Introduction
Enhancer of zeste homologue 2 (EZH2), a catalytic
subunit in the polycomb repressive complex 2 (PRC2), is involved in
methylating histone H3 at lysine 27 (H3K27) and silencing
tumor-suppressor genes (1–3). Accumulating evidence suggests that
EZH2 is associated with increased tumor cell proliferation, local
invasiveness and distant metastasis (3–6). In
breast cancer cells, EZH2 was found to promote tumor invasion by
transcriptionally repressing the metastasis suppressor RKIP
(7). Ectopic expression of EZH2
maintained the differentiation state of basal-like breast cancer
cells and promoted the expression of progenitor-associated genes,
leading to reduced luminal differentiation, which is a hallmark of
the aggressive phenotype in breast cancer (8). Indeed, aberrant expression of EZH2 was
identified in a variety of solid tumors and might be correlated
with the aggressive features of breast cancer such as higher
histological grade, increased tumor cell proliferation, lymph node
invasion and larger tumor size (4,9–15).
Although the aggressive effects of EZH2 have been confirmed
(4,16,17),
the prognostic value of EZH2 in breast cancer remains unclear
(9,16–18).
Kleer et al found that high levels of EZH2 mRNA were
correlated with poor distant metastasis-free survival (DMFS) and
that overexpression of EZH2 protein predicted inferior overall
survival (OS) and disease-free survival (DFS) (9). In a nested case-control study, a close
correlation between EZH2 and Ki67 was identified, but there was no
significant prognostic value after the final multivariate analysis
(16).
The activation of hypoxia-inducible factor α
(HIF-1α), the main regulator of the cellular response to hypoxia
(19), is linked with tumor
angiogenesis and metastasis (20).
Its aberrant expression was also found to predict an unfavorable
prognosis in lymph node-positive breast cancer (21). Importantly, the aggressive
clinicopathological effects of HIF-1α may be attributed to its
interaction with several important cellular proteins, such as
growth factor β (20), Beclin 1
(22,23), EZH2 (24), Aurora-A (25) and AKT (26). For example, HIF-1α transcriptionally
upregulates EZH2 by binding to the hypoxia reaction element (HRE)
in the EZH2 promoter region, enhancing the activation of
RAF1-ERK-β-catenin signaling to promote cancer progression in
CD44+CD24−/low breast cancer initiating cells
(27). Nevertheless, the
clinicopathological effects of EZH2 and HIF-1α in breast cancer
have not yet been characterized.
This study was conducted to assess the
clinicopathological value of EZH2 and its relationship with HIF-1α
in breast cancer patients.
Materials and methods
Patients and eligibility
Tumor samples were harvested from 410 breast cancer
patients who underwent mastectomy (n=398) or lumpectomy (n=12) with
axillary lymph node dissection from April 1999 to October 2008. The
patient clinicopathological characteristics were obtained from
archived records (Table I).
Patients who met the following inclusion criteria were enrolled in
the study: pathologically confirmed breast cancer; no history of
ontological surgery, chemotherapy, or radiotherapy; and complete
follow-up information and paraffin-embedded specimens were
available. Patients were excluded if they previously received any
anticancer therapy, had a prior malignancy, or were pregnant and
lactating. Histological grade was classified according to the
Elston-Ellis modification of the Scarff-Bloom-Richardson grading
system (28). Estrogen receptor
(ER), progesterone receptor (PR) and human epidermal growth factor
receptor type 2 (HER2) status was determined by
immunohistochemistry (IHC). The Human Ethics Committee of the Third
Affiliated Hospital, Sun Yat-sen University approved this study.
Written informed consent was obtained from all patients prior to
treatment.
 | Table IEZH2 status in relation to the
clinicopathological characteristics of 410 breast cancer
patients. |
Table I
EZH2 status in relation to the
clinicopathological characteristics of 410 breast cancer
patients.
| | EZH2
expression | |
|---|
| |
| |
|---|
|
Characteristics | n | High | Low | P-value |
|---|
| N | 410 | 99 | 311 | |
| Age (years) | | | | |
| <50 | 236 | 56 | 180 | 0.818 |
| ≥50 | 174 | 43 | 131 | |
| Menopausal
status | | | | |
|
Post-menopausal | 164 | 41 | 123 | 0.742 |
|
Pre-menopausal | 246 | 58 | 188 | |
| Histological
type | | | | |
| Ductal | 391 | 97 | 294 | 0.359 |
| Lobular | 11 | 1 | 10 | |
| Other | 8 | 1 | 7 | |
| Tumor size
(cm) | | | | |
| <2 | 173 | 35 | 138 | 0.138 |
| 2–5 | 184 | 53 | 131 | |
| >5 | 53 | 11 | 42 | |
| Histological
grade | | | | |
| 1–2 | 155 | 35 | 120 | 0.419 |
| 3 | 65 | 18 | 47 | |
| Unknown | 190 | | | |
| Lymph node
metastasis | | | | |
| Negative | 186 | 45 | 141 | 0.984 |
| Positive | 224 | 54 | 170 | |
| Lymphatic
invasion | | | | |
| No | 390 | 90 | 300 | 0.025 |
| Yes | 20 | 9 | 11 | |
| ER status | | | | |
| Negative | 3 | 1 | 2 | 0.565 |
| Positive | 407 | 98 | 309 | |
| PR status | | | | |
| Negative | 18 | 3 | 15 | 0.581 |
| Positive | 392 | 96 | 296 | |
| HER2 status | | | | |
| Negative | 379 | 85 | 293 | 0.005 |
| Positive | 31 | 14 | 17 | |
| HIF-1α
expression | | | | |
| Low | 113 | 13 | 100 | <0.001 |
| High | 272 | 81 | 191 | |
| Unknown | 25 | | | |
Adjuvant therapy
All the patients received standard postoperative
adjuvant therapy according to the National Comprehensive Cancer
Network (NCCN) Guidelines. Briefly, patients with tumors >1 cm
and/or lymph node metastasis, were administered postoperative
adjuvant anthracycline-containing chemotherapy, such as FAC
(5-fluorouracil/doxorubicin/cyclophosphamide), TAC
(docetaxel/doxorubicin/cyclophosphamide), or AC
(doxorubicin/cyclophosphamide) followed by paclitaxel/docetaxel.
Adjuvant radiotherapy was performed in patients with four or more
positive axillary lymph node metastases and/or with tumors >5
cm. Patients with tumors positive for ER or PR received adjuvant
endocrine therapy (tamoxifen or aromatase inhibitors) for 5
years.
Tissue microarray (TMA) construction
Prior to TMA construction, all hematoxylin and
eosin-stained tissues were reviewed anew. A tissue array machine
(Beecher Instruments, Silver Spring, MD, USA) was then used to
harvest three malignant cores and two normal adjacent cores per
case to construct the TMAs. Briefly, a hollow needle was used to
pinch and remove bipartite cylinder tissue cores (1.0 mm in
diameter) from selected regions of donor tissues. The pinched
tissue cores were then inserted into a paraffin block in a
precisely spaced array pattern (29).
Semi-quantitative assessment of IHC
staining
TMAs were sectioned at a 4-μm thickness, dewaxed
with xylene, rehydrated with graded ethanol and immersed in sodium
citrate (pH 6.0) for antigen retrieval using a microwave. After
blocking in hydrogen peroxide and goat serum albumin, sections were
incubated with primary rabbit polyclonal antibodies for EZH2
(1:200; BD Pharmingen, 612666) and mouse polyclonal antibodies for
HIF-1α (1:100; Millipore, MAB5382) at 4°C overnight, followed by
the appropriate secondary antibody for 30 min at room temperature.
Slides were then processed further with diaminobenzidine and
counterstained with hematoxylin. Slides with known high expression
of EZH2 and HIF-1α were used as the positive control. Replacing the
specific primary antibody with phosphate-buffered saline served as
a negative control.
Two pathologists, who were blinded to the
clinicopathological and follow-up information, evaluated IHC
staining independently. Visible brown nuclear staining was
considered to indicate positive staining for EZH2 and HIF-1α.
Immunoreactivity was assessed based on both the intensity and
extent of the staining as we previously described (22). The staining intensity was scored as
0 (negative), 1 (bordering), 2 (weak), 3 (moderate) and 4 (strong).
The staining extent was categorized as 0 (negative), 1 (0–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%) according to the percentage of
positive staining cells in the field. The final score was obtained
by multiplying the intensity and extent scores.
Cell cultures
The human breast cancer cell lines MCF-7, MDA-MB-231
and BT-474 were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal bovine serum. The human mammary
epithelial cell line MCF-10A was grown in DMEM/F-12 medium
supplemented with 5% horse serum, 20 ng/ml epidermal growth factor,
10 μg/ml human insulin, 0.5 μg/ml hydrocortisone, 100 U/ml
penicillin and 100 μg/ml streptomycin. All cells were maintained in
a humidified incubator at 37°C with 5% CO2.
Western blotting
Proteins were extracted from the cultured cells and
liquid nitrogen-preserved tissues using RIPA buffer (Takara Bio) on
ice. After determining the protein concentrations using the
Bradford method with bovine serum albumin (Sigma-Aldrich) as a
standard, 50 μg of protein was loaded onto each lane of 12%
SDS-polyacrylamide gels and transferred to nitrocellulose membranes
(Bio-Rad). The membranes were then blocked and incubated with mouse
anti-EZH2 (1:1,000; BD Pharmingen, 612666), mouse anti-HIF-1α
(1:1,000; Millipore, MAB5382), or mouse anti-β-actin (1:1,000;
Santa Cruz, sc-81178) antibody.
Receiver operating characteristic (ROC)
analysis
ROC curve analysis was used to identify the IHC
cutoff score for EZH2 expression as we previously reported
(30). Briefly, the sensitivity and
specificity for the prognosis being studied at each IHC score were
plotted to generate a ROC curve. The score closest to the point of
maximum sensitivity and specificity, the point (0.0, 1.0) on the
curve, was fixed as the cutoff score to classify the patients as
having or not having the outcome. Prior to ROC analysis, the
survival status was dichotomized: survival [death from cancer vs.
others (censored, alive, or death from other causes)], local
failure (with vs. without) and distant metastasis (with vs.
without).
Clinical outcome assessment
All patients were followed up using a strict
protocol. After the completion of surgery, patients were observed
every 4–6 months for 5 years and every 12 months thereafter. OS was
defined as the time from the date of surgery to the date of death
due to breast cancer, or the date of last follow-up if patients
were still alive. DFS was measured from the date of surgery to the
date of local recurrence/distant metastasis, date of death, or the
latest date when censored. LFFS was calculated from the date of
surgery to the date of local failure, date of death, or the latest
date when censored. DMFS was defined as the time from the date of
surgery to the date of distant metastases, the date of death, or
the latest date when censored (22).
Statistical analysis
The relationship between EZH2 expression and
clinicopathological variables was assessed using the Chi-square
test. The probability of survival was estimated using the
Kaplan-Meier method and the difference between curves was assessed
using the log-rank test. The prognostic value of multiple factors
on survival was evaluated in a Cox proportional hazards model.
Statistical analyses were performed using SPSS v. 17.0 (SPSS, Inc.,
Chicago, IL, USA). A two-tailed P<0.05 was considered to
indicate a statistically significant result.
Results
Patient characteristics
A total of 410 breast cancer patients were included
in the study. The median age was 49 years (range, 26–84 years). The
clinicopathological characteristics of the 410 patients are shown
in Table I. After a median
follow-up of 78.8 months (range, 36.6–144.7 months), 76 of the 410
patients (18.5%) suffered tumor relapse (10 locoregional
recurrences and 66 distant metastases) and 55 patients (13.4%)
ultimately died of tumor progression. The 5-year OS, DFS, LFFS and
DMFS were 93.1, 88.7, 97.0, and 89.9%, respectively.
EZH2 and HIF-1α expression and ROC
analysis
IHC staining showed that both EZH2 and HIF-1α
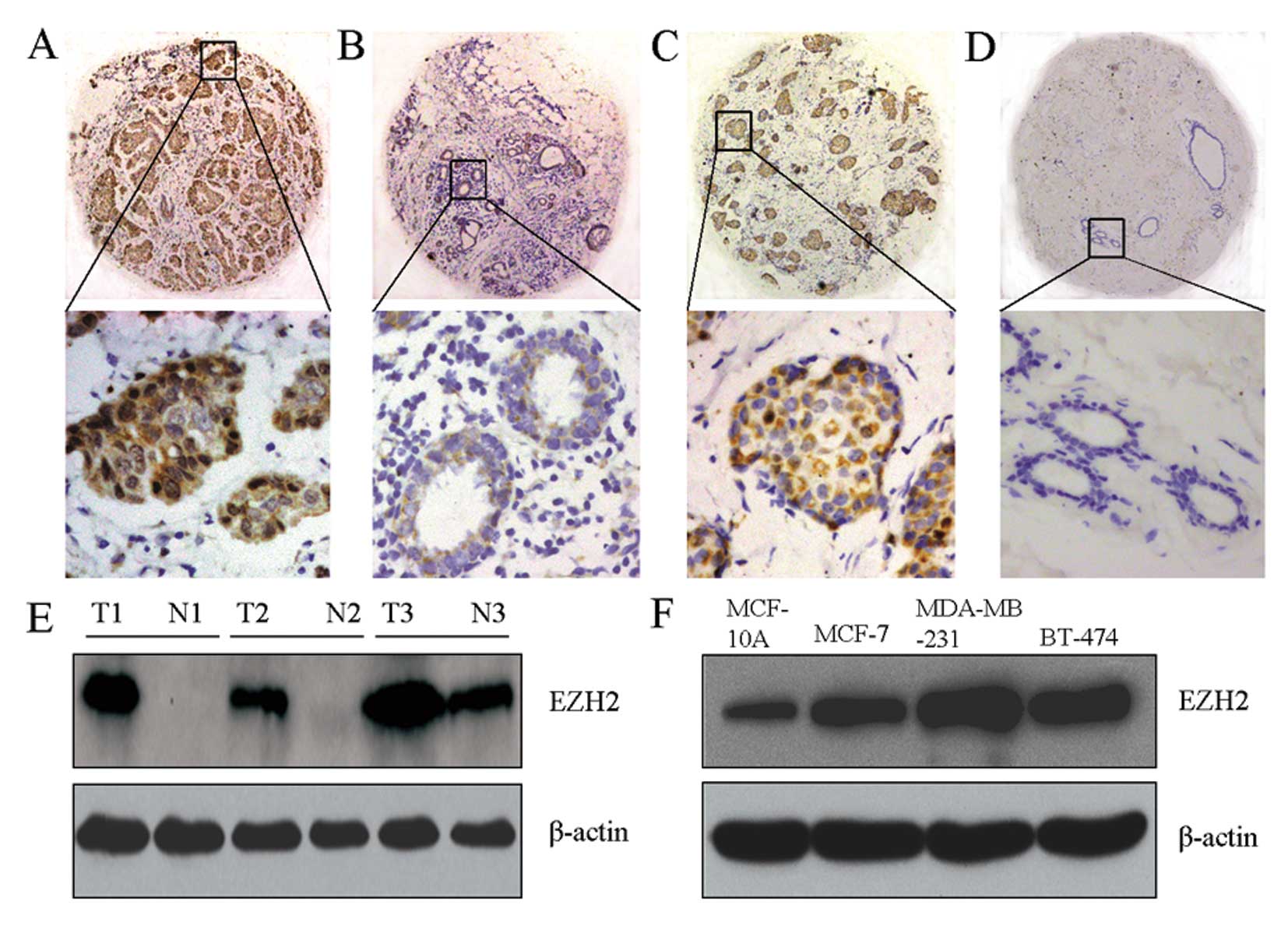
displayed strong nuclear staining in the tumors (Fig. 1A and C), but were weakly or
negatively expressed in normal breast glandular epithelia (Fig. 1B and D). Western blotting further
confirmed that, compared with adjacent tissues and normal breast
cells (MCF-10A), EZH2 was overexpressed in tumors and breast cancer
cell lines (MCF-7, MDA-MB-231 and BT-474) (Fig. 1E and F).
For EZH2, the ROC analysis-generated IHC cutoff
scores for predicting OS, DFS, LFFS and DMFS were 8.0, 8.0, 4.0,
and 8.0, respectively. Therefore, we defined an EZH2 IHC score ≤8.0
as low expression and >8.0 as high expression. For HIF-1α, a
cutoff point of 3.0 separated patients into high and low expression
subgroups.
According to the cutoff scores generated from ROC
analyses, EZH2 had nuclear overexpression in 24.1% (99/410) of
patients, whereas HIF-1α was aberrantly expressed in 70.7%
(272/385) of patients. Moreover, there was a positive correlation
between EZH2 and HIF-1α levels (r=0.299, P<0.001).
EZH2 and HIF-1α expression and
clinicopathological characteristics
As shown in Table I,
EZH2 overexpression was significantly correlated with aggressive
clinicopathological features, including lymphatic invasion
(P=0.025), HER2 expression (P=0.005) and hypoxia (defined by HIF-1α
expression) (P<0.001). Other potential factors including age,
menopausal status, histological type, tumor size, histological
grade, lymph node metastasis and ER and PR status were not
significantly correlated with EZH2 expression (all P>0.05).
EZH2 and HIF-1α expression and patient
outcome
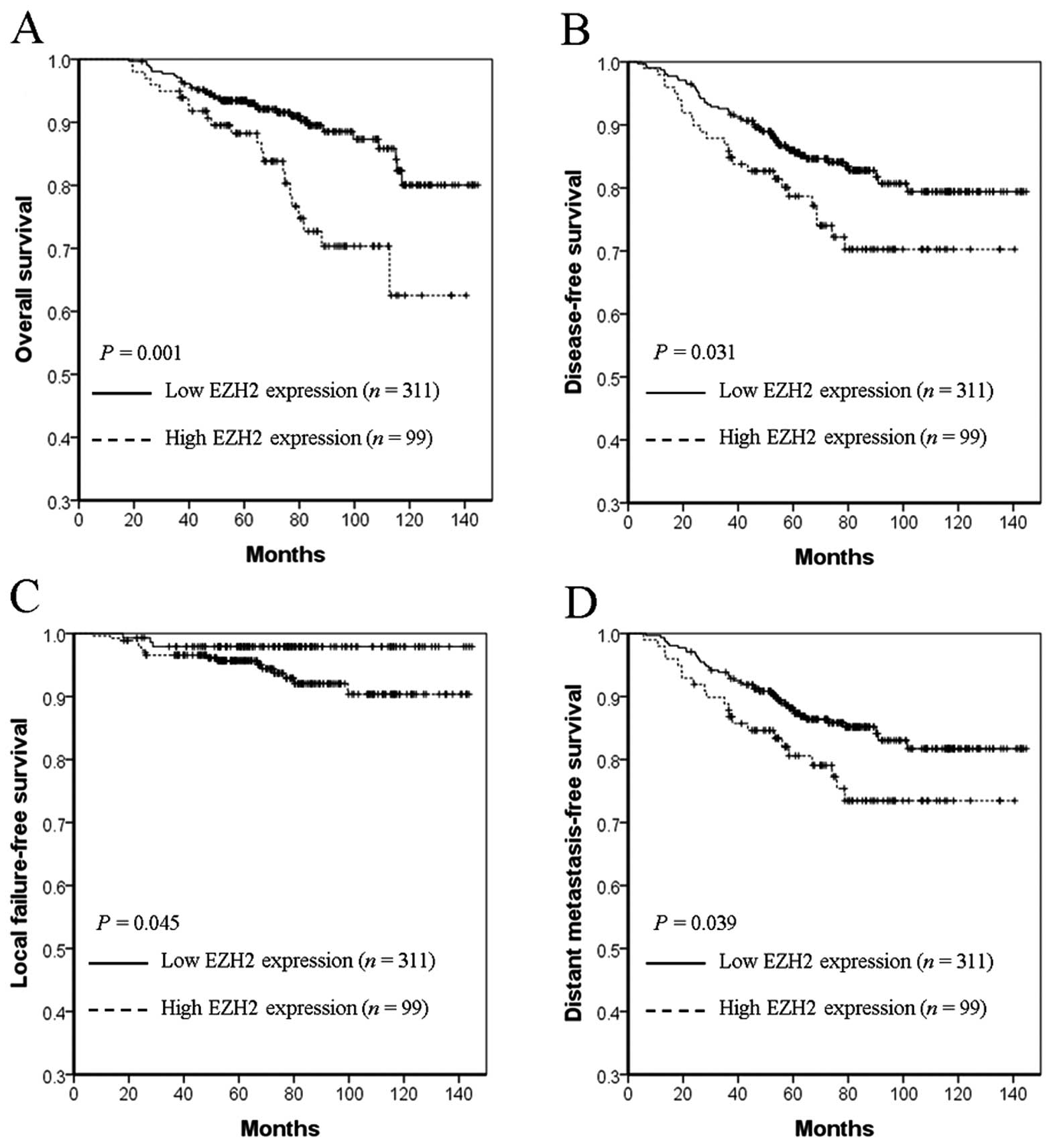
As shown in Fig. 2,
EZH2 overexpression was associated with an inferior 5-year OS (74.8
vs. 93.4%, P=0.001, Fig. 2A), DFS
(72.2 vs. 88.6%, P=0.031, Fig. 2B),
LFFS (95.7 vs. 97.9%, P=0.045, Fig.
2C) and DMFS (75.4 vs. 90.5%, P=0.039, Fig. 2D) in breast cancer. In contrast,
there was no predictive value of HIF-1α in OS (93.1 vs. 90.8%,
P=0.858), DFS (85.2 vs. 86.5%, P=0.633), LFFS (96.0 vs. 97.3%,
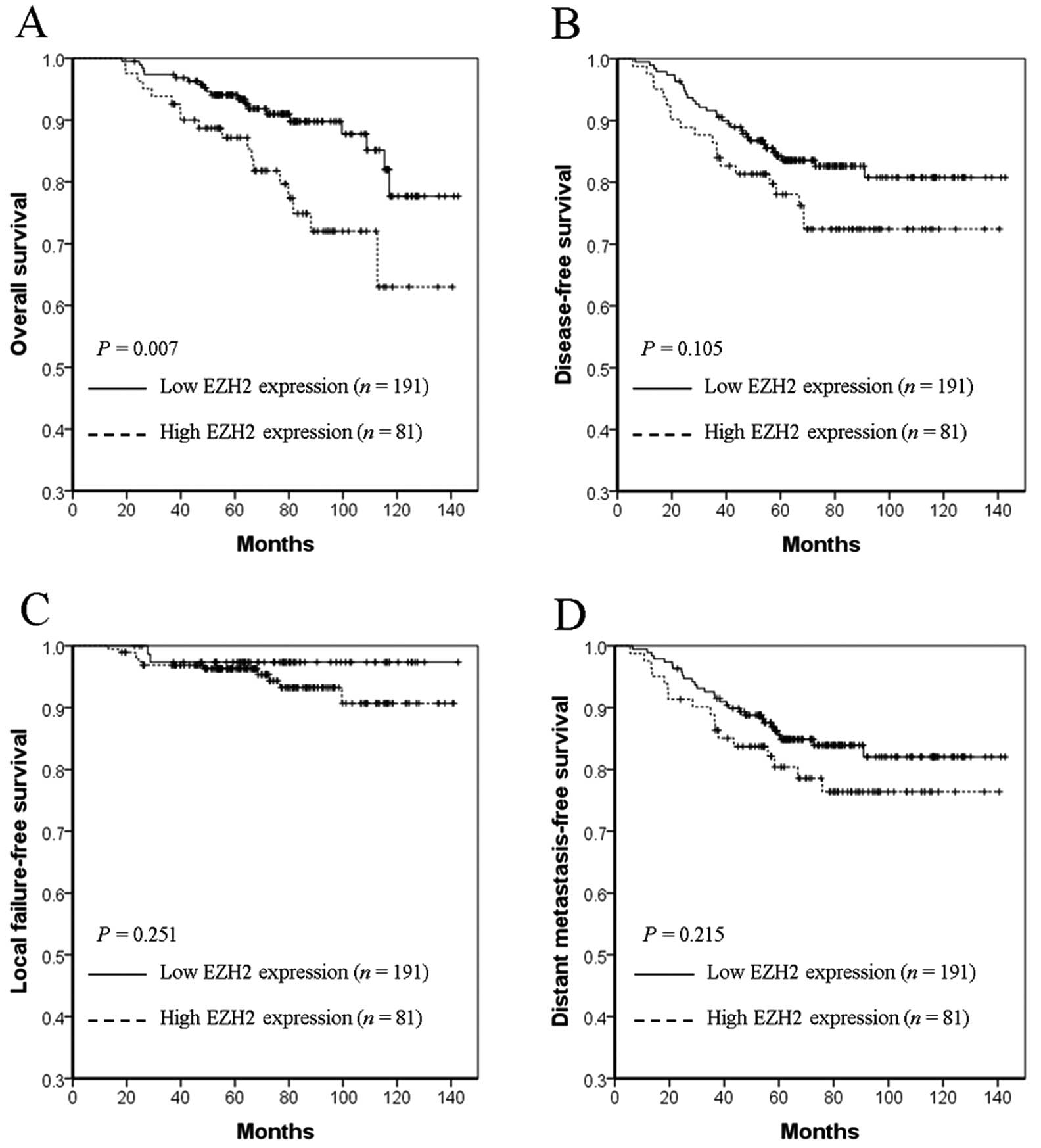
P=0.490) and DMFS (87.5 vs. 88.4%, P=0.578). However, in the HIF-1α
overexpressing subgroup, high EZH2 expression significantly
worsened 5-year OS (72.0 vs. 93.4%, P=0.007, Fig. 3A), but not DFS (72.4 vs. 84.9%,
P=0.105, Fig. 3B), LFFS (96.3 vs.
97.3%, P=0.251, Fig. 3C), or DMFS
(76.4 vs. 86.3%, P=0.215, Fig. 3D)
compared with low EZH2 expression.
Univariate and multivariate analysis
As shown in Table
II, poor OS and DMFS were associated with tumor size
(P<0.001 and P<0.001, respectively), lymph node metastasis
(P<0.001 and P<0.001, respectively), HER2 status (P=0.041 and
P=0.019, respectively) and EZH2 expression (P=0.001 and P=0.039,
respectively). Moreover, tumor size (P<0.001), lymph node
metastasis (P<0.001) and EZH2 expression (P=0.031) might predict
poor DFS. For LFFS, only lymph node metastasis (P=0.001) and EZH2
expression (P=0.045) were potential prognostic factors.
Multivariate analysis confirmed that tumor size, lymph node
metastasis status and EZH2 levels were independent prognostic
factors for patient outcome (Table
III). Specifically, EZH2 was an independent poor prognostic
biomarker for OS [hazard ratio (HR), 2.250; 95% confidence interval
(CI): 1.297–3.903, P=0.004)], DFS (HR, 1.635; 95% CI: 1.002–2.667,
P=0.049) and LFFS (HR, 4.165; 95% CI: 1.181–14.689, P=0.027), but
not for DMFS (HR, 1.572; 95% CI: 0.930–2.659, P=0.091). As
expected, tumor size was an independent predictor for poor OS (HR,
1.777; 95% CI: 1.178–2.680, P=0.006), DFS (HR, 1.557, 95% CI:
1.109–2.188, P=0.011) and DMFS (HR, 1.584, 95% CI: 1.099–2.283,
P=0.014). Lymph node metastasis status was also a negative
independent prognostic factor for OS (HR, 3.499; 95% CI:
1.720–7.117, P=0.001), DFS (HR, 3.395; 95% CI: 1.899–6.070,
P<0.001), LFFS (HR, 8.372; 95% CI: 1.904–36.817, P=0.005) and
DMFS (HR, 2.944; 95% CI: 1.604–5.405, P<0.001).
 | Table IIUnivariate analysis of OS, DFS, LFFS,
and DMFS in 410 breast cancers |
Table II
Univariate analysis of OS, DFS, LFFS,
and DMFS in 410 breast cancers
| P-values |
|---|
|
|
|---|
| Variables | OS | DFS | LFFS | DMFS |
|---|
| Age | 0.953 | 0.902 | 0.823 | 0.550 |
| Menopause
status | 0.449 | 0.207 | 0.315 | 0.181 |
| Tumor size | <0.001 | <0.001 | 0.211 | <0.001 |
| Histological
grade | 0.789 | 0.160 | 0.057 | 0.608 |
| Lymph node
metastasis | <0.001 | <0.001 | 0.001 | <0.001 |
| Lymphatic
invasion | 0.090 | 0.093 | 0.819 | 0.156 |
| ER status | 0.321 | 0.425 | 0.692 | 0.458 |
| PR status | 0.768 | 0.888 | 0.962 | 0.666 |
| HER2 status | 0.041 | 0.083 | 0.663 | 0.019 |
| HIF-1α
expression | 0.363 | 0.416 | 0.815 | 0.224 |
| EZH2
expression | 0.001 | 0.031 | 0.045 | 0.039 |
 | Table IIIMultivariate Cox proportional-hazards
analysis in 410 breast cancers. |
Table III
Multivariate Cox proportional-hazards
analysis in 410 breast cancers.
| OS | DFS | LFFS | DMFS |
|---|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age | 1.288 | 0.741–2.238 | 0.369 | 1.227 | 0.770–1.957 | 0.389 | 1.337 | 0.531–3.362 | 0.537 | 1.099 | 0.663–1.821 | 0.714 |
| Tumor size | 1.777 | 1.178–2.680 | 0.006 | 1.557 | 1.109–2.188 | 0.011 | 1.610 | 0.804–3.227 | 0.179 | 1.584 | 1.099–2.283 | 0.014 |
| Lymph node
metastasis | 3.499 | 1.720–7.117 | 0.001 | 3.395 | 1.899–6.070 | <0.001 | 8.372 | 1.904–36.817 | 0.005 | 2.944 | 1.604–5.405 | <0.001 |
| Lymphatic
invasion | 1.343 | 0.471–3.832 | 0.581 | 1.212 | 0.515–2.850 | 0.660 | 0.680 | 0.087–5.296 | 0.713 | 1.167 | 0.460–2.963 | 0.745 |
| HER2 status | 1.277 | 0.590–2.765 | 0.535 | 1.252 | 0.613–2.560 | 0.537 | 0.662 | 0.145–3.026 | 0.594 | 1.601 | 0.776–3.304 | 0.202 |
| EZH2
expression | 2.250 | 1.297–3.903 | 0.004 | 1.635 | 1.002–2.667 | 0.049 | 4.165 | 1.181–14.689 | 0.027 | 1.572 | 0.930–2.659 | 0.091 |
Discussion
EZH2 is a core subunit of PRC2 that plays an
essential role in catalyzing the trimethylation of H3K27 and
mediating transcriptional repression. Therefore, it is involved in
cell cycle regulation, deciding cell fate, senescence and cancer
(31). Although EZH2 has been
linked with an aggressive phenotype in breast cancer, its
clinicopathological value remains unclear. In the present study, we
examined the expression of EZH2 in 410 breast cancer patients and
found that EZH2 levels were closely associated with lymphatic
invasion status, HER2 expression and tumor hypoxia (Table I). EZH2 overexpression predicted a
poor 5-year OS, DFS, LFFS and DMFS (Fig. 2). Importantly, although no
prognostic value of HIF-1α was detected, the overexpression of both
HIF-1α and EZH2 significantly worsened OS in the HIF-1α
overexpression subgroup of patients (Fig. 3). Moreover, univariate and
multivariate analyses demonstrated that EZH2 is an independent
prognostic marker for breast cancer (Tables II and III).
A number of studies have reported that EZH2
expression levels might vary in different breast cancer subtypes.
In early-stage breast cancer, EZH2 was found to be overexpressed in
57.6% of patients (18). An
inflammatory breast cancer cohort study revealed that EZH2 was
positively expressed in 75.7% of patients (32). In a subgroup of triple-negative
patients, EZH2-positive staining was detected in 85.7% of patients
(33). When all breast cancer
subtypes were combined, the EZH2 expression rate was 47.4–64.0%
(16,17). The corresponding mean mRNA levels of
EZH2 in luminal A, luminal B, HER2+, basal-like and
normal-like subtypes were −0.476, 0.145, 0.186, 0.778 and −0.853,
respectively (34). These results
suggest that the EZH2 level might be expressed in a
subtype-dependent manner. However, the EZH2 expression levels were
not characterized in luminal subtypes. In the present study, most
patients were ER-positive (99.3%), PR-positive (95.6%) and
HER2-negative (92.4%), suggesting that the subtypes in the present
cohort were mainly luminal A and luminal B. The EZH2 expression
rate was 24.1%, which is similar to the 33.2% mRNA expression rate
identified in 235 ER-positive breast cancer patients (35). Therefore, the current and previous
studies demonstrated that EZH2 might have a relatively low
expression level in luminal subtype breast cancer.
It was reported that high levels of EZH2 are
associated with ER-negative, PR-negative and HER2-overexpressing
breast cancer (9,16–18).
Consistent with this, we found that enhanced EZH2 was closely
correlated with HER2, rather than ER and PR status (Table I). The underlying mechanism for this
could be that EZH2 can repress or activate nuclear factor-κB
(NF-κB) signaling in different breast cancer subtypes. In
ER-negative basal-like breast cancer, EZH2 functioned by
transactivating NF-κB signaling molecules such as interleukin 6 and
tumor necrosis factor. Conversely, EZH2 might repress other NF-κB
signaling molecules such as GATA3 and FOXA1 in ER-positive
luminal-like breast cancer (36).
Importantly, NF-κB signaling played an essential role in regulating
ER, PR and HER2 expression in breast cancer (37). Consistent with this, the present
study found that EZH2 levels were ranked from low to high in an
ER/PR/HER2-dependent manner: MCF-10A (human mammary epithelial cell
line) < MCF-7 (ER-positive) < BT-474 (HER2-positive) <
MDA-MB-231 (ER-negative). This suggests that EZH2 might be a
determining factor in establishing breast cancer subtypes.
Although luminal breast cancer is associated with a
relatively favorable clinical outcome, ~15% of patients ultimately
develop cancer-related mortality (38). Therefore, developing additional
prognostic biomarkers will greatly benefit the subgroup of patients
at high risk of disease progression in luminal subtypes. The
present study showed that EZH2 could predict the outcome of luminal
subtypes. More importantly, we found that EZH2 had a comparable
hazard ratio to tumor size (1.777 and 1.557, respectively) and
lymph node metastasis (3.499 and 3.395, respectively) for
predicting the risk of death and relapse (Table III). This suggests that combining
EZH2 and the TNM staging system would lead to a more accurate
prognosis prediction and risk definition for breast cancer. In
addition, we also found that the expression of EZH2 and HIF-1α were
positively correlated in luminal breast cancer subtypes (r=0.299,
P=0.039). Moreover, HIF-1α and EZH2 co-overexpression was a
predictor of poorer OS (Fig. 3A).
These findings suggest that EZH2 could be a useful negative
prognostic predictor and that hypoxia might lead to a more worsened
OS in luminal breast cancer patients.
HIF-1α activation is an aggressive
clinicopathological biomarker and might be a poor prognostic factor
in breast cancer (21). However,
the clinicopathological value of HIF-1α in luminal-subtype breast
cancer remains unclear. Similar to EZH2, HIF-1α expression was
tumor subtype-dependent: luminal-type tumors expressed lower levels
of HIF-1α than basal-like and HER2-positive tumors (39). Therefore, HIF-1α was reported to be
a negative prognostic biomarker in non-luminal subtype breast
cancer, but was not an independent prognostic factor for the
luminal subtype in the present study.
In conclusion, this study demonstrated that high
levels of EZH2 expression predicted poor OS, PFS, LFFS and DMFS in
luminal-subtype breast cancer patients. Importantly, EZH2 and
HIF-1α co-overexpression predicted poorer OS, leading to refined
risk stratification for luminal breast cancer subtypes.
Acknowledgements
The National Natural Science Foundation of China
(no. 81370060 to X-B.W., no. 81372374 to X-Y.W.) and the Science
and Technology Foundation of Guangdong Province (no. 2012B091100460
to X-Y.W., no. 2011B031800014 to M.D.) supported this work.
References
|
1
|
Fujii S, Ito K, Ito Y and Ochiai A:
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by
increasing histone H3 methylation. J Biol Chem. 283:17324–17332.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Du J, Li L, Ou Z, et al: FOXC1, a target
of polycomb, inhibits metastasis of breast cancer cells. Breast
Cancer Res Treat. 131:65–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Q, Yu J, Dhanasekaran SM, et al:
Repression of E-cadherin by the polycomb group protein EZH2 in
cancer. Oncogene. 27:7274–7284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bachmann IM, Halvorsen OJ, Collett K, et
al: EZH2 expression is associated with high proliferation rate and
aggressive tumor subgroups in cutaneous melanoma and cancers of the
endometrium, prostate, and breast. J Clin Oncol. 24:268–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richter GH, Plehm S, Fasan A, et al: EZH2
is a mediator of EWS/FLI1 driven tumor growth and metastasis
blocking endothelial and neuro-ectodermal differentiation. Proc
Natl Acad Sci USA. 106:5324–5329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Au SL, Wong CC, Lee JM, et al: Enhancer of
zeste homolog 2 epigenetically silences multiple tumor suppressor
microRNAs to promote liver cancer metastasis. Hepatology.
56:622–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren G, Baritaki S, Marathe H, et al:
Polycomb protein EZH2 regulates tumor invasion via the
transcriptional repression of the metastasis suppressor RKIP in
breast and prostate cancer. Cancer Res. 72:3091–3104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Granit RZ, Gabai Y, Hadar T, et al: EZH2
promotes a bi-lineage identity in basal-like breast cancer cells.
Oncogene. 32:3886–3895. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mimori K, Ogawa K, Okamoto M, Sudo T,
Inoue H and Mori M: Clinical significance of enhancer of zeste
homolog 2 expression in colorectal cancer cases. Eur J Surg Oncol.
31:376–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yonemitsu Y, Imazeki F, Chiba T, et al:
Distinct expression of polycomb group proteins EZH2 and BMI1 in
hepatocellular carcinoma. Hum Pathol. 40:1304–1311. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao ZY, Cai MY, Yang GF, et al: EZH2
supports ovarian carcinoma cell invasion and/or metastasis via
regulation of TGF-beta1 and is a predictor of outcome in ovarian
carcinoma patients. Carcinogenesis. 31:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao W, Feng Z, Cui Z, et al: Up-regulation
of enhancer of zeste homolog 2 is associated positively with cyclin
D1 overexpression and poor clinical outcome in head and neck
squamous cell carcinoma. Cancer. 118:2858–2871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee H, Yoon SO, Jeong WY, Kim HK, Kim A
and Kim BH: Immunohistochemical analysis of polycomb group protein
expression in advanced gastric cancer. Hum Pathol. 43:1704–1710.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huqun, Ishikawa R, Zhang J, et al:
Enhancer of zeste homolog 2 is a novel prognostic biomarker in
nonsmall cell lung cancer. Cancer. 118:1599–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Collett K, Eide GE, Arnes J, et al:
Expression of enhancer of zeste homologue 2 is significantly
associated with increased tumor cell proliferation and is a marker
of aggressive breast cancer. Clin Cancer Res. 12:1168–1174. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Athanassiadou AM, Tsipis A, Patsouris E,
et al: Enhancer of zeste homologue 2 expression in breast carcinoma
smears in relationship with p53, Ki-67 and other prognostic
parameters. Acta Cytol. 55:180–186. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alford SH, Toy K, Merajver SD and Kleer
CG: Increased risk for distant metastasis in patients with familial
early-stage breast cancer and high EZH2 expression. Breast Cancer
Res Treat. 132:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Semenza GL: Regulation of mammalian
O2 homeostasis by hypoxia-inducible factor 1. Annu Rev
Cell Dev Biol. 15:551–578. 1999.
|
|
20
|
Schito L, Rey S, Tafani M, et al:
Hypoxia-inducible factor 1-dependent expression of platelet-derived
growth factor B promotes lymphatic metastasis of hypoxic breast
cancer cells. Proc Natl Acad Sci USA. 109:E2707–E2716. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schindl M, Schoppmann SF, Samonigg H, et
al: Overexpression of hypoxia-inducible factor 1alpha is associated
with an unfavorable prognosis in lymph node-positive breast cancer.
Clin Cancer Res. 8:1831–1837. 2002.PubMed/NCBI
|
|
22
|
Wan XB, Fan XJ, Chen MY, et al: Elevated
Beclin 1 expression is correlated with HIF-1alpha in predicting
poor prognosis of nasopharyngeal carcinoma. Autophagy. 6:395–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong M, Wan XB, Yuan ZY, et al: Low
expression of Beclin 1 and elevated expression of HIF-1α refine
distant metastasis risk and predict poor prognosis of ER-positive,
HER2-negative breast cancer. Med Oncol. 30:3552013.PubMed/NCBI
|
|
24
|
Cao P, Deng Z, Wan M, et al: MicroRNA-101
negatively regulates Ezh2 and its expression is modulated by
androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 9:1082010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan XB, Fan XJ, Huang PY, et al: Aurora-A
activation, correlated with hypoxia-inducible factor-1α, promotes
radiochemoresistance and predicts poor outcome for nasopharyngeal
carcinoma. Cancer Sci. 103:1586–1594. 2012.
|
|
26
|
Park JH, Lee JY, Shin DH, Jang KS, Kim HJ
and Kong G: Loss of Mel-18 induces tumor angiogenesis through
enhancing the activity and expression of HIF-1alpha mediated by the
PTEN/PI3K/Akt pathway. Oncogene. 30:4578–4589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang CJ, Yang JY, Xia W, et al: EZH2
promotes expansion of breast tumor initiating cells through
activation of RAF1-β-catenin signaling. Cancer Cell. 19:86–100.
2011.PubMed/NCBI
|
|
28
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|
|
29
|
Fan XJ, Wan XB, Yang ZL, et al: Snail
promotes lymph node metastasis and Twist enhances tumor deposit
formation through epithelial-mesenchymal transition in colorectal
cancer. Hum Pathol. 44:173–180. 2013. View Article : Google Scholar
|
|
30
|
Wan XB, Zhao Y, Fan XJ, et al: Molecular
prognostic prediction for locally advanced nasopharyngeal carcinoma
by support vector machine integrated approach. PLoS One.
7:e319892012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sauvageau M and Sauvageau G: Polycomb
group proteins: multi-faceted regulators of somatic stem cells and
cancer. Cell Stem Cell. 7:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong Y, Huo L, Liu P, et al: Polycomb
group protein EZH2 is frequently expressed in inflammatory breast
cancer and is predictive of worse clinical outcome. Cancer.
117:5476–5484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Brot M, Rocha RM, Soares FA and Gobbi
H: Prognostic impact of the cancer stem cell related markers ALDH1
and EZH2 in triple negative and basal-like breast cancers.
Pathology. 44:303–312. 2012.PubMed/NCBI
|
|
34
|
Pietersen AM, Horlings HM, Hauptmann M, et
al: EZH2 and BMI1 inversely correlate with prognosis and TP53
mutation in breast cancer. Breast Cancer Res. 10:R1092008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jansen MP, Reijm EA, Sieuwerts AM, et al:
High miR-26a and low CDC2 levels associate with decreased EZH2
expression and with favorable outcome on tamoxifen in metastatic
breast cancer. Breast Cancer Res Treat. 133:937–947. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee ST, Li Z, Wu Z, et al:
Context-specific regulation of NF-κB target gene expression by EZH2
in breast cancers. Mol Cell. 43:798–810. 2011.
|
|
37
|
Shapochka DO, Zaletok SP and Gnidyuk MI:
Relationship between NF-κB, ER, PR, Her2/neu, Ki67, p53 expression
in human breast cancer. Exp Oncol. 34:358–363. 2012.
|
|
38
|
Wang Y, Yin Q, Yu Q, et al: A
retrospective study of breast cancer subtypes: the risk of relapse
and the relations with treatments. Breast Cancer Res Treat.
130:489–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gatza ML, Kung HN, Blackwell KL, Dewhirst
MW, Marks JR and Chi JT: Analysis of tumor environmental response
and oncogenic pathway activation identifies distinct basal and
luminal features in HER2-related breast tumor subtypes. Breast
Cancer Res. 13:R622011. View Article : Google Scholar
|