Introduction
The tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a member of the TNF
superfamily, and is an attractive anticancer agent. The ability of
TRAIL includes the selective killing of a variety of cancer cells
without affecting normal cells (1–4). TRAIL
is one of the most promising experimental cancer therapeutic drugs
and is presently being tested in clinical trials (5–8). To
date, five different TRAIL receptors have been identified: death
receptor DR4 (TRAIL-R1) and DR5 (TRAIL-R2), decoy receptor DcR1 and
DcR2 and osteoprotegerin (OPG). DR4 and DR5 are able to transduce
an apoptotic signal, whereas the other three (DcR1, DcR2 and OPG)
are decoy receptors to impede TRAIL-induced apoptosis, and play a
dominant-negative role by competing with DR4 and DR5 for
interaction with TRAIL (9–12).
The binding of TRAIL to two closely related
receptors, DR4 and DR5, leads to recruitment of the adaptor
protein, Fas-associated protein with death domain (FADD) and
initiator caspase-8 to form the death-inducing signaling complex
(DISC). This process leads to the cleavage and activation of
caspase-8, which in turn activates the downstream caspase cascade,
such as caspase-9 and -3 in the presence or absence of
mitochondrial amplification machinery (13–18).
However, human cancer cell lines and primary tumor cells are found
to develop resistance to TRAIL through intrinsic or acquired
resistance mechanisms. This resistance is mediated through
deregulation of apoptotic-related signaling molecules, such as
downregulation of DR4, DR5, caspase-8, or Bax and enhanced
expression of antiapoptotic molecules such as survivin, or
overexpression of the Bcl-2 family proteins (19–21).
FLICE causes the activation of caspase-8, and FLICE-like inhibitors
such as cFLIP have been reported to bind to caspase-8 and impede
the activation of downstream incidents leading to apoptosis,
including TRAIL-mediated apoptosis (22–25).
Consequently, the relationship between tumors and TRAIL has caused
a large interest in understanding the effector mechanisms and the
search for novel compounds which can resensitize tumor cells to
TRAIL-induced apoptosis.
Reactive oxygen species (ROS), such as superoxide,
H2O2 and hydroxyl radicals, trigger a variety
of cellular responses leading to cell growth, differentiation, or
cell death (26–31). Mitogen-activated protein kinases
(MAPKs), such as stress activated protein kinase/c-Jun N-terminal
kinase (JNK), extracellular signal-regulated kinase (ERK) and p38
are principal mediators of the ROS-induced signaling pathway
(31–35). In addition, when MAPKs are
activated, this triggers diverse signaling cascades resulting in
cell proliferation, differentiation or cell death in various tumor
cells (30,34–39).
Several 6,7-substituted 2-phenylquinolin-4-ones
(2PQs) have been synthesized and identified as novel antimitotic
agents (40). Recently, novel
2-selenophenyl quinolin-4-ones and their isosteric compounds were
designed, synthesized and evaluated for their in vitro
anticancer activity. The most promising target compound,
2-(5-methylselenophen-2-yl)-6,7-methylenedioxyquinolin-4-one
(CCT327) excibited highly selective and potent growth inhibition
against MDA-MB-435 melanoma. CCT327 regulates the expression of
mitotic phase- and apoptosis-associated proteins. CCT327 was found
to decrease the expression of cyclin B1 and CDK1 proteins in a
concentration-dependent manner in HL-60 cells. In addition, CCT327
was found to activate caspase-3 and poly(ADP-ribose) polymerase
(PARP) cleavage (41). However,
whether CCT327 can sensitize tumor cells to TRAIL-induced apoptosis
is not known. In this study, we attempted to ascertain the effect
of CCT327 on TRAIL-induced apoptosis in TRAIL-resistance human
leukemia cells. Our investigation showed that CCT327 sensitized
TRAIL-induced apoptosis through the upregulation of DR4 and DR5
expression and the downregulation of cFLIP, and other antiapoptotic
proteins. Furthermore, JNK and p38 regulated the expression of DR4
and DR5 via a ROS-mediated mechanism.
Materials and methods
Chemicals and reagents
CCT327 was synthesized in our laboratory (Fig. 1A). Recombinant soluble human TRAIL
was purchased from PeproTech (Rocky Hill, NJ, USA). Primary
antibodies against caspase-3, caspase-8 and caspase-9, PARP, Bcl-2,
survivin, JNK, phospho-JNK, ERK1/2, phospho-ERK1/2, p38 and
phospho-p38 were purchased from Cell Signaling Technology (Danvers,
MA, USA). DcR1 and DcR2 antibodies were purchased from ProSci Inc.
(Poway, CA, USA). Antibodies against Bax,
FLIPS/FLIPL and Bid were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Primary DR4 and DR5 were
purchased from Abcam Inc. (Cambridge, MA, USA) and Novus
Biologicals (Littleton, CO, USA), respectively. Secondary
antibodies, HRP-conjugated goat anti-mouse IgG and goat anti-rabbit
IgG, were obtained from Millipore (Billerica, MA, USA). Cell
culture materials were obtained from Invitrogen Corp. (Carlsbad,
CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), propidium iodide (PI) and antibodies for β-actin
were purchased form Sigma (St. Louis, MO, USA). PD98059, SB203580
and SP600125 were obtained from Calbiochem (San Diego, CA,
USA).
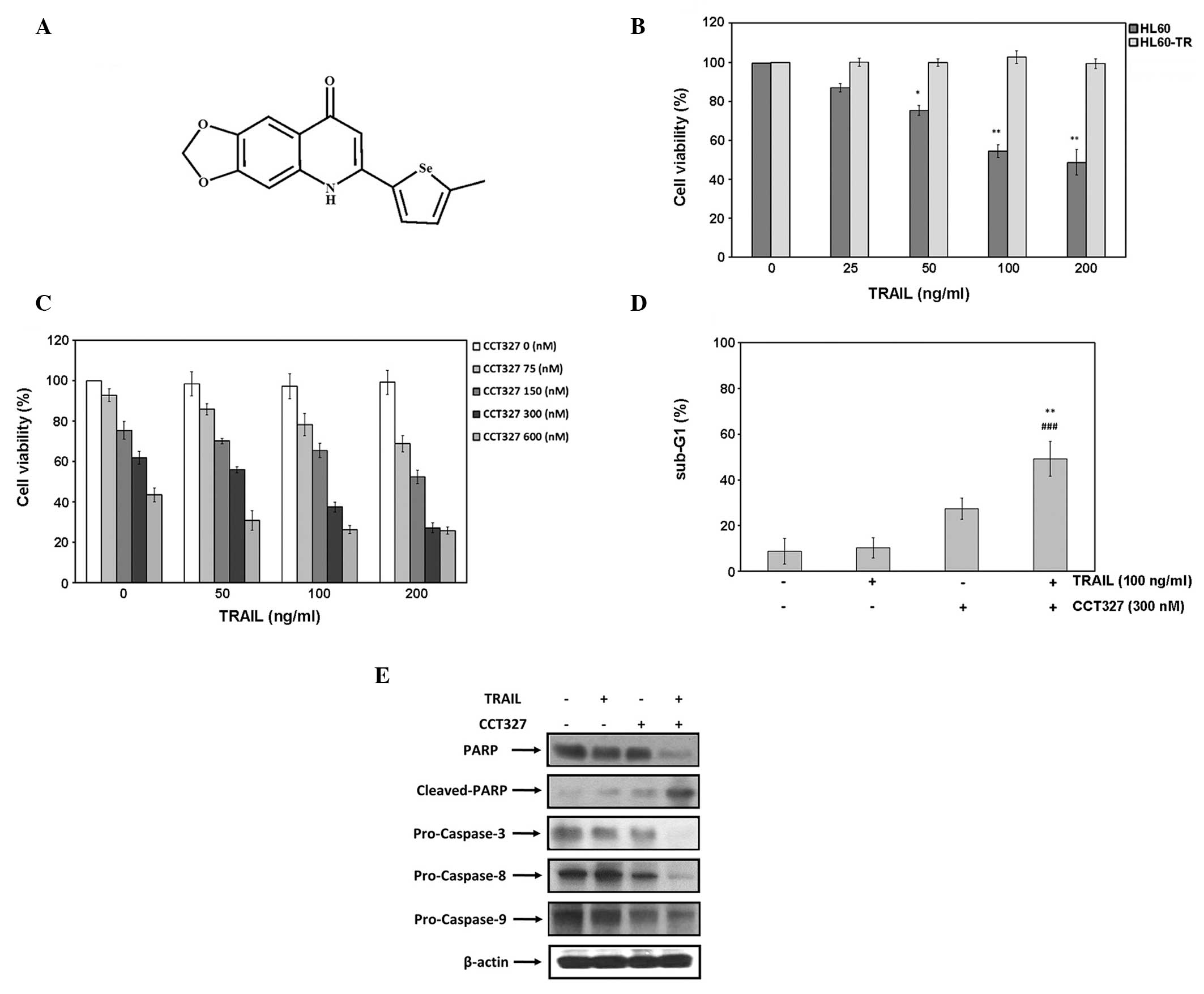 | Figure 1CCT327-potentiated as TRAIL-induced
apoptosis of HL60-TR cells. (A) Chemical structure of
2-(5-methylselenophen-2-yl)-6,7-methylenedioxyquinolin-4-one
(CCT327). (B) Dose-response curves for HL60 and HL60-TR cells
treated with TRAIL. Cells (1×104) were treated with
TRAIL (0, 25, 50, 100, 200 ng/ml) for 48 h, and cell viability was
quantitated by MTT assay. In comparison with HL60 cells, HL60-TR
cells were resistant to induction of apoptosis by TRAIL. Data are
expressed as mean ± SEM. *P<0.05 and
**P<0.01, significant difference as compared to
HL60-TR. (C) HL60-TR cells (1×104/well) were plated in
24-well plates for 48 h and treated with various concentrations of
CCT327 (0, 75, 150, 300 or 600 nM) and TRAIL (0, 50, 100 or 200
ng/ml). Cell viability was determined by MTT assay, as described in
Materials and methods. Data are expressed as mean ± SEM. (D)
HL60-TR cells (5×105) were treated with TRAIL (100
ng/ml) and with CCT327 (300 nM) or without for 48 h. Cells were
stained with PI, and the sub-G1 fraction was analyzed using flow
cytometry. Data are expressed as mean ± SEM.
**P<0.01, significant difference as compared to
CCT327; ###P<0.001, significant difference as
compared to TRAIL. (E) HL60-TR cells were treated with CCT327 (300
nM) and with TRAIL (100 ng/ml) or without for 48 h. Whole-cell
extracts were prepared and analyzed by western blotting using
antibodies against pro-caspase-3, -8, -9 and PARP. The same blots
were stripped and reprobed with β-actin antibody to verify equal
protein loading. TRAIL, tumor necrosis factor (TNF)-related
apoptosis-inducing ligand; PARP, poly(ADP-ribose) polymerase; PI,
propidium iodide. |
Cell lines and cell cultures
The human leukemia cancer cell line used in this
study was HL60 (CCL 240) from the parental cell line obtained from
the American Type Culture Collection (Manassas, VA, USA). HL60
cells derived from a human acute promyelocytic leukemia are usually
sensitive to chemotherapeutic drugs and TRAIL. TRAIL-resistant HL60
cells (HL60-TR) were selected by exposure of HL60 cells to
escalating doses of TRAIL (10 ng/ml, 20 ng/ml, 50 ng/ml, 100 ng/ml,
500 ng/ml, 1 μg/ml, 5 μg/ml and 10 μg/ml) for 2 to 3 days. After
each exposure, surviving cells were recovered and cultured in fresh
medium for 3 days and then treated with the subsequent dose
(42). HL60-TR cells were routinely
maintained in RPMI-1640 (Invitrogen). Medium was supplemented with
2 mM L-glutamine, 100 μg streptomycin, 100 U penicillin and 10%
fetal bovine serum (FBS) (Invitrogen). Cells were grown in a
humidified incubator at 37°C under 5% CO2 in air.
Cytotoxicity assay
In brief, cells were seeded on a 24-well plate
(1×104 cells/well) overnight and then treated with
different concentrations of CCT327 and TRAIL as indicated in the
figure legends and then incubated for 48 h. Following treatments,
80 μl of MTT (stock concentration 2 mg/ml) was added to each well
and incubated for 2 h under 5% CO2 at 37°C. The cell
viability was measured by MTT, which is converted by succinate
dehydrogenase in the mitochondria of viable cells to form a purple
formazan dye by metabolically viable cells. The formazan dye was
dissolved in dimethyl sulfoxide (DMSO). To measure the absorbance,
an enzyme-linked immunosorbent assay (ELISA) reader was used at OD
570 nm.
Flow cytometric analysis
To determine the effect of CCT327 plus TRAIL on the
cell cycle distribution, treated and untreated cells were stained
with PI as mentioned earlier. Briefly, 5×105 cells were
treated with CCT327 plus TRAIL for 48 h at 37°C and subjected to PI
staining. Cells were collected by trypsinization, fixed with 70%
(v/v) ethanol at 4°C for 30 min and washed with phosphate-buffered
saline (PBS). After centrifugation, cells were resuspended in 500
μl of PI solution comprising Triton X-100 (0.1%, v/v), RNase (100
mg/ml) and PI (80 mg/ml) and then analyzed with FACScan and the
Cell Quest software (Becton-Dickinson, Mountain View, CA, USA)
(43).
Western blotting
HL60-TR cells on 100-mm culture dishes
(1×106 cells/dish) were treated with various agents as
indicated in the figure legends and were then incubated for 48 h.
Cells were harvested and the protein fraction was extracted by
adding 50 μl of Gold lysis buffer (50 mM Tris-HCl, pH 7.4; 1 mM
phenylmethylsulfonyl fluoride; 1 mM NaF; 1% NP-40; 150 mM NaCl; 1
mM EGTA and 10 mg/ml leupeptin) to the cell pellets. Lysate protein
was measured by the Lowry protein assay (Bio-Rad Laboratories,
Berkeley, CA, USA). Proteins between 50 and 100 μg were used for
the sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF)
membrane (Biotrace, UK). The blotted membrane was blocked with 5%
skim milk for 1 h at room temperature and probed with the primary
antibody overnight at 4°C. Finally, HRP-conjugated appropriated
secondary antibodies were used for 1 h.
Statistical analysis
One-way analysis of variance (ANOVA) was used for
the comparison of more than 2 mean values. The results represent at
least two to three independent experiments and are shown as
averages ± SEM. Results with a P-value <0.05 were considered
statistically significant (P<0.05, P<0.01, P<0.001 as
indicated in the figure legends).
Results
CCT327 sensitizes HL60-TR cells to
TRAIL-mediated apoptosis
We first examined the sensitivity of HL60 and
HL60-TR cells to TRAIL. HL60 and HL60-TR cells were treated with
increasing doses of recombinant TRAIL and were then assessed for
cell viability using the MTT method. The dose-response of HL60 and
HL60-TR cells to TRAIL is shown in Fig.
1B. The HL60 cells were found to be highly sensitive to TRAIL
whereas the HL60-TR cells were completely resistant (Fig. 1B). Therefore, the HL60-TR cells were
used for a detailed investigation of the resistance mechanisms.
We next aimed to ascertain whether CCT327 enhances
TRAIL-induced apoptosis in HL60-TR cells. HL60-TR cells were
treated with CCT327 (0–600 nM) and then exposed to TRAIL (0–200
ng/ml) for 48 h. Treatment with TRAIL had no effect on cell
viability. However, combination treatment with CCT327 and TRAIL
significantly enhanced TRAIL-induced cytotoxicity (Fig. 1C). To further confirm the effect of
CCT327 on TRAIL-induced apoptosis, we also investigated the
distribution of cells by PI staining. The percentage of apoptotic
cells in the sub-G1 peak as evidenced by the increase in subdiploid
fraction of treated cells was measured by flow cytometry. We found
that TRAIL-induced apoptosis was increased from 9.7 to 50.8% in the
HL60-TR cells (Fig. 1D). Thus, our
results indicated that CCT327 converted the TRAIL-resistant HL60-TR
cells to TRAIL-sensitive cells.
Activation of caspases is an important hallmark of
apoptosis induced by most agents. We next investigated whether the
effect of CCT327 on TRAIL-induced cell death was through activation
of caspase-8, -9 and -3 and PARP cleavage. Treatment with 300 nM
CCT327 alone had little effect on the cleavage of procaspase-3, -8,
-9 and PARP (Fig. 1E). Moreover,
TRAIL alone did not induce processing of any caspases. Co-treatment
with CCT327 and TRAIL effectively induced activation of all three
caspases, thus leading to enhanced PARP cleavage. These results
suggest that CCT327 can enhance TRAIL-induced apoptosis. The
activation of caspases by CCT327 was essential for the stimulation
of TRAIL-mediated apoptosis.
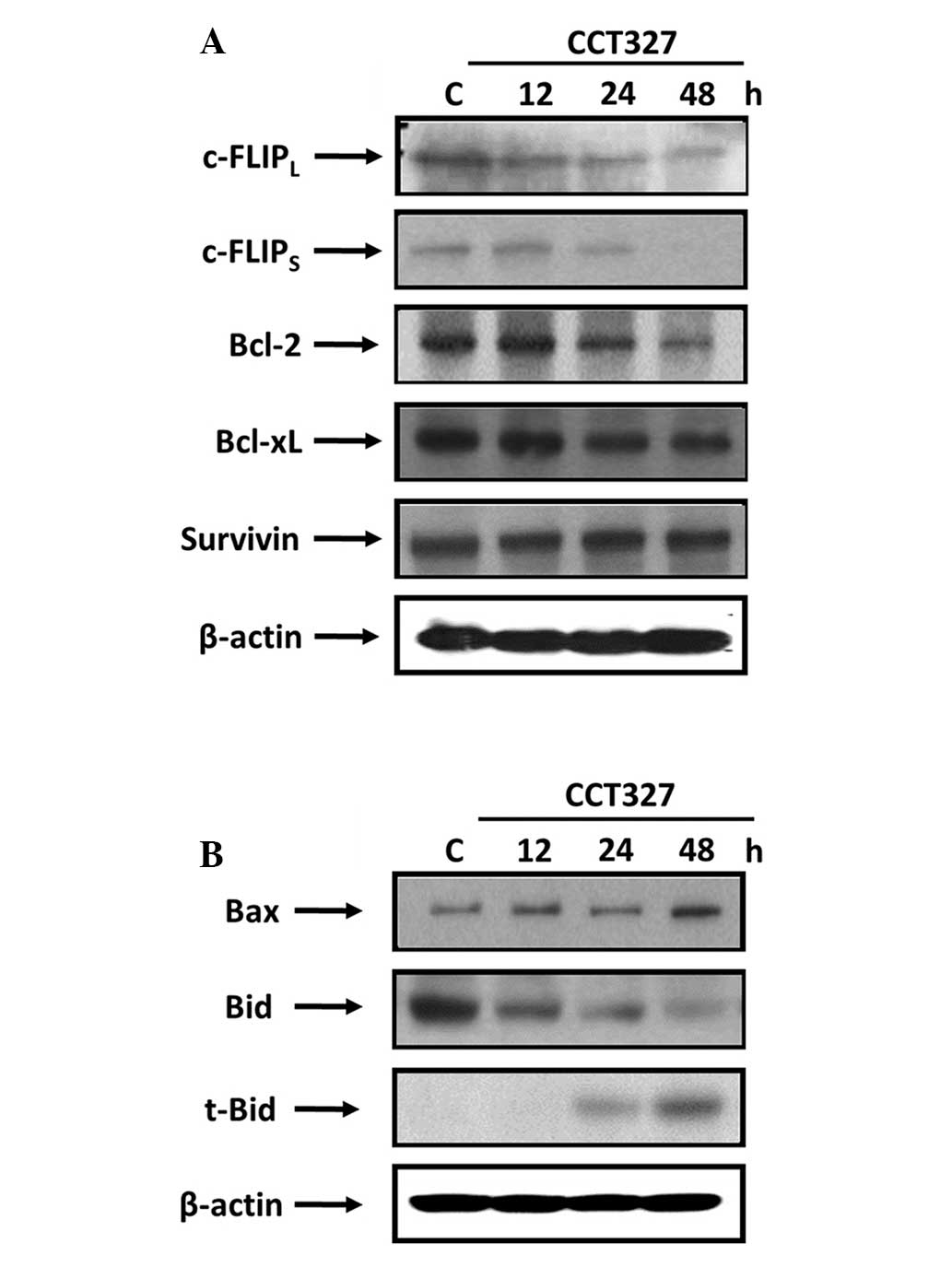
CCT327 inhibits the expression of
antiapoptotic proteins
Several antiapoptotic proteins are known to suppress
TRAIL-induced apoptosis. The mechanism by which CCT327 enhances
TRAIL-induced apoptosis was next investigated. HL60-TR cells were
exposed to 300 nM CCT327 for different times and were then examined
for the expression of cFLIPL and cFLIPS (long
and short), Bcl-2, Bcl-xL and survivin. CCT327 suppressed
expression of the antiapoptotic proteins such as Bcl-2 and both the
short and long forms of cFLIP (Fig.
2A). It had no effect on the expression of survivin. Expression
of Bcl-xL was not distinct. Our results suggest that downregulation
of antiapoptotic proteins is another mechanism by which CCT327
sensitizes TRAIL-induced apoptosis.
CCT327 regulates the expression of
proapoptotic proteins
Whether CCT327 affects the expression of
proapoptotic proteins was next examined. CCT327 caused the cleavage
of bid protein and enhanced the expression of proapoptotic protein
bax (Fig. 2B). Induction of bid and
bax by CCT327 suggests that these proteins may disrupt
mitochondrial homeostasis, which further contributes to the
enhancement of the apoptotic effects of TRAIL.
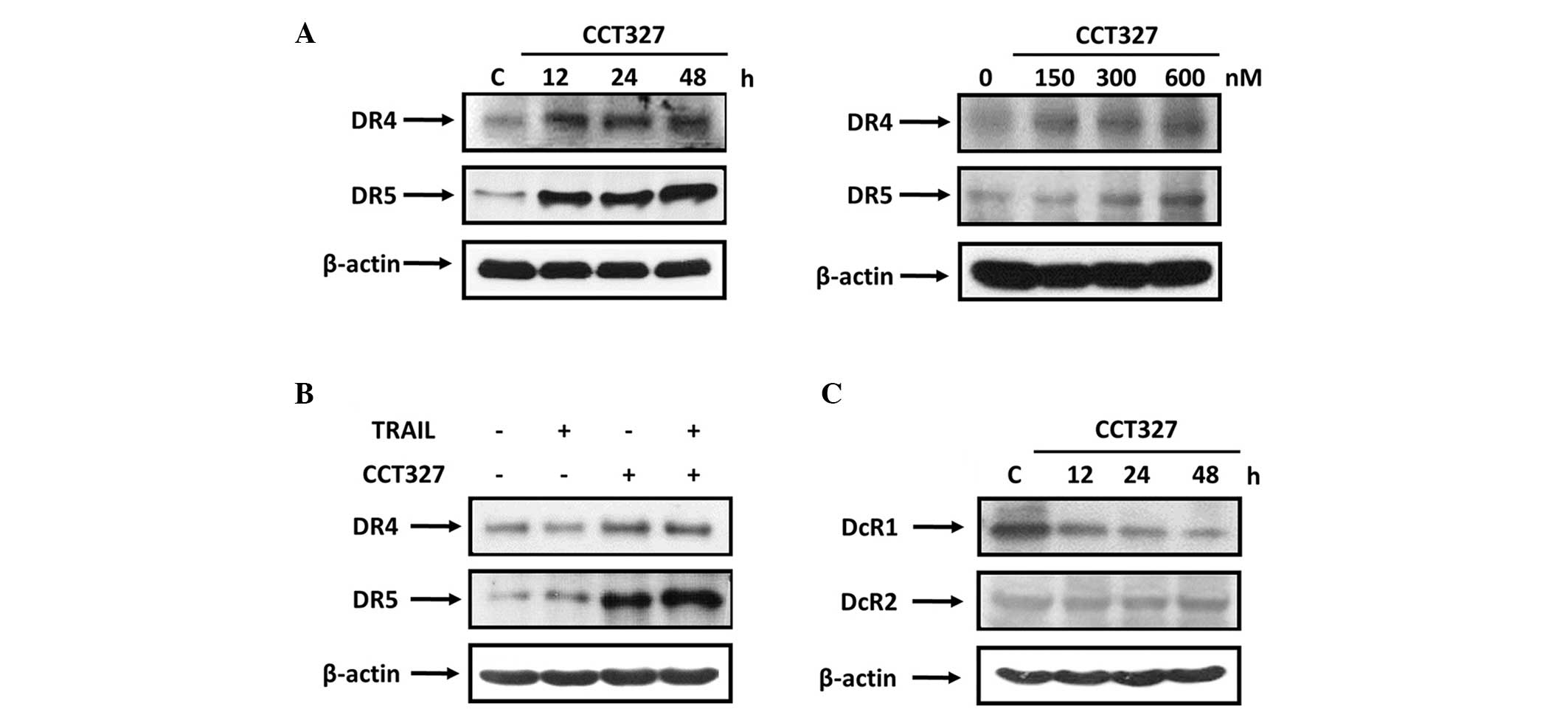
CCT327 induces the expression of DR4 and
DR5 in HL60-TR cells
To understand how CCT327 enhances TRAIL-induced
apoptosis, we investigated its effect on DR4 and DR5 in HL60-TR
cells. Treatment of HL60-TR cells with 300 nM CCT327 induced
expression of DR4 and DR5 in a time-dependent manner (Fig. 3A, left). Treatment with different
concentrations of CCT327 to HL60-TR cells for 48 h induced both DR4
and DR5 in a dose-dependent manner (Fig. 3A, right). Fig. 3B shows that treatment with TRAIL
alone had no effect on DR4 and DR5. However, combination treatment
with CCT327 (300 nM) and TRAIL (100 ng/ml) significantly enhanced
the expression of DR4 and DR5 in HL60-TR cells. These data show
that CCT327 regulated DR4 and DR5 that both play a major role in
TRAIL-induced apoptosis. This is another mechanism by which CCT327
enhanced the proapoptotic effects of TRAIL in HL60-TR cells.
CCT327 downregulates decoy receptors
Decoy receptors compete with the death receptors for
ligand binding and thereby inhibit ligand-induced apoptosis
(9–12). Therefore, we next examined whether
CCT327 modulates the expression of DcRs. We found that CCT327
decreased the expression of DcR1, but did not influence the level
of DcR2 (Fig. 3C). Thus, CCT327 may
potentiate TRAIL-induced apoptosis by inhibition of DcR1.
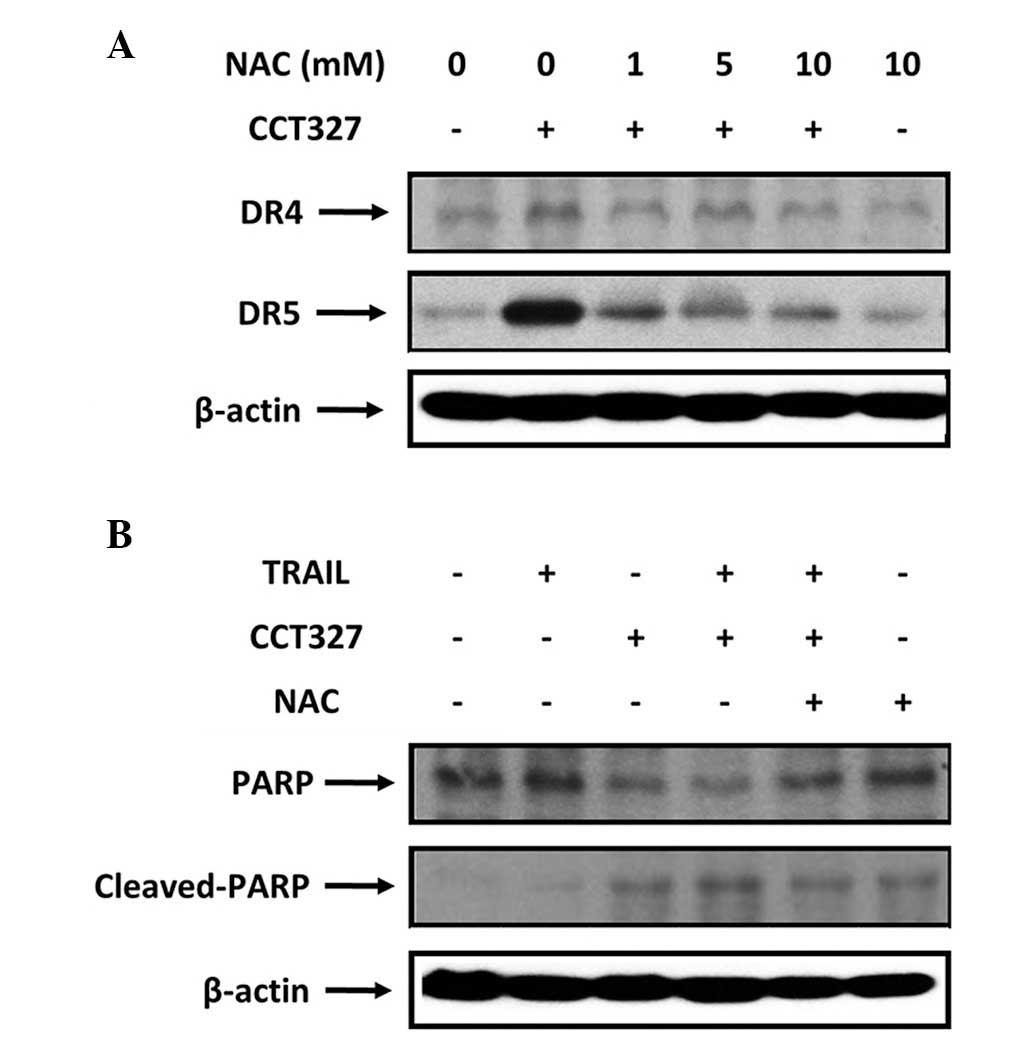
CCT327-induced upregulation of TRAIL
receptors is dependent on ROS
Several studies have reported that TRAIL-induced
apoptosis is regulated by ROS (47–51).
We attempted to ascertain whether CCT327-induced TRAIL receptors
are also regulated by ROS. Our data showed that pretreatment of
HL60-TR cells with the ROS scavenger N-acetylcysteine (NAC) reduced
the CCT327-induced upregulation of both DR5 and DR4 expression in a
dose-dependent manner (Fig. 4A).
This suggests that ROS is involved in the induction of TRAIL
receptors by CCT327. Next, we examined whether ROS is needed for
potentiation of TRAIL-induced apoptosis by CCT327. As shown in
Fig. 4B, we found that pretreatment
with NAC abolished the effect of CCT327 on TRAIL-induced cleavage
of PARP. These results show that CCT327 potentiated TRAIL-induced
apoptosis through ROS.
CCT327-induced upregulation of TRAIL
receptors is mediated through the activation of MAPKs
MAPKs, including ERK1/2, p38 and JNK, have been
reported to mediate induction of TRAIL receptors (47,49).
Therefore, we ascertained whether CCT327 activates ERK1/2, p38 and
JNK. Cells were pretreated with CCT327 for different times and were
then examined for phosphorylated ERK, JNK and p38. We found that
CCT327 activated ERK1/2 in a time-dependent manner (Fig. 5A). No activation of JNK was noted.
In addition, activation of p38 was observed (Fig. 5A). Our result showed that induction
of TRAIL receptors by CCT327 required ERK1/2 and p38. Next, we also
determined whether these MAPKs have any effect on CCT327-induced
TRAIL receptors. Cells were pretreated with 20 μM of the ERK1/2
inhibitor (PD98059), 20 μM of the JNK inhibitor (SP600125) and 10
μM of the p38 inhibitor (SB202190), respectively (52). Both the ERK1/2 inhibitor (Fig. 5B) and the p38 inhibitor (Fig. 5D) suppressed the CCT327-induced
upregulation of DR4 and DR5. No effect of the JNK inhibitor was
observed on CCT327-induced DR4 and DR5 expression. Upregulation of
TRAIL receptors by CCT327 was reversed by inhibitors of ERK1/2 and
p38. Thus, the activation of ERK1/2 and p38 is consistent with the
results obtained with the effect of their inhibitors on the
CCT327-induced expression of TRAIL receptors.
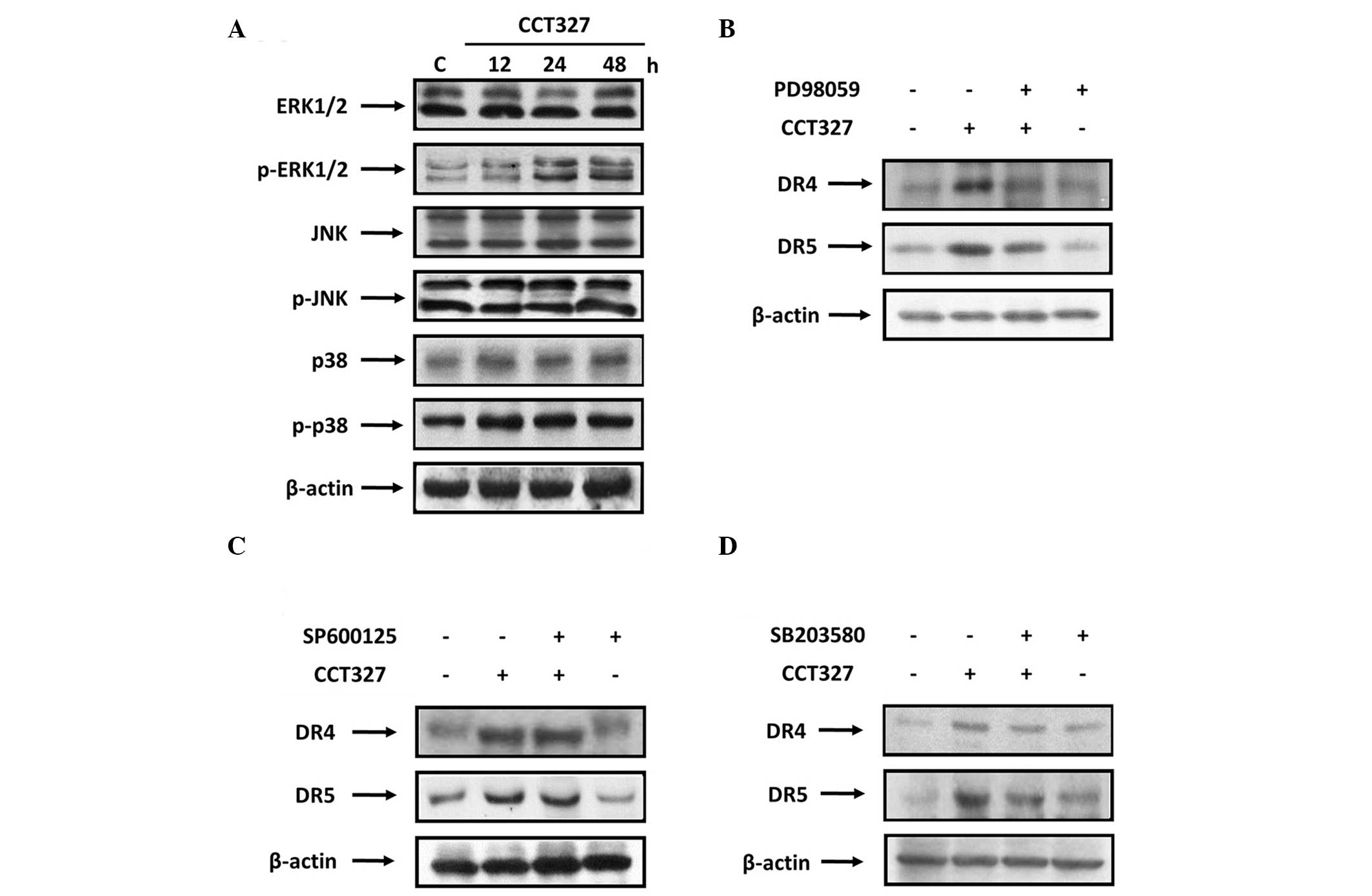 | Figure 5Upregulation of death receptors is
ERK1/2- and p38-dependent. (A) HL60-TR cells (1×106
cells) were treated with 300 nM CCT327 as indicated, and whole-cell
extracts were subjected to western blotting for phosphorylated
ERK1/2, p38 and JNK. The same blots were stripped and reprobed with
ERK1/2, p38 and JNK to ensure equal loading. HL60-TR cells were
pretreated with (B) 20 μM of the ERK1/2 inhibitor, PD98059; (C) 20
μM of the JNK inhibitor, SP600125; and (D) 10 μM of the p38
inhibitor, SB203580 for 1 h and were then treated with 300 nM
CCT327 for 48 h as indicated. Whole-cell extracts were prepared and
analyzed by western blotting using DR4 and DR5 antibodies. β-actin
was used as a loading control. ERK, extracellular signal-regulated
kinase; CCT327,
2-(5-methylselenophen-2-yl)-6,7-methylenedioxyquinolin-4-one; JNK,
c-Jun N-terminal kinase; DR, death receptor. |
Discussion
TRAIL is the only cytokine that is being explored as
an anticancer agent among all the apoptosis-inducing cytokines. The
unique property of triggering apoptosis in a variety of human
cancer cells while sparing normal cells makes TRAIL a highly
promising cancer therapeutic agent (2,3). Both
TRAIL and the agonistic antibodies against the receptor are
presently in phase II clinical trial (53). TRAIL induces apoptosis through
recognizing and binding to its cognate death receptors, DR4 and DR5
(also named as TRAIL-R1 and TRAIL-R2), on the cell surface. Upon
ligand stimulation, DRs (Fas or death receptor 4/5, DR4/5) recruit
FADD and the initiator caspases, caspase-8 or caspase-10, resulting
in the formation of DISC, thereby inducing death signaling and the
apoptosis pathway (9,10). However, TRAIL resistance is a major
limitation to its clinical application as a cancer therapeutic
agent. Nevertheless, a previous study demonstrated that the
resistance of cancer cells to TRAIL is one of the major roadblocks
to the development of this therapy (21). Thus, efforts to identify agents that
activate DRs or block antiapoptotic effectors may improve
therapeutic design.
In our previous report, we introduced and described
a novel compound, CCT327, which has been shown to induce apoptosis
in human leukemia cancer cells (41). Research has shown convincing data
that the upregulation of DR4 or/and DR5 could sensitize
TRAIL-resistant cells to TRAIL-induced cell death (49–52).
We showed that CCT327 sensitized TRAIL-induced apoptosis through
modulation of death receptors. Our results also indicate that DR4
and DR5 are involved in the reversal of TRAIL-resistance by
CCT327.
Research has shown that resistance to TRAIL can be
due to several mechanisms, including overexpression of
antiapoptotic proteins and decoy receptors (21). CCT327 decreased the expression of
DcR1, but it did not influence the level of DcR2. In addition to
the induction of DcR1, we also found that CCT327 downregulated
expression of antiapoptotic proteins including cFLIP (long and
short), Bcl-2, Bcl-xL and survivin. The effect was most pronounced
on cFLIPS and Bcl-2. Recently, c-FLIPS was
shown to be correlated with TRAIL resistance in various tumor
types, and c-FLIP downregulation has been implicated in
chemotherapy-sensitized TRAIL-induced apoptosis (22,55).
Several studies have shown that Bcl-2 blocks apoptosis by
maintaining mitochondrial function (56). Taken together, our results indicate
that c-FLIPS and Bcl-2 downregulation contributes to
CCT327-facilitated TRAIL-mediated apoptosis.
ROS trigger a variety of cellular responses leading
to cell growth, differentiation or cell death (54). ROS generation has been proposed to
be involved in death receptor upregulation by cancer
chemopreventive agents (51,52).
In the present study, we found that induction of ROS was critical
for the sensitization of cells to TRAIL by CCT327. Our data
revealed that the mechanism by which CCT327 induces DR upregulation
is through production of ROS. The antioxidant NAC abolished the
upregulation of DR by CCT327. The effect of CCT327 on TRAIL-induced
apoptosis was also neutralized by the antioxidant. This reversal
was apparently due to inhibition of induction of TRAIL receptors.
An important downstream mediator of ROS-induced signaling is the
MAPKs (31,34). MAPKs, including ERK1/2, p38 and JNK,
have been reported to mediate induction of TRAIL receptors
(47,49). Recent studies have shown that
activation of ERK, JNK or p38 is also associated with
TRIAL-induced-apoptosis via upregulation of DR4/5 (49,50).
CCT327 activated ERK1/2 p38 in a time-dependent manner. We
questioned whether the activation of p38 and ERK1/2 was the cause
or a downstream effect of the upregulation of the TRAIL receptors.
Both ERK1/2 inhibitor (Fig. 5B) and
p38 inhibitor (Fig. 5D) suppressed
the CCT327-induced upregulation of DR4 and DR5. Notably, the
presence of the JNK inhibitor had no effect on CCT327-induced DR4
and DR5 expression. CCT327 induced the expression of TRAIL
receptors dependent on MAPK, particularly ERK1/2 and p38.
Overall, we demonstrated that CCT327 can sensitize
TRAIL-induced apoptosis through the upregulation of DRs mediated by
JNK and p38 and the downregulation of cFLIP and other antiapoptotic
proteins. CCT327 has potential for application in the treatment of
cancer by TRAIL, particularly for tumors that develop resistance to
TRAIL.
Acknowledgements
The authors want to thank Dr Ho for his kind and
constructive advice.
Abbreviations:
|
CCT327
|
2-(5-methylselenophen-2-yl)-6,7-methylenedioxyquinolin-4-one
|
|
2PQs
|
6,7-substituted
2-phenylquinolin-4-ones
|
|
TRAIL
|
tumor necrosis factor (TNF)-related
apoptosis- inducing ligand
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI
|
propidium iodide
|
|
NAC
|
N-acetylcysteine
|
|
DR
|
death receptor
|
|
DcR
|
decoy receptor
|
|
OPG
|
osteoprotegerin
|
|
FADD
|
Fas-associated protein with death
domain
|
|
DISC
|
death-inducing signaling complex
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
ROS
|
reactive oxygen species
|
|
FLICE
|
FADD-like interleukin-1β converting
enzyme
|
|
c-FLIP
|
FLICE inhibitory protein
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
JNK
|
c-Jun N-terminal kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
FBS
|
fetal bovine serum
|
|
SDS-PAGE
|
sodium dodecyl
sulfate-polyacrylamide
|
|
ECL
|
enhanced chemiluminescence
|
|
PBS
|
phosphate-buffered saline
|
|
RPMI-1640
|
Roswell Park Memorial
Institute-1640
|
References
|
1
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C and Smith
CA: Identification and characterization of a new member of the TNF
family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen
H, Shahrokh Z and Schwall RH: Safety and antitumor activity of
recombinant soluble Apo2 ligand. J Clin Invest. 104:155–162. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C,
Smolak P, Goodwin RG, Rauch CT, Schuh JC and Lynch DH: Tumoricidal
activity of tumor necrosis factor-related apoptosis-inducing ligand
in vivo. Nat Med. 5:157–163. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plummer R, Attard G, Pacey S, Li L, Razak
A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey
A, Calvert H and de Bono J: Phase 1 and pharmacokinetic study of
lexatumumab in patients with advanced cancers. Clin Cancer Res.
13:6187–6194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotte SJ, Hirte HW, Chen EX, Siu LL, Le
LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL and Oza AM: A
phase 1 study of mapatumumab (fully human monoclonal antibody to
TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer
Res. 14:3450–3455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Camidge DR, Herbst RS, Gordon MS, Eckhardt
SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Sager J, Ashkenazi A,
Bray G and Mendelson D: A phase I safety and pharmacokinetic study
of the death receptor 5 agonistic antibody PRO95780 in patients
with advanced malignancies. Clin Cancer Res. 16:1256–1263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forero-Torres A, Infante JR, Waterhouse D,
Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J,
Jin X, Wang Q, Austin T, Rosen M, Beckman R, von Roemeling R,
Greenberg J and Saleh M: Phase 2, multicenter, open-label study of
tigatuzumab (CS-1008), a humanized monoclonal antibody targeting
death receptor 5, in combination with gemcitabine in
chemotherapy-naive patients with unresectable or metastatic
pancreatic cancer. Cancer Med. 2:925–932. 2013. View Article : Google Scholar
|
|
9
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan G, Ni J, Wei YF, Yu G, Gentz R and
Dixit VM: An antagonist decoy receptor and a death
domain-containing receptor for TRAIL. Science. 277:815–818. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan G, Ni J, Yu G, Wei YF and Dixit VM:
TRUNDD, a new member of the TRAIL receptor family that antagonizes
TRAIL signalling. FEBS Lett. 424:41–45. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheridan JP, Marsters SA, Pitti RM, Gurney
A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood
WI, Goddard AD, Godowski P and Ashkenazi A: Control of
TRAIL-induced apoptosis by a family of signaling and decoy
receptors. Science. 277:8181997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muzio M, Chinnaiyan AM, Kischkel FC,
O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M,
Gentz R, Mann M, Krammer PH, Peter ME and Dixit VM: FLICE, a novel
FADD-homologous ICE/CED-3-like protease, is recruited to the CD95
(Fas/APO-1) death - inducing signaling complex. Cell. 85:817–827.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaudhary PM, Eby M, Jasmin A, Bookwalter
A, Murray J and Hood L: Death receptor 5, a new member of the TNFR
family, and DR4 induce FADD-dependent apoptosis and activate the
NF-κB pathway. Immunity. 7:821–830. 1997.PubMed/NCBI
|
|
15
|
Aggarwal BB: Signalling pathways of the
TNF superfamily: a double-edged sword. Nat Rev Immunol. 3:745–756.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashkenazi A, Holland P and Eckhardt SG:
Ligand-based targeting of apoptosis in cancer: the potential of
recombinant human apoptosis ligand 2/Tumor necrosis factor-related
apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol.
26:3621–3630. 2008. View Article : Google Scholar
|
|
17
|
Srivastava RK: TRAIL/Apo-2L: mechanisms
and clinical applications in cancer. Neoplasia. 3:535–546. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suliman A, Lam A, Datta R and Srivastava
RK: Intracellular mechanisms of TRAIL: apoptosis through
mitochondrial-dependent and -independent pathways. Oncogene.
20:2122–2133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsujimoto Y and Shimizu S: Bcl-2 family:
life-or-death switch. FEBS Lett. 466:6–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irmler M, Thome M, Hahne M, Schneider P,
Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C,
Rimoldi D, French LE and Tschopp J: Inhibition of death receptor
signals by cellular FLIP. Nature. 388:190–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griffith TS, Chin WA, Jackson GC, Lynch DH
and Kubin MZ: Intracellular regulation of TRAIL-induced apoptosis
in human melanoma cells. J Immunol. 161:2833–2840. 1998.PubMed/NCBI
|
|
24
|
Krueger A, Baumann S, Krammer PH and
Kirchhoff S: FLICE-inhibitory proteins: regulators of death
receptor mediated apoptosis. Mol Cell Biol. 21:8247–8254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): a novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cimino F, Esposito F, Ammendola R and
Russo T: Gene regulation by reactive oxygen species. Curr Top Cell
Regul. 35:123–148. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dalton TP, Shertzer HG and Puga A:
Regulation of gene expression by reactive oxygen. Annu Rev
Pharmacol Toxicol. 39:67–101. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakon S, Xue X, Takekawa M, Sasazuki T,
Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T and Nakano
H: NF-kappaB inhibits TNF-induced accumulation of ROS that mediate
prolonged MAPK activation and necrotic cell death. EMBO J.
22:3898–3909. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ventura JJ, Cogswell P, Flavell RA,
Baldwin AS Jr and Davis RJ: JNK potentiates TNF-stimulated necrosis
by increasing the production of cytotoxic reactive oxygen species.
Gene Dev. 18:2905–2915. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamata H, Honda S, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou JY, Liu Y and Wu GS: The role of
mitogen-activated protein kinase phosphatase-1 in oxidative
damage-induced cell death. Cancer Res. 66:4888–4894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hara H, Ohta M, Ohta K, Kuno S and Adachi
T: Increase of antioxidative potential by tert-butylhydroquinone
protects against cell death associated with
6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y
cells. Brain Res Mol Brain Res. 119:125–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang H, Ren Y, Zhao J and Feng J: Parkin
protects human dopaminergic neuroblastoma cells against
dopamine-induced apoptosis. Hum Mol Genet. 13:1745–1754. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kyriakis JM, Banerjee P, Nikolakaki E, Dai
T, Rubie EA, Ahmad MF, Avruch J and Woodgett JR: The
stress-activated protein kinase subfamily of c-Jun kinases. Nature.
369:156–160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raingeaud J, Gupta S, Rogers JS, Dickens
M, Han J, Ulevitch RJ and Davis RJ: Pro-inflammatory cytokines and
environmental stress cause p38 mitogen-activated protein kinase
activation by dual phosphorylation on tyrosine and threonine. J
Biol Chem. 270:7420–7426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dérijard B, Hibi M, Wu IH, Barrett T, Su
B, Deng T, Karin M and Davis RJ: JNK1: a protein kinase stimulated
by UV light and Ha-Ras that binds and phosphorylates the c-Jun
activation domain. Cell. 76:1025–1037. 1994.PubMed/NCBI
|
|
37
|
Eliopoulos AG, Gallagher NJ, Blake SM,
Dawson CW and Young LS: Activation of the p38 mitogen-activated
protein kinase pathway by Epstein-Barr virus-encoded latent
membrane protein 1 coregulates interleukin-6 and interleukin-8
production. J Biol Chem. 274:16085–16096. 1999. View Article : Google Scholar
|
|
38
|
Wang WH, Gregori G, Hullinger RL and
Andrisani OM: Sustained activation of p38 mitogen-activated protein
kinase and c-Jun N-terminal kinase pathways by hepatitis B virus X
protein mediates apoptosis via induction of Fas/FasL and tumor
necrosis factor (TNF) receptor 1/TNF-alpha expression. Mol Cell
Biol. 24:10352–10365. 2004. View Article : Google Scholar
|
|
39
|
Nakshatri H, Rice SE and Bhat-Nakshatri P:
Antitumor agent parthenolide reverses resistance of breast cancer
cells to tumor necrosis factor-related apoptosis-inducing ligand
through sustained activation of c-Jun N-terminal kinase. Oncogene.
23:7330–7344. 2004. View Article : Google Scholar
|
|
40
|
Kuo SC, Lee HZ, Juang JP, Lin YT, Wu TS,
Chang JJ, Lednicer D, Paull KD, Lin CM, Hamel E, et al: Synthesis
and cytotoxicity of 1,6,7,8-substituted 2-(4′-substituted
phenyl)-4-quinolones and related compounds: identification as
antimitotic agents interacting with tubulin. J Med Chem.
36:1146–1156. 1993.
|
|
41
|
Chen CT, Hsu MH, Cheng YY, Liu CY, Chou
LC, Huang LJ, Wu TS, Yang X, Lee KH and Kuo SC: Synthesis and in
vitro anticancer activity of 6,7-methylenedioxy (or
5-hydroxy-6-methoxy)-2-(substituted selenophenyl) quinolin-4-one
analogs. Eur J Med Chem. 46:6046–6056. 2011.
|
|
42
|
Cheng J, Hylander BL, Baer MR, Chen X and
Repasky EA: Multiple mechanisms underlie resistance of leukemia
cells to Apo2 Ligand/TRAIL. Mol Cancer Ther. 5:1844–1853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lin VC, Chou CH, Lin YC, Lin JN, Yu CC,
Tang CH, Lin HY and Way TD: Osthole suppresses fatty acid synthase
expression in HER2-overexpressing breast cancer cells through
modulating Akt/mTOR pathway. J Agric Food Chem. 58:4786–4793. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R,
Ebner R, Ni J and Dixit VM: The receptor for the cytotoxic ligand
TRAIL. Science. 276:111–113. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Walczak H, Degli-Esposti MA, Johnson RS,
Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA,
Smith CA, Goodwin RG and Rauch CT: TRAIL-R2: a novel
apoptosis-mediating receptor for TRAIL. EMBO J. 16:5386–5397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Daniel PT, Wieder T, Sturm I and
Schulze-Osthoff K: The kiss of death: promises and failures of
death receptors and ligands in cancer therapy. Leukemia.
15:1022–1032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ohtsuka T and Zhou T: Bisindolylmaleimide
VIII enhances DR5-mediated apoptosis through the MKK4/JNK/p38
kinase and the mitochondrial pathways. J Biol Chem.
277:29294–29303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Izeradjene K, Douglas L, Tillman DM,
Delaney AB and Houghton JA: Reactive oxygen species regulate
caspase activation in tumor necrosis factor-related
apoptosis-inducing ligand-resistant human colon carcinoma cell
lines. Cancer Res. 65:7436–7445. 2005. View Article : Google Scholar
|
|
49
|
Shenoy K, Wu Y and Pervaiz S: LY303511
enhances TRAIL sensitivity of SHEP-1 neuroblastoma cells via
hydrogen peroxide-mediated mitogen-activated protein kinase
activation and up-regulation of death receptors. Cancer Res.
69:1941–1950. 2009. View Article : Google Scholar
|
|
50
|
Yodkeeree S, Sung B, Limtrakul P and
Aggarwal BB: Zerumbone enhances TRAIL-induced apoptosis through the
induction of death receptors in human colon cancer cells: Evidence
for an essential role of reactive oxygen species. Cancer Res.
69:6581–6589. 2009. View Article : Google Scholar
|
|
51
|
Prasad S, Yadav VR, Ravindran J and
Aggarwal BB: ROS and CHOP are critical for dibenzylideneacetone to
sensitize tumor cells to TRAIL through induction of death receptors
and downregulation of cell survival proteins. Cancer Res.
71:538–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang CH, Moon DO, Choi YH, Choi IW, Moon
SK, Kim WJ and Kim GY: Piceatannol enhances TRAIL-induced apoptosis
in human leukemia THP-1 cells through Sp1- and ERK-dependent DR5
up-regulation. Toxicol In Vitro. 25:605–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Johnstone RW, Frew AJ and Smyth MJ: The
TRAIL apoptotic pathway in cancer onset, progression and therapy.
Nat Rev Cancer. 8:782–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jacobson MD: Reactive oxygen species and
programmed cell death. Trends Biochem Sci. 21:83–86. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|