Introduction
Osteosarcoma is the most common malignant primary
bone tumor that occurs in children and adolescents, and accounts
for 20% of all bone tumors and approximately 5% of pediatric tumors
overall (1). Osteosarcoma is
characterized by a highly malignant and metastatic potential, and
the leading cause of death of osteosarcoma patients is distant
metastases (2). In recent years,
although improvements in patient survival rates have been achieved
through multimodal therapeutic approaches (3), the overall relapse-free survival rate
over 5 years still remains at 65%–75% (4). Therefore, to the development of new
effective therapeutic strategies for osteosarcoma is required.
In recent years, much research concerning
osteosarcomagenesis has been carried out; however, the pathogenesis
of osteosarcoma is not fully understood. There is increasing
evidence that genetic alterations are responsible for the
tumorigenesis, including anomalous activation of oncogenes and/or
inactivation of tumor suppressors. Recently, yes-associated protein
1 (YAP1) has been suggested to be a candidate oncogene in multiple
tumors, and it was found to be highly expressed in a variety of
human cancers, such as liver, colon, prostate, ovarian, lung and
breast cancers (5–7), suggesting that YAP1 plays an important
role in tumorigenesis (8,9). YAP1, a 65-kDa proline-rich
phosphoprotein, is the main downstream effector of the Hippo
pathway and functions as a transcriptional coactivator which can
bind transcription factor Sd to enhance the expression of several
proliferation and anti-apoptosis-related genes (10), including cycE, diap1 and cylinD1 and
therefore regulates cell proliferation and apoptosis depending on
the cellular context (11,12). YAP1 was originally identified by
virtue of its binding to the Src family member non-receptor
tyrosine kinase YES (13). A number
of studies have shown that the upregulation of YAP1 promotes
tumorigenesis in most but not all tumor types evaluated (14), and the overexpression of YAP1 is an
independent poor prognostic factor in hepatocellular and urothelial
carcinoma of the bladder (15,16).
In addition, transgenic mice with liver-specific YAP1
overexpression were found to exhibit a marked increase in liver
size and eventually developed tumors (17). However, to date, our understanding
of the molecular mechanism of the tumor promotion by YAP1 is still
quite limited.
Apart from the Hippo signaling pathway, it was
reported that YAP1 is also implicated in the Wnt signaling pathway
(18), which plays a key role in
the pathogenesis of colon and other cancers (19). Binding of Wnts to frizzled (Fz)
receptor and low-density lipoprotein 5 or 6 (LRP5/6) co-receptors
inactivates destruction complex composed of AXIN1, GSK3β and
adenomatous polyposis coli (APC), leading to β-catenin accumulation
and subsequent translocation into the nucleus. In the nucleus,
β-catenin forms a complex with TCF/LEF transcription factors to
drive the transcription of genes that contribute to cell
proliferation (19,20). Increasing evidence indicates that
Wnt signaling increases osteosarcoma cell proliferation and their
development (21,22). Furthermore, it was reported that
YAP1 and the transcription factor TBX5 form a complex with
β-catenin and then phosphorylation of YAP1 leads to localization of
this complex to the promoters of anti-apoptotic genes including
BCL2L1 and BIRC5 (9). However, the
role of YAP1 in osteosarcoma initiation and development still
remains unclear. Therefore, we hypothesized that YAP1 is likely to
play a key role in the progression of osteosarcoma.
In this study, for the first time, we investigated
the contribution of YAP1 in osteosarcoma cell proliferation and
tumor formation through shRNA-based technique. We found that
knockdown of endogenous YAP1 inhibited the cell proliferation and
tumor formation of osteosarcoma, and the underlying mechanism was
associated with the inhibition of the Wnt signaling pathway. Our
findings provide a potential target for osteosarcoma
therapeutics.
Materials and methods
Cell lines and cell culture
Human osteosarcoma cell lines, MG-63 and HOS, were
purchased from the American Type Culture Collection (Rockville, MD,
USA) and were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad,
USA) at 37°C in a humidified incubator with 95% air and 5%
CO2.
Western blot analysis
The lysates from the cell lines were separated by
SDS-PAGE and transferred onto PVDF membranes by wet transfer. After
being blocked with 5% fat-free milk, the membranes were incubated
with the primary antibody for YAP1 (1:500; Abcam, Cambridge, UK),
β-actin (1:1000; Santa Cruz, CA, USA), cyclinD1 (1:500; Santa Cruz)
and c-myc (1:500; Santa Cruz) overnight at 4°C, and then rinsed
thrice with Tris-buffered saline containing 0.05% Tween-20 (TBST),
followed by secondary incubation with anti-rabbit or anti-mouse IgG
(1:5000; Cell Signaling) linked with horseradish peroxidase (HRP)
for 1 h at room temperature. Blots were developed with enhanced
chemiluminescence reagent (Millipore, Billerica, MA, USA) on X-ray
film.
RNA interference
RNA interference was performed using short hairpin
RNA (shRNA), and the shRNA expression vector containing the
YAP1-specific sequence was generated by GeneChem Co., Ltd.
(Shanghai, China). The targeting sequence of the shRNA was
5′-UUAUAUAGUAAAUUUCUCC-3′ and 5′-UUAAGAAGUAUCUCUGACC-3′. A
scrambled shRNA was used as a negative control. The shRNA
expression vectors were transfected into MG-63 and HOS cells,
respectively, using Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer’s protocols. Transfected cells were
treated with G418 (Invitrogen) for 2 weeks, and drug-resistant
colonies were collected by trypsinization and used without
cloning.
Cell proliferation assay
The anti-proliferative effect of YAP1 silencing on
osteosarcoma cells was assessed through cell count and MTT assay,
respectively. Briefly, MG-63 and HOS cells with YAP1 silencing were
seeded in 6-well plates at 5×104 cells/well and
incubated as described above. The cells were harvested and counted
continuously for 7 day using a hemocytometer.
For the MTT assay, the cells were seeded in 96-well
plates at 2×103 cells/well and incubated for 1, 3, 5 and
7 days. At each time point, MTT (Sigma-Aldrich) was added into each
well. After 4 h of incubation, the resulting formazan was then
dissolved in 100 μl of dimethyl sulfoxide (DMSO; Sigma-Aldrich),
and the absorbance was recorded at 490 nm using a Bio-Rad 3350
microplate reader.
Colony formation assay
Cells were plated in 60-mm culture dishes at 200
cells/well and cultured in DMEM supplemented with 10% FBS for 3
weeks. Then the colonies were rinsed with PBS, fixed with absolute
methanol for 15 min, and stained with Giemsa solution for 30 min.
The colonies containing ≥50 cells were counted as positive for
growth.
Tumorsphere formation assay
To obtain tumorspheres, cells were seeded in 24-well
ultra-low attachment plates at a concentration of 103
cells/well and cultured in serum-free DMEM/F12 medium supplemented
with 1X B-27 (Invitrogen), 20 ng/ml epidermal growth factor (EGF;
Sigma-Aldrich) and 10 ng/ml basic fibroblast growth factor (bFGF;
PeproTech, USA) at 37°C under 5% CO2 for 2 weeks. The
formed tumorspheres were quantified using an inverted
microscope.
Cell cycle analysis
Cells were cultured in 6-well plates until they
reached 60–70% confluency. Cells (1×106 ) were harvested
and washed twice with ice-old PBS and then fixed overnight with 70%
ethanol at 4°C. Following incubation with 50 mg/ml RNase A and 20
mg/ml propidium iodide (PI; Sigma) for 30 min at room temperature,
the cell cycle was analyzed by FACSCalibur flow cytometry
(Becton-Dickinson, Franklin Lakes, USA) using CellQuest
software.
β-catenin reporter assay
β-catenin transcriptional activity was determined by
TOP/FOP-Flash assays. Briefly, TOP/FOP-Flash plasmids were
transfected into cells with Lipofectamine 2000 in 24-well plates.
Firefly and Renilla luciferase assays were performed using
Dual-Glo Luciferase assay kit (Promega, Madison, WI, USA) according
to the manufacturer’s recommendations.
Tumor xenograft assay
Six week old BALB/c nude mice were purchased from
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Cells
(1×106) were subcutaneously injected into the dorsum of
the mice, and tumor size was measured every week. The tumor volume
(V) was determined by the length (a) and width (b) as: V =
ab2/2. At the end of the experiment, the mice were
sacrificed, and the formed tumors were dissected out and their wet
weights were determined. Animals were treated humanely in
accordance with institutional policies, and all studies performed
were approved by the Animal Care and Use Committee of Xi’an
Jiaotong University.
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software. The experiments were performed in triplicate and repeated
three times independently. Comparisons among all groups were
performed using one-way analysis of variance (ANOVA), and the
Student’s t-test was used for comparison of differences between two
groups. Differences were considered significant at P<0.05.
Results
Knockdown of YAP1 inhibits the
proliferation and colony formation of osteosarcoma cells in
vitro
Recently, a number of studies have reported that
YAP1 protein is highly expressed in human osteosarcoma tissues
(23,24); however, the role of YAP1 in
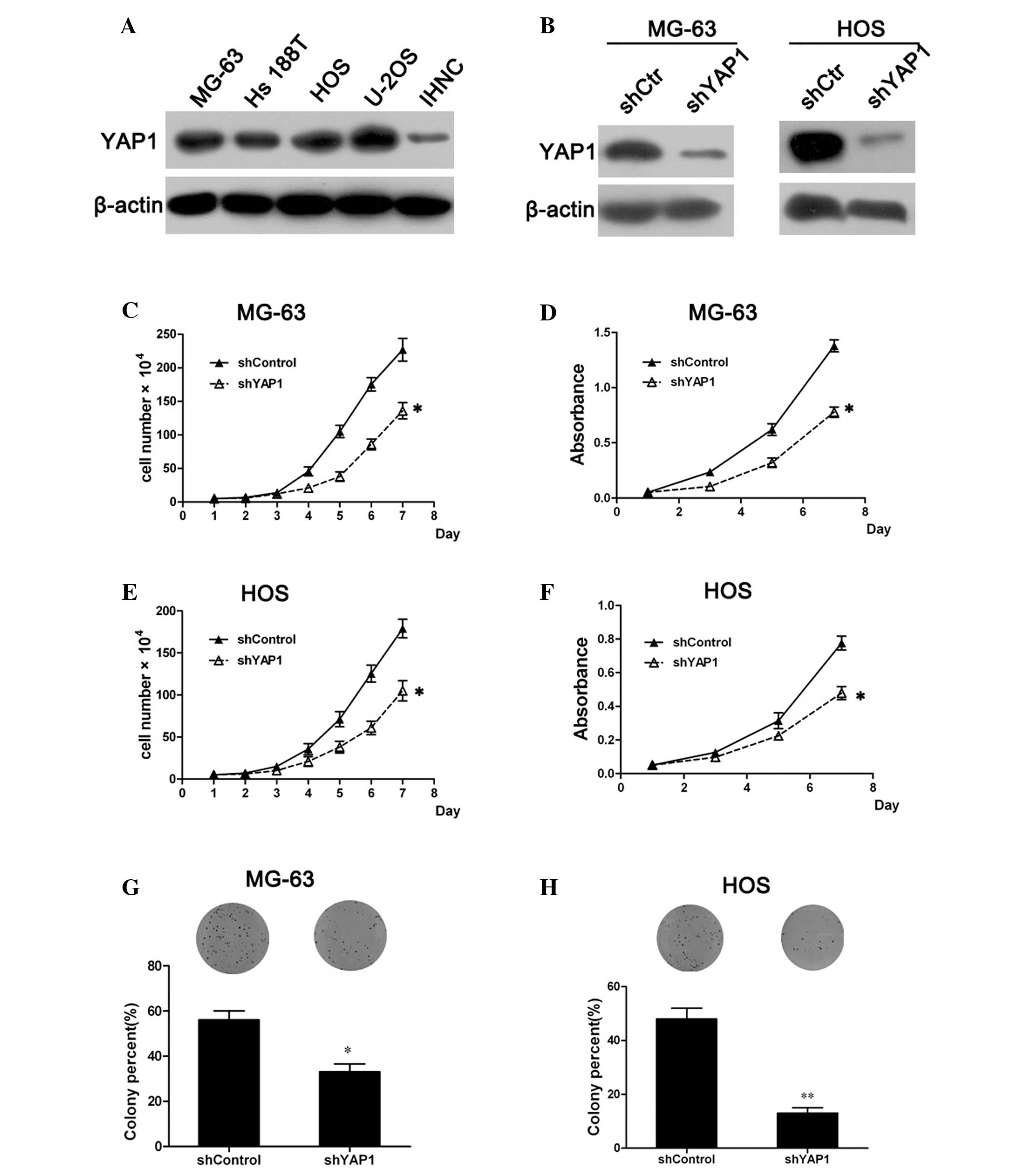
osteosarcoma tumorigenesis is unclear. Western blot analysis showed
that YAP1 expression in osteosarcoma cell lines MG-63, Hs 188T, HOS
and U-2OS was much higher than that in normal human osteoblasts
(IHNC), therefore we established shRNA-mediated YAP1-knockdown
MG-63 and HOS cell lines to examine the effect of YAP1 on
osteosarcoma cell proliferation (Fig.
1A). The level of YAP1 expression in the YAP1-knockdown cells
was confirmed to be markedly downregulated compared with the
control cells (Fig. 1B).
Subsequently, the cell growth curve showed that the knockdown of
YAP1 resulted in a significant inhibition of MG-63 cell growth
(Fig. 1C). Moreover, the cell
viability assay by MTT revealed that the viability of the
YAP1-knockdown MG-63 cells was markedly decreased when compared
with that of the control cells (Fig.
1D). Similar results were observed in the YAP1-knockdown HOS
cells (Fig. 1E and F). These
results suggest that the knockdown of YAP1 inhibits the
proliferation of osteosarcoma cells.
Furthermore, colony formation assays were performed
to confirm the inhibitory effect of YAP1 knockdown on osteosarcoma
cells. Compared with the control cells, the percentage of colony
formation in the YAP1-knockdown cells (MG-63 and HOS) was
significantly decreased (P<0.05; Fig. 1G and H), suggesting that knockdown
of YAP1 also inhibited the colony formation ability of osteosarcoma
cells. Collectively, all the results demonstrated that YAP1
knockdown inhibits the proliferation of osteosarcoma cells.
Knockdown of YAP1 suppresses the tumor
formation of osteosarcoma cells in vitro and in vivo
Since the knockdown of YAP1 inhibited the
proliferation of osteosarcoma cells in vitro, we
hypothesized that YAP1 knockdown may affect the tumorigenic
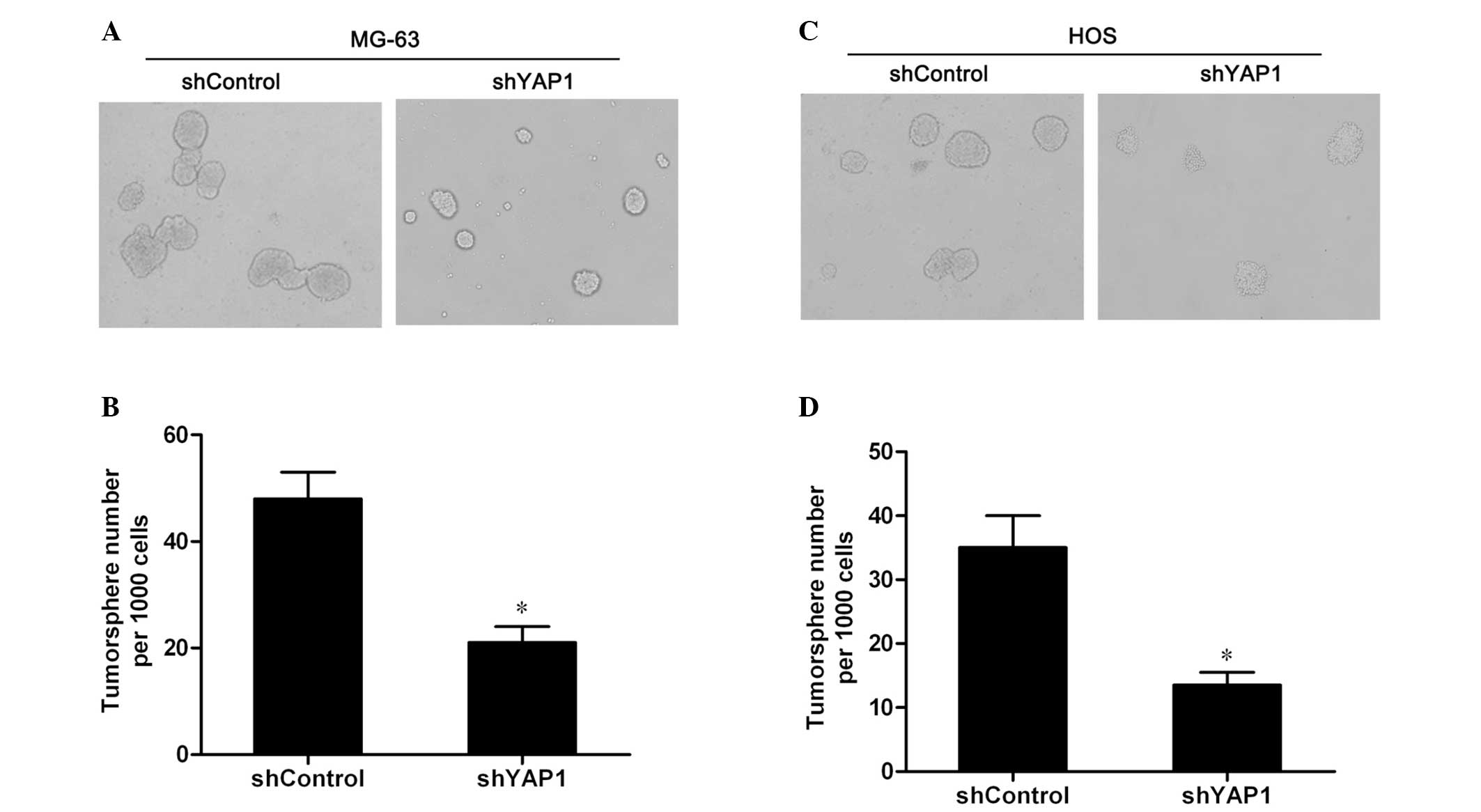
potential of osteosarcoma cells. Therefore, firstly, the
tumorsphere formation in vitro was assessed to examine the
role of YAP1 in the tumorigenesis of osteosarcoma. As shown in
Fig. 2A and C, the YAP1-knockdown
MG-63 and HOS cells formed fewer and smaller tumorspheres compared
to the control cells. Further quantitative analysis showed that the
numbers of tumorspheres formed by the YAP1-knockdown cells were
significantly decreased compared to the control cells (P<0.05;
Fig. 2B and D). The results
indicate that the knockdown of YAP1 inhibits the tumorsphere
formation of MG-63 and HOS cells.
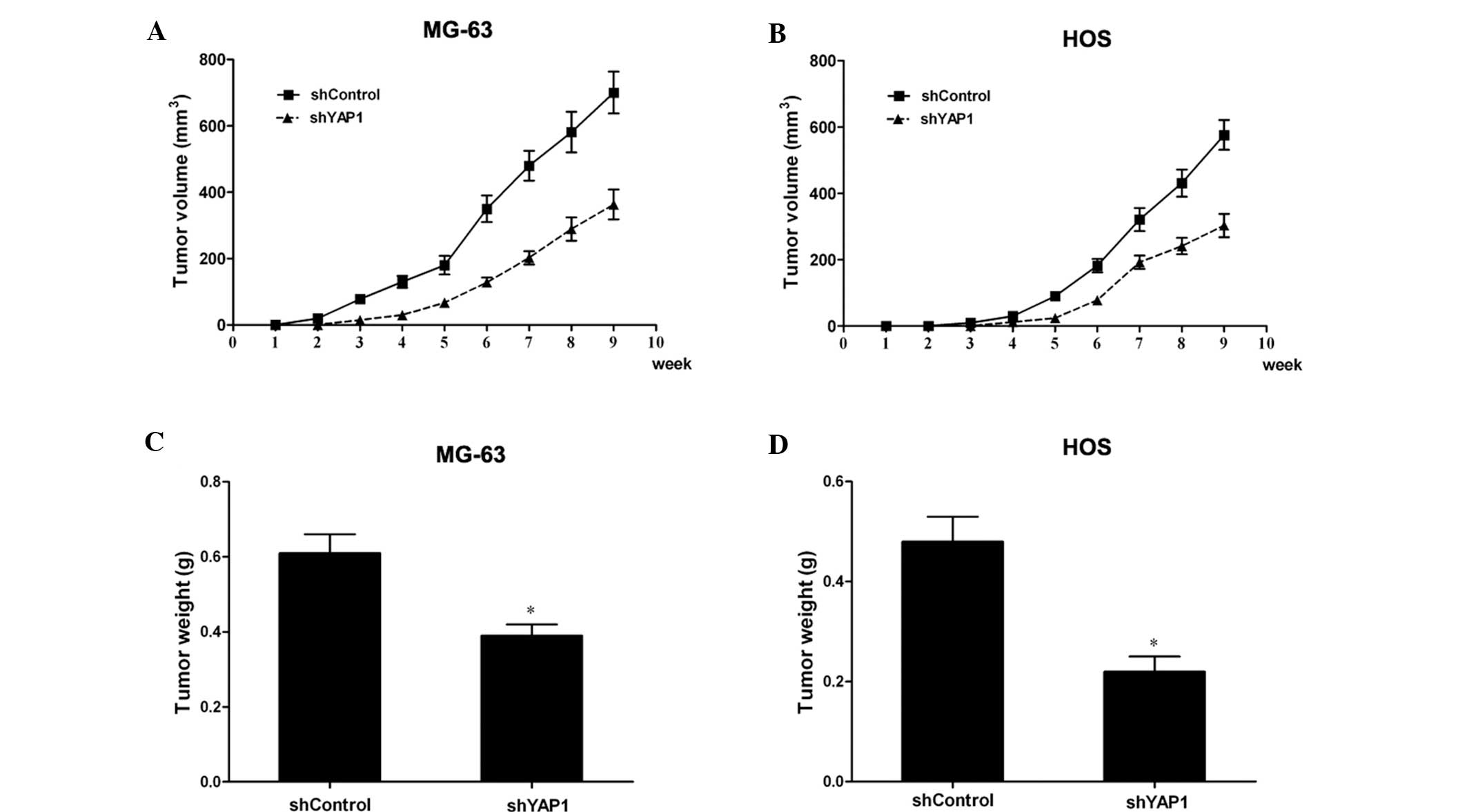
Next, YAP1-knockdown MG-63 and HOS cells were
injected into nude mice, and the development and growth of tumors
were monitored every week in terms of tumor volume. During the
course of the study, the knockdown of YAP1 led to a significant
inhibition of xenograft tumor growth (Fig. 3A and B). Moreover, the weights of
the tumors generated from the YAP1-knockdown MG-63 and HOS cells
were reduced significantly compared with the control cells
(P<0.05; Fig. 3C and D). All
these data suggest that knockdown of YAP1 also inhibits tumor
growth in vivo. Taken together, these results demonstrated
that knockdown of YAP1 inhibits the tumor formation ability of
osteosarcoma cells.
YAP1 regulates the cell cycle progression
of osteosarcoma cells
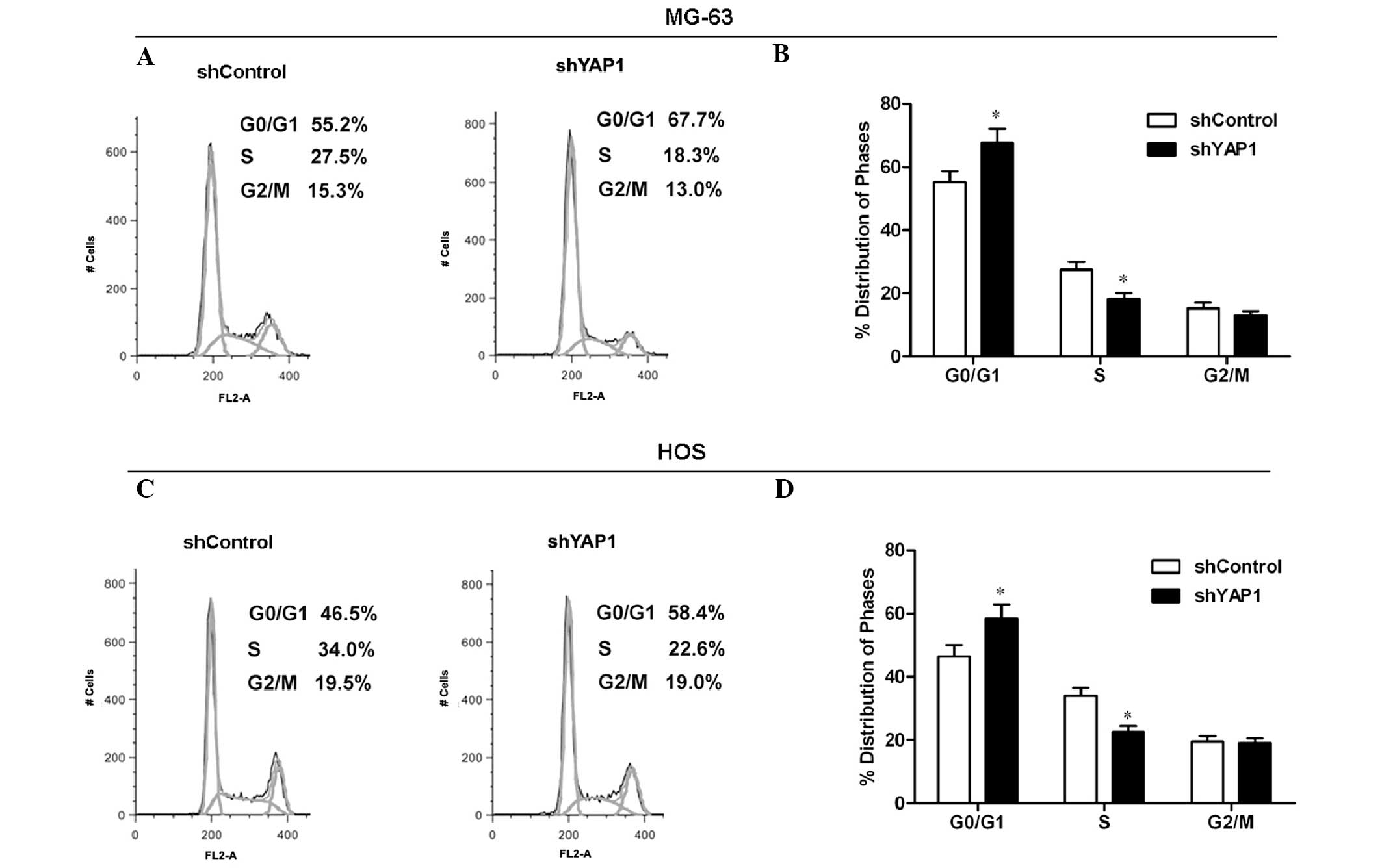
It is well known that the alteration in cell cycle
progression is frequently involved in cell proliferation. To
explore the mechanism by which YAP1 knockdown inhibits tumor
growth, cell cycle distribution was analyzed by flow cytometry.
Representative histograms are shown in Fig. 4A and C. The knockdown of YAP1 led to
a marginal accumulation of MG-63 cells in the G0/G1 phase and a
significant decrease in cells in the S phase of the cell cycle
(P<0.05; Fig. 4B). Similar to
the MG-63 cell results, the percentage of YAP1-knockdown HOS cells
distributed in the S phase was sharply decreased compared to the
control cells (Fig. 4D). These
results suggest that the knockdown of YAP1 arrests the cell cycle
progression of osteosarcoma cells.
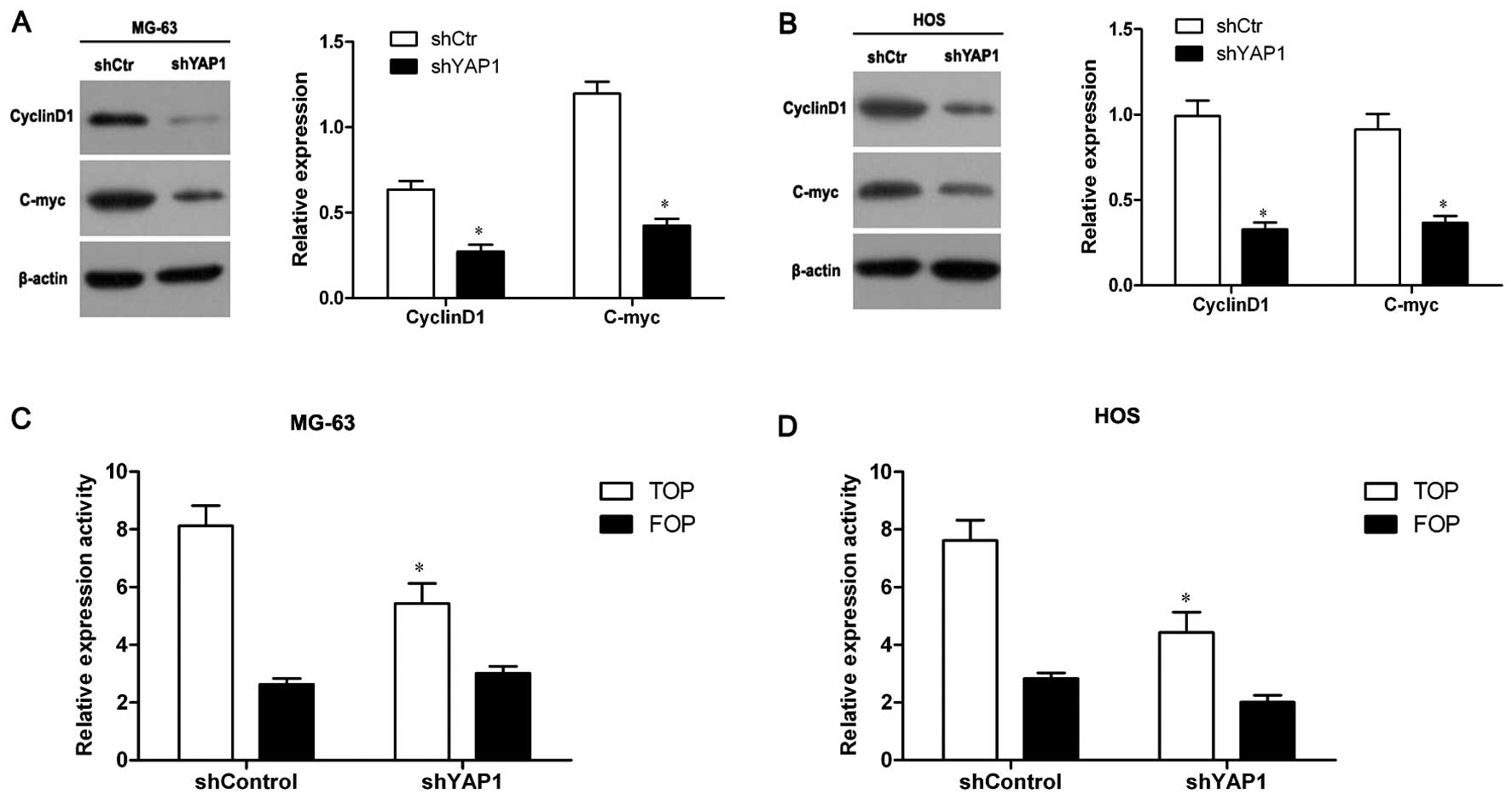
Knockdown of YAP1 inhibits the Wnt
signaling pathway in osteosarcoma cells
It has been reported that YAP1 potentiates the Wnt
signaling pathway through forming a complex with β-catenin
(9). To test whether YAP1 also
affects the Wnt signaling pathway in osteosarcoma cells, we
detected the expression of cyclinD1 and c-myc proteins, target
genes of the Wnt signaling pathway, in YAP1-knockdown MG-63 and HOS
cells through western blot assay. As shown in Fig. 5A and B, the levels of cyclinD1 and
c-myc expression in the YAP1-knockdown MG-63 and HOS cells were
significantly decreased compared to the control cells (P<0.05),
suggesting that the Wnt signaling pathway was inhibited in the
YAP1-knockdown cells.
Furthermore, TOP/FOP-Flash reporter assay was
performed, a canonical experiment for the detection of the activity
of the Wnt signaling pathway. The results showed that TOP-Flash
reporter activity in the YAP1-knockdown MG-63 cells was
significantly decreased compared to the control cells (P<0.05),
and FOP-Flash reporter activity had no obvious difference between
the YAP1-knockdown and the control cells (Fig. 5C). Similar results were also
observed in the YAP1-knockdown HOS cells (Fig. 5D). These results confirmed the
inhibitory effect of YAP1 knockdown on the Wnt signaling pathway.
Therefore, all these data demonstrated that knockdown of YAP1
inhibits the Wnt signaling pathway in osteosarcoma cells.
Discussion
YAP1, a downstream component of the Hippo pathway,
is a key regulator of organ size and tissue expansion and regulates
the balance between cell proliferation and apoptosis to maintain
the steady state of the cellular environment (25,26).
Recently, research has revealed that YAP1 is implicated in
tumorigenesis and promotes the progression of various tumors,
including hepatoma, colorectal and ovarian cancer (7,27).
However, the function of YAP1 in osteosarcoma is unclear. In the
present study, we for the first time demonstrated the crucial role
of YAP1 in the tumor growth and tumorigenesis of osteosarcoma.
Firstly, we found that YAP1 expression in
osteosarcoma cell lines was higher compared with that in the normal
human osteoblasts (IHNC) through western blot assay. Then, to
investigate the specific role of YAP1 in osteosarcoma tumor
development, YAP1 expression in MG-63 and HOS cell lines was
knocked down through shRNA. The results showed that knockdown of
YAP1 resulted in significant suppression of cell proliferation and
reduction in colony formation (Fig.
1). This evidence suggests a potential role of YAP1 in the
regulation of osteosarcoma cell growth and proliferation. Our
findings are in agreement with previous studies that found that
overexpression of YAP1 in other types of cancer cell lines resulted
in a marked increase in cell growth rate (28) and was positively associated with
Ki67 expression, a marker for cell proliferation (29). As cell proliferation is usually
regulated by cell cycle progression, consequently, the cell cycle
distribution of YAP1-knockdown MG-63 and HOS cells was analyzed
using flow cytometry. The results showed that knockdown of YAP1
induced cell cycle arrest in the G0/G1 phase (Fig. 4), suggesting that YAP1 might have
important impact on the cell cycle. Previous studies have reported
that increased expression of YAP1 is significantly correlation with
cell cycle progression and contributes to pulmonary adenocarcinoma
growth (30). Therefore, our data
indicated that the inhibitory effect of YAP1 knockdown on cell
proliferation was probably associated with regulation of the cell
cycle.
Recently, accumulating evidence supports that a
subpopulation of cancer cells with stem-like properties (CSCs)
exist in bone sarcomas (31), which
have high tumorigenic potential and are resistant to chemotherapy
and irradiation. CSCs have self-renewal and differentiation
abilities and may be responsible for tumor development and relapse
(32,33). Moreover, it was reported that YAP1
is associated with intestinal stem cell proliferation and colonic
tumorigenesis (34). Accordingly,
the role of YAP1 in osteosarcoma tumorigenesis was assessed through
tumorsphere formation assay in vitro. The knockdown of YAP1
significantly decreased the number and size of tumorspheres formed
in the culture conditions that allowed the proliferation of only
CSCs and progenitor cells (Fig. 2).
Hence, YAP1 may confer some of the properties of stem cells to
tumor cells and then regulate the growth of osteosarcoma by
promoting the proliferation of CSCs. In addition, to further
determine the role of YAP1 in tumor formation in vivo, tumor
xenograft experiments in nude mice were conducted. We observed that
YAP1 knockdown significantly retarded the development and growth of
implanted osteosarcoma tumors (Fig.
3). Moreover, the weight of the tumor xenografts derived from
the YAP1-knockdown cells was much lighter than that of the control
cells. Our result is consistent with a previous report which showed
that YAP1-overexpressing NIH3T3 cells resulted in tumor formation
when transplanted into nude mice (35). Collectively, these data confirmed
the crucial role of YAP1 in the development and growth of
osteosarcoma tumors.
Notably, some researchers have noted that YAP1 is
closely related to the Wnt signaling pathway (36), which plays a critical role in the
modulation of stem cell proliferation and tumorigenesis (37). Moreover, the Wnt canonical pathway
is known to be involved in cell proliferation and fate. McQueen
et al reported that the Wnt signaling pathway is
dysregulated in osteosarcoma (38).
In the present study, we found that YAP1 knockdown in the
osteosarcoma cell lines (MG-63 and HOS) resulted in a significant
reduction in cyclinD1 and c-myc protein expression, target genes of
the Wnt signaling pathway, implying that the Wnt signaling pathway
may be inhibited. TOP/FOP-Flash reporter assay further confirmed
that the knockdown of YAP1 in MG-63 and HOS cells suppressed the
activities of the Wnt signaling pathway (Fig. 5). Therefore, these data suggest that
the suppression of the proliferation of osteosarcoma cells
following YAP1 knockdown was probably associated with the
inhibition of the Wnt signaling pathway.
In summary, our study demonstrated that the
knockdown of YAP1 resulted in suppression of osteosarcoma cell
proliferation and tumor growth in vitro and in vivo,
and the mechanism was associated with the Wnt signaling pathway.
However, the precise mechanisms that are ultimately involved in the
interaction between YAP1 and Wnt signaling molecules remain to be
elucidated. Nevertheless, our findings suggest the potential
important role of YAP1 in the regulation of osteosarcoma growth and
also provide a new target for gene therapy of osteosarcoma.
Acknowledgements
The present study was supported by the Science and
Technology Research and Development Projects of Shanxi Province of
China (no. 2013K12-17-03).
References
|
1
|
Gatta G, Capocaccia R, Stiller C, Kaatsch
P, Berrino F and Terenziani M: Childhood cancer survival trends in
Europe: a EUROCARE Working Group study. J Clin Oncol. 23:3742–3751.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buddingh EP, Anninga JK, Versteegh MI, et
al: Prognostic factors in pulmonary metastasized high-grade
osteosarcoma. Pediatr Blood Cancer. 54:216–221. 2010.PubMed/NCBI
|
|
3
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
4
|
Lewis IJ, Nooij MA, Whelan J, et al:
Improvement in histologic response but not survival in osteosarcoma
patients treated with intensified chemotherapy: a randomized phase
III trial of the European Osteosarcoma Intergroup. J Natl Cancer
Inst. 99:112–128. 2007. View Article : Google Scholar
|
|
5
|
Lau AN, Curtis SJ, Fillmore CM, et al:
Tumor-propagating cells and Yap/Taz activity contribute to lung
tumor progression and metastasis. EMBO J. 33:468–481. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Wang W, Zhu B, et al: A functional
variant rs1820453 in YAP1 and breast cancer risk in Chinese
population. PLoS One. 8:e790562013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu MZ, Yao TJ, Lee NP, et al:
Yes-associated protein is an independent prognostic marker in
hepatocellular carcinoma. Cancer. 115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Orr BA, Bai H, Odia Y, Jain D, Anders RA
and Eberhart CG: Yes-associated protein 1 is widely expressed in
human brain tumors and promotes glioblastoma growth. J Neuropathol
Exp Neurol. 70:568–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosenbluh J, Nijhawan D, Cox AG, et al:
β-catenin-driven cancers require a YAP1 transcriptional complex for
survival and tumorigenesis. Cell. 151:1457–1473. 2012.
|
|
10
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tapon N, Harvey KF, Bell DW, et al:
salvador promotes both cell cycle exit and apoptosis in
Drosophila and is mutated in human cancer cell lines. Cell.
110:467–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai ZC, Wei X, Shimizu T, et al: Control
of cell proliferation and apoptosis by mob as tumor suppressor,
mats. Cell. 120:675–685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sudol M: Yes-associated protein (YAP65) is
a proline-rich phosphoprotein that binds to the SH3 domain of the
Yes proto-oncogene product. Oncogene. 9:2145–2152. 1994.PubMed/NCBI
|
|
14
|
Fernandez-L A and Kenney AM: The Hippo in
the room: a new look at a key pathway in cell growth and
transformation. Cell Cycle. 9:2292–2299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu MZ, Yao TJ, Lee NP, et al:
Yes-associated protein is an independent prognostic marker in
hepatocellular carcinoma. Cancer. 115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu JY, Li YH, Lin HX, et al:
Overexpression of YAP 1 contributes to progressive features and
poor prognosis of human urothelial carcinoma of the bladder. BMC
Cancer. 13:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camargo FD, Gokhale S, Johnnidis JB, et
al: YAP1 increases organ size and expands undifferentiated
progenitor cells. Curr Biol. 17:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gilgenkrantz H: The world according to
YAP: a continuous cross-talk between Wnt and Hippo pathways. Med
Sci. 29:868–874. 2013.(In French).
|
|
19
|
Clevers H: Wnt/β-catenin signaling in
development and disease. Cell. 127:469–480. 2006.
|
|
20
|
Wend P, Holland JD, Ziebold U and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Semin
Cell Dev Biol. 21:855–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: implications for therapy
in osteosarcoma. Expert Rev Anticancer Ther. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kansara M, Tsang M, Kodjabachian L, et al:
Wnt inhibitory factor 1 is epigenetically silenced in human
osteosarcoma, and targeted disruption accelerates
osteosarcomagenesis in mice. J Clin Invest. 119:837–851. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YH, Li B, Shen L, Shen Y and Chen
XD: The role and clinical significance of YES-associated protein 1
in human osteosarcoma. Int J Immunopathol Pharmacol. 26:157–167.
2013.PubMed/NCBI
|
|
24
|
Chan LH, Wang W, Yeung W, Deng Y, Yuan P
and Mak KK: Hedgehog signaling induces osteosarcoma development
through Yap1 and H19 overexpression. Oncogene. Oct 21–2013.(Epub
ahead of print).
|
|
25
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao B, Wei X, Li W, et al: Inactivation
of YAP oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, George J, Deb S, et al: The Hippo
pathway transcriptional co-activator, YAP, is an ovarian cancer
oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Dong Q, Zhang Q, Li Z, Wang E and
Qiu X: Overexpression of yes-associated protein contributes to
progression and poor prognosis of non-small-cell lung cancer.
Cancer Sci. 101:1279–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu JY, Li YH, Lin HX, et al:
Overexpression of YAP 1 contributes to progressive features and
poor prognosis of human urothelial carcinoma of the bladder. BMC
Cancer. 13:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JM, Kang DW, Long LZ, et al:
Differential expression of Yes-associated protein is correlated
with expression of cell cycle markers and pathologic TNM staging in
non-small-cell lung carcinoma. Hum Pathol. 42:315–323. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martins-Neves SR, Lopes AO, do Carmo A, et
al: Therapeutic implications of an enriched cancer stem-like cell
population in a human osteosarcoma cell line. BMC Cancer.
12:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang CJ, Yang JY, Xia W, et al: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011.PubMed/NCBI
|
|
33
|
Cordenonsi M, Zanconato F, Azzolin L, et
al: The Hippo transducer TAZ confers cancer stem cell-related
traits on breast cancer cells. Cell. 147:759–772. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou D, Zhang Y, Wu H, et al: Mst1 and
Mst2 protein kinases restrain intestinal stem cell proliferation
and colonic tumorigenesis by inhibition of Yes-associated protein
(Yap) overabundance. Proc Natl Acad Sci USA. 108:E1312–E1320. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ota M and Sasaki H: Mammalian Tead
proteins regulate cell proliferation and contact inhibition as
transcriptional mediators of Hippo signaling. Development.
135:4059–4069. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Konsavage WM Jr, Kyler SL, Rennoll SA, Jin
G and Yochum GS: Wnt/β-catenin signaling regulates Yes-associated
protein (YAP) gene expression in colorectal carcinoma cells. J Biol
Chem. 287:11730–11739. 2012.
|
|
37
|
Burgess AW, Faux MC, Layton MJ and Ramsay
RG: Wnt signaling and colon tumorigenesis - a view from the
periphery. Exp Cell Res. 317:2748–2758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi
X and Hoang BH: The Wnt signaling pathway: implications for therapy
in osteosarcoma. Expert Rev Anticancer Ther. 11:1223–1232. 2011.
View Article : Google Scholar : PubMed/NCBI
|