Introduction
Colorectal cancer (CRC) is the third most frequent
malignancy, and represents the fourth most common cause of
cancer-related deaths in the world (1). Despite many advances in the management
of early and advanced CRC, the 5-year survival rate of CRC patients
remains much lower than expected (2). In the past 20 years, the encouraging
improvement in patient outcome has been followed by a plethora of
markers of prognosis and response to anticancer therapy, although
most are not clinically viable. Nevertheless, several protein and
genetic markers that predict prognosis and treatment benefit have
been validated (3). The development
and progression of CRC is a multistep process that occurs due to
the accumulation of numerous genetic alterations, including
epigenetic modifications, chromosomal abnormalities and mutations
in genes that regulate proliferation, differentiation, apoptosis
and angiogenesis (4).
Genes that are altered by amplification and result
in concomitant overexpression in tumors are considered candidate
oncogenes (5). Human ZNF703
(also known as NocA-like zinc finger protein 1, NLZ1) is a
member of the NET gene family, which is located on chromosome 8
(8p11.23). ZNF703 protein contains six evolutionarily conserved
domains, three of which have not been previously described and are
specific for NET proteins (6).
Several studies have revealed an amplification of the 8p11–12
chromosomal region associated with human breast cancers,
particularly with the luminal B subtype (7–16). In
addition, two recent studies have strongly suggested the possible
role of ZNF703 as a potential breast oncogene (15,16).
For example, ZNF703 expression is correlated with poor
clinical prognosis in estrogen receptor-positive (ER+)
breast cancer patients. Overexpression of ZNF703 induces cell
proliferation and interferes with transforming growth factor β
(TGF-β) signaling in breast epithelial cells (15). Furthermore, ZNF703 is overexpressed
in luminal B-type breast cancer cell lines, MDA-MB-134 and HCC1500,
while its expression is low in the normal mammary epithelial cell
line MCF-10A (17). In addition,
relatively high expression of ZNF703 was found in luminal breast
cancers, and is associated with an intermediate tumor grade
(17), and high ZNF703 mRNA
expression is correlated with poor survival in patients with
ER+ luminal B tumors (18). In addition, Znf703 (Zeppo1,
zinc finger elbow-related proline domain protein 1) was identified
as a human ZNF703 ortholog in mice, which has been shown to
regulate proliferation, migration and cell adhesion in mouse
mammary epithelial cells (19).
Morever, Znf703 was found to regulate transcription by repressing
E-cadherin, Wnt and TGF-β reporter expression, and to increase lung
metastases in a mouse breast cancer model (19).
Based on these findings, clinical correlations and
experimental data, it appears that ZNF703 meets the classical
definition of an oncogene in luminal B breast tumors. However, to
the best of our knowledge, there are no reports on its role in the
development and progression of gastrointestinal malignancies. In
the present study, we examined the expression of ZNF703 in
primary CRC tissues and, using cell line models, aimed to determine
its biological role in CRC.
Materials and methods
Cell lines
CRC cell lines (LS174T, SW480, HT29, SW620, DLD1,
SW1116, LoVo and CaCo2) were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). All cells were
cultured in RPMI-1640 (HyClone, Logan, UT, USA) supplemented with
10% fetal bovine serum (FBS; HyClone) in a humidified atmosphere of
5% CO2 at 37°C. Protein and RNA samples were extracted
from subconfluent cells during the exponential phase of growth.
Patients and samples
Fresh frozen CRC tissue samples and their paired
normal mucosal samples (10-cm distance from the tumor), as well as
CRC metastatic lymph nodes, were collected at the Department of
General Surgery, Nanfang Hospital, Guangzhou, China. In addition,
formalin-fixed and paraffin-embedded tumor tissues (including 58
paired normal mucosal tissues) from 138 patients with a
pathological diagnosis of CRC, who had undergone colonoscopy at the
Department of Gastroenterology between 2008 and 2009, were also
included in the present study. All patients had undergone elective
surgery for CRC at Nanfang Hospital. The comprehensive set of
clinicopathological data were obtained from the Tumor Tissue Bank
of Nanfang Hospital. Approval for the study was obtained from the
Ethics Committee of Guangzhou Southern Medical University in China,
and written informed consent was obtained from all patients.
RNA extraction, reverse-transcription and
semi-quantitative RT-PCR
Total RNA was extracted from the colorectal
specimens using TRIzol reagent (Invitrogen, Carlsbad, CA, USA).
Reverse transcription was performed in a total volume of 25 μl with
3 μg of total RNA using a RevertAid First Strand cDNA Synthesis kit
(Promega, Madison, WI, USA). Next, 2 μl of cDNA was used as a
template to amplify the ZFN703 fragment using the following primers
(F, 5′-GATCAGGGTCCTG AAGATGC-3′ and R, 5′-CCGAGTTGAGTTTGGAGGAG-3′)
and the GoTaq® Green Mix kit (Promega), under the
following conditions: 95°C for 2 min; 35 cycles of 95°C for 30 sec,
56°C for 30 sec, and 73°C for 30 sec; with final extension of 73°C
for 5 min. The GAPDH gene was used as an internal control and
amplified using the following primers: F, 5′-TATGATGATA
TCAAGAGGGTAGT-3′ and R, 5′-TGTATCCAAACTCATT GTCATAC-3′.
Western blot analysis
Tissues and cells were lysed in RIPA. Cytosolic and
nuclear extract were prepared with a protocal of ProteoExtract
subcellular proteome extraction kit (Calbiochem, Darmstadt,
Germany). The supernatant was collected, and the protein
concentration was quantified using a protein assay reagent (Bio-Rad
Laboratories, Hercules, CA, USA).
After boiling, the proteins (25 μg) were separated
by polyacrylamide gel electrophoresis (PAGE) under denaturing
conditions and transferred to a polyvinylidene fluoride membrane
(PVDF) (Millipore Corp., Billerica, MA, USA). Membranes were
blocked with 5% skim milk in TBS containing 0.1% Tween-20 (TBS-T),
and then incubated for 1 h at room temperature with rabbit
polyclonal antibody against human ZNF703 (GTX107721; GeneTex, Inc.,
Irvine, CA, USA) (1:1,000). Mouse monoclonal anti-GAPDH (CW0100A;
CWBIO, Beijing, China) or TOPO I (GTX63013; Genetex Inc., Irvine,
CA, USA) was used as loading control. Next, membranes were
incubated for 1 h with a 1:3,000 dilution of horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G (sc-45106; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) and anti-mouse
immunoglobulin G (sc-2962; Santa Cruz Biotechnology). The membranes
were developed with a horseradish peroxidase chemiluminescence
detection reagent (ECL Plus System; Millipore Corp.) and then
exposed to Hyperfilm ECL (Millipore Corp.).
Immunohistochemical analysis
Formalin-fixed and paraffin-embedded tissues were
cut into 4-μm sections, deparaffinized with xylene and rehydrated
through a graded series of ethanol/water. Next, the slides were
subjected to heat-induced antigen retrieval in 10 mM sodium citrate
buffer (pH 6.0) in a water bath for 15 min at 100°C. All of the
specimens were then preincubated with 10% normal bovine serum and
incubated with a primary polyclonal rabbit antibody against human
ZNF703 (1:100) (GTX107721; GeneTex) overnight at 4°C. The slides
were next incubated with a biotinylated goat anti-rabbit
immunoglobulin G antibody, and the reaction products were
visualized using diaminobenzidine (DAB; Dako, Carpinteria, CA, USA)
with methyl green as a counterstain. For negative controls, the
primary antibodies were omitted, but otherwise the protocol was the
same.
Immunostaining in each tumor was classified into
four categories depending on the intensity of staining of cancer
cells as negative (0), weak (1) moderate (2) or strong (3). The
percentage of stained cells was determined using the following
scale: <5% (0), 5–25% (1), 26–50% (2), 51–75% (3) and >75%
(4). The final score was obtained by multiplying these two values.
This resulted in an overall ZNF703 immunohistochemical score of 0,
1, 2, 3, 4, 6, 9 or 12. ZNF703 expression was considered low when
scores were ≤4, and high when scores were ≥6.
Small interfering RNA (siRNA)-mediated
ZNF703 silencing
Expression of human ZNF703 was knocked down with
siRNA (Shanghai GenePharma Co., Ltd., Shanghai, China) duplexes
5′-CCACACACUUUGGGCCUAAdTdT-3′ (forward) and
5′-dTdTCCACACACUUUGGGCCUAA-3′ (reverse) targeting the 3′UTR of
endogenous ZNF703. The negative control siRNA
5′-UUCUCCGAACGUGUCACGUTT-3′ (forward) and
5′-ACGUGACACGUUCGGAGAATT-3′ (reverse) targeting an unknown mRNA
sequence was used as a control. Exponential growth phase cells were
plated in 6-well plates at a density of 2×105 cells/ml,
cultured for 48 h and transfected with 1 μg of siRNA in reduced
serum medium (OPTI-MEM-I; Invitrogen) according to the
manufacturer’s protocol at 30–50% confluency.
In vitro cell growth assay
Fourty-eight hours after siRNA transfection, cells
were prepared at a concentration of 1×104 cells/ml.
Aliquots (100 μl) were dispensed into 96-well plates, and cells
were incubated for 1, 2, 3, 4, 5 or 6 days. Following incubation,
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay was performed by adding 20 ml of MTT (5 mg/ml; Promega)
for 4 h. Next, the supernatants were removed, and 150 μl of
dimethylsulfoxide (Sigma, St. Louis, MO, USA) was added to each
well. Fifteen minutes later, the absorbance value [optical density
(OD)] of each well was measured at 490 nm with a microplate reader.
All experiments were repeated three times.
Wound closure assay
Cells transfected with siRNA-ZNF703 and negative
control were seeded on 6-well culture plates. When cell confluency
reached ~80% at 48 h post-transfection, cells were scratched with a
sterile 1-ml pipette tip and rinsed with medium to remove any
free-floating cells and debris. Complete culture medium was then
added, and plates were incubated at 37°C. Wound closure was
observed at 0, 24 and 48 h, and representative scrape lines were
photographed. Duplicate wells were examined for each condition, and
each experiment was repeated three times.
Transwell migration assay
Cells in serum-free medium (2×105
cells/200 μl) were added to the upper chamber of 8-μm pore size
Transwell chambers (Corning Star, Cambridge, MA, USA). The bottom
chambers contained 10% FCS as a chemoattractant. Cells were allowed
to migrate through the porous membrane for 24 h at 37°C.
Non-migrating cells that remained on the upper surface of the
filter were removed with cotton swabs, and the remaining cells on
the lower surface of the filter were fixed with 100% methanol,
stained with crystal violet and counted under a bright field
microscope in eight different fields (Nikon E400, ×100). Each
experiment was independently performed three times.
Statistical analysis
Statistical software SPSS 16.0 (Statistical Package
for the Social Sciences; SPSS, Inc., Chicago, IL, USA) was used for
statistical analyses. The association between ZNF703 expression and
CRC clinicopathological features was analyzed using a χ2
test. The Kaplan-Meier method was used to analyze cumulative
survival rate, and differences among groups were estimated using
the log-rank test. P<0.05 was considered to indicate a
statistically significant result.
Results
ZNF703 mRNA expression in the CRC
tissues
We analyzed ZNF703 mRNA expression in CRC
tissues and adjacent normal colorectal mucosal tissues by
semi-quantitative RT-PCR. ZNF703 mRNA expression was
elevated in the majority of CRC tissues compared with their normal
pairs (16/22, 72.72%); representative RT-PCR results of ZNF703 mRNA
expression are presented in Fig.
1.
ZNF703 protein expression in CRC
tissues
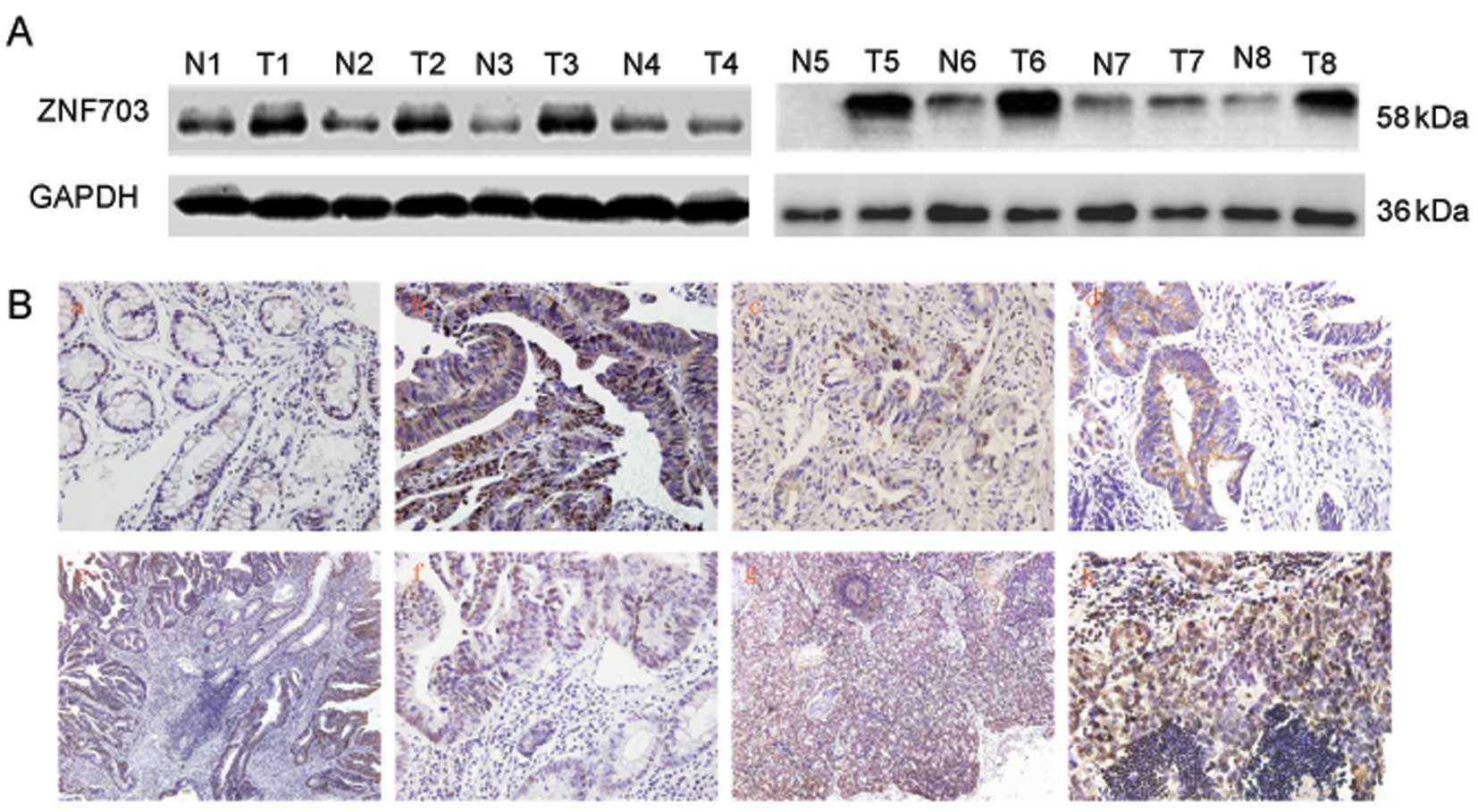
We also examined expression of ZNF703 protein in the
CRC tissues and adjacent normal colorectal mucosal tissues by
western blot analysis. Despite the interindividual variations, the
expression of ZNF703 protein was significantly upregulated in the
CRC tissues (17/26, 65.38%) (Fig.
2A). The change in ZNF703 protein expression pattern was
similar to that observed at the mRNA level.
In order to further investigate ZNF703 protein
expression and subcellular localization, we performed IHC analysis
in paraffin-embedded CRC tissues, paired adjacent normal colorectal
mucosal tissues and metastatic lymph nodes from the same CRC
patient. Normal colorectal mucosa typically expressed low ZNF703
protein (54/58, 93.10%). Contrary to this, expression of ZNF703 in
CRC tissues was detected in a large proportion of the samples
(68/138, 49.27%) and was mainly localized in the nucleus (58/138,
42.02%) and cytoplasm. In addition, the majority of metastatic
lymph nodes were positive for ZNF703 (21/23, 91.30%).
Representative results of the ZNF703 IHC analyses are shown in
Fig. 2B.
ZNF703 expression and its relation to CRC
progression and clinicopathological parameters
To elucidate the role of ZNF703 in the progression
of CRC, we examined the correlation between ZNF703 expression and
clinicopathological features of the CRC patients including age,
gender, tumor size, tumor location, pathological differentiation,
serosal invasion, lymph node metastasis and AJCC stage (Table I). Expression of ZNF703 was closely
associated with tumor size (P=0.016), pathological grading
(P=0.011), serosal invasion (P=0.023), lymph node metastasis
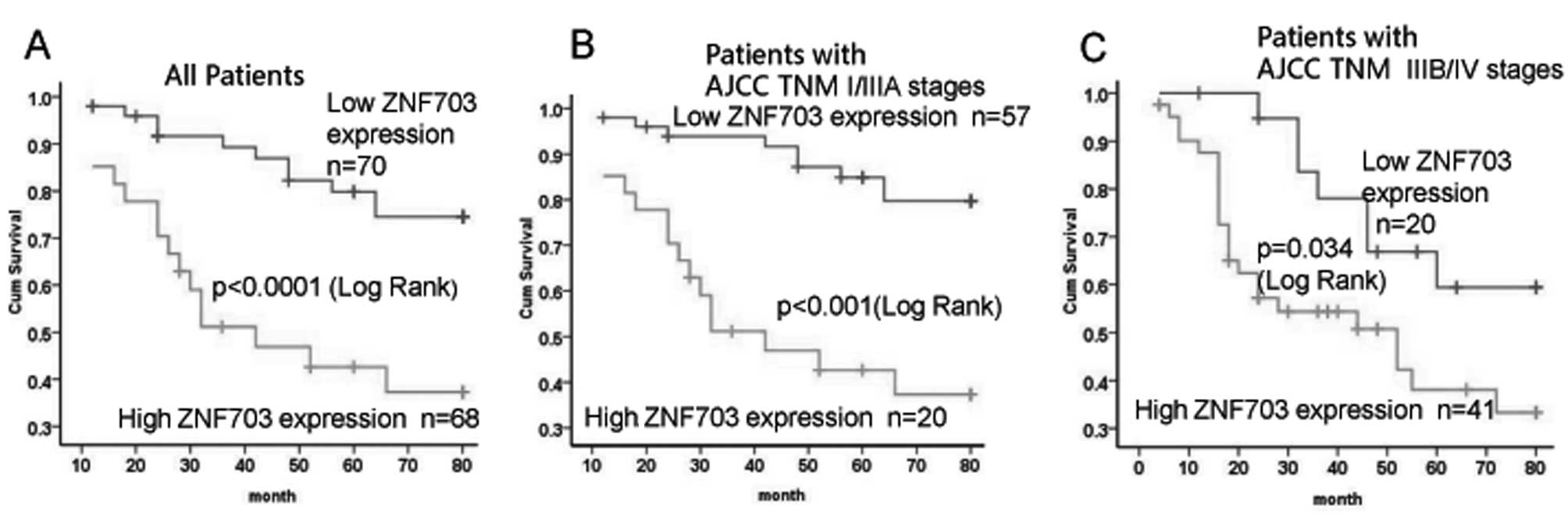
(P=0.004) and AJCC stage (P<0.001). In addition, we examined the
association between ZNF703 expression and cancer-specific survival
using a Kaplan-Meier curve and log-rank test. The difference in
patient survival between ZNF703-negative and weakly positive tumors
was not significant. Patients in these two categories were then
combined and resubjected to Kaplan-Meier analysis in comparison
with patients with high ZNF703 expression. Patients with high
ZNF703 expression had significantly shorter cancer-specific
survival than patients with either negative or low ZNF703
expression (P<0.001 for all patients in Fig. 3A). This relationship was more
obvious in patients with AJCC stage I–IIIA CRC (P<0.001;
Fig. 3B) than in AJCC stage IIIB–IV
patients (P=0.034; Fig. 3C).
Unfortunately, the prognostic value of ZNF703 expression was not
evident in patient subgroups stratified according to AJCC stage
IIIB–IV (Fig. 3). Furthermore,
multivariate analysis failed to confirm overexpression of ZNF703 as
an independent prognostic factor for CRC.
 | Table IZNF703 expression and
clinicopathological parameters of the 138 CRC patients. |
Table I
ZNF703 expression and
clinicopathological parameters of the 138 CRC patients.
| | ZNF703
expression | | |
|---|
| |
| | |
|---|
| Parameters | Total n | Score ≤4 n (%) | Score >6 n
(%) | χ2 | P-value |
|---|
| Age (years) | | 70 | 68 | 2.585 | 0.108 |
| ≤60 | 84 | 38 (45.2) | 46 (54.8) | | |
| >60 | 54 | 32 (59.3) | 22 (40.7) | | |
| Gender | | | | | |
| Male | 88 | 48 (54.5) | 40 (45.5) | 1.419 | 0.234 |
| Female | 50 | 22 (44.0) | 28 (56.0) | | |
| Tumor location | | | | | |
| Colonic and
ileocecal | 77 | 41 (53.2) | 36 (46.8) | 0.443 | 0.5006 |
| Rectal | 61 | 29 (47.5) | 32 (52.5) | | |
| Tumor size (cm) | | | | | |
| >3 | 75 | 31 (41.3) | 44 (58.7) | 5.797 | 0.016 |
| ≤3 | 63 | 39 (61.9) | 24 (38.1) | | |
| Pathological
grading | | | | | |
| Well | 76 | 46 (60.5) | 30 (39.5) | 6.502 | 0.011 |
| Moderate/poor | 62 | 24 (38.7) | 38 (61.3) | | |
| Serosal
invasion | | | | | |
| Present | 80 | 34 (42.5) | 46 (57.7) | 5.151 | 0.023 |
| Absent | 58 | 36 (62.1) | 22 (37.9) | | |
| Lymph node
metastasis | | | | | |
| Present | 62 | 23 (37.1) | 39 (62.9) | 8.365 | 0.004 |
| Absent | 76 | 47 (61.8) | 29 (38.2) | | |
| AJCC TNM
stages | | | | | |
| I–IIIA | 78 | 50 (64.1) | 27 (35.9) | 14.074 | 0.000 |
| IIIB–IV | 60 | 20 (33.3) | 41 (66.7) | | |
| Tissues | | | | | |
| Carcinoma | 138 | 70 (50.7) | 68 (49.3) | 31.558 | 0.000 |
| Normal colorectal
mucosa | 58 | 54 (93.1) | 4 (6.9) | | |
ZNF703 protein expression in cell
lines
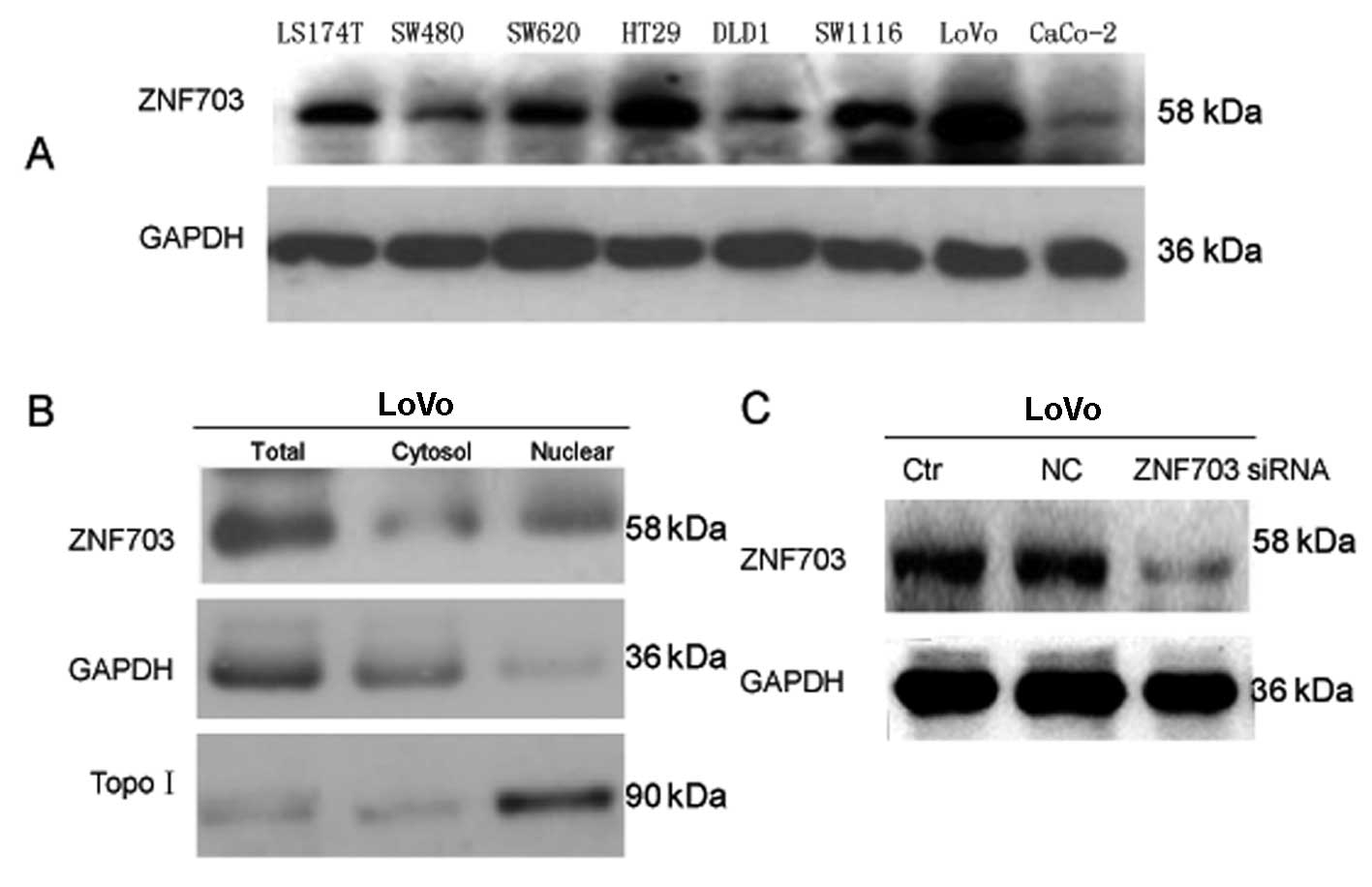
Next, we analyzed ZNF703 expression and function at
the cellular level. Expression of ZNF703 in CRC cell lines (LS174T,
SW480, HT29, SW620, DLD1, SW1116, LoVo and CaCo-2) was examined by
western blot analysis (Fig. 4A).
The highest ZNF703 expression was detected in LoVo cells (Fig. 4A). Therefore, LoVo cells were
selected for futher experiments.
Based on the results of IHC staining in tumor cells,
we decided to examine expression of ZNF703 in protein extracts of
LoVo cell, cytoplasm and nuclei by western blot analysis (Fig. 4B). As expected, ZNF703 was expressed
in LoVo cell, cytoplasm and nuclei.
In vitro silencing of ZNF703 affects the
function of LoVo cells
We investigated the role of ZNF703 in the display of
aggressive phenotypes of CRC cells in vitro. siRNA
transfection was employed to knock down ZNF703 expression in LoVo
cells with high endogenous ZNF703 expression, and high
proliferation and invasion capability. Western blot analysis showed
80% knockdown of the ZNF703 protein when compared to cells treated
with negative control siRNA or to untreated cells (Fig. 4C).
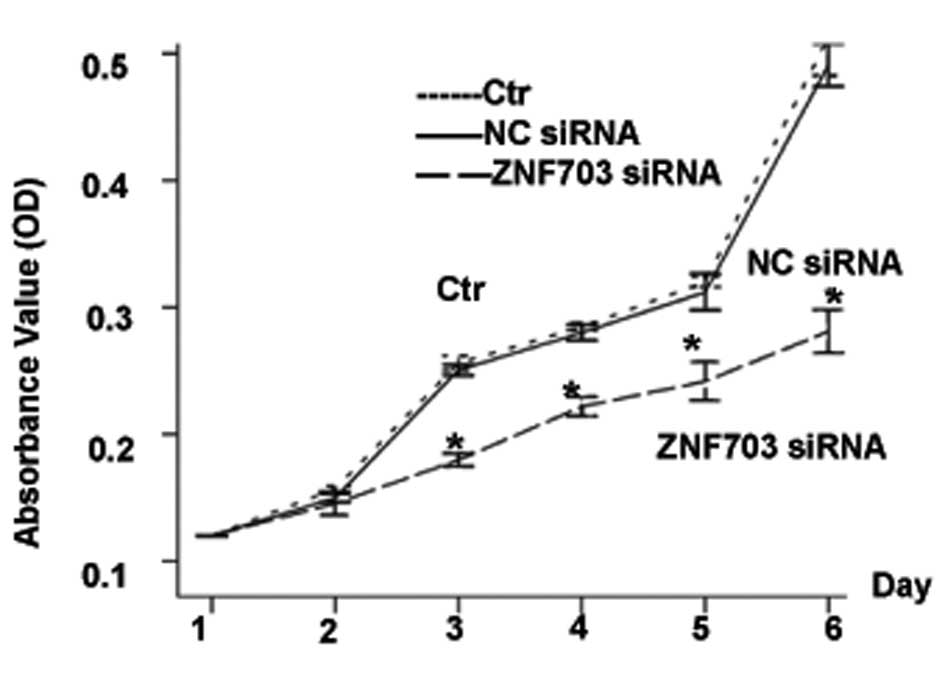
As shown by MTT assays, ZNF703 knockdown inhibited
cell proliferation when compared with the control cells
(P<0.0001) (Fig. 5). We also
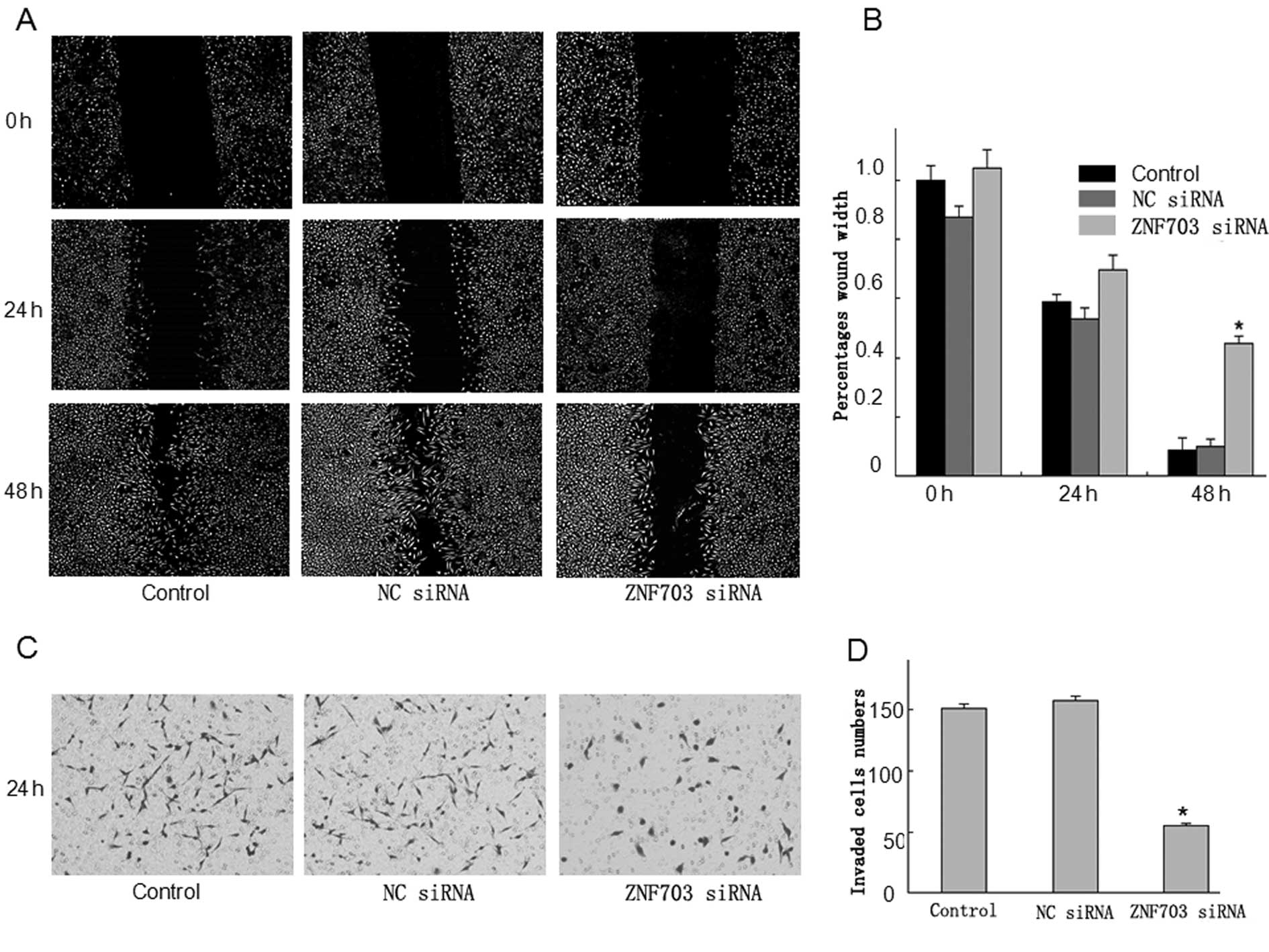
used a wound-healing assay to examine the impact of ZNF703
expression on migration of LoVo cells. ZNF703 inhibited the
migration of LoVo cells that had been physically wounded and
incubated for 24 or 48 h (Fig. 6A).
To analyze invasiveness, another important feature of malignant
cells, we performed Transwell invasion assays using cell culture
inserts covered with extracellular matrix components;
ZNF703/RNAi-transfected cells had relatively weak invasive
abilities (Fig. 6C). Collectively,
the wound-healing and invasion assays indicated that downregulation
of ZNF703 expression inhibited migration and invasion of LoVo
cells.
Discussion
ZNF703 is an oncogenic transcription factor that
regulates expression of numerous genes involved in multiple aspects
of the cancer phenotype, including proliferation, increased
self-renewal and invasion (13–17).
The aim of the present study was to examine the role
of ZNF703 in CRC. According to our results, ZNF703
expression was higher in CRC tissues than in normal colorectal
mucosa at both the mRNA and protein levels. IHC staining revealed
that the subcellular localization of ZNF703 was mainly in the
nucleus, and partly in the cytoplasm or membrane of CRC cells. In
addition, elevated ZNF703 expression was correlated with serosal
invasion, lymph node metastasis and AJCC stage. CRC patients with
low ZNF703 expression had higher survival rates. At the cellular
level, CRC cell lines had higher ZNF703 expression than the normal
297T cell line. Due to their relatively high ZNF703 expression,
LoVo cells were chosen for siRNA silencing of ZNF703. Based on
these knockdown experiments, ZNF703 silencing led to reduced
cell proliferation and migration. Collectively, these findings
indicated that ZNF703 may not be important in the differentiation
of cancer cells, but may play an important role in the progression
and metastasis of CRC.
Our findings in regards to the role of ZNF703 in CRC
are in agreement with the results of a previous study examining the
role of ZNF703 in breast cancer. According to the study of Zhang
et al (17), ZNF703 is
overexpressed in a number of breast cancer cell lines, while its
expression is low in normal mammary epithelial cells. In addition,
high ZNF703 expression contributes to tumor aggressiveness
(17). The mechanisms leading to
ZNF703 overexpression in human tumors are not well established, and
it still remains to be determined how ZNF703 expression or function
is regulated. In addition, major downstream effectors of oncogenic
functions of ZNF703 still remain elusive.
ZNF703 plays a role in tamoxifen resistance induced
by activation of the Akt/mTOR signaling pathway and downregulation
of ERα, providing a potential mechanism of its action in
tumorigenesis (18). In addition,
ZNF703 regulates transcription in mouse EpH4.9 cells, complexing
with Groucho and repressing E-cadherin, Wnt and TGF-β reporter
expression (19). Possible
mechanisms of its activation include point mutation, gene
amplification, gene rearrangement and insertion of strong promoter
or enhancer. Epigenetic modifications including demethylation and
deacetylation may also be responsible for activating ZNF703 in
tumorigenesis. Nevertheless, the exact mechanisms leading to ZNF703
oncogenic activation have yet to be examined.
In conclusion, ZNF703 was upregulated in CRC
patients, particularly in those with metastatic disease, implying
an involvement in poor clinical outcomes. Although the molecular
mechanism of ZNF703 action in carcinogenesis remains
unexplored, we found that silencing ZNF703 inhibited cancer cell
growth and migration in vitro. Hence, ZNF703 should be
considered as a potential therapeutic target for metastatic
colorectal disease.
Acknowledgements
The present study was supported by grants from the
Guangdong Provincial Science and Technology Projects (nos.
2010B031600243, 2011B05040009 and 2012B050600020) and the National
Natural Science Foundation of China (no. 81272761).
Abbreviations:
|
ZNF703
|
zinc finger protein 703
|
|
CRC
|
colorectal cancer
|
|
NLZ1
|
NocA-like zinc finger protein 1
|
|
ER+
|
estrogen receptor-positive
|
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 27:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Tänzer M, Liebl M and Quante M: Molecular
biomarkers in esophageal, gastric, and colorectal adenocarcinoma.
Pharmacol Ther. 140:133–147. 2013.PubMed/NCBI
|
|
4
|
Vogelstein B, Fearon ER, Hamilton SR, et
al: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santarius T, Shipley J, Brewer D, Stratton
MR and Cooper CS: A census of amplified and overexpressed human
cancer genes. Nat Rev Cancer. 10:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pereira-Castro I, Costa AM, Oliveira MJ,
Barbosa I, Rocha AS, Azevedo L and da Costa LT: Characterization of
human NLZ1/ZNF703 identifies conserved domains essential for proper
subcellular localization and transcriptional repression. J Cell
Biochem. 114:120–133. 2013. View Article : Google Scholar
|
|
7
|
Garcia MJ, Pole JC, Chin SF, et al: A1 Mb
minimal amplicon at 8p11–12 in breast cancer identifies new
candidate oncogenes. Oncogene. 24:5235–5245. 2005.PubMed/NCBI
|
|
8
|
Gelsi-Boyer V, Orsetti B, Cervera N, et
al: Comprehensive profiling of 8p11–12 amplification in breast
cancer. Mol Cancer Res. 3:655–667. 2005.
|
|
9
|
Chin K, DeVries S, Fridlyand J, et al:
Genomic and transcriptional aberrations linked to breast cancer
pathophysiologies. Cancer Cell. 10:529–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adélaïde J, Finetti P, Bekhouche I, et al:
Integrated profiling of basal and luminal breast cancers. Cancer
Res. 67:11565–11575. 2007.PubMed/NCBI
|
|
11
|
Kwek SS, Roy R, Zhou H, Climent J,
Martinez-Climent JA, Fridlyand J and lbertson DG: Co-amplified
genes at 8p12 and 11q13 in breast tumors cooperate with two major
pathways in oncogenesis. Oncogene. 28:1892–1903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melchor L, Garcia MJ, Honrado E, et al:
Genomic analysis of the 8p11–12 amplicon in familial breast cancer.
Int J Cancer. 120:714–717. 2007.
|
|
13
|
Haverty PM, Fridlyand J, Li L, et al:
High-resolution genomic and expression analyses of copy number
alterations in breast tumors. Genes Chromosomes Cancer. 47:530–542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sircoulomb F, Nicolas N, Ferrari A, et al:
ZNF703 gene amplification at 8p12 specifies luminal B breast
cancer. EMBO Mol Med. 3:153–166. 2011. View Article : Google Scholar
|
|
15
|
Holland DG, Burleigh A, Git A, et al:
ZNF703 is a common Luminal B breast cancer oncogene that
differentially regulates luminal and basal progenitors in human
mammary epithelium. EMBO Mol Med. 3:167–180. 2011. View Article : Google Scholar
|
|
16
|
Curtis C, Shah SP, Chin SF, Turashvili G,
Rueda OM and Dunning MJ: The genomic and transcriptomic
architecture of 2,000 breast tumours reveals novel subgroups.
Nature. 486:346–352. 2012.PubMed/NCBI
|
|
17
|
Zhang X, Mu X, Huang O, Xie Z, Jiang M,
Geng M and Shen K: Luminal breast cancer cell lines overexpressing
ZNF703 are resistant to tamoxifen through activation of Akt/mTOR
signaling. PLoS One. 8:e720532013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reynisdottir I, Arason A, Einarsdottir BO,
et al: High expression of ZNF703 independent of
amplification indicates worse prognosis in patients with luminal B
breast cancer. Cancer Med. 2:437–446. 2013.
|
|
19
|
Slorach EM, Chou J and Werb Z: Zeppo1 is a
novel metastasis promoter that represses E-cadherin expression and
regulates p120-catenin isoform expression and localization. Genes
Dev. 25:471–484. 2011. View Article : Google Scholar : PubMed/NCBI
|