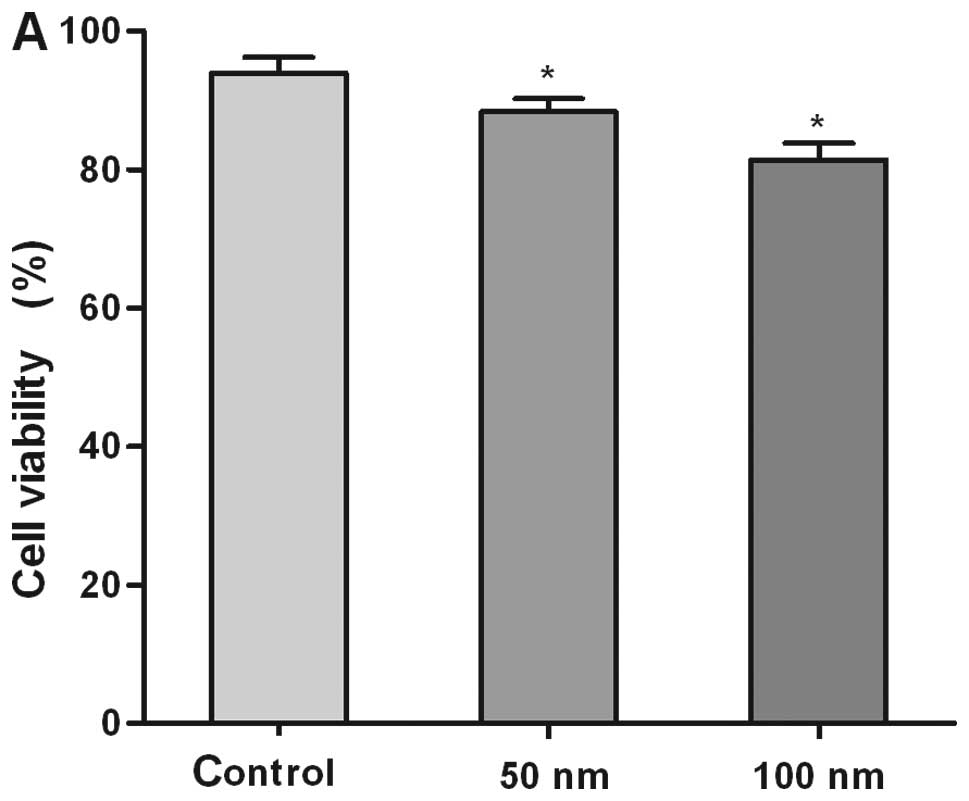
Using CASY and EdU assay, we evaluated the function
of high level miR-155 in gastric carcinoma cells by measuring cell
viability and proliferation in SGC-7901 cells which were
transfected with the miR-155 mimic of different concentrations.
CASY measurement showed a significant decrease in the viability of
SGC-7901 cells transfected by miR-155 mimics when compared with the
negative control group. Cells treated with 50 nM or higher
concentrations of miR-155 showed significantly lower rates of
viability when compared with control miRNA-treated cells (Fig. 2A).
Cell adhesion assays (Fig. 2B) showed similar results; high level
of mir-155 reduced the adhesion ability, and the inhibition rate
correspondingly increased according to the increased concentration
of mir-155 mimics. The mir-155 mimics 100 nm group showed the most
obvious change when compared with the control groups.
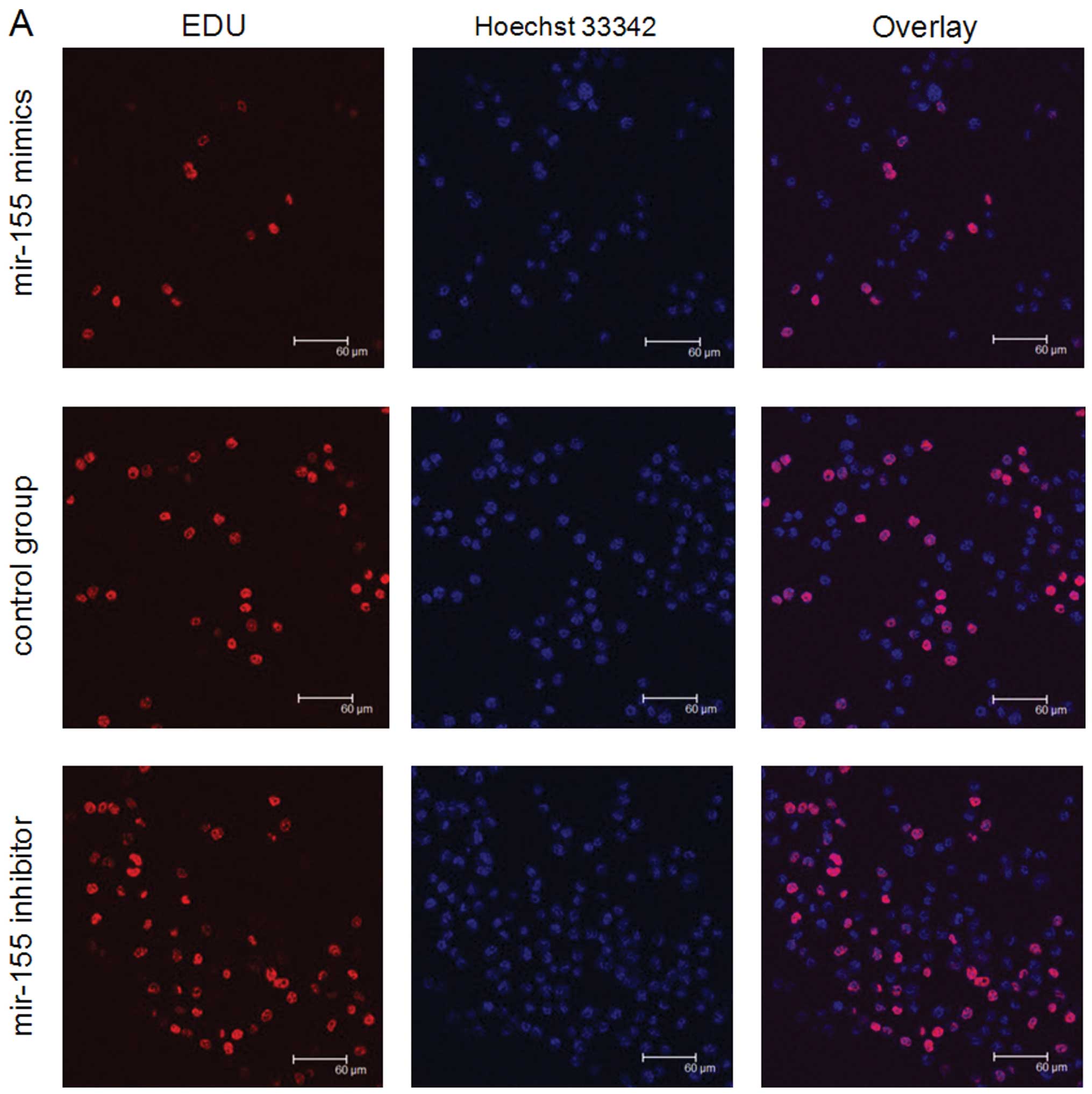
The result of the EdU assay (Fig. 3) also showed the level of miR-155
modulated SGC-7901 cell proliferation. EdU experiments were
performed to assess proliferation after SGC-7901 cells were
transfected with mir-155 inhibitor or mimics (100 nm). Under these
conditions, the level of proliferation in the miR-155
mimics-transfected cells was reduced, compared with cells
transfected with the control group. On the contrary, compared with
cells in the control group, proliferation of SGC-7901 cells which
were transfected by miR-155 inhibitor was increased. The results
support the theory that high level of miR-155 is able to prevent
SGC-7901 proliferation.
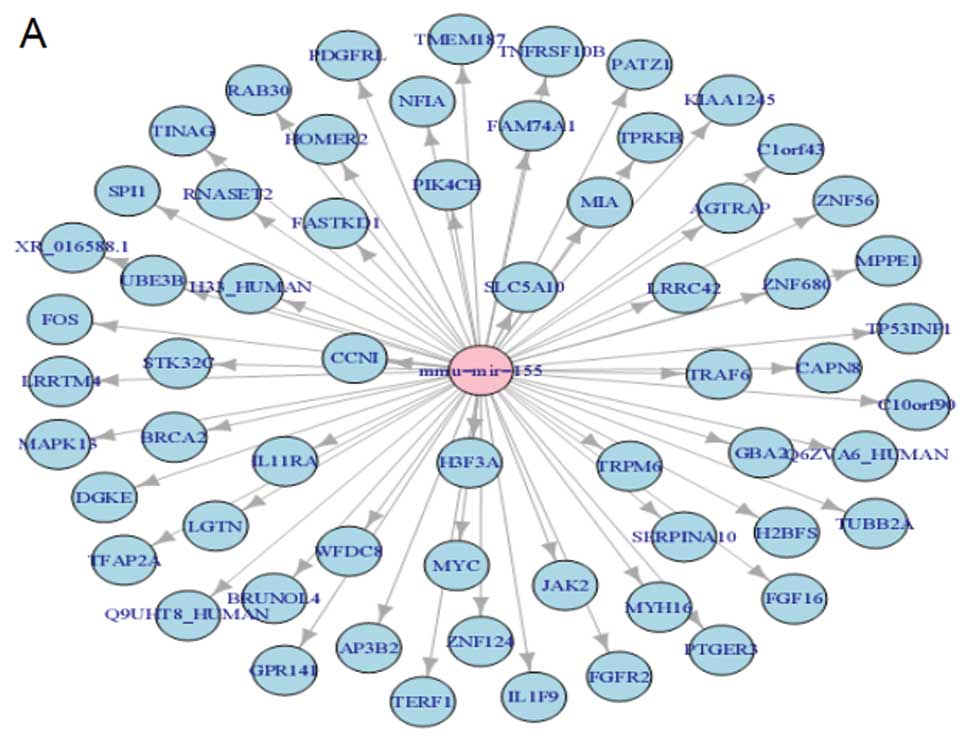
Target assay of miR-155
The functional significance of miR-155
downregulation in cancer shown here needs to be understood. First,
in silico prediction of targets was performed by using
Sanger database (version 5) of conserved 3′-UTR miRNA targets
(13). Sanger contained 991
predictions for miR-155. Some well known cancer genes were
predicted as targets for miR-155 (Fig.
4A). We then experimentally confirmed the in silico
predictions for one cancer gene, myc (proto-oncogene protein). To
experimentally validate the computational data, the myc 3′-UTR
(2,356 bp) was subcloned downstream of the f-luc open reading frame
(HindIII and SpeI sites). This reporter construct
(pMIR/2356/5′3′) was cotransfected in the HEK293 cell line with
pMIR-REPORT™ (to normalize for transfection differences) and a
control non-targeting RNA oligonucleotide (miR-control) or miR155
precursor RNA oligonucleotide (Ambion). The relative luciferase
activity was markedly diminished (61.3±4.2%) in cells cotransfected
with the pMIR/2356/5′3′ construct and miR-155 (50 nM final
concentration; Fig. 4B). The
experiment indicated that miR-155 may decrease c-myc expression by
inhibiting the translation process.
Endogenous c-myc is suppressed by
miR-155
miR-155 decreases c-myc expression in human gastric
cancer SGC-7901 cells. SGC-7901 cells demonstrated the endogenous
expression of the myc (3). We
transfected miR-155 mimics (50 nm) into SGC-7901 cells. As
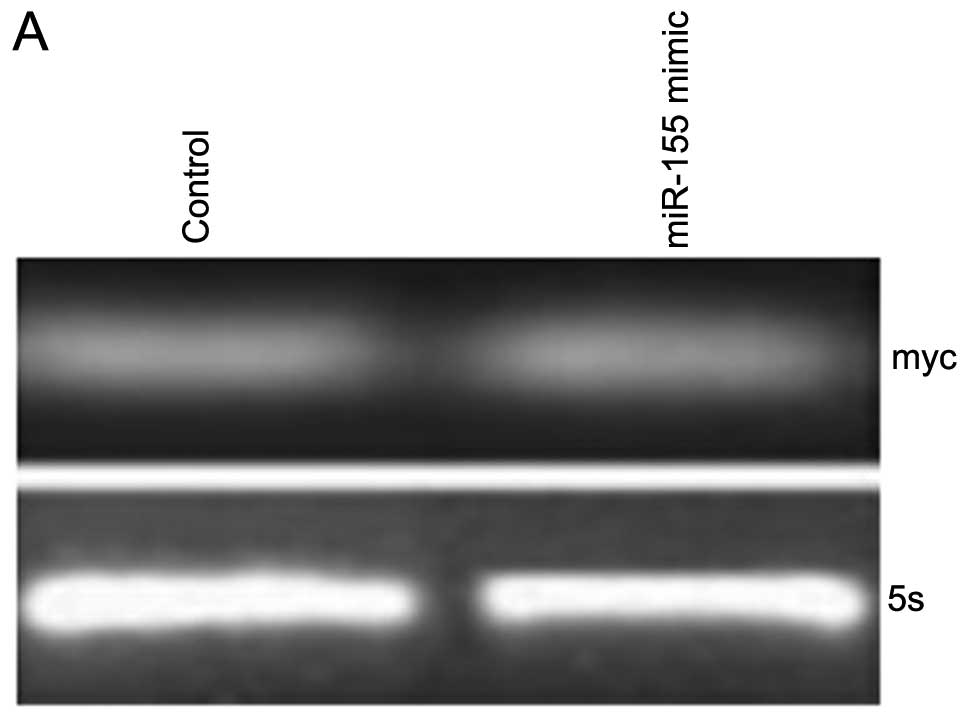
expected, myc mRNA levels were unaffected by miR-155 (Fig. 5A). In contrast, c-myc protein levels
decreased substantially following treatment with miR-155, as shown
by western blotting (Fig. 5B)
indicating that miR-155 regulation is specific to myc.
Discussion
MicroRNAs (miRNAs) are endogenous short non-coding
RNA molecules that regulate cell differentiation, proliferation and
apoptosis through post-transcriptional suppression of gene
expression by binding to the complementary sequence in the
3′-untranslated region (3′-UTR) of target messenger (28,29).
Gastric carcinoma develops through the accumulation
of multiple genetic lesions that involve oncogenes, tumor
suppressor genes and DNA mismatch repair genes (30,31).
miRNAs have been proved to be closely related to the development of
gastric cancer in several studies (32–34),
and it is believed that they may offer a new approach for early
diagnosis and individual therapy of human cancer. The relationship
between gastric carcinoma genesis and the expression of miR-155 is
rarely reported. In the present study, we examined, using real-time
PCR, the expression of mature miR-155 in SGC-7901 cells and 52
matched pairs of gastric tumoral and non-tumoral tissues from
patients. The results showed miRNA was significantly downregulated
in SGC-7901 cells as well as in 32 of the 52 patients with gastric
cancer. Similarly, the expression of miR-155 was reduced in solid
tumors (19).
EdU has been widely used to measure the novel
synthesis of DNA, and already takes the place of bromodeoxyuridine;
it is simple and reproducible and may keep excellent preservation
of nuclear structure due to the simple detection systems. In this
study, we used EdU assay to observe the proliferation of the
SGC-7901 cells transfected by mir-155 mimics or inhibitor, and the
results showed that high level of mir-155 may inhibit the
proliferation of SGC-7901 cells. On the contrary, the low level may
lead to high proliferation. Some studies also reported that the
level of mir-429 and -214 lead to similar effects of tumor cells
(33,35). Meanwhile, the data showed that high
level of mir-155 may reduce attachment to fibronectin and the
viability of SGC-7901 cells, attachment and viability closely
relate to the migration and invasion of cancer cells. These suggest
that downregulation of miR-155 in solid tumor or SGC-7901 cells may
play a considerable role in the development of gastric cancer
through enhancing cell proliferation and cell invasion.
It is well known that the function of miRNA is
decided by its target genes. Prediction of target genes found that
for miR-155 there may be >900 target genes of which only a small
portion has been confirmed by experiments (36). Xie et al reported transient
ectopic expression of miR-155 was able to decrease endogenous
levels of p21 by targeting SOX6 in hepatocellular carcinoma
(37). Relevant targets for miR-155
can be dominant (oncogene) cancer genes, such as myc, FGFR,
according to the Sanger miRbase (Fig.
4A). c-myc is overexpressed in various types of human cancers
and a previous study (38) showed
c-myc directly or indirectly influences cancer cell growth,
proliferation and metastasis. c-myc has previously been shown to be
a direct downstream target of miR-145 in non-small cell lung cancer
cells (39), and miR-145 has also
been shown to be an intermediate in the p53-mediated downregulation
of c-myc in HCT116 cells (40) and
we believe c-myc also plays an important role in the progression of
gastric carcinoma. We then experimentally confirmed the predictions
for myc cancer gene and was found to be positive in a luciferase
assay by showing a significant reduction of protein translation in
respect to the scrambled control oligoRNAs (Fig. 5B). This finding suggests a
post-transcriptional mechanism for regulation of myc that could be
well explained by the concomitant miR-155 downregulation we
detected in gastric carcinoma for the first time. Our finding
showed that high level of miR-155 may lead to the downregulation of
c-myc, and serving as a pro-oncogene, c-myc is closely related to
tumor cell proliferation, transformation and the induction of
apoptosis, that may provide further insight into how mir-155
affected cell proliferation and viability. This experimental
evidence also reinforces the hypothesis that key cancer genes are
regulated by aberrant expression of miRNAs in solid cancers
(41). These data add to the list
of miRNAs with important cancer gene targets as previously shown by
Johnson et al (42) (let-7,
Ras interaction), O’Donnell et al (43) (miR-17-5p, E2F1) and Cimmino et
al (44) (miR-16, Bcl2).
In summary, the present study identified miR-155 as
a miRNA which was downregulated in solid cancer and SGC-7901 cells,
and which plays a considerable role in the proliferation and
viability of cancer cells. The present study indicates that miR-155
is involved in gastric carcinoma and supports its function in a
recessive manner, by controlling the expression of protein-coding
oncogenes. It is noteworthy that we demonstrated for the first time
that c-myc is a target of miR-155, and mir-155 directly targets the
3′-UTR of c-myc and inhibits the translation process.
Although the function and expression of miRNA
regulation mechanism remains unclear, miRNA in many tumor tissues
indicates that its expression in cancer diagnosis and treatment is
important (45). Perhaps the
tissues and fluids of miR-155 expression levels can also be used as
diagnostic and prognostic tumor markers (46). However, these should be confirmed
with further in vivo experiments and clinical analyses of
larger sample sizes.
One important limitation of the present study was
that only 52 patients were enrolled in the analysis of the
expression level of miR-155 and the findings should be confirmed in
a larger patient population. Additionally, more assays should be
performed to validate the mechanism of miR-155 in tumor migration
and invasion.
Acknowledgements
This study was supported by the Scientific Research
Fund of Heilongjiang Provincial Education Department (No.
12541508).
References
|
1
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cullen BR: Transcription and processing of
human microRNA precursors. Mol Cell. 16:861–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eder M and Scherr M: MicroRNA and lung
cancer. N Engl J Med. 352:2446–2448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji J, Shi J, Budhu A, et al: MicroRNA
expression, survival, and response to interferon in liver cancer. N
Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
8
|
Hammond SM: MicroRNAs as oncogenes. Curr
Opin Genet Dev. 16:4–9. 2006. View Article : Google Scholar
|
|
9
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Huang J, Yang N, et al: microRNAs
exhibit high frequency genomic alterations in human cancer. Proc
Natl Acad Sci USA. 103:9136–9141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam EJ, Yoon H, Kim SW, et al: MicroRNA
expression profiles in serous ovarian carcinoma. Clin Cancer Res.
14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L, Thomson JM, Hemann MT, et al: A
microRNA polycistron as a potential human oncogene. Nature.
435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng SR, Guo GL, Zhai Q, et al: Effects
of miR-155 antisense oligonucleotide on breast carcinoma cell line
MDA-MB-157 and implanted tumors. Asian Pac J Cancer Prev.
14:2361–2366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pu J, Bai D, Yang X, et al: Adrenaline
promotes cell proliferation and increases chemoresistance in colon
cancer HT29 cells through induction of miR-155. Biochem Biophys Res
Commun. 428:210–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinha AU, Kaimal V, Chen J and Jegga AG:
Dissecting microregulation of a master regulatory network. BMC
Genomics. 9:882008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolesnikov NN, Titov SE, Veriaskina IA, et
al: MicroRNA, evolution and cancer. Tsitologiia. 55:159–164.
2013.(In Russian).
|
|
21
|
Hede K: Studies define role of microRNA in
cancer. J Natl Cancer Inst. 97:1114–1115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen C, Ridzon DA, Broomer AJ, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Y, Arora A, Min W, et al: EdU
incorporation is an alternative non-radioactive assay to
[3H]thymidine uptake for in vitro measurement of mice
T-cell proliferations. J Immunol Methods. 350:29–35. 2009.
|
|
26
|
Diermeier-Daucher S, Clarke ST, Hill D, et
al: Cell type specific applicability of 5-ethynyl-2′-deoxyuridine
(EdU) for dynamic proliferation assessment in flow cytometry.
Cytometry A. 75:535–546. 2009.PubMed/NCBI
|
|
27
|
Kohlmeier F, Maya-Mendoza A and Jackson
DA: EdU induces DNA damage response and cell death in mESC in
culture. Chromosome Res. 21:87–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwarzenbacher D, Balic M and Pichler M:
The role of microRNAs in breast cancer stem cells. Int J Mol Sci.
14:14712–14723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Jia XJ, Jiang HJ, et al: MicroRNAs
185, 96, and 223 repress selective high-density lipoprotein
cholesterol uptake through posttranscriptional inhibition. Mol Cell
Biol. 33:1956–1964. 2013. View Article : Google Scholar
|
|
30
|
Yasui W, Yokozaki H, Fujimoto J, et al:
Genetic and epigenetic alterations in multistep carcinogenesis of
the stomach. J Gastroenterol. 35(Suppl 12): 111–115.
2000.PubMed/NCBI
|
|
31
|
Tahara E: Molecular biology of gastric
cancer. World J Surg. 19:484–490. 1995. View Article : Google Scholar
|
|
32
|
Liu Y, Xing R, Zhang X, et al: miR-375
targets the p53 gene to regulate cellular response to ionizing
radiation and etoposide in gastric cancer cells. DNA Repair.
12:741–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang TS, Yang XH, Wang XD, et al: MiR-214
regulate gastric cancer cell proliferation, migration and invasion
by targeting PTEN. Cancer Cell Int. 13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sacconi A, Biagioni F, Canu V, et al:
miR-204 targets Bcl-2 expression and enhances responsiveness of
gastric cancer. Cell Death Dis. 3:e4232012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Wang C, Xing J and Wu D: miR-429
modulates the expression of c-myc in human gastric carcinoma cells.
Eur J Cancer. 47:2552–2559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voorhoeve PM and Agami R: Classifying
microRNAs in cancer: the good, the bad and the ugly. Biochim
Biophys Acta. 1775:274–282. 2007.PubMed/NCBI
|
|
37
|
Xie Q, Chen X, Lu F, et al: Aberrant
expression of microRNA 155 may accelerate cell proliferation by
targeting sex-determining region Y box 6 in hepatocellular
carcinoma. Cancer. 118:2431–2442. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eilers M and Eisenman RN: Myc’s broad
reach. Genes Dev. 22:2755–2766. 2008.
|
|
39
|
Chen Z, Zeng H, Guo Y, et al: miRNA-145
inhibits non-small cell lung cancer cell proliferation by targeting
c-Myc. J Exp Clin Cancer Res. 29:1512010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sachdeva M, Zhu S, Wu F, et al: p53
represses c-Myc through induction of the tumor suppressor
miR-145. Proc Natl Acad Sci USA. 106:3207–3212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tibshirani R, Hastie T, Narasimhan B and
Chu G: Diagnosis of multiple cancer types by shrunken centroids of
gene expression. Proc Natl Acad Sci USA. 99:6567–6572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family.
Cell. 120:635–647. 2005. View Article : Google Scholar
|
|
43
|
O’Donnell KA, Wentzel EA, Zeller KI, et
al: c-Myc-regulated microRNAs modulate E2F1 expression. Nature.
435:839–843. 2005.PubMed/NCBI
|
|
44
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2.
Proc Natl Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar
|
|
45
|
Krützfeldt J, Rajewsky N, Braich R, et al:
Silencing of microRNAs in vivo with ‘antagomirs’. Nature.
438:685–689. 2005.
|
|
46
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|