Introduction
Acute myeloid leukemia (AML) is morphologically
defined by an abnormal increase in myeloblasts in the bone marrow
(BM). Since AML is heterogeneous in regards to morphologic and
cytogenetic features, patient prognoses are extremely variable. To
improve understanding of the biological features of human AML,
several mouse models have been developed, including a xenograft
model and a genetically engineered mouse model (1,2). The
xenograft model has been shown to better mimic the therapeutic
responses and tumor microenvironment observed in the human
condition, as well as being rapidly produced within several weeks
(3). The xenograft model of
leukemic progression in human beings has been gradually improved.
Successful transplantation of human hematopoietic cells into
immunodeficient mice was first reported in studies from the late
1980s that used homozygous severe combined immunodeficient
(C.B.17-SCID) mice (4,5). Then, modified SCID model studies
showed that, irrespective of the morphologic subtypes, only a small
fraction of leukemic cells, the putative leukemic stem cells
(LSCs), could recapitulate leukemia (6–8). Among
the many types of mouse strains used for the xenograft model, the
most advanced strain is the nonobese diabetic, severe combined
immunodeficiency (NOD/SCID) mouse with targeted deletion of the
interleukin (IL-2) receptor with the common γ-chain
(IL-2Rγnull), termed the NSG mouse. This mouse has a
stable lack of mature T, B and NK cells, a prolonged survival
beyond 16 months of age and is acceptable for engraftment of
primary human cells. Ishikawa et al showed the efficient
development of functional human hemato-lymphopoiesis in the
NOD/SCID/IL2γnull newborn model (9). After these reports, he continuously
demonstrated that LSCs exclusively recapitulate AML and retain
self-renewal capacity in vivo (10), suggesting the importance of LSCs.
Because other immunodeficient mice have a short life-span and a
disturbed long-term evaluation in in vivo studies, NSG mice
were used to establish a leukemic xenograft model with long-term
survival. Although many investigators have reported established
mouse models using mononuclear cells (MNCs), such models have shown
low rates of success due to individual variation in LSC potential.
Therefore, some scientists often decide to use BM-MNCs equivalent
to more than 10,000 LSCs after calculation in a xenograft model
(11,12). CD34+CD38−
cells, known as LSCs, are the main cell population responsible for
producing leukemia due to their self-renewing properties (13). Lapidot et al reported that
AML cells with the CD34+CD38− phenotype are
capable of producing leukemia in immunodeficient mice (14). Although the identification of
functional LSCs is still debated, CD34+CD38−
are currently accepted as representative markers for LSCs in
vivo as well as in vitro (13,15).
Recently, the capacity of aldehyde dehydrogenase dim
(ALDHdim)-positive cells to repopulate following
injection into NSG mice with leukemic properties was addressed by
Gerber et al (16). Hence,
ALDHdim cells in leukapheresed peripheral blood (LPB)
that are also CD34+CD38− were the main focus
in the present study, and we investigated whether LPB from AML
patients possesses a high level of LSCs with an abundant
ALDHdim population compared to that of the BM
counterpart. We found that LPB, which displayed a high proportion
of ALDHdim-expressing CD34+CD38−
cells, contributes as much as BM to establishing a leukemic
xenograft model repetitively and can be used as an alternative cell
source without having the limitations of volume and a short
life-span. Collectively, this study is the first to report the
comparison between using BM and LPB cells in a leukemic xenograft
model and provides beneficial information for investigators who
attempt the xenograft model using primary leukemic cells.
Materials and methods
Human primary cells and cell lines
All experiments were performed with authorization
from the Institutional Review Board for Human Research at the
Catholic University of Korea. AML blood samples were obtained from
the Catholic Blood and Marrow Transplantation Center at Seoul St.
Mary’s Hospital. A total of 16 AML samples were prospectively
collected and examined. Samples were obtained from both newly
diagnosed and relapsed patients. These patients showed diverse FAB
subtypes, including M0 (1 case), M1 (2 cases), M2 (3 cases), M3 (1
case), M4 (5 cases) and M5 (4 cases). BM and LPB samples were
frozen in fetal bovine serum with 10% DMSO and stored in liquid
nitrogen. BM-derived mononuclear cells (BM-MNCs) and PB-derived
MNCs (PB-MNCs) were fractionated by density gradient centrifugation
using Ficoll-Paque™ Plus (17-1440-03; GE Healthcare Life Sciences,
Piscataway, NJ, USA). The clinical characteristics and experimental
information of the AML patients enrolled in the present study are
listed in Table I. The cell lines
TF-1a, K562 and Kasumi-6 were originally obtained from the American
Type Culture Collection (ATCC, Rockville, MD, USA). These cells
were grown in the appropriate culture media recommended by the
ATCC.
 | Table IClinical and laboratory features of
the AML patients. |
Table I
Clinical and laboratory features of
the AML patients.
| Patients | FAB subtype | Age (years) | Gender | Cell source | WBCs/mm3
at diagnosis | Cytogenetic
anomalies | Molecular
defects |
|---|
| 1 | M5b | 56 | F | PB | 147,800 | 46,XY[20] | Negative |
| 2 | M4 | 56 | F | PB | 18,510 |
46,XX,add(12)(p13),der(16)inv(16)
(p13.1q22)del(16)(q22)[28]/46,XX[2] | CBFb/MYH11 |
| 3 | M4 | 58 | M | PB | 40,800 | 46,XY[20] | Negative |
| 4 | M2 | 15 | F | PB | 15,310 | 46,XX[20] | Negative |
| 5 | M1 | 58 | M | PB | 15,300 | 46,XY[20] | Negative |
| 6 | M5 | 27 | M | PB | 149,550 | 46,XY[20] | Negative |
| 7 | M4 | 41 | F | PB | 4,970 |
46,XX,inv(3)(q21q26.3)[20] | Negative |
| 8 | M1 | 27 | M | PB | 2,150 |
46,XY,t(9;11)(p22;q23)[26]/46,idem,
add(1)(p36.1)[4] | MLL/AF9 |
| 9 | M3 | 45 | M | PB | 2,400 | 46,XY[20] | Negative |
| 10 | M4 | 63 | F | PB | 35,370 | 46,XX[19] | Negative |
| 11 | M1 | 17 | M | PB | 18,230 |
45,X,−Y,t(8;21)(q22;q22)[6]/46,XY[14] | AML1/ETO |
| 12 | M1 | 45 | F | PB | 8,000 |
48,XX,+8,+10[25]/46,XX[5] | Negative |
| 13 | M2 | 27 | M | PB | 11,560 |
46,XY,del(9)(q22q31),del(11)(q13q23)[22]/46,
XY[8] | Negative |
| 14 | M4 | 28 | F | PB | 30,900 |
46,XX,inv(16)(p13.1q22)[20] | CBFb/MYH11 |
| 15 | M1 | 39 | M | PB | 10,700 |
46,XY,t(10;11)(q22;q23)[10]/47,idem,+21[30] | Negative |
| 16 | AML from ET | 73 | M | PB | 20,120 | 47,XY,+8[20] | Negative |
| 17 | M2 | 50 | F | PB | 1,200 |
46,XX,t(11;17)(q23;q21)[22]/46,XX[3] | Negative |
| 18 | M2 | 63 | F | PB | 1,240 | 46,XX[20] | Negative |
| 19 | M2 | 34 | F | PB | 6,860 |
46,XX,del(2)(q33),add(5)(q31),del(6)(p23),
del(7)(q32),t(8;21)(q22;q22),add(10)(q26),
add(11)(p15),t(?11;12)(q21;p13)[cp17]/46,XX[3] | Negative |
| 20 | M1 | 34 | M | BM | 28,180 |
46,X,idic(Y)(q12)x2,dup(1)(q12q42),−16,
der(21)t (16;21)(p11.2;q22)[7]/47,idem,+idic(Y)[13] | TLS/ERG |
| 21 | M2 | 49 | M | BM | 4,500 |
46,XY,t(1;11)(q21;q23)[20] | MLL/AF1q |
| 22 | M1 | 19 | M | BM | 139,610 |
46,XY,inv(16)(p13.1q22)[20] | CBFb/MYH11 |
| 23 | M3 | 56 | F | BM | 11,650 | 45
XX,add(3q),del(17q),−18 | Negative |
| 24 | M5b | 15 | F | BM | 302,010 |
48,XX,+8,+13[7]/48,idem,del(13)(q12q14)[17]/52,
idem,+4,+8,+10,+13[5]/46,XX[1] | Negative |
| 25 | M5 | 51 | F | BM | 45,790 | 46,XY[20] | Negative |
| 26 | M4 | 50 | F | BM | 52,920 |
47,XX,+add(1)(p13)[10]/46,XX[10] | Negative |
| 27 | M4 | 56 | F | BM | 18,510 |
46,XX,add(12)(p13),der(16)inv(16)(p13.1q22)
del(16)(q22)[28]/46,XX[2] | CBFb/MYH11 |
| 28 | M2 | 15 | F | BM | 15,310 | 46,XX[20] | Negative |
| 29 | M7 | 27 | M | BM | 239,400 |
47,XY+8[17]/46,XY[5] | Negative |
| 30 | M4 | 50 | F | BM | 195,280 | 46,XX[20] | Negative |
| 31 | M3 | 45 | M | BM | 2,400 | 46,XY[20] | Negative |
| 32 | M5 | 27 | M | BM | 149,550 | 46,XY[20] | Negative |
| 33 | M4 | 43 | M | BM | 1,920 | 46,XY[20] | Negative |
| 34 | M5 | 27 | M | BM | 2,150 |
46,XY,t(9;11)(p22;q23)[26]/46,idem,
add(1)(p36.1)[4] | MLL/AF9 |
| 35 | M3 | 41 | M | LPB | 43,010 |
46,XY,t(15;17)(q22;q12)[20] | PML/RARA |
| 36 | M3 | 46 | M | LPB | 31,770 |
46,XY,t(15;17)(q22;q21)[20] | AML M3
PML/RARa(+)
F (TKD+)NC(−) |
| 37 | M2 | 17 | M | LPB | 120,160 | 46,XY[20] | Negative |
| 38 | M3 | 60 | F | LPB | 83,650 |
46,XX,t(15;17)(q22;q12)[20] | Negative |
| 39 | MRC | 17 | M | LPB | 115,970 |
6,XY,der(9)del(p13p22)inv(p12q13)[18]/46,XY[2] | Negative |
| 40 | M1 | 52 | F | LPB | 100,250 | 46,XX[20] | Negative |
| 41 | M4 | 53 | M | LPB | 13,800 | 46,XY[20] | Negative |
| 42 | M1 | 31 | M | LPB | 138,940 | 46,XY[20] | Negative |
| 43 | M2 | 58 | M | LPB | 145,690 | 46,XX[20] | Negative |
| 44 | M2 | 27 | M | LPB | 30,090 |
46,XX,t(9;11)(p22;q23)[20] | MLL/AF9 |
| 45 | M4 | 45 | M | LPB | 218,610 |
47,XY,+mar[3]/46,XY[22] | Negative |
| 46 | M5b | 60 | F | LPB | 143,720 |
46,XX,t(6;11)(q27;q23)[20] | Negative |
| 47 | M0 | 57 | M | LPB/BM | 4,390 | 46,XX[20] | Negative |
| 48 | M4 | 58 | M | LPB/BM | 142,700 |
46,XX,t(6;11)(q27;q23)[30] | MLL/AF6 |
| 49 | M2 | 58 | M | LPB | 13,530 | 46,XY[20] | Negative |
| 50 | M4 | 58 | M | LPB | 23,140 | 46,XY[20] | Negative |
| 51 | M4e | 15 | F | LPB | 154,500 |
46,XY,t(9;22)(q34;q11.2),inv(16)(p13.1q22)[13]/47,
idem,+17[15]/48,idem,+8,+17[2] | Negative |
Mice and human xenograft model
All mice were bred by the Department of Laboratory
Animal at the Catholic University of Korea.
NOD/ShiLtSz-scid/IL2Rγnull (NOD.
Cg-PrkdcscidIl2rgtm1Wjl/SzJ,
termed NSG) mice were purchased from the Jackson Laboratory and
housed in ventilated micro-isolator cages in a high-barrier
facility under specific pathogen-free conditions. Autoclaved water
and irradiated food were provided ad libitum. All protocols
for animal experiments were approved by the Institutional Animal
Care and Use Committee of the Catholic University of Korea. For the
xenograft model, 8-week-old mice were sublethally irradiated with
300 cGy of total body irradiation 24 h before intravenous injection
of leukemic cells. AML blood samples were thawed at 37°C, washed
twice in PBS, and cleared of aggregates and debris using a 40-μm
cell filter. For the i.v. injection, cells were suspended in PBS at
a final concentration of 1×107 cells per 200 ml of PBS
per mouse. Mice were monitored daily for symptoms of disease,
including ruffled coat, hunched back, weakness and reduced
motility. Once injected animals showed signs of distress, they were
sacrificed. If no signs of stress were observed, mice were analyzed
at 15 weeks following transplantation. The time from
transplantation to sacrifice varied from 8 to 15 weeks with an
average of 10 weeks.
Gross examination and survival
monitoring
After injection, mice were sacrificed at signs of
sickness and observed for tumor burden, characterized by tumor
cluster in liver, suppression of erythropoiesis in BM and enlarged
spleen. Femur (BM), spleen, and blood from NSG mice were collected
and analyzed for lodgments of leukemia cells.
PCR and DNA fingerprinting
Total RNA isolation and DNA synthesis were performed
as previously described (17).
Human MLL/AF9 primers (forward, 5′-aatagaggaggcagccgaag-3′ and
reverse, 5′-gtccagcgagcaaagatcaa-3′) were used. PCR work for
fingerprinting was performed using a universal fingerprinting kit
(JK Biotech Korea, cat no. JK090016) according to the
manufacturer’s protocol. UPF 2, 5, 13 primers were used to confirm
origination, and PCR reactions were performed in a 50-μl PCR
mixture containing 100 ng of each primer, 1X TE buffer, 100 ng of
template DNA, 2.5 units HQ Taq polymerase and 2.5 mM of
dNTP. PCR amplification was performed in a conventional PCR machine
(Px2 Thermal Cycler; Thermo Electron Corp., Marietta, OH, USA)
using the following profile: one cycle of 4 min at 94°C; 38 cycles
of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C; one final
extension cycle of 7 min at 72°C. PCR products were electrophoresed
in a 2.0% agarose gel at 12 V/cm with TAE buffer. DNA fragments in
the gel were visualized by staining with ethidium bromide and
photographed under a UV transilluminator.
Flow cytometry
FACS staining and analysis of MNCs were performed as
previously described (18).
Briefly, cells were resuspended in 100 μl of rinsing buffer and
incubated with antibodies. After washing, the cells were analyzed
using a FACSCalibur flow cytometer equipped with Cell
Quest® software (BD Biosciences, San Diego, CA, USA). We
used phycoerythrin (PE)-conjugated mouse anti-human CD34 and
PEcy5-conjugated mouse anti-human CD38 primary antibodies (555822
and 555461, respectively; both from BD Pharmingen) to examine LSCs.
For engraftments, FITC-conjugated mouse anti-human CD45 and
allophycocyanin (APC)-conjugated rat anti-mouse CD45 (555482 and
559864 respectively, both from BD Pharmingen) were used. For
secondary Abs, proper isotype-matched IgG and unstained controls
were used to detect primary signals. ALDH activity was measured in
MNCs according to the manufacturer’s instructions (Aldefluor™;
StemCo Biomedical Inc., San Diego, CA, USA).
Histology
BM samples were fixed in PFA, decalcified with 5%
formic acid and embedded in paraffin. Prepared slides were
counterstained with Meyer’s hematoxylin. Hematoxylin and eosin
(H&E) staining was used after fixation to confirm leukemic
blast infiltration in BM.
Statistical analysis
Results are presented as the means ± SE. Data were
compared using the Mann-Whitney U test. GraphPad Prism®
software, ver. 4 (GraphPad software, La Jolla, CA, USA) was used
for analyses. Values of P<0.05 were considered to indicate
statistically significant differences.
Results
LPB in AML patients shows a high level of
leukemic stem cells, CD34+CD38− cells
We investigated whether a difference in the
frequency of CD34+CD38− cells exists between
PB, LPB and BM samples. AML is propagated by self-renewing leukemic
stem cells characterized by the CD34+CD38−
phenotype. FACS analysis was performed using PB, LPB and BM samples
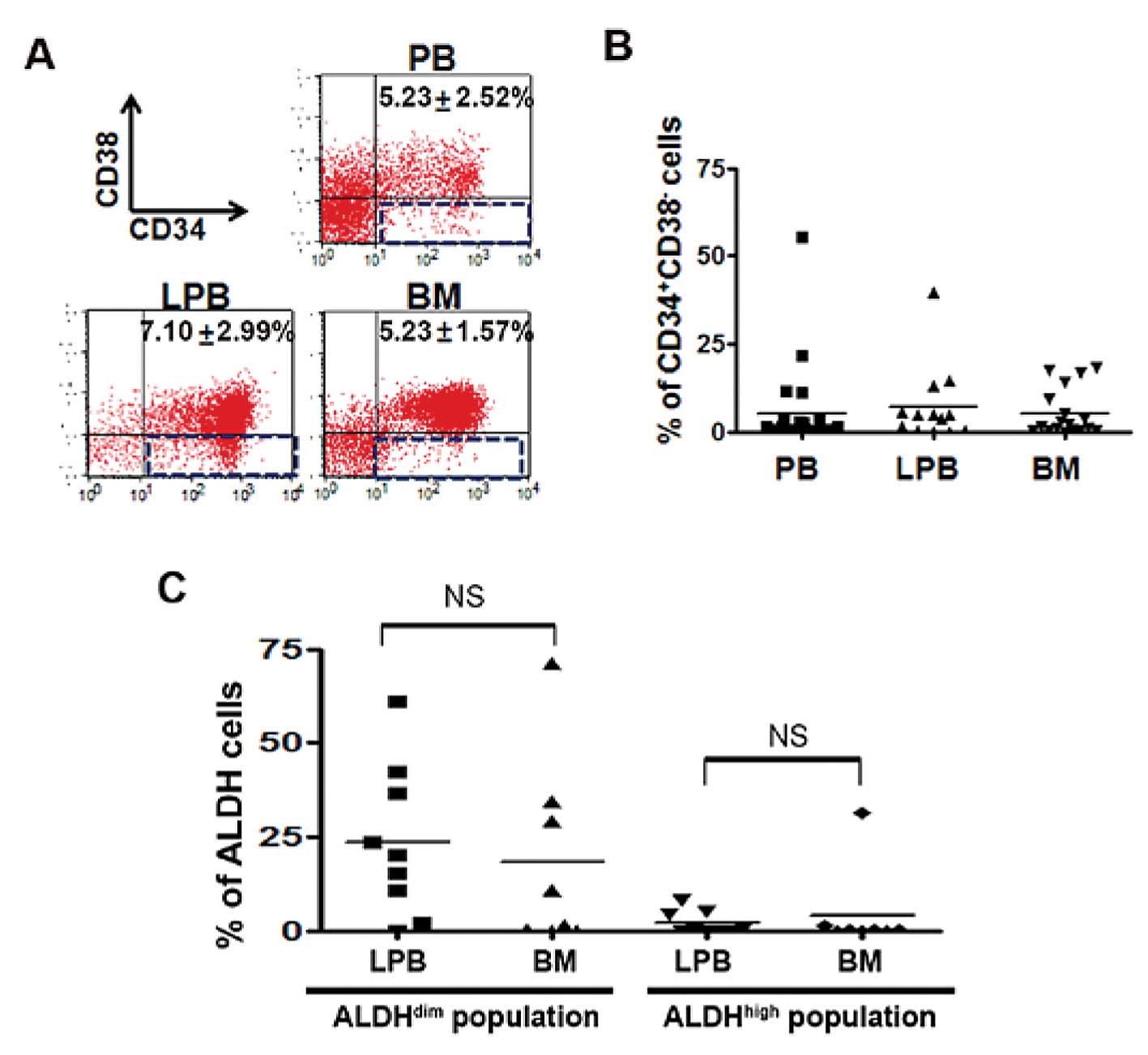
from patients and the cell lines TF-1a, K562 and Kasumi-6. No
significant difference in the proportion of
CD34+CD38− cells was detected in PB, LPB and
BM (PB, 5.23±2.52%; LPB, 7.10±2.99%; BM, 5.23±1.57%; Fig. 1A), suggesting the possibility of LPB
as a cell source for the xenograft model. Some samples showed high
levels of the LSC population and frequencies of abnormal blasts in
PB; however, no significant difference was detected among the three
groups (Fig. 1B and data not
shown). In contrast, the cell lines K562 and Kasumi-6 displayed low
levels of the LSC population (K562, 0.16%; Kasumi-6, 0.02%),
whereas 7.72% of the CD34+ cell line, TF-1a, showed a
CD34+CD38− phenotype (data not shown). Next,
we checked the ALDH level in CD34+CD38− cells
from LPB and BM. As shown in Fig.
1C, no difference in the ALDHdim population was
found between the two samples, implying that similar leukemic
properties exist between both cell sources in AML. The LSCs of AML
patients revealed that the ALDHdim population was higher
than the ALDHhigh population regardless of cell type
(ALDHdim population in BM, 18.43%; ALDHdim
population in LPB, 23.54%; ALDHhigh population in BM,
4.17%; ALDHhigh population in LPB, 2.03%; Fig. 1C). These results suggest that the
leukemic characteristics, high ALDHdim population and
low ALDHhigh population, in LPB are similar to those
observed in BM.
A successful human xenograft model was
accompanied by a stable lodgment of injected LPBs
Next, we investigated the engraftment of human
cells, gross examination and infiltration of human leukemic cells
in leukemic mouse tissues. In the normal humanized mouse model, the
mouse model was completed by injecting CD34+ cells from
normal human cord blood and HSCs; humanized NSG mice displayed
normal physiologic condition without virulence (19,20).
However, gross appearance from the leukemia humanized mouse clearly
showed a significant difference in tumor infiltration when
1×107 MNCs from LPB were injected into the NSG mice via
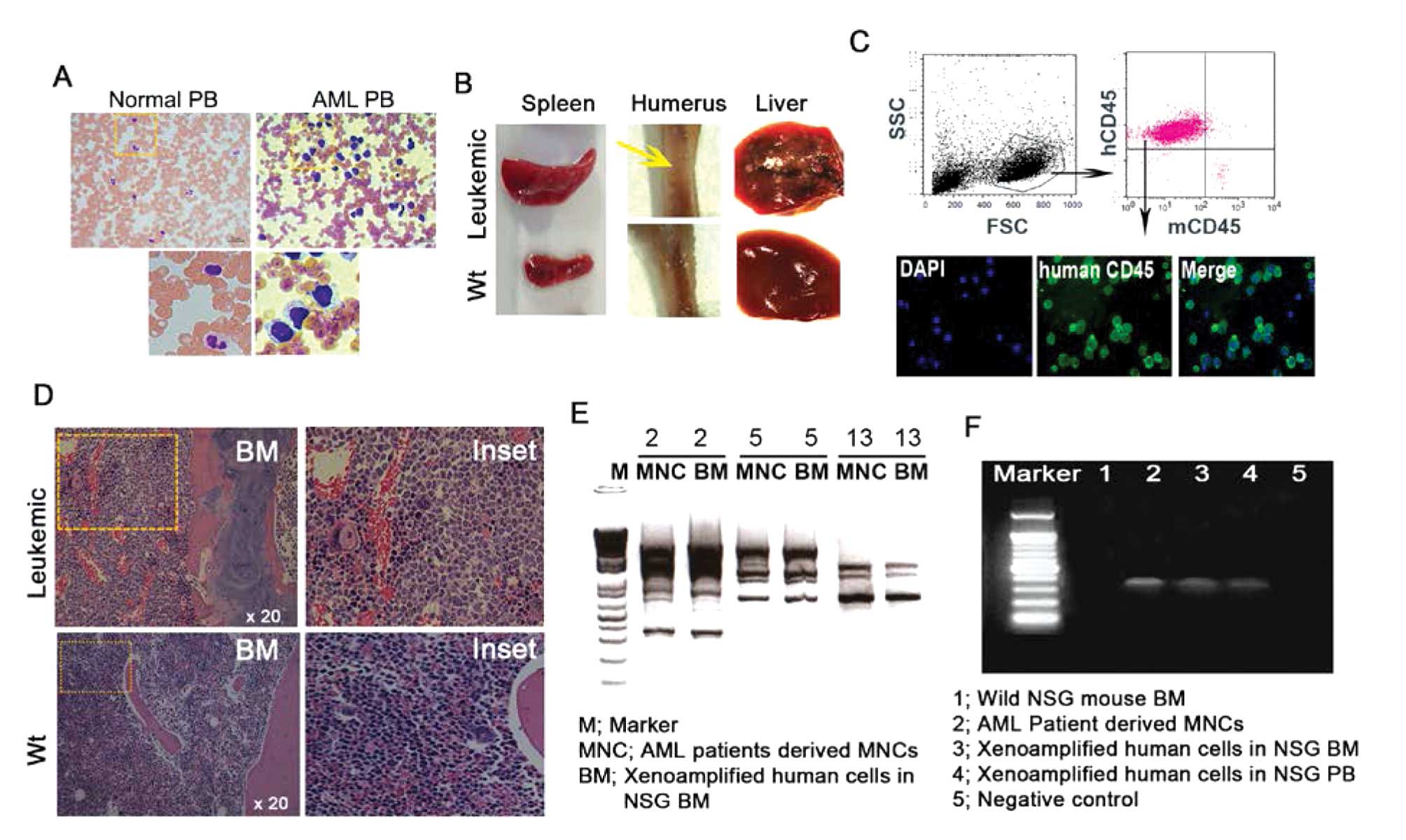
the tail vein. First, we checked the existence of AML blasts in
patient-derived LPB, and immature blasts were easily detected in
the ideal zone from LPB smearing regardless of WHO type (Fig. 2A). In addition, our leukemic
xenograft model displayed aberrant and morbid phenomena including
liver with disseminating masses, enlarged spleen, and suppression
of erythropoiesis in the humerus, suggesting disease induction
(Fig. 2B). To confirm human cell
infiltration, FACS analysis was carried out on PB and flushed BM.
The level of human CD45dim cells in PB from NSG mice at
13 weeks exclusively increased with little existence of murine CD45
cells. Consistently, microscopic imaging also clearly showed
FITC-conjugated human CD45 expression in the PB samples, which was
performed for FACS analysis (Fig.
2C). BM tissue sections showed immature blasts of human origin
with large size and faint hematoxylin staining in vascular regions.
The morphology of human cells was easily distinguished from mouse
cells in the leukemic mouse BM, and no human cells were found in BM
from wild-type mice (Fig. 2D),
indicating engraftment of human cells. To confirm whether
patient-derived hematopoietic cells can contribute to the leukemic
xenograft, DNA fingerprinting and conventional PCR were performed
using genomic DNA and specific mutant gene from patient-derived
MNCs. DNA fingerprinting allowed the identification of a specific
type of individual DNA sequence, known as a ‘microsatellite’. LPB
from patients who displayed high efficiency of engraftment (93.5%)
in BM tissues was used for the DNA fingerprint. As shown in
Fig. 2E and F, our data revealed
that PCR bands from patient-derived PB-MNCs and BM-MNCs from NSG
demonstrated the same pattern of UPF primer of 2, 5, 13 numbers,
suggesting patient-derived cells infiltrated the mouse BM (Fig. 2E), and indicated that xenoamplified
cells originated from primary MNCs from the AML patients. Leukemic
engraftment was monitored by the detection of the MLL/AF9 human
mutation gene by PCR. PCR amplifications were positive in primary
patient MNCs and xenoamplified human LPB cells in mouse BM and PB
(Fig. 2F). Although not all samples
readily showed complete accordance between the original blood
sample and the mouse derived MNCs, most likely due to various
factors such as evolution and mutation in vivo, we found AML
patient-derived cells grafted in the NSG mouse, indicating leukemia
manifestation. A xenograft model developed by injecting sorted LSCs
can successfully establish advanced leukemia with fewer cell
numbers compared to LPB MNCs (data not shown). Without LSC sorting,
sufficient numbers of LPB cells can successfully achieve the
xenograft model if an adequate number of LSCs are contained in the
LPB cell population.
Leukemic xenograft model using LPB cells
shows high level of abnormal blasts with CD45dim
compared to BM cells
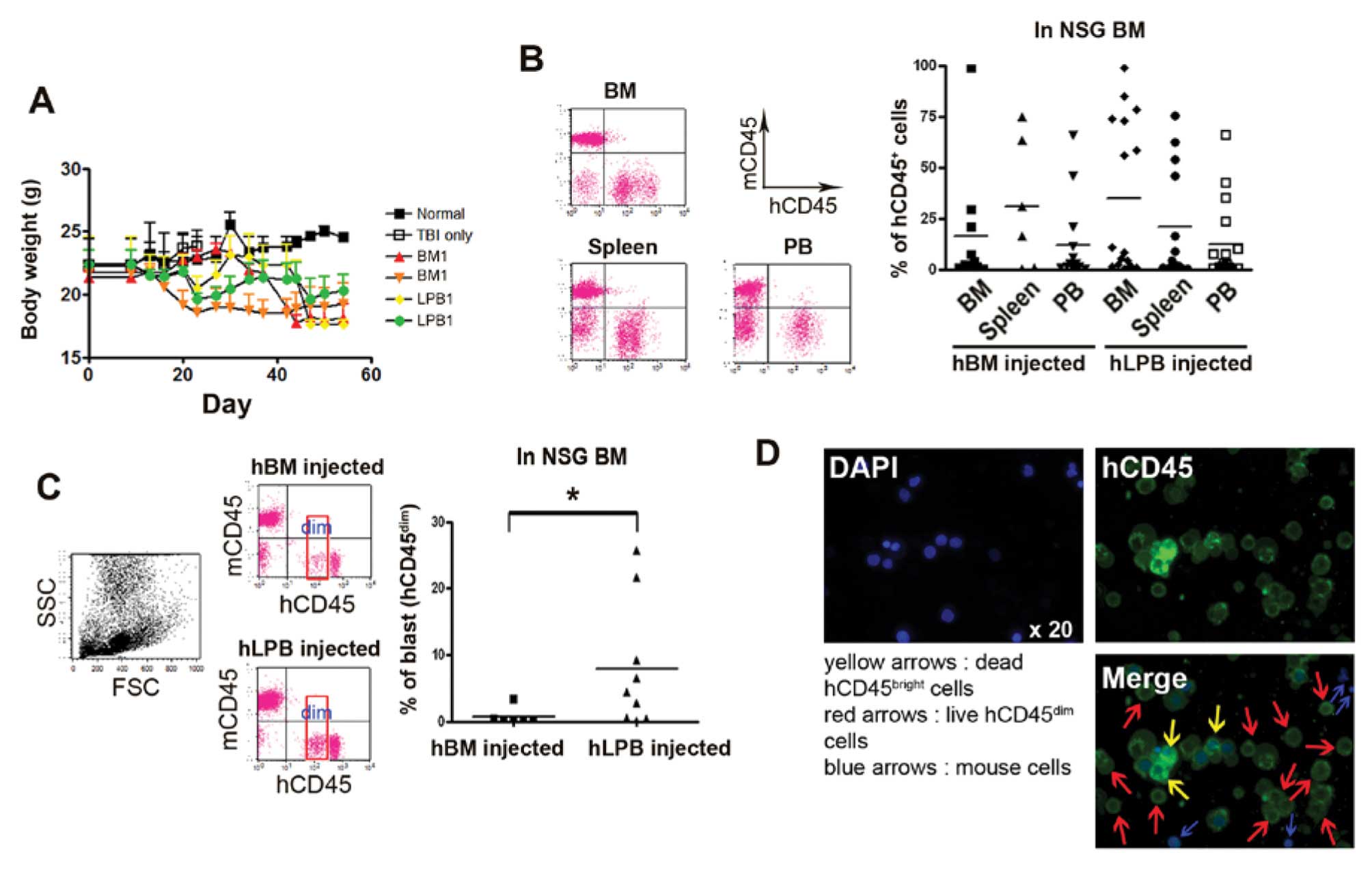
To further investigate whether LPB displays a
similar level of engraftment and weight loss compared to BM cells,
LPB cells (1×107 cells in 200 ml PBS), including
functional LSCs, were intravenously (i.v.) injected into NSG mice
via the tail vein. As expected, NSG mice, which received two types
of cells, BM and LPB, had a similar pattern of weight loss with no
significant difference. However, mice receiving patient cells
noticeably lost body weight compared to wild-type mice (Fig. 3A). Furthermore, BM, PB and spleens
from NSG mice clearly showed high engraftment of CD45+
human cells with cancerous symptoms. Because all mice showed
increased permissiveness when irradiated before cell
transplantation, irradiated female NSG mice were used in the
present study (21). Consistently,
our data also showed a moderate difference in grafting between
irradiated NSG and non-irradiated NSG mice (data not shown). The
human CD45 distribution in the BM from NSG mice following the
injection of human BM or LPB cells varied (BM cells injected, 0.37
to 99.04%; LPB cells injected, 0.09 to 99.05%). In PB and spleen,
the number of human BM or LPB cells also varied (in PB: BM cells
injected, 0.27 to 65.68%; LPB cells injected, 0.01 to 65.80%; in
spleen: BM cells injected, 0.43 to 74.49%; LPB cells injected, 0.26
to 75.47%). Although no significant difference in BM engraftment
was found between the BM- and LPB-injected groups, the LPB-injected
group revealed a slighly higher average engraftment when LPB cells
were injected into NSG mice compared to mice that received BM cells
(LPB cells injected into NSG BM, 34.9±9.39%; BM cells injected into
NSG BM, 16.56±9.68%; Fig. 3B). Mice
were sacrificed and xenoamplified leukemic cells were harvested
from the tissues when signs of sickness became evident or the hCD45
in PB exceeded 70% during the time from 2 to 11 weeks
post-injection. We then examined the percentage of human
CD45dim abnormal blasts in BM tissues. As AML blasts
show a dim intensity of staining with the leukocyte common antigen
CD45 antibody, CD45 has been used to distinguish AML cells from
normal white blood cells (22).
FACS data certainly showed a high engraftment of abnormal blasts in
NSG mouse BM 2 weeks after LPB cells were injected (Fig. 3C). BM cell-injected mice displayed a
significantly low CD45dim cell population in the BM
compared to the LPB cell-injected mice (Fig. 3C). The intensity of human CD45 cells
in gated cells was divided into two fractions, a ‘dim’ and a ‘high’
population. Notably, human CD45dim cells in the
LPB-injected NSG mice were clearly distinguished from
CD45high cells (Fig. 3B and
C). We only counted the ‘dim’ population of CD45-positive cells
to calculate abnormal cells. Moreover, fluorescence microscopic
images also showed that CD45high cells and
CD45dim cells were distinguishable in the BM cells. A
small nucleus without the human CD45 marker identified mouse cells
and DAPI-positive dead human CD45high and live human
CD45dim cells were present in the BM flushed cells
(Fig. 3D). Surprisingly, three LPB
samples, which showed above average expression of
ALDHdim and LSCs, had a significant increase in fold
change of CD45dim cells in NSG BM at 2 weeks
post-transplantation, as well as high engraftment in the NSG mice
(Fig. 1A and C and Fig. 3C). We found that human cell
engraftment fully relied on individual variations and was dependent
on whether a high level of ALDHdim-expressing LSCs was
present or not. The strength of LPB as a cell source in the
leukemic xenograft mouse model was apparent when
ALDHdim-expressing LSCs were selected.
Leukemic xenograft model using LPB cells
displays longevity with stable engraftment
In general, leukemic xenograft mouse models have
been used to study biological features in leukemia and to
investigate responses to antitumor drugs. Therefore, to maintain
the longevity of a mouse with cancer is one important factor by
which to elicit experimental results in vivo. Unfortunately,
since it is not acceptable to acquire huge amounts of leukemic stem
cells from patient BM, many in vivo studies using leukemic
xenograft models are defeated before the start of the experiment,
or have difficulty acquiring consistent data interpretation due to
individual diversity. Therefore, we also addressed the differential
efficacy of human cell lodgment in a time-dependent manner with
longevity. Time was divided into an early time phase (<2 weeks)
and a late time phase (>5 weeks). From 3 to 9 heads of mice were
used for each sample. Lodgment of human CD45 cells in BM was not
significantly different between BM and LPB cell sources in a time
phase manner (Fig. 4A).
CD45-positive cells in the early time phase were fewer in number
than that observed in the late time phase, and CD45-positive cells
gradually increased in a time-dependent manner for both primary
human samples studied, BM and LPB. Next, we sought to address the
stable longevity of abnormal blasts in engrafted mice. Three to 6
heads of mice that received human BM cells, and 2 to 4 heads of
mice which received human LPB cells, were used to examine they
longevity. Although unexpected mortality was monitored in the BM
cell-injected NSG mice due to individual characteristics, we found
that BM and LPB cells that were grafted into NSG mice in the
presence of abnormal blasts continuously maintained a stable
survival at >50% until 8 weeks post-injection. Mice injected
with LPB cells showed low mortality (Fig. 4B), suggesting that LPB may maintain
the model for a longer time in which to examine the in vivo
study.
Discussion
Xenograft models using human AML cells are important
tools by which to study the pathophysiology of AML, including the
tumor microenvironment, leukemic stem cells and drug resistance,
in vivo. Moreover, advanced xenograft models help to screen
individualized molecular treatment modalities in vivo. AML
is a stem cell-mediated disease, and a variable population of LSCs
has been associated with the disease. Therefore, we hypothesized
that LPB may be a preferable cell source to generate the xenograft
model if the frequency of ALDHdim-expressing LSCs is
high. Similarly, BM cells from AML patients are considered to be a
superior cell source due to its high level of LSCs compared to PB
cells. However, an important limitation of BM is that it can only
be obtained in small volumes from patients. Because a synchronized
mouse model prepared from a single sample without variation has
yielded reproducible data in disease biology, we attempted to
accumulate evidence from this limited experiment, which can
contribute towards completing the human xenograft model using LPB
cells. The leukemic xenograft model is also hampered by individual
patient variation and the short life-span of immunodeficient mice.
A large amount of material from the same patient can minimize these
deficits. In addition, the leukemic xenograft model using LPB cells
shows no significant difference in graft and cell amplification
with genetic abnormalities when compared to the model using BM
cells. LPB is also capable of producing numerous models at one
time. Importantly, LPB cells can support a longer life-span without
sudden death, and can maintain more than 60% survival 5 weeks after
human AML cell injection. We cannot exclude that BM cells may
aggressively progress with AML features and graft-versus-host
disease induction by co-infused T cells in the graft (23). The use of LPB cells prevents high
mortality. Consistent with previous reports, stem cell quantity and
quality of LPB appeared to vary and was difficult to estimate among
patients (24). Some patients have
more than sufficient stem cells in LPB, while others do not. The
frequency of LSCs in LPB cells depends on multiple parameters, such
as mobilization timing, chemotherapy type, and cytokine addition.
Because a variety of reasons affect the status of circulating blood
cells, it is difficult to determine the best sample without a
proper indicator in the xenograft model. To overcome the frailty of
reproducibility and volume, some investigators have used leukemic
cell lines, such as TF-1a and K562, instead of primary AML cells
(25–27). However, leukemic cell lines are
fundamentally different from primary leukemic cells in grafting.
Our data also revealed low efficacy of the CD34+ cell
line TF-1a with at least a 2- to 3-fold reduction in grafting
compared to that of primary cells (data not shown). To develop a
more reasonable protocol with which to produce a xenograft model,
our results suggest using LPB with high levels of
ALDHdim-expressing LSCs. Moreover, one of the advantages
of LPB is that the sample naturally occurs during the course of
treatment. Blood collection does not require anesthesia or
antibiotic treatment. We also demonstrated the potential of LPB as
an advantageous cell source with which to generate a xenograft
model with a long life-span. Progenitor cells from LPB displayed
rapid hematopoietic recovery after conditioning regimens with high
dose therapy (28). This rapid
recovery may reduce mortality and morbidity. Xenograft models are
also very diverse in many factors, including recipient
permissiveness, age and gender (21). Ultimately, the goal of xenograft
models is to be clinically relevant, mimic the situation of the
patients and be sufficient to perform a wide spectrum of
experiments. Depending on these goals, we found that human LPB is a
beneficial cell source for a xenograft model to satisfy the patient
setting. To our knowledge, this direct comparison between using BM
and LPB cells in a leukemic xenograft model has not been previously
reported. We compared the efficiency of both BM and LPB cell
engraftment using NSG mice and retrospectively found that
ALDHdim-expressing LSCs may belong in the category of
cells which can induce a successful graft model. Our data are
informative in deciding which cell sources to use to accomplish a
successful xenograft model. A xenograft model with stable leukemic
properties is necessary to determine the effects of antitumor drugs
and immune cell therapies, such as those using cytotoxic T cells
and natural killer cells.
In conclusion, LPB cells, which contain high levels
of ALDHdim-expressing CD34+CD38−
cells, can serve as a suitable alternative cell source to BM cells
for the generation of leukemic xenograft models.
Acknowledgements
This research was partly supported by a grant from
the National R&D Program for Cancer Control, Ministry for
Health and Welfare, Republic of Korea (1020370).
References
|
1
|
Gopinathan A and Tuveson DA: The use of
GEM models for experimental cancer therapeutics. Dis Model Mech.
1:83–86. 2012. View Article : Google Scholar
|
|
2
|
Richmond A and Su Y: Mouse xenograft
models vs GEM models for human cancer therapeutics. Dis Model Mech.
1:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kelland LR: Of mice and men: values and
liabilities of the athymic nude mouse model in anticancer drug
development. Eur J Cancer. 40:827–836. 2004.PubMed/NCBI
|
|
4
|
McCune JM, Namikawa R, Kaneshima H, Shultz
LD, Lieberman M and Weissman IL: The SCID-hu mouse: murine model
for the analysis of human hematolymphoid differentiation and
function. Science. 241:1632–1639. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mosier DE, Gulizia RJ, Baird SM and Wilson
DB: Transfer of a functional human immune system to mice with
severe combined immunodeficiency. Nature. 335:256–259. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lubin I, Faktorowich Y, Lapidot T, Gan Y,
Eshhar Z, Gazit E, et al: Engraftment and development of human T
and B cells in mice after bone marrow transplantation. Science.
252:427–431. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lapidot T, Pflumio F, Doedens M, Murdoch
B, Williams DE and Dick JE: Cytokine stimulation of multilineage
hematopoiesis from immature human cells engrafted in SCID mice.
Science. 255:1137–1141. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung AM, Wan TS, Leung JC, Chan LY,
Huang H, Kwong YL, et al: Aldehyde dehydrogenase activity in
leukemic blasts defines a subgroup of acute myeloid leukemia with
adverse prognosis and superior NOD/SCID engrafting potential.
Leukemia. 21:1423–1430. 2007. View Article : Google Scholar
|
|
9
|
Ishikawa F, Yasukawa M, Lyons B, Yoshida
S, Miyamoto T, Yoshimoto G, et al: Development of functional human
blood and immune systems in NOD/SCID/IL2 receptor {gamma}
chain(null) mice. Blood. 106:1565–1573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishikawa F, Yoshida S, Saito Y, Hijikata
A, Kitamura H, Tanaka S, et al: Chemotherapy-resistant human AML
stem cells home to and engraft within the bone-marrow endosteal
region. Nat Biotechnol. 25:1315–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Will B, Kawahara M, Luciano JP, Bruns I,
Parekh S, Erickson-Miller CL, et al: Effect of the nonpeptide
thrombo-poietin receptor agonist Eltrombopag on bone marrow cells
from patients with acute myeloid leukemia and myelodysplastic
syndrome. Blood. 114:3899–3908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanchez PV, Perry RL, Sarry JE, Perl AE,
Murphy K, Swider CR, et al: A robust xenotransplantation model for
acute myeloid leukemia. Leukemia. 23:2109–2117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goardon N, Marchi E, Atzberger A, Quek L,
Schuh A, Soneji S, et al: Coexistence of LMPP-like and GMP-like
leukemia stem cells in acute myeloid leukemia. Cancer Cell.
19:138–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, et al: A cell initiating human acute
myeloid leukaemia after transplantation into SCID mice. Nature.
367:645–648. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarry JE, Murphy K, Perry R, Sanchez PV,
Secreto A, Keefer C, et al: Human acute myelogenous leukemia stem
cells are rare and heterogeneous when assayed in
NOD/SCID/IL2Rγc-deficient mice. J Clin Invest. 121:384–395.
2011.PubMed/NCBI
|
|
16
|
Gerber JM, Smith BD, Ngwang B, Zhang H,
Vala MS, Morsberger L, et al: A clinically relevant population of
leukemic CD34(+)CD38(−) cells in acute myeloid leukemia. Blood.
119:3571–3577. 2012.PubMed/NCBI
|
|
17
|
Hong SH, Nah HY, Lee JY, Lee YJ, Lee JW,
Gye MC, et al: Estrogen regulates the expression of the small
proline-rich 2 gene family in the mouse uterus. Mol Cells.
17:477–484. 2004.PubMed/NCBI
|
|
18
|
Lee JY, Park C, Cho YP, Lee E, Kim H, Kim
P, et al: Podoplanin-expressing cells derived from bone marrow play
a crucial role in postnatal lymphatic neovascularization.
Circulation. 122:1413–1425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shultz LD, Ishikawa F and Greiner DL:
Humanized mice in translational biomedical research. Nat Rev
Immunol. 7:118–130. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi B, Chun E, Kim M, Kim SY, Kim ST,
Yoon K, et al: Human T cell development in the liver of humanized
NOD/SCID/ IL-2Rγ(null)(NSG) mice generated by intrahepatic
injection of CD34(+) human (h) cord blood (CB) cells. Clin Immunol.
139:321–335. 2011.PubMed/NCBI
|
|
21
|
Notta F, Doulatov S and Dick JE:
Engraftment of human hematopoietic stem cells is more efficient in
female NOD/SCID/ IL-2Rgc-null recipients. Blood.
115:3704–3707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lacombe F, Durrieu F, Briais A, Dumain P,
Belloc F, Bascans E, et al: Flow cytometry CD45 gating for
immunophenotyping of acute myeloid leukemia. Leukemia.
11:1878–1886. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whartenby KA, Straley EE, Kim H, Racke F,
Tanavde V, Gorski KS, et al: Transduction of donor hematopoietic
stem-progenitor cells with Fas ligand enhanced short-term
engraftment in a murine model of allogeneic bone marrow
transplantation. Blood. 100:3147–3154. 2002. View Article : Google Scholar
|
|
24
|
Breems DA, van Hennik PB, Kusadasi N,
Boudewijn A, Cornelissen JJ, Sonneveld P and Ploemacher RE:
Individual stem cell quality in leukapheresis products is related
to the number of mobilized stem cells. Blood. 87:5370–5378.
1996.PubMed/NCBI
|
|
25
|
Zhang J, Yang WH, Yang XD, Shi ZX, Wang
XL, Yu WJ, et al: Establishment and identification of CML model via
injection of K562 cells into the murine caudal vein. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 20:773–776. 2012.(In Chinese).
|
|
26
|
Park J, Kim KI, Koh Y, Won NH, Oh JM, Lee
DS, et al: Establishment of a new Glivec-resistant chronic myeloid
leukemia cell line, SNUCML-02, using an in vivo model. Exp Hematol.
38:773–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee J, Li M, Milwid J, Dunham J, Vinegoni
C, Gorbatov R, et al: Implantable microenvironments to attract
hematopoietic stem/cancer cells. Proc Natl Acad Sci USA.
109:19638–19643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elias AD, Ayash L, Anderson KC, Hunt M,
Wheeler C, Schwartz G, et al: Mobilization of peripheral blood
progenitor cells by chemotherapy and granulocyte-macrophage
colony-stimulating factor for hematologic support after high-dose
intensification for breast cancer. Blood. 79:3036–3044. 1992.
|