Introduction
The invasion and metastasis of malignant tumors are
the primary factors leading to treatment failure and poor prognosis
or death in cancer patients. Recent studies have shown that
epithelial-mesenchymal transition (EMT) is closely associated with
the invasion and metastasis of malignant tumors (1). EMT is a complex molecular program in
which epithelial cells lose their polarity and acquire mesenchymal
characteristics through cytoskeletal remodeling. Also during this
process, the cellular phenotype changes, with the lack of
E-cadherin expression serving as the most important marker of EMT
(2). The occurrence of EMT enables
tumor cells to acquire the ability to infiltrate surrounding
tissues and invade adjacent vasculature; as a result, these cells
can metastasize to other tissues or organs to form metastatic foci
(3). Indeed, it has been confirmed
that EMT can promote the invasion and metastasis of many solid
tumors including bladder, liver, colorectal, ovarian, esophageal,
breast and prostate cancer (4).
Pancreatic cancer is the fourth leading cause of
tumor-related death in the USA and is associated with a high degree
of malignancy and poor prognosis (5). Lymphatic, vascular and distant organ
metastases can occur at an early stage; therefore, when pancreatic
cancer is confirmed, most patients are at intermediate or advanced
stages and have passed the optimal time for surgical treatment
(6).
Currently, it is believed that EMT is closely
associated with invasion, metastasis and drug resistance in
pancreatic cancer (7). Among the
pancreatic cancer cell lines examined in previous studies, 78%
expressed Snail, 50% expressed Slug, and Twist was activated under
hypoxic stimulation (8). Studies
have further shown that in PANC-1 cells, treatment with
transforming growth factor (TGF) promotes EMT through the promotion
of N-cadherin and vimentin expression and the inhibition of
E-cadherin expression (9).
Furthermore, immunohistochemical analysis of a large number of
histopathological sections of pancreatic cancer showed that EMT
occurrence was significantly correlated with poor prognosis in
pancreatic cancer (10). In
addition, the characteristics of the EMT phenotype were also
observed in gemcitabine-resistant pancreatic cancer cells (11). These results indicate that EMT not
only participates in the progression of pancreatic cancer but is
also closely associated with drug resistance in pancreatic
cancer.
USP22 is a ubiquitin-specific peptidase that belongs
to the deubiquitinating enzyme (DUB) family and serves as a subunit
of the hSAGA complex (12). USP22
deubiquitinates the H2A and H2B histone proteins and acetylates the
H4 histone protein (13). In
addition, USP22 can activate BMI-1-, c-Myc- and FBP-1-mediated
target gene transcription and plays a key role in cell cycle
regulation, embryonic development and telomere homeostasis
(14–17). USP22 expression in normal tissues is
low, but its expression is significantly higher in tumors (12). Recently, it has been shown that
USP22 is closely associated with the metastatic potential and
prognosis of many solid tumors. However, the mechanism underlying
the role of abnormal USP22 expression in the occurrence and
development of tumors and its regulatory aspects remain poorly
defined; moreover, the association between USP22 and pancreatic
cancer has not yet been reported.
The present study showed that USP22 is closely
associated with EMT occurrence in PANC-1 cells. In particular,
upregulation of USP22 expression promoted the redistribution and
phosphorylation of Ezrin protein through the focal adhesion kinase
(FAK) signaling pathway, which led to cytoskeletal remodeling, the
promotion of EMT, upregulated MMP2/MMP9 expression, and the
increased invasion and migration of PANC-1 cells. In contrast,
interference with USP22 expression resulted in the opposite
effects. Overall, our results suggest that USP22 represents a novel
regulatory protein of EMT in pancreatic cancer, which may provide a
new approach for the targeted therapy of pancreatic cancer.
Materials and methods
Cell culture
PANC-1 and AsPC-1 cells were cultured in DMEM/F12
medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS) (Invitrogen, Karlsruhe, Germany); CFPAC-1 cells
were maintained in IMDM medium supplemented with 10% FBS. BxPC-3
cells were maintained in RPMI-1640 (both from Gibco) supplemented
with 10% FBS. All cells were cultured in cell-culture flasks or
Petri dishes in a humidified incubator at 37°C in an atmosphere of
5% CO2.
Plasmid preparation and cell
transfection
The USP22 and FAK overexpression plasmids and
USP22-shRNA plasmids and FAK-siRNA were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). All transfection
reactions were performed using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) in accordance with the manufacturer’s
instructions. Stable transfectants were selected with 800 μg/ml
G418 (Sigma-Aldrich, St. Louis, MO, USA), and individual clones
were isolated.
Western blotting
Proteins were separated by SDS-PAGE and transferred
to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes
were blocked in a buffer (TBS: 50 mM Tris-HCl, 150 mM NaCl, pH 7.4)
containing 5% bovine serum albumin and 0.1% Tween-20, followed by
incubation with the primary antibodies. The immunoreactive proteins
were visualized using the ECL Western Blotting System (Bio-Rad),
and densitometric analysis was performed using BioImaging systems
(LabWorks™, version 4.6; UVP). Mean values of the data obtained
from three separate chambers are presented.
Immunofluorescence
PANC-1 cells were seeded onto coverslips, fixed with
4% paraformaldehyde and permeabilized with 0.3% Triton X-100 for 10
min. Slides were blocked with 1% bovine serum albumin and incubated
with the primary antibodies overnight at 4°C. After washing in PBS,
the cells were stained with secondary antibodies and incubated for
1 h at room temperature, followed by nuclear counterstaining with
DAPI. Images were captured with the 3i Marianas XL spinning disk
confocal microscope (SDCM).
In vitro wound-healing migration
assay
Cells were seeded in 6-well culture plates in
DMEM/F12 containing 10% FBS. After 24 h, the cell monolayers were
wounded by manually scratching them with a pipette tip, and this
was followed by washing with PBS. The monolayers were then
incubated at 37°C for 24 h. The monolayers were photographed at 0
and 24 h. Images were captured with the Olympus BX51 fluorescence
microscope. Mean values of the data obtained from three separate
chambers are presented.
Transfilter invasion assay
Transfilter assays were performed with 8.0-μm pore
inserts in 24-well BioCoat chambers (Becton-Dickinson) using
5×104 cells in serum-free Dulbecco’s modified Eagle’s
medium (DMEM). The DMEM with 10% FBS was placed in the lower
chambers as a chemoattractant. For the invasion assays,
Matrigel-coated Transwell chambers were used. Cells were removed
from the upper surface of the filter by scraping with a cotton swab
after 24 h in culture, respectively. Invasive cells were fixed and
stained with the crystal violet reagent. Images were captured with
the Olympus BX51 fluorescence microscope. Mean values of the data
obtained from three separate chambers are presented.
Statistical analysis
The quantitative data derived from three independent
experiments are expressed as means (± SD). Unpaired Student’s
t-tests were used to analyze between group differences that is
repeated. P<0.05 was considered to indicate a statistically
significant result.
Results
Establishment of USP22 overexpression
monoclones and silencing of the USP22 gene in PANC-1 cells
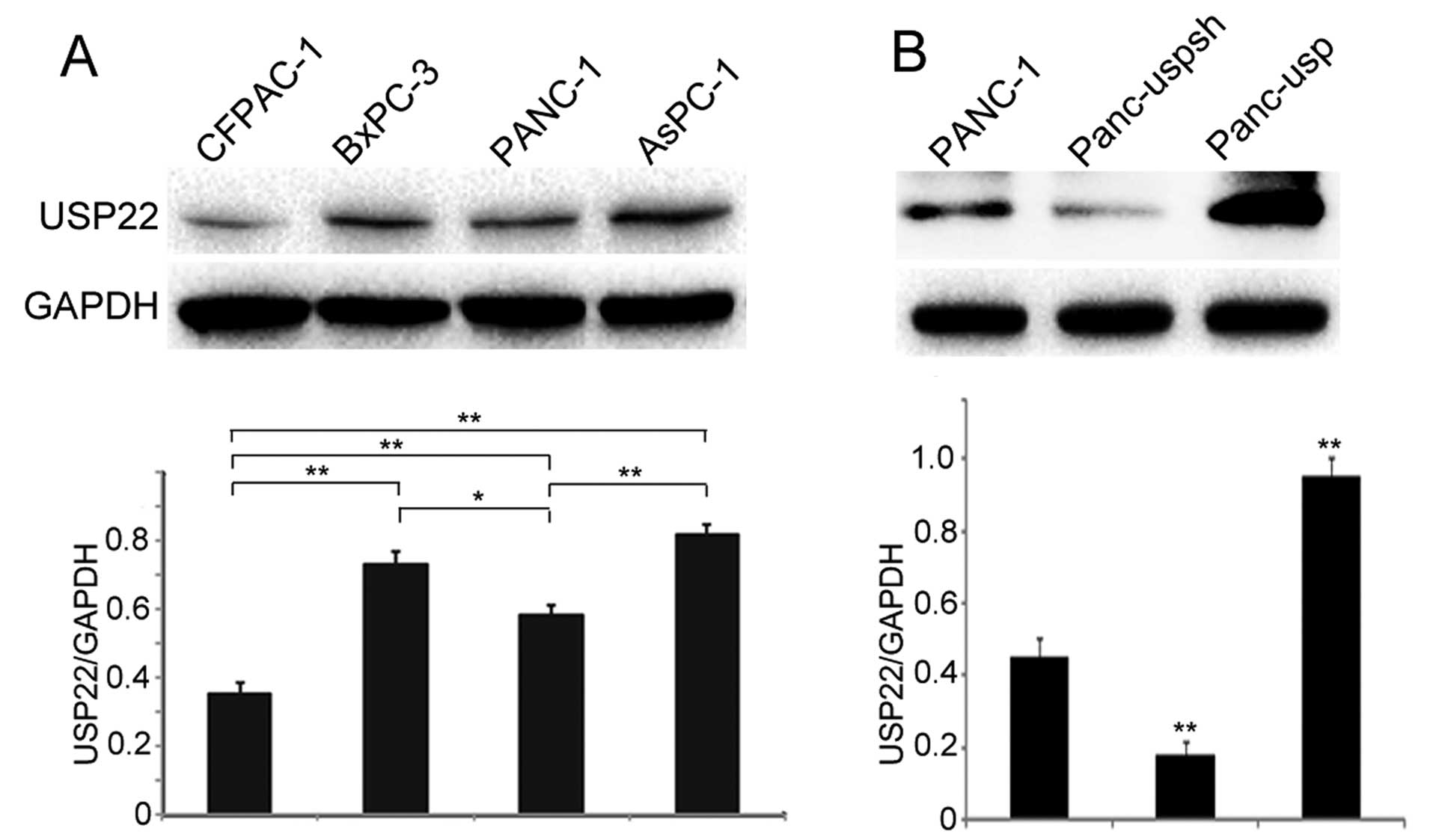
We first detected USP22 protein expression in 4
different pancreatic cancer cell lines that were stored in our
laboratory. The results showed that USP22 expression in poorly
differentiated cell lines, including BxPC-3, AsPC-1 and PANC-1, was
significantly higher than that observed in highly differentiated
CFPAC-1 cells (Fig. 1A). In
addition, USP22 expression in the PANC-1 cells was significantly
lower than that detected in the BxPC-3 and AsPC-1 cells. Therefore,
the PANC-1 cell line demonstrated a moderate expression level and
was selected for use in subsequent experiments.
To study the function of USP22 in pancreatic cancer
cells, a USP22 overexpression plasmid and an shRNA were stably
transfected into PANC-1 cells. After G418 selection, the cell
clones Panc-usp and Panc-uspsh, which were stably transfected with
the corresponding plasmids, were selected for future studies.
Western blot results showed that the expression levels of USP22 in
the Panc-usp and Panc-uspsh cells were 2.11-fold greater and 30.6%
of the level observed in the PANC-1 cells, respectively (Fig. 1B). In addition, the expression level
of USP22 in the Panc-usp cells was 5.3-fold greater than that
detected in the Panc-uspsh cells.
USP22 promotes the redistribution and
phosphorylation of Ezrin and cytoskeletal remodeling in PANC-1
cells
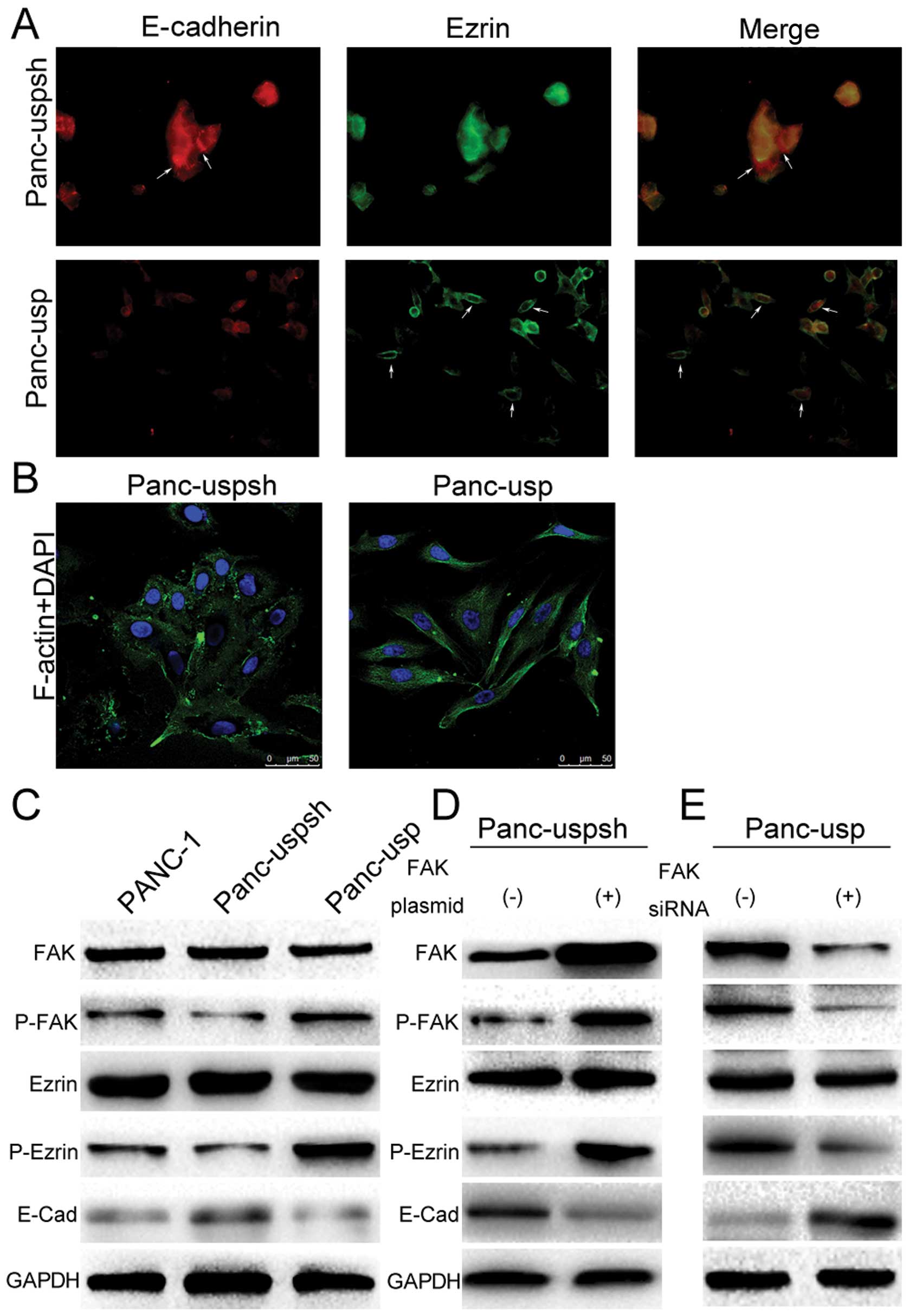
Cytoskeletal remodeling is a key step in EMT. To
examine whether USP22 promotes EMT in PANC-1 cells, detection of
F-actin expression in Panc-usp and Panc-uspsh cells was performed
using laser-scanning confocal immunofluorescence. The results
showed that there was a large amount of longitudinally oriented
actin filaments in the cytoplasm of the Panc-usp cells, whereas in
the Panc-uspsh cells, actin showed punctate expression without
actin filaments in the cytoplasm (Fig.
2B). Next, the expression of Ezrin protein, an important
regulatory and cell adhesion protein for connections to the cell
membrane (CD44 and intercellular adhesion molecule-2) and the
cytoskeleton (actin), was determined. The results showed that Ezrin
expression in the Panc-uspsh cells was mainly concentrated in the
cytoplasm (Fig. 2A). Interestingly,
in USP22 stably expressing Panc-usp cells, Ezrin expression was
significantly closer to the cell membrane and almost absent in the
cytoplasm. The subsequent western blot results showed that although
the expression levels of Ezrin in the PANC-1, Panc-uspsh and
Panc-usp cells did not change, the levels of Ezrin phosphorylation
(P-Ezrin) were significantly altered (Fig. 2C). For example, the expression level
of P-Ezrin in the Panc-usp cells was 6.4-fold greater than that
observed in the Panc-uspsh cells. These results indicate that USP22
significantly affects cytoskeletal remodeling in pancreatic cancer
cells, which may be associated with the redistribution and
phosphorylation of Ezrin.
USP22 promotes Ezrin phosphorylation
through the FAK signaling pathway
To clarify the potential mechanism underlying the
promotion of Ezrin phosphorylation by USP22 overexpression, the FAK
signaling pathway, which is loosely associated with invasion,
metastasis and adhesion of tumor cells, was studied. Western blot
results showed that the expression level of P-FAK in the Panc-usp
cells was 3.6-fold greater than that in the Panc-uspsh cells. To
further confirm the function of the FAK signaling pathway,
control-siRNA and FAK-siRNA were separately transfected into the
Panc-usp cells. The results showed that with the decrease in FAK
expression, P-PAK and P-Ezrin expression was also significantly
decreased (Fig. 2E). Furthermore,
transfection of an empty plasmid or an FAK overexpression plasmid
into the Panc-uspsh cells showed that with the increase in FAK
expression, P-FAK and P-Ezrin expression also increased (Fig. 2D). These results showed that USP22
promoted Ezrin protein phosphorylation through activation of the
FAK signaling pathway.
Overexpression of USP22 induces
phenotypic changes and lead to the acquisition of mesenchymal
markers and reduction of epithelial markers in PANC-1 cells
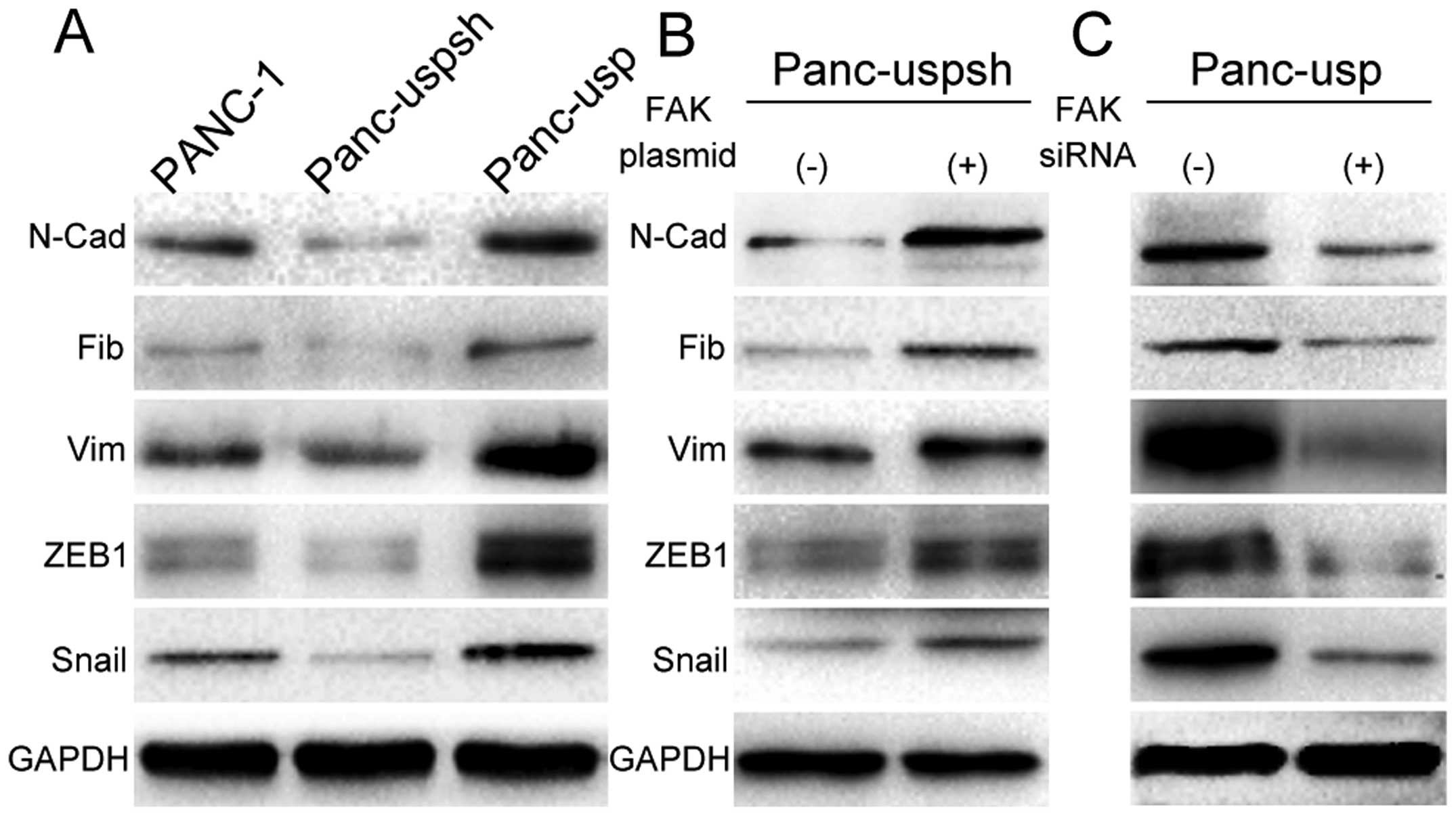
To clarify whether USP22 promotes EMT, the
expression of EMT-associated molecular markers and transcription
factors, including ZEB1 and Snail, in PANC-1 cells was evaluated.
Western blot results for Panc-usp cells showed that the expression
levels of the mesenchymal markers N-cadherin, vimentin and
fibronectin were increased 5.8-, 4.2- and 2.7-fold compared to
those in Panc-uspsh cells (Fig.
3A), while the expression level of the epithelial marker
E-cadherin was decreased by 75.3% compared to that in Panc-uspsh
cells. The evaluation of EMT-associated transcription factors
showed that the expression levels of ZEB1 and Snail in the Panc-usp
cells were 5.6- and 5.3-fold greater than those in the Panc-uspsh
cells.
The expression of E-cadherin protein was also
determined using laser-scanning confocal immunofluorescence. The
results showed that USP22 stably downregulated Panc-uspsh cells
were closely connected and showed a large amount of E-cadherin
aggregated at the cell membrane. In contrast, USP22 stably
overexpressing Panc-usp cells were loosely connected, extended and
spindle-shaped, and E-cadherin protein expression was significantly
downregulated and scattered throughout the cytoplasm (Fig. 2A). Thus, these results strongly
suggest that USP22 induces EMT in PANC-1 cells through the
upregulation of ZEB1 and Snail, accompanied by loss of the EMT
marker E-cadherin and gain of the mesenchymal markers vimentin and
fibronectin.
USP22 alters the expression of various
EMT markers in association with upregulation of the transcription
factors ZEB1 and Snail via the FAK pathway
To further confirm the role of the FAK signaling
pathway in USP22 overexpression-induced EMT, FAK-siRNA was
transfected into Panc-usp cells. The results showed that with the
downregulation of P-FAK, the expression levels of the mesenchymal
markers N-cadherin, vimentin and fibronectin were decreased by
63.8, 81.3 and 64.5%, respectively (Fig. 3C), while the expression level of the
epithelial marker E-cadherin was increased 4.2-fold. With the
downregulation of P-FAK, the expression levels of the
EMT-associated transcription factors ZEB1 and Snail were decreased
to 86.2 and 78.4%, respectively. Next, Panc-uspsh cells were
transfected with the FAK-overexpressing plasmid, and with the
upregulation of P-FAK, the expression levels of the mesenchymal
markers N-cadherin, vimentin and fibronectin were increased 5.2-,
1.4- and 3.6-fold, respectively (Fig.
3B), while the expression level of the epithelial marker
E-cadherin was decreased by 87.5%. In addition, the expression
levels of the EMT-associated transcription factors ZEB1 and Snail
were increased 2.8- and 3.6-fold, respectively. These results
further indicate that the FAK signaling pathway participates in the
USP22 overexpression-induced EMT in PANC-1 cells.
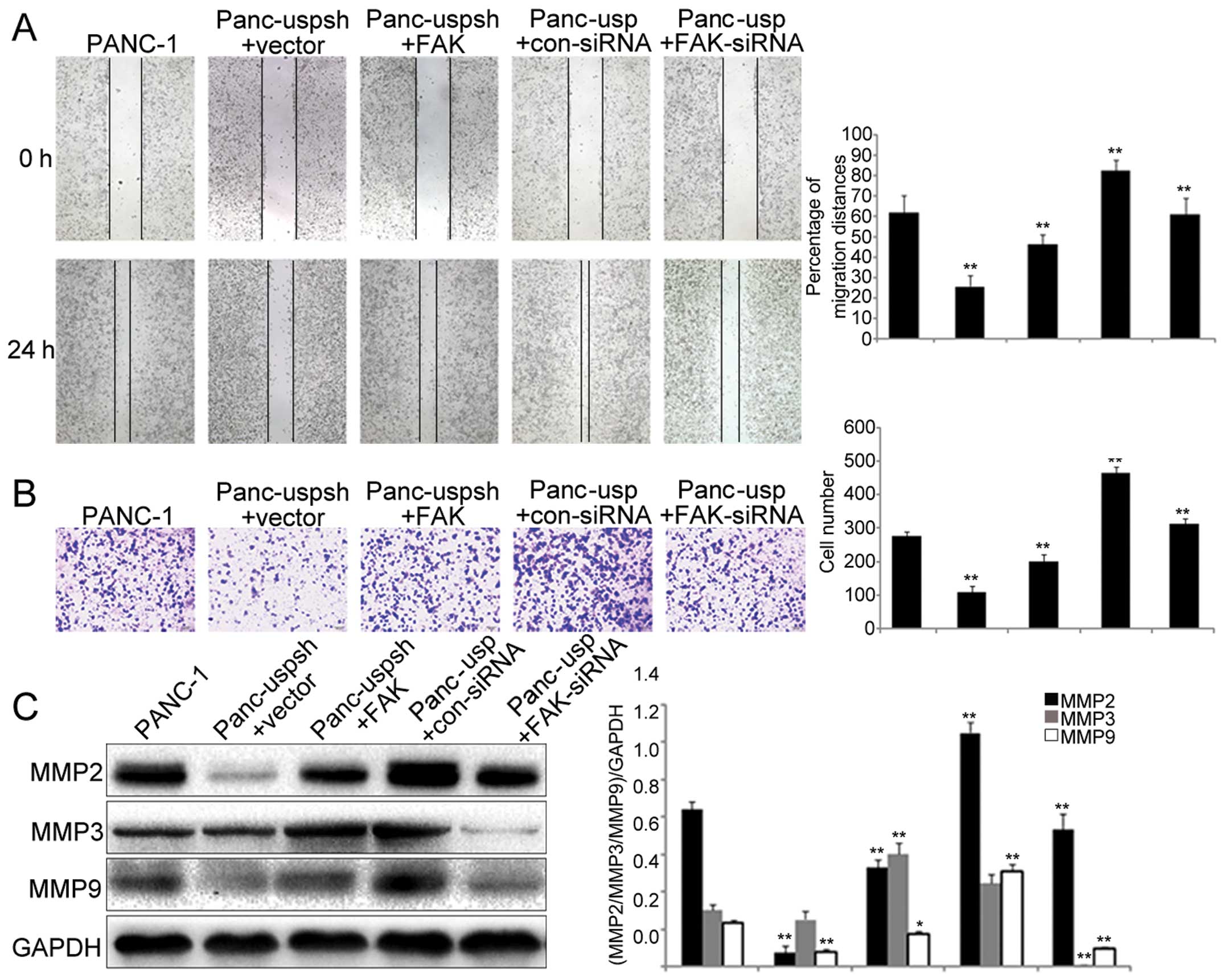
USP22 promotes PANC-1 cell invasion and
migration through the FAK signaling pathway
As a result of EMT, the biological behaviors of
cells are altered, and cells acquire increased migration and
invasion abilities. Using scratch and Transwell assays, we showed
that the migration and invasion abilities of the Panc-usp cells
were significantly greater than those of the Panc-uspsh cells
(Fig. 4A and B). In addition,
downregulation of FAK in the Panc-usp cells significantly decreased
the migration and invasion rates, while overexpression of FAK in
the Panc-uspsh cells significantly increased the migration and
invasion rates.
The expression of MMP2, MMP3 and MMP9 has been shown
to play an important role in the process of EMT in various tumors.
Our study showed that the expression levels of MMP2 and MMP9 in the
Panc-usp cells were significantly increased (by 9.6 and 5.2-fold,
respectively) compared to those in the Panc-uspsh cells, while the
expression of MP3 was not significantly different (Fig. 4C). In addition, downregulation of
FAK in the Panc-usp cells led to significantly downregulated
expression of MMP2, MMP3 and MMP9, while overexpression of FAK in
the Panc-uspsh cells led to significantly upregulated expression of
MMP2, MMP3 and MMP9. These results demonstrated that although the
FAK signaling pathway regulated the expression of these three
matrix metal-loproteinases (MMP2, MMP3 and MMP9), the EMT process
in PANC-1 cells induced by USP22 was associated with the expression
of MMP2 and MMP9, but not that of MMP3.
Discussion
EMT was first discovered during the study of
embryonic development (18).
In-depth studies in human tumors and experimental animal models
further revealed that EMT was not only associated with the normal
process of embryonic development but was also closely associated
with organic fibrosis and tumor invasion and metastasis (3). Yang and Weinberg (19) summarized the following
characteristics of changes associated with EMT: i) morphological
changes, including loss of cell polarity, spindle-like cell
morphology, cytoskeletal remodeling, and appearance of pseudopods;
ii) molecular marker changes, including the upregulation of
epithelial adhesion-associated molecules such as E-cadherin and
downregulation of molecules associated with mesenchymal origin such
as N-cadherin and fibronectin; iii) changes in biological
behaviors, including the acquisition of mesenchymal phenotypes such
as increased migration and invasion and resistance to apoptosis. As
one of the protein components of the SAGA complex, USP22
participates in proto-oncogene c-Myc-mediated target gene
transcriptional regulation, thus playing a key role in the
promotion of malignant transformation of tumors and the regulation
of the cell cycle (16,20). Therefore, USP22 is considered to be
a tumor stem cell marker. Furthermore, USP22 is also involved in
the early developmental process of mouse embryos (21). As a tumor marker, USP22 has recently
been shown to be highly expressed in many solid tumors and is
closely associated with metastatic potential and poor prognosis.
However, recent studies on USP22 have mainly focused on its role in
cell cycle regulation, while the potential mechanism underlying the
promotion of tumor invasion and metastasis by abnormal USP22
expression has not been reported.
During the process of EMT, epithelial cells
downregulate their adhesive structures between cells, change their
polarity, and remodel their cytoskeleton to become isolated and
mobile anti-apoptotic cells. After EMT is complete, epithelial
cells lose their polarity, and the morphology of these cells change
from a cubic shape to a spindle-shaped fibroblast-like morphology.
As the first discovered ezrin/radixin/moesin (ERM) protein family
member, Ezrin is mainly distributed and concentrated at protruding
parts of the cell surface such as microvilli and cell folds
(22). Ezrin is considered an
important regulatory protein and adhesion protein between cell
membrane molecules (CD44 and intercellular adhesion molecule-2) and
the cellular cytoskeleton (actin), and this protein plays an
important role in cytoskeletal remodeling (23). Ezrin is also a regulatory factor for
cell motility; studies have shown that stimulation of epithelial
tumor cells with hepatocyte growth factor/scatter factor could
promote the tyrosine phosphorylation of Ezrin, the movement of
Ezrin from the cytoplasm to cell microfolds, and the enhancement of
tumor cell mobility (24). The
present study showed that USP22 overexpression resulted in the
appearance of a large amount of longitudinally oriented actin
filaments in the cytoplasm of PANC-1 cells; in contrast, USP22
stably downregulated Panc-uspsh cells exhibited punctate actin
expression and the absence of actin filament in the cytoplasm. At
the same time, with the increase in USP22 expression, Ezrin
expression moved from the cytoplasm to the cell membrane. Moreover,
our dynamic observations of cell morphology showed that PANC-1
cells with stable downregulation of USP22 were closely connected
and polygonal in shape. In contrast, USP22 stably overexpressing
Panc-usp cells were loosely connected and extended and
spindle-shaped. These results helped to confirm that USP22
significantly downregulated adhesion between PANC-1 cells,
remodeling of the cytoskeleton, and generated cells with an
isolated, spindle-shaped, fibroblast-like morphology. Moreover,
these changes may be associated with the redistribution and
phosphorylation of Ezrin.
The most important process in the early stage of EMT
is the conversion between E-cadherin and N-cadherin, which is
referred to as cadherin conversion. In addition, transcription
factors such as Snail1 (Snail), Snail2 (Slug), Snail3, ZEB
(including ZEB1 and ZEB2) and Twist can bind to the common box
sequences of the promoter region of the E-cadherin gene, thereby
downregulating E-cadherin expression (25). Moreover, activated Ezrin protein at
the cell membrane can block the translocation of E-cadherin from
the cytoplasm to the cell membrane, which indirectly decreases the
connections between tumor cells; as a result, tumor cells can more
easily leave the primary tumor focus to cause distant metastasis
(26). The present study showed
that the upregulation of USP22 in PANC-1 cells significantly
downregulated E-cadherin expression in the cell membrane at
cell-cell junctions and upregulated the expression of mesenchymal
markers, including N-cadherin, vimentin and fibronectin. In
addition, the expression levels of the transcription factors ZEB1
and Snail were also significantly increased. Together, these
results revealed that USP22 promotes the transition of PANC-1 cells
from the epithelial phenotype to the mesenchymal phenotype, and
this process may be associated with the activation of Ezrin.
The final step in EMT is the acquisition of
migration and invasion capacity. Many studies have reported that in
gastrointestinal cancers, EMT promotes the occurrence and
metastasis of esophageal, gastric, colorectal and pancreatic cancer
and is associated with poor prognosis. Matrix metal-loproteinases
constitute a large family of enzymes that can degrade almost all
protein components in the extracellular matrix (ECM), thereby
removing a barrier for tumor cell invasion consequently promoting
metastasis (27). Among these
enzymes, the type IV collagenases represent an important group that
includes two major forms: glycosylated MMP9 and non-glycosylated
MMP2. Recent studies have shown that the expression of MMP2, MMP3
and MMP9 plays an important role in the EMT process in many types
of tumors (28,29). Using scratch and Transwell assays,
the present study found that USP22 overexpression significantly
promoted the invasion and migration abilities of PANC-1 cells. In
particular, the expression of MMP2 and MMP9, but not MMP3, was
associated with USP22-induced EMT.
FAK is a non-receptor type protein tyrosine kinase
(30). Previous studies have shown
that FAK participates in biological processes such as cell
proliferation, adhesion, invasion and apoptosis through its effects
on many signal transduction pathways. In particular, FAK plays an
important role in regulation of the cytoskeleton and cell motility;
through regulating the activation of Rho family proteins, FAK can
regulate cell proliferation, adhesion and directional movement
(31). Furthermore, Ezrin
activation can result in Rho activation and lead to cascading
effects in corresponding Rho signal transduction systems (32). These results led us to speculate
that FAK may also play an important role in USP22-induced EMT in
PANC-1 cells, and the following findings confirmed this
speculation. First, P-PAK expression in the USP22 stably expressing
Panc-usp cells was significantly higher than that in the
USP22-downregulated Panc-uspsh cells. Second, FAK downregulation in
the Panc-usp cells significantly reduced the expression of
mesenchymal markers, whereas FAK overexpression in the Panc-uspsh
cells significantly upregulated the expression of mesenchymal
markers. Third, FAK downregulation in the Panc-usp cells
significantly downregulated migration and invasion, whereas FAK
overexpression in the Panc-uspsh cells significantly upregulated
migration and invasion. These results indicate that future research
should address the pathway through which USP22 promotes FAK
phosphorylation during EMT in PANC-1 cells as well as the
relationship between FAK phosphorylation and the redistribution and
phosphorylation of Ezrin.
In summary, the present study revealed that USP22
mediated the occurrence of EMT in PANC-1 cells. This process was
achieved through activation of the FAK signaling pathway, which
promoted the redistribution and phosphorylation of Ezrin protein,
cytoskeletal remodeling, EMT occurrence, upregulation of the
expression of MMP2/MMP9 and increased invasion and migration in
PANC-1 cells. Therefore, downregulation of USP22 may represent an
effective treatment method for inhibiting the invasion and
migration of pancreatic cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China Research grant (no. 30870719 to Z.W.,
no. 30672753 to J.L.) and China 973 grant (no. 2012CB822100 to
Q.Y.).
References
|
1
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brabletz T: EMT and MET in metastasis:
where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
6
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
7
|
Pan JJ and Yang MH: The role of
epithelial-mesenchymal transition in pancreatic cancer. J
Gastrointest Oncol. 2:151–156. 2011.PubMed/NCBI
|
|
8
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takano S, Kanai F, Jazag A, Ijichi H, Yao
J, Ogawa H, Enomoto N, Omata M and Nakao A: Smad4 is essential for
down-regulation of E-cadherin induced by TGF-βin pancreatic cancer
cell line PANC-1. J Biochem. 141:345–351. 2007.
|
|
10
|
Krantz SB, Shields MA, Dangi-Garimella S,
Bentrem DJ and Munshi HG: Contribution of epithelial-mesenchymal
transition to pancreatic cancer progression. Cancers. 2:2084–2097.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM
and Baek KH: The expression patterns of deubiquitinating enzymes,
USP22 and Usp22. Gene Expr Patterns. 6:277–284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang XY, Pfeiffer HK, Thorne AW and
McMahon SB: USP22, an hSAGA subunit and potential cancer stem cell
marker, reverses the polycomb-catalyzed ubiquitylation of histone
H2A. Cell Cycle. 7:1522–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Atanassov BS and Dent SY: USP22 regulates
cell proliferation by deubiquitinating the transcriptional
regulator FBP1. EMBO Rep. 12:924–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park IK1, Qian D, Kiel M, Becker MW,
Pihalja M, Weissman IL, Morrison SJ and Clarke MF: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 423:302–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XY, Varthi M, Sykes SM, Phillips C,
Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL and McMahon SB: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang DD, Cui BB, Sun LY, Zheng HQ, Huang
Q, Tong JX and Zhang QF: The co-expression of USP22 and BMI-1 may
promote cancer progression and predict therapy failure in gastric
carcinoma. Cell Biochem Biophys. 61:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shook D and Keller R: Mechanisms,
mechanics and function of epithelial-mesenchymal transitions in
early development. Mech Dev. 120:1351–1383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Glinsky GV: Genomic models of metastatic
cancer: functional analysis of death-from-cancer signature genes
reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype
with altered cell cycle control and activated Polycomb Group (PcG)
protein chromatin silencing pathway. Cell Cycle. 5:1208–1216.
2006.
|
|
21
|
Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao
B, Dong H, Wei J, Song J, Zhang DD and Fang D: USP22 antagonizes
p53 transcriptional activation by deubiquitinating Sirt1 to
suppress cell apoptosis and is required for mouse embryonic
development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruce B, Khanna G, Ren L, Landberg G,
Jirström K, Powell C, Borczuk A, Keller ET, Wojno KJ, Meltzer P,
Baird K, McClatchey A, Bretscher A, Hewitt SM and Khanna C:
Expression of the cytoskeleton linker protein ezrin in human
cancers. Clin Exp Metastasis. 24:69–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hunter KW: Ezrin, a key component in tumor
metastasis. Trends Mol Med. 10:201–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fievet BT, Gautreau A, Roy C, Del Maestro
L, Mangeat P, Louvard D and Arpin M: Phosphoinositide binding and
phosphorylation act sequentially in the activation mechanism of
ezrin. J Cell Biol. 164:653–659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thompson EW and Williams ED: EMT and MET
in carcinoma - clinical observations, regulatory pathways and new
models. Clin Exp Metastasis. 25:591–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pujuguet P, Del Maestro L, Gautreau A,
Louvard D and Arpin M: Ezrin regulates E-cadherin-dependent
adherens junction assembly through Rac1 activation. Mol Biol Cell.
14:2181–2191. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egeblad M and Werb Z: New functions for
the matrix metal-loproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duong TD and Erickson CA: MMP-2 plays an
essential role in producing epithelial-mesenchymal transformations
in the avian embryo. Dev Dyn. 229:42–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Borghaei RC, Rawlings PL Jr, Javadi M and
Woloshin J: NF-κB binds to a polymorphic repressor element in the
MMP-3 promoter. Biochem Biophys Res Commun. 316:182–188. 2004.
|
|
30
|
Hanks SK and Polte TR: Signaling through
focal adhesion kinase. Bioessays. 19:137–145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schaller MD: Cellular functions of FAK
kinases: insight into molecular mechanisms and novel functions. J
Cell Sci. 123:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Wang D, Guo Z, Zhao J, Wu B, Deng
H, Zhou T, Xiang H, Gao F, Yu X, Liao J, Ward T, Xia P, Emenari C,
Ding X, Thompson W, Ma K, Zhu J, Aikhionbare F, Dou K, Cheng SY and
Yao X: Rho kinase phosphorylation promotes ezrin-mediated
metastasis in hepatocellular carcinoma. Cancer Res. 71:1721–1729.
2011. View Article : Google Scholar : PubMed/NCBI
|