Introduction
Chemokine-like factor (CKLF) was first
isolated from PHA-stimulated U937 cells (1). Since then, eight novel genes of the
CKLF family have been identified through BLAST searches in
combination with expressed sequence tag assembly and have been
experimentally validated. They have been designated as
chemokine-like factor superfamily (CKLFSF) members and have
been approved by the HUGO Gene Nomenclature Committee (2). In 2005, CKLFSF1–8 were renamed
CKLF-like MARVEL transmembrane domain containing 1–8
(CMTM1–8). CKLF and CMTM1–8 represent a novel
protein family linking the chemokine and TM4SF families that may
play multiple roles in a wide range of physiological and
pathological processes. CKLF1 has chemotactic effects on a
wide spectrum of leukocytes, while CKLF2 stimulates the
proliferation and differentiation of C2C12 cells (3). Rat and mouse cklf have similar
RNA splice forms and functions as human CKLF (4,5).
Our laboratory cloned and characterized
CMTM1, which is highly expressed in testis tissue. The
CMTM1 gene consists of seven exons and six introns. Most of
the intron-exon boundaries agreed with the GT/AG rule and two
alternative transcription start sites, 1A and 1B, were identified.
Site 1A predicted a complete open reading frame ORF1. Site 1B,
located inside the putative exon 1, predicted a shorter open
reading frame ORF2. cDNA sequencing revealed that CMTM1
contains at least 23 alternatively spliced isoforms, which were
designated CMTM1_v1–v23 (suggested by the HUGO Gene
Nomenclature Committee). The proteins of CMTM1_v1–16 are
encoded by ORF1 and ORF2 encodes CMTM1_v17–23.
CMTM1 is most abundant in spermatocytes of
human testicular tissues (6) and is
highly upregulated and greatly susceptible to the effect of hrIL-30
in PC3 cells (7). CMTM2 is a
secreted protein that may be functionally relevant during
spermatogenesis (8). CMTM8
is a novel negative regulator of epidermal growth factor-induced
signaling via facilitation of ligand-induced receptor endocytosis
and subsequent desensitization (9).
CMTM3 is highly conserved and highly expressed in both the
immune system and the male reproductive system (10). It was reported that CMTM3–5
(11–13) and CMTM7 (14) all exhibited inhibitory effects on
the growth of tumor cells. However, the functions of another member
of the CKLFSF family, CMTM6, are largely
undefined.
In the present study, we found that CMTM1_v17
was highly expressed in human testis and many human tumor tissues,
and cell lines but was almost undetectable in human normal tissues.
MDA-MB-231 breast cancer cells overexpressing CMTM1_v17 had
enhanced proliferation and resisted tumor necrosis factor-α
(TNF-α)-induced apoptosis. We suggest that CMTM1_v17 may be
a novel potential therapeutic target in breast cancer patients and
it may also be a novel potential cancer/testis antigen.
Materials and methods
Cell culture and transfection
The human cell lines HEK293T (a gift from T.
Matsuda, Japan), HEK293, MDA-MB-231, MCF-7, HepG2, SGC7901, AGS,
Caov3, HeLa and H1299 were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Life Technologies, USA) containing 10% fetal bovine
serum (FBS; HyClone, USA), 100 U/ml penicillin, and 100 μg/ml
streptomycin at 37°C in a humidified incubator with 5%
CO2. The suspension cell lines K562, Raji and U937 were
maintained in RPMI-1640 medium supplemented with FBS and
antibiotics as above. MDA-MB-231 cells were plated in fresh culture
medium prior to initiating experiments. The indicated amount of
siRNA plus plasmid or plasmid alone was transfected into MDA-MB-231
cells using Lipofectamine 2000 (Invitrogen, USA) according to the
manufacturer’s protocol.
cDNA preparation and real-time qPCR
cDNA preparations of normal tissues were purchased
from Clontech (Mountain View, CA, USA). Tumor tissues were gifts
from Beijing Shijitan Hospital. Three pathologists evaluated all
the tissues to establish the presence of tumors. Tumor tissues were
homogenated and pellet cells were suspended in TRIzol reagent
(Invitrogen) for the isolation of total RNA according to the
manufacturer’s instructions. cDNA was synthesized from 3 μg of
total RNA using the ThermoScript™ RT-PCR System (Invitrogen). We
prepared cDNA from multiple cell lines. The resulting cDNA products
were used to amplify the fragment of CMTM1_v17 (forward,
5′-ATG TTG AAG ATC CTG AGA CT-3′ and reverse, 5′-CAA TGT AAA TAG
GTC AGC AA GTG GTG-3′) by real-time qPCR using the Power SYBR-Green
PCR Master Mix on a AB7500 System (both from Applied Biosystems,
USA) as follows: 95°C for 30 sec, 40 cycles of 95°C for 15 sec,
60°C for 1 min. The mRNA levels of CMTM1_v17 were normalized
using GAPDH mRNA levels. Tissues that were utilized for RNA or
protein extraction were frozen in liquid nitrogen immediately after
harvesting. All samples were obtained from patients with their
informed consent and the approval of the Ethics Committee.
cDNA cloning and vector construction
The CMTM1_v17 gene was amplified from a cDNA
library of the human breast cancer cell line MDA-MB-231 by PCR
using the primers: forward, 5′-ATG TTG AAG ATC CTG AGA CT-3′ and
reverse, 5′-GCA CGT GTC TGT CGA ATC GCT-3′. The purified PCR
product was ligated into the pGEM-T Easy vector (Promega, Madison,
WI, USA) and the insert was released by EcoRI digestion and
ligated into the EcoRI site of pcDNA.3.1/myc-His(−)B
(Invitrogen) to construct the plasmid pcDB/CMTM1_v17. The
reverse primer (5′-TCGGATCCACGTCTCGTAAAA-3′) was fused with a
C-terminal GFP tag in pEGFP-N1 (Clontech) vector by BamHI to
construct the plasmid CMTM1_v17-EGFP. A C-terminal human
CMTM1_v17 cDNA fragment encoding a 31 amino acid hydrophilic
region was amplified from the human breast cancer cell line
MDA-MB-231 cDNA library using the primers forward, 5′-GAA AAG ATT
CCT GGG AGT CG-3′ and reverse, 5′-GCA CGT GTC TGT CGA ATC GCT-3′.
The PCR product was cloned into pGEX-4T-2 (Pharmacia, USA) for
expression in E. coli. All clones were confirmed by
sequencing. All plasmids were purified using Qiagen Plasmid kit
(Qiagen, Germany) columns.
Confocal microscopy
To determine the subcellular localization of
CMTM1_v17, HEK293T cells transfected with control EGFP or
CMTM1_v17-EGFP were fixed in 4% paraformaldehyde for 15 min and
permeabilized with 0.2% Triton X-100 for 1 h at room temperature.
Cells were rinsed with phosphate-buffered saline (PBS) and stained
with DAPI for nuclear visualization. Fluorescence was detected by
confocal microscopy (Leica TCS SP5; Confocal System, Germany).
Synthesis of CMTM1_v17 small interfering
RNAs
Human CMTM1_v17 double-stranded small
interference RNA was synthesized from GeneChem Corporation
(Shanghai, China). The sense oligonucleotide, 5′ ACC ACU UGC UGA
CCU AUU UdTdT and the antisense oligonucleotide, 5′ AAA UAG GUC AGC
AAG UGG UdTdT were utilized.
Antibody preparation
The GST fusion protein was expressed and purified as
described in the GST Gene Fusion System manual (Pharmacia).
Polyclonal anti-CMTM1_v17 antiserum was raised in rabbits
immunized with the purified GST fusion protein. Immunoreactive
serum was identified by ELISA and western blotting (data not
shown). The GST tag of the fusion protein was removed with thrombin
and the purified antigen was coupled to a chromatographic matrix
(Sepharose 4B; Pharmacia). The column was used to purify
highly-specific, polyclonal anti-CMTM1_v17 antibodies from
the antiserum.
Western blotting
Lysates were harvested from primary tissues and cell
lines. Protein lysates (30–50 μg) were separated by SDS-PAGE and
transferred to nitrocellulose membranes. After transferring, the
membranes were blocked in 5% non-fat dry milk in TBS-T for 2 h at
room temperature followed by incubation overnight at 4°C with
primary antibodies that were specific for CMTM1_v17,
phospho-IKK, IKKβ and IκBα (1:1,000; Cell Signaling). Blots were
washed with TBS-T and then incubated at room temperature for 1 h
with the appropriate IRDye™ 800-conjugated secondary antibodies
(LI-COR Biosciences, USA). Immunocomplexes were detected using
Odyssey Infrared Imager System (LI-COR Biosciences). Membranes were
re-probed with β-actin monoclonal antibody to confirm equal
loading.
Tissue microarrays and
immunohistochemistry
Three tissue microarray slides (lot nos.
CC08-02-004, CC08-11-001 and CC08-11-002) containing tumor, normal
and non-cancerous specimens were purchased from Cybrdi (Xi’an,
Shaanxi, China). The slides were dewaxed by rinsing with xylene
followed by graded ethanol washes and then heated twice in 10
mmol/l sodium citrate (pH 6.0) for 5 min each in a microwave oven
for antigen retrieval. Endogenous peroxidase activity was blocked
in methanol containing 3% hydrogen peroxide for 10 min at room
temperature. After washing with PBS, the slides were incubated at
room temperature for 10 min in 10% normal goat serum/1X PBS. Then,
the slides were incubated with rabbit polyclonal antibody to human
CMTM1_v17 for 1 h at room temperature. A monoclonal
anti-cancer antigen 15-3 (CA15-3) antibody (Zhongshan, Beijing,
China) was used as a positive control. After adequate washing, they
were subjected to HRP-labeled polymer for 30 min and DAB +
substrate − chromogen solution for 5 min (ChemMate™ DAKO EnVision™
System; Dako, USA). All stained sections were counterstained with
hematoxylin, then dehydrated and mounted with coverslips.
Cell growth analysis
Cell viability was assessed by trypan blue staining
followed by counting of the unstained cells. Relative cell numbers
were determined using the MTT colorimetric assay. Transfected cells
were plated in 96-well plates at a density of 2,000 cells/well in
100 μl medium. At the indicated time points, MTT solution was added
and samples were incubated for 4 h. After that, the absorbance was
measured at a wavelength of 570 nm. Each group was assayed in
triplicate and each experiment was repeated three times.
Detection of phosphatidylserine
externalization
At the indicated time points after transfection, the
detached and adherent (trypsinized) cells were collected, washed
twice with PBS and resuspended in 200 μl Annexin V binding buffer
(10 mM HEPES pH 7.4, 140 mM NaCl, 1 mM MgCl2, 5 m MKCl,
2.5 mM CaCl2). FITC-conjugated Annexin V was added to a
final concentration of 0.5 μg/ml. After incubation for 30 min at
4°C in the dark, propidium iodide (PI) was added to samples at 1
μg/ml. Phosphatidylserine (PS) externalization analysis was
performed on a FACSCalibur flow cytometer (Becton-Dickinson,
USA).
Dual luciferase assay
Transient transfection of HEK293T cells was
performed using VigoFect (Vigorous) according to the manufacturer’s
instructions. The relative luciferase activity was determined with
a Dual-Luciferase Reporter Assay System (Promega) using a Veritas
Microplate Luminometer (USA) by measuring firefly luciferase
activity normalized by Renilla luciferase activity.
Statistical analysis
A Chi-square test was used to compare the expression
of CMTM1_v17 between normal and tumor tissues. A p-value
(two-sided) of 0.05 was considered to indicate a statistically
significant result. Statistical analysis was performed using
SigmaStat 2.03 software (SPSS, Inc.).
Results
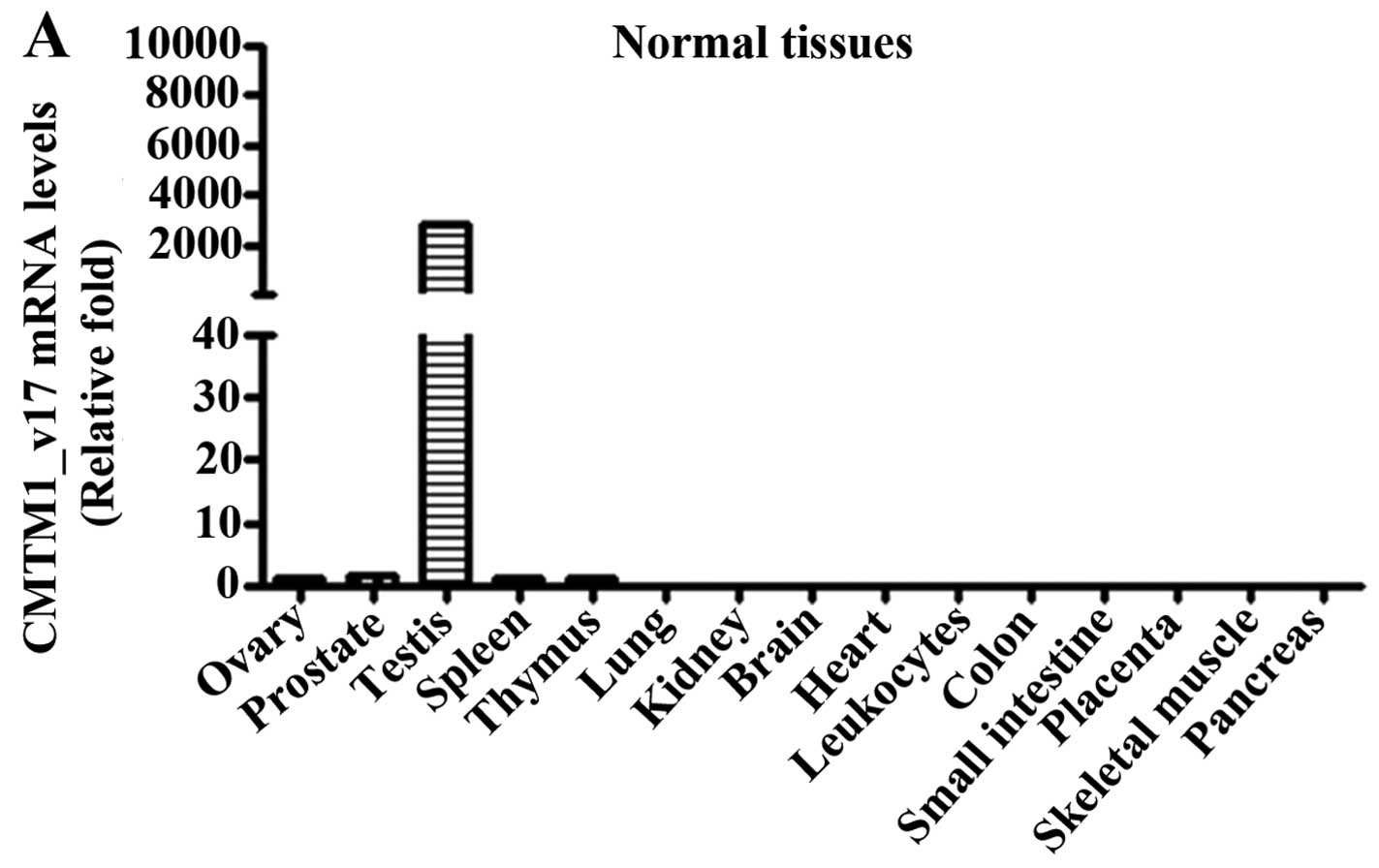
Expression pattern of CMTM1_v17 mRNA
The expression pattern of human CMTM1_v17
mRNA transcripts was determined by real-time qPCR. CMTM1_v17
mRNA was highly expressed in testicular tissue, but was expressed
at a low or undetectable level in other tissues (Fig. 1A). However, expression of
CMTM1_v17 mRNA was detected in tumor tissues from breast,
kidney, lung, liver and ovarian cancers, but not colon or rectal
cancers (Fig. 1B). We also found
that the expression level of CMTM1_v17 mRNA was high in K562
and U937 cells, moderate in MDA-MB-231 and Caov3, and relatively
low in other cells lines (Fig.
1C).
Subcellular localization of
CMTM1_v17
To investigate the subcellular localization of the
human CMTM1-v17 protein, we examined the localization of the
CMTM1_v17-EGFP construct using confocal fluorescence
microscopy. In the cells that overexpressed the control EGFP
vector, the fluorescence was distributed throughout the transfected
cells with no specific subcellular localization while in the cells
overexpressing CMTM1_v17-EGFP, moderate fluorescence was
detected only in the cytoplasm (Fig.
1D).
Expression of CMTM1_v17 is more prevalent
in breast tumor than in normal breast tissues
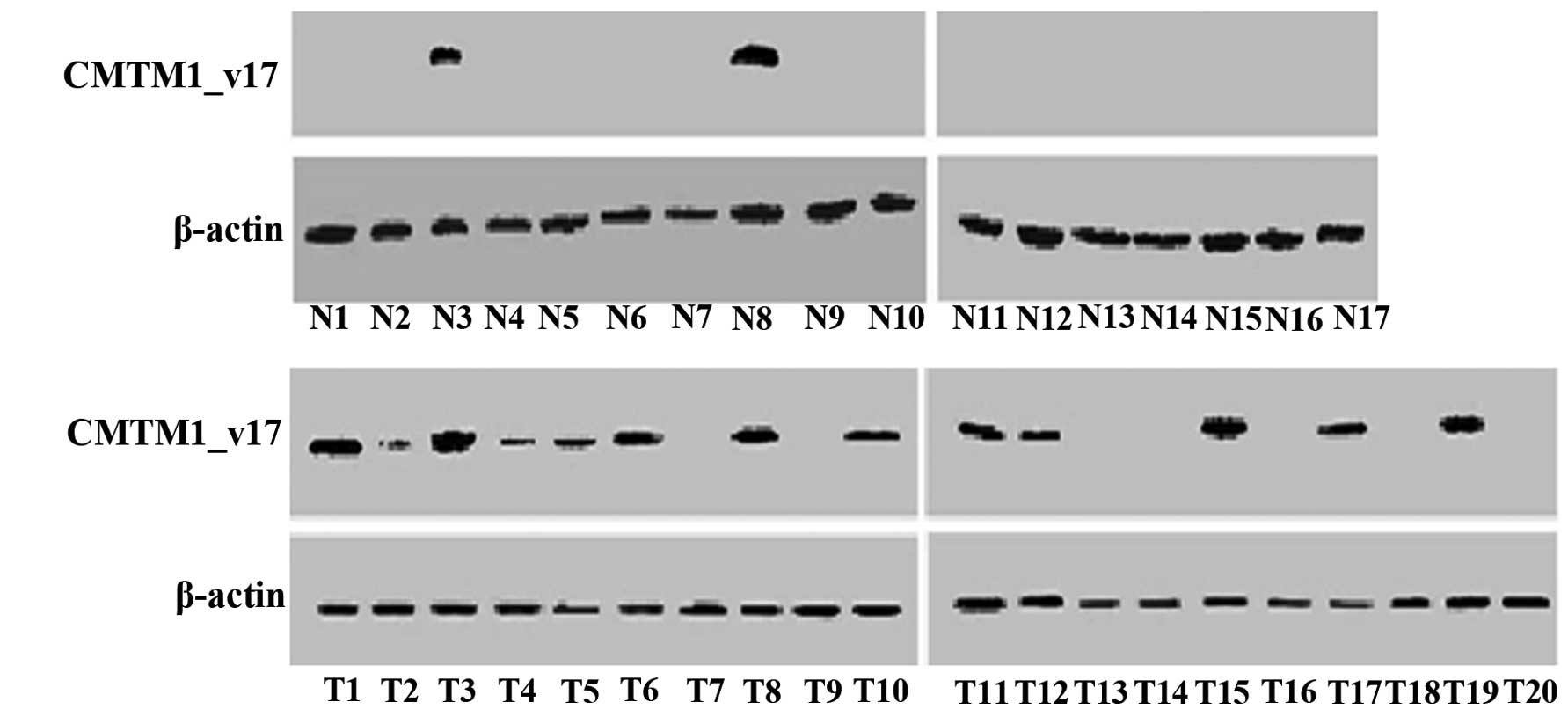
CMTM1_v17 protein was detected in both breast tumor
and adjacent non-cancerous mammary tissues by western blotting. In
these samples, CMTM1_v17 expression was more prevalent in
tumor than in normal breast tissues (Fig. 2). CMTM1_v17 was detected in
13/20 (65.00%) tumors compared to only 2/17 (11.76%) non-cancerous
breast tissues (Table I).
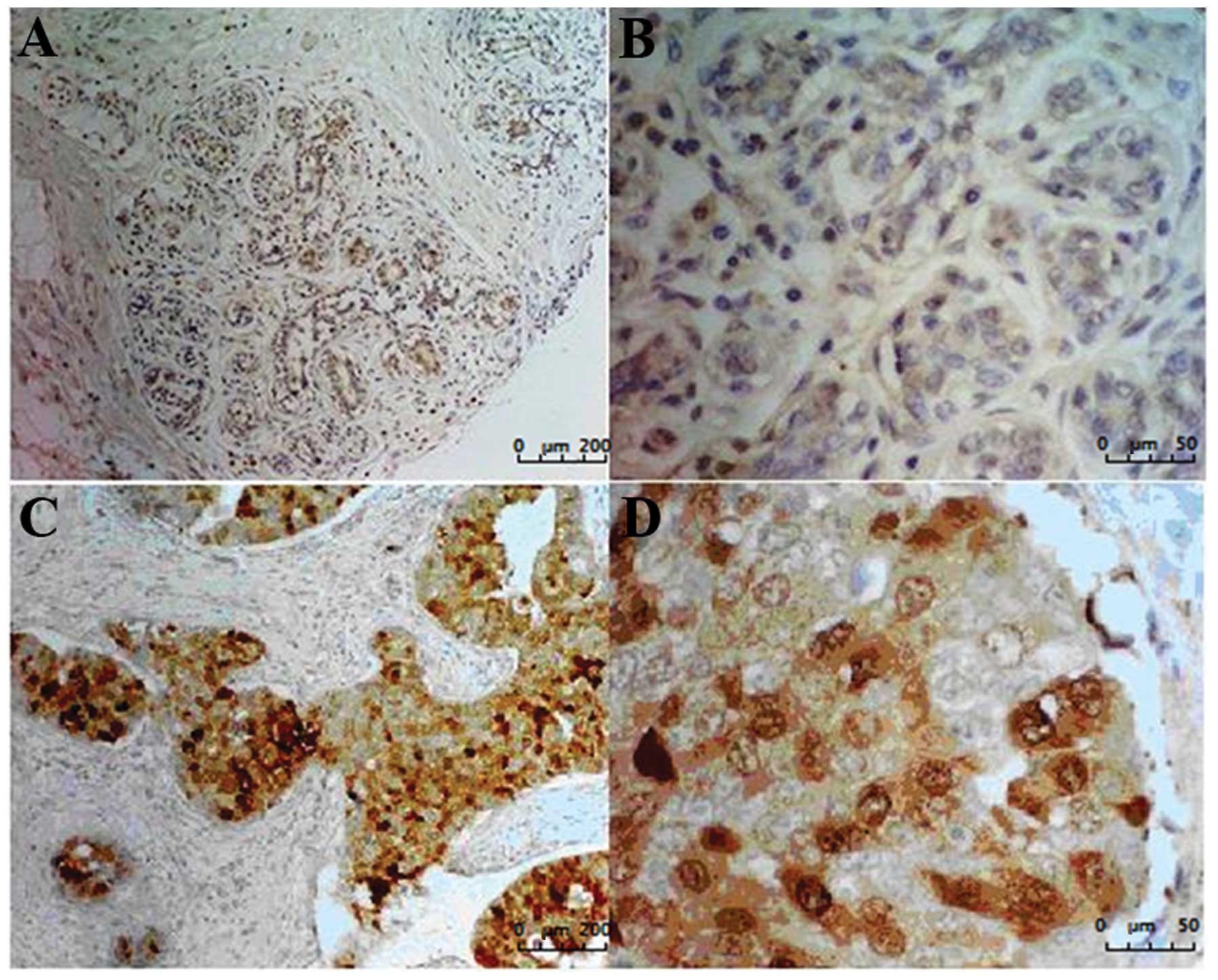
Immunohistochemistry was performed using commercially available
tissue microarrays. Results showed that only 23/127 (18.11%) normal
breast or non-cancerous tissues expressed CMTM1_v17 at a
high level. Most of the normal breast sections (82/100, 82.0%) and
non-cancerous breast tissues (22/27, 81.48%) showed only faint
staining. Of the 105 tumor samples, 71 (67.6%) showed moderate or
strong expression of CMTM1_v17 (Fig. 3 and Table IIA). CA15-3 is a cell surface
marker expressed by various tumor cells including breast cancer.
The tissue microarray comprised of tumor (n=105) and
normal/non-cancerous (n=127) tissues was subjected to
immunohistochemical staining with anti-CA15-3 antibody.
Approximately 67/105 (63.81%) tumor and 26/127 (20.47%)
normal/non-cancerous tissues expressed CA15-3 (Table IIB). Table III shows the correlation analysis
(McNemar’s test, p>0.05) between CMTM1_v17 and CA15-3 in
normal/non-cancerous samples (Table
IIIA) or in tumor samples (Table
IIIB). The co-staining of CMTM1_v17 and CA15-3 increased
the positive rate to 83.81%.
 | Table IWestern blot analysis of CMTM1_v17 in
breast cancer and non-cancerous mammary tissues. |
Table I
Western blot analysis of CMTM1_v17 in
breast cancer and non-cancerous mammary tissues.
| Negative n (%) | Positive n (%) | P-value |
|---|
| Non-cancerous
mammary tissues (n=17) | 15 (88.24) | 2 (11.76) | <0.01 |
| Breast cancer
tissues (n=20) | 7 (35.00) | 13 (65.00) | |
 | Table IIExpression of CMTM1_v17 and CA15-3 in
tissue microarray analysis. |
Table II
Expression of CMTM1_v17 and CA15-3 in
tissue microarray analysis.
| A, Expression of
CMTM1_v17 in tissue microarray analysis |
|---|
|
|---|
| Samples | Weak staining n
(%) | Moderate/strong
staining n (%) | P-value |
|---|
|
Normal/non-cancerous (n=127) | 104 (81.89) | 23 (18.11) | <0.01 |
| Tumor (n=105) | 34 (32.38) | 71 (67.62) | |
|
| B, Expression of
CA15-3 in tissue microarray analysis |
|
| Samples | Faint staining n
(%) | Moderate/strong
staining n (%) | P-value |
|
|
Normal/non-cancerous (n=127) | 101 (79.53) | 26 (20.47) | <0.01 |
| Tumor (n=105) | 38 (36.19) | 67 (63.81) | |
 | Table IIICorrelation analysis between
CMTM1_v17 and CA15-3 in normal/non-cancerous and tumor samples. |
Table III
Correlation analysis between
CMTM1_v17 and CA15-3 in normal/non-cancerous and tumor samples.
| A, Correlation
analysis between CMTM1_v17 and CA15-3 in normal/non-cancerous
samples (McNemar’s test, p>0.05). |
|---|
|
|---|
|
Normal/non-cancerous samples (n=127) | CA15-3 |
|---|
|
|---|
| Negative | Positive |
|---|
| CMTM1_v17 |
| Negative | 86 | 18 |
| Positive | 13 | 10 |
|
| B, Correlation
analysis between CMTM1_v17 and CA15-3 in tumor samples (McNemar’s
test, p>0.05). |
|
| Tumor samples
(n=105) | CA15-3 |
|
| Negative | Positive |
|
| CMTM1_v17 |
| Negative | 17 | 18 |
| Positive | 20 | 50 |
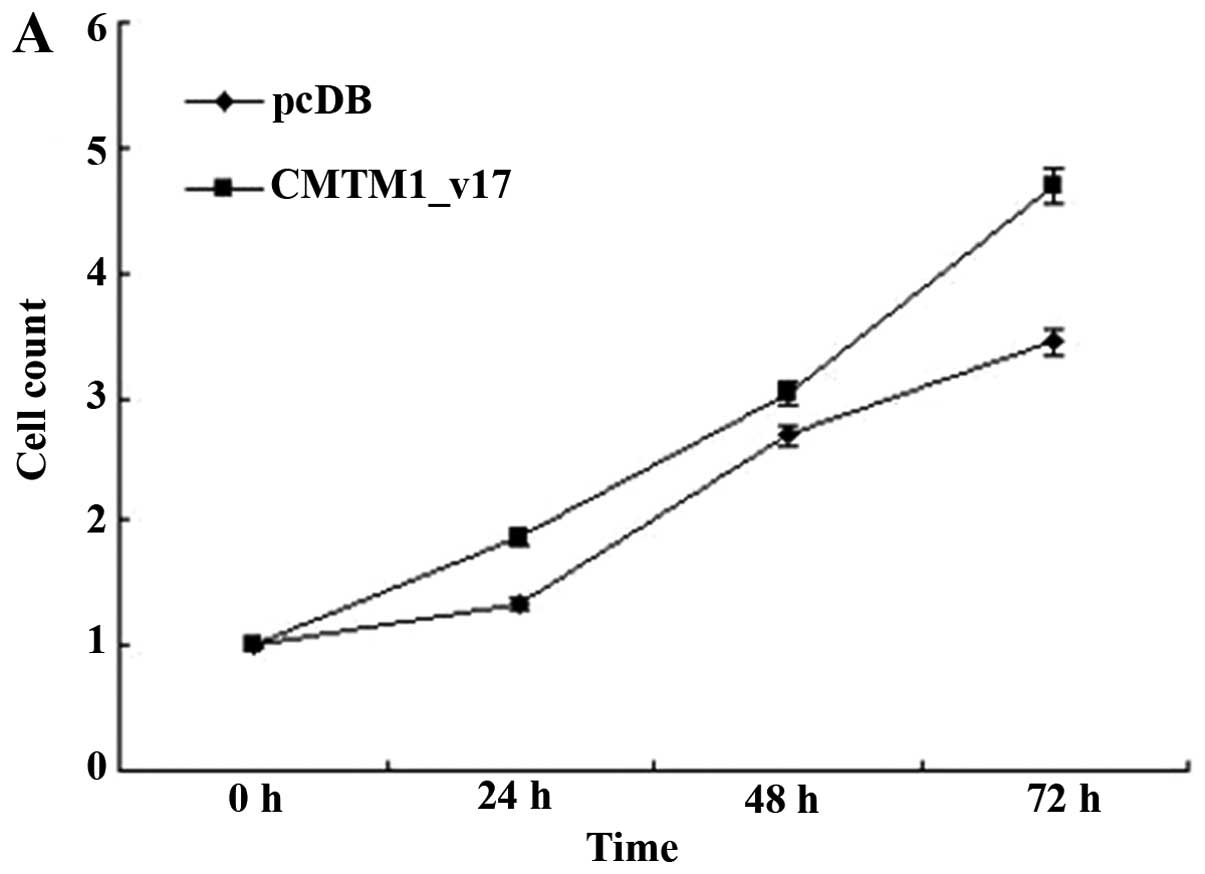
CMTM1_v17 promotes the proliferation of
MDA-MB-231 cells
The breast cancer cell line MDA-MB-231 was
transfected with CMTM1_v17 or control vector and then the
cells were assayed for proliferation. Results obtained from cell
counting and MTT analysis showed that CMTM1_v17 had an
obviously positive effect on the growth of MDA-MB-231 cells
(Fig. 4).
CMTM1_v17 increases the resistance of
MDA-MB-231 cells to TNF-α-induced apoptosis
Since CMTM1_v17 promoted proliferation of
MDA-MB-231 cells, we considered whether it also played a role in
cellular apoptosis. To investigate this possibility, MDA-MB-231
cells were transiently transfected with CMTM1_v17 or control
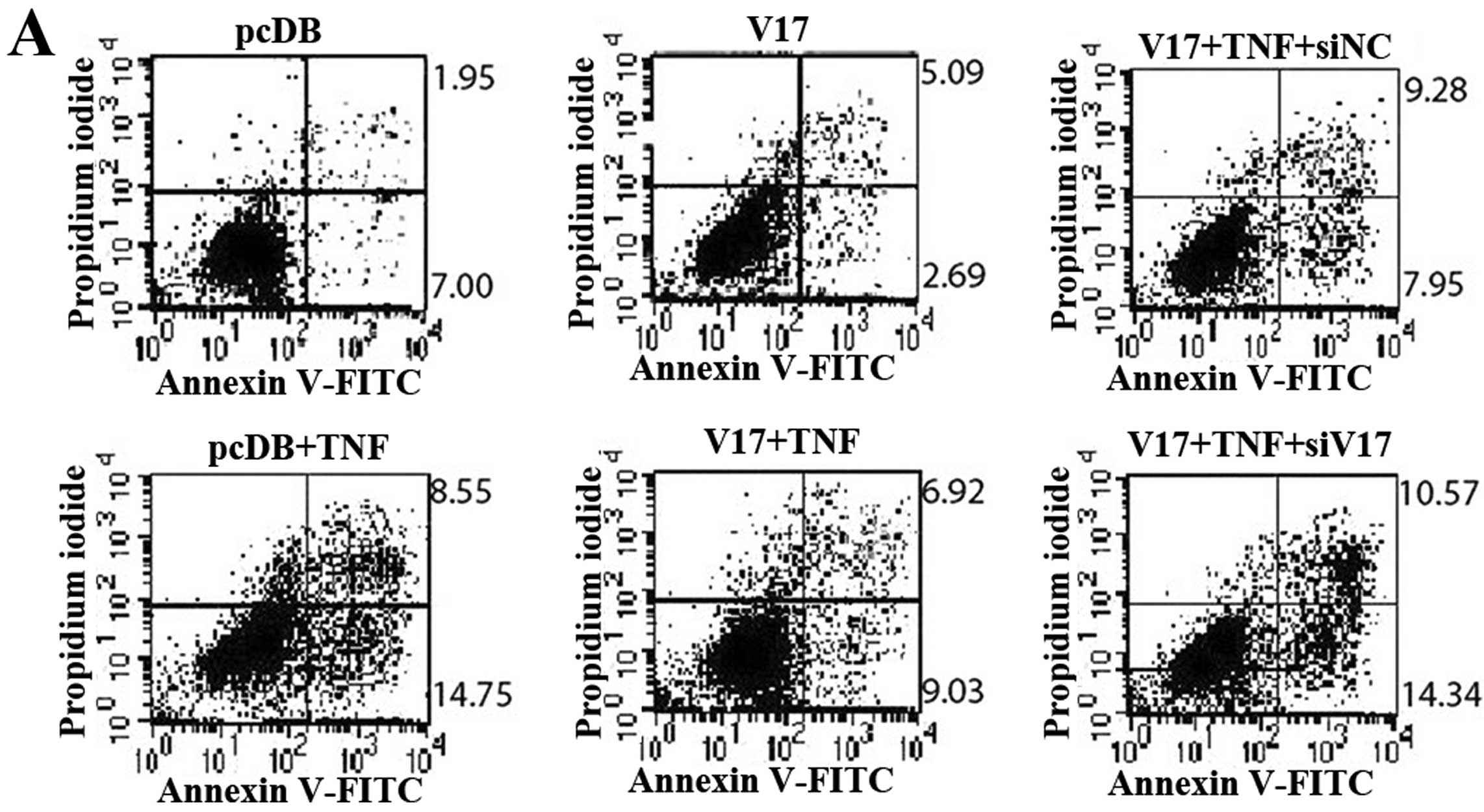
vector and stimulated with TNF-α. The percentage of Annexin
V-positive cells (15.95%) in CMTM1_v17 overexpressing cells
was much lower than that in cells transfected with the control
vector (23.30%) at 24 h (Fig. 5A).
The result was more marked at 48 h (Fig. 5B). Similar results were obtained
with human MCF-7 cells (data not shown).
Silencing of CMTM1_v17 expression
sensitizes MDA-MB-231 cells to TNF-α-induced apoptosis
We next investigated whether the silencing of
CMTM1_v17 expression sensitized MDA-MB-231 cells to
TNF-α-induced apoptosis. CMTM1-v17 expression was silenced
using targeted siRNA and non-silencing siRNA as a control. Annexin
V/PI staining was used to detect apoptotic cells after exposure to
TNF-α. We found that siRNA-mediated silencing of CMTM1-v17
restored the sensitivity of MDA-MB-231 cells to TNF-α-induced
apoptosis (Fig. 5). These data
further demonstrate that CMTM1-v17 promotes cellular
resistance to TNF-α-induced apoptosis.
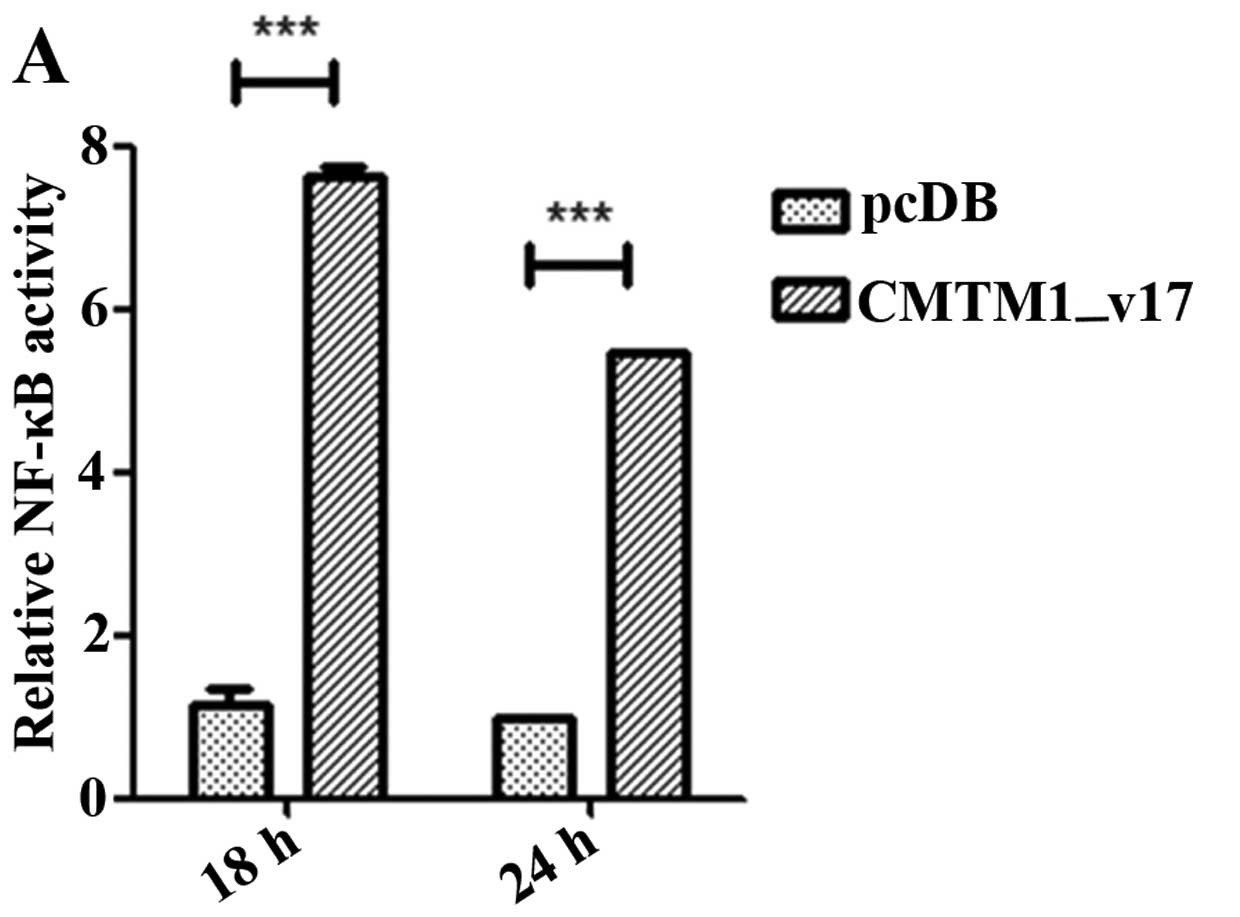
CMTM1_v17 may function via activation of
the NF-κB signaling pathway
To investigate the mechanisms underlying the
enhanced proliferation and resistance to TNF-α-induced apoptosis in
cells overexpressing CMTM1_v17, we performed luciferase
reporter assays in HEK293T cells for NF-κB activity by transiently
transfecting pcDB or CMTM1_v17 with NF-κB-luciferase and
control Renilla reporter plasmids. As is shown,
CMTM1_v17 significantly increased the transcriptional
activity of NF-κB in a time- (Fig.
6A) and dose- (Fig. 6B)
dependent manner. These data suggest that overexpression of
CMTM1_v17 plays a tonic effect on the activation of NF-κB
signaling. To provide further experimental evidence, we performed
western blot analysis to detect the key members of NF-κB signaling
pathway. In cells that overexpressed CMTM1_v17, we detected
increased IKK phosphorylation and decreased expression of total
IκBα. Total IKKβ and β-actin expression levels served as a loading
control (Fig. 6C). Taken together,
the data from luciferase assays and western blot analysis suggest
that activation of the NF-κB pathway by CMTM1_v17 was likely
mediated via phosphorylation of IKK and decreased levels of total
IκBα.
Discussion
In the present study, we found that CMTM1_v17
was specifically highly expressed in human testes, many human tumor
tissues and cell lines but was largely undetectable in the other
normal tissues tested. This was consistent with our previous study
(6), in which northern blot
analysis revealed that CMTM1 was highly expressed in
testicular tissue while it was hardly detectable in other normal
tissues. The distinctive expression profile of CMTM1_v17 in
human tissues, namely its high expression in multiple neoplastic
tissues and cell lines and its absence in normal tissues except for
the testes, suggests that the CMTM1_v17 may contribute to
tumorigenesis by increasing cellular proliferation.
In recent years, it has became evident that breast
cancer is one of the most common malignancies and a leading cause
of mortality among women. The sporadic (non-inherited) breast
cancer that constitutes >90% of all breast cancers is a complex
and heterogeneous disease at both the clinical and molecular
levels. Several genetic aberrations and changes in gene expression
have been shown to occur during malignant transformation,
development and progression of breast cancers (15). TNF-α can induce multiple mechanisms
to initiate apoptosis in many tumor cell lines and causes tumor
necrosis in certain animal models by binding its membrane receptor
TNF-R1 (16). Our findings showed
that, compared to normal breast tissues, the expression of
CMTM1_v17 was higher in breast tumors. Moreover, ectopic
expression of CMTM1_v17 in breast cancer cells promoted the
proliferation as well as the resistance to TNF-α-induced apoptosis
of breast cancer cell MDA-MB-231 while silenced CMTM1_v17
expression sensitized cells to TNF-α-induced apoptosis. We
hypothesized that the abnormal expression of CMTM1_v17 in
breast cancer might have clinical applications; in particular, it
might be a novel marker of diagnosis and a therapeutic target in
breast cancer.
It is well known that NF-κB induces a variety of
anti-apoptotic factors (17). To
provide further mechanism evidence, the dual-luciferase reporter
assay and western blot analysis were performed and the results
suggested that overexpression of CMTM1_v17 protein induced robust
NF-κB activation. Therefore, we demonstrated that CMTM1_v17 could
promote the proliferation and lead to partial resistance to TNF-α
induced apoptosis likely via activation of the NF-κB signaling
pathway.
In summary, we found that CMTM1_v17 was
highly expressed in a variety of tumors including breast cancer.
Overexpression of CMTM1_v17 in the cell line MDA-MB-231
promoted proliferation and enhanced the resistance to TNF-α-induced
apoptosis likely via activation of the NF-κB pathway. Moreover,
silencing of CMTM1_v17 restored the sensitivity of the cells
to TNF-α-induced apoptosis. These data indicate that
CMTM1_v17 is a novel potential therapeutic target in breast
cancer and may play an important role in its diagnosis.
CMTM1_v17 may also be a new cancer/testis antigen, as it is
also highly expressed in human testes while it is almost
undetectable in other normal human tissues. Further studies are
required to confirm the therapeutic efficacy of targeting
CMTM1_v17 in breast cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 91129707 and
81172001).
References
|
1
|
Han W, Lou Y, Tang J, et al: Molecular
cloning and characterization of chemokine-like factor 1 (CKLF1), a
novel human cytokine with unique structure and potential
chemotactic activity. Biochem J. 357:127–135. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han W, Ding P, Xu M, et al: Identification
of eight genes encoding chemokine-like factor superfamily members
1–8 (CKLFSF1–8) by in silico cloning and experimental
validation. Genomics. 81:609–617. 2003.PubMed/NCBI
|
|
3
|
Xia D, Li X, Lou Y, et al: Overexpression
of chemokine-like factor 2 promotes the proliferation and survival
of C2C12 skeletal muscle cells. Biochim Biophys Acta. 1591:163–173.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lou Y, Xia D, Han W, et al: Molecular
cloning and characterization of rat chemokine-like factor 1 and 2.
Gene. 307:125–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rui M, Xia D, Zhang Y, et al: Molecular
cloning and characterization of four isoforms of mCKLF, mouse
homologues of human chemokine-like factor. Mol Biol Rep.
30:229–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Wu C, Zheng Y, et al: Molecular
cloning and characterization of chemokine-like factor super family
member 1 (CKLFSF1), a novel human gene with at least 23
alternative splicing isoforms in testis tissue. Int J Biochem Cell
Biol. 36:1492–1501. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Meo S, Airoldi I, Sorrentino C, Zorzoli
A, Esposito S and Di Carlo E: Interleukin-30 expression in prostate
cancer and its draining lymph nodes correlates with advanced grade
and stage. Clin Cancer Res. 20:585–594. 2014.PubMed/NCBI
|
|
8
|
Shi S, Rui M, Han W, et al: CKLFSF2 is
highly expressed in testis and can be secreted into the
seminiferous tubules. Int J Biochem Cell Biol. 37:1633–1640. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin C, Ding P, Wang Y and Ma D: Regulation
of EGF receptor signaling by the MARVEL domain-containing protein
CKLFSF8. FEBS Lett. 579:6375–6382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong J, Wang Y, Qiu X, et al:
Characterization and expression profile of CMTM3/CKLFSF3. J Biochem
Mol Biol. 39:537–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plate M, Li T, Wang Y, et al:
Identification and characterization of CMTM4, a novel gene with
inhibitory effects on HeLa cell growth through inducing G2/M phase
accumulation. Mol Cells. 29:355–361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao L, Cui Y, Li H, et al: CMTM5
exhibits tumor suppressor activities and is frequently silenced by
methylation in carcinoma cell lines. Clin Cancer Res. 13:5756–5762.
2007. View Article : Google Scholar
|
|
13
|
Wang Y, Li J, Cui Y, et al: CMTM3,
located at the critical tumor suppressor locus 16q22.1, is silenced
by CpG methylation in carcinomas and inhibits tumor cell growth
through inducing apoptosis. Cancer Res. 69:5194–5201. 2009.
View Article : Google Scholar
|
|
14
|
Li H, Li J, Su Y, et al: A novel 3p22.3
gene CMTM7 represses oncogenic EGFR signaling and inhibits cancer
cell growth. Oncogene. 33:3109–3118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JC, Voisin V, Bader GD, et al:
Seventeen-gene signature from enriched Her2/Neu mammary
tumor-initiating cells predicts clinical outcome for human
HER2+:ERα− breast cancer. Proc Natl Acad Sci
USA. 109:5832–5837. 2012. View Article : Google Scholar
|
|
16
|
Locksley RM, Killeen N and Lenardo MJ: The
TNF and TNF receptor superfamilies: integrating mammalian biology.
Cell. 104:487–501. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baldwin AS: Regulation of cell death and
autophagy by IKK and NF-κB: critical mechanisms in immune function
and cancer. Immunol Rev. 246:327–345. 2012.
|