Introduction
The incidence of biliary tract carcinoma has
considerable geographic variations. High standardized mortality
ratios of biliary tract carcinomas are found in cancer registries
for Asian countries such as Japan and Thailand and for South
American countries such as Chile, Peru and Colombia (1). Japan has one of the world’s highest
age-adjusted cancer death rates related to biliary tract
carcinomas, and the rate appears to be steadily increasing,
particularly for women (2).
Nearly two thirds of biliary tract carcinomas arise
in the gallbladder, making it the most common biliary tract
carcinoma. Gallbladder carcinoma has always been associated with a
dismal overall prognosis (3,4). This
is essentially attributed to slow and asymptomatic growth of
gallbladder carcinoma infiltrating surrounding structures, and the
disease is therefore usually detected at an advanced stage with a
high frequency of distant organ metastasis. Details of
tumorigenesis as well as growth and progression of the disease are
complex and not completely understood. Certain predisposing factors
such as dietary habits (5), chronic
cholecystitis (6) and the presence
of an anomalous pancreaticobiliary junction (7) have been reported to be linked to the
disease. Overexpression of erbB2, an erbB2 receptor tyrosine kinase
family member, has been reported in a significant percentage of
gallbladder carcinomas (8,9).
BK5.erbB2 transgenic mice (BK5.erbB2 mice) that
overexpress wild-type rat erbB2 under the control of the bovine
keratin 5 (BK5) promoter have been generated (10) and found to develop adenocarcinoma of
the gallbladder with high incidence (10). Alterations in erbB2 signaling have
been implicated in neoplastic transformation in vitro
(11) and in vivo (12,13),
and this model provides evidence that erbB2 signaling plays a role
in gallbladder carcinogenesis.
Several studies have shown the involvement of
membrane mucins, including Muc4, in cell signaling (14–16).
Muc4 is itself a heterodimeric glycoprotein composed of a mucin
subunit, ascites sialoglycoprotein (ASGP1), and a transmembrane
subunit, ASGP2. ASGP2 contains two epidermal growth factor (EGF)
domains with conserved amino acid residues of active EGF-like
growth factors, one of which reportedly acts as a ligand for erbB2
(16). Thus, Muc4 potentially acts
as a novel transmembrane ligand for the tyrosine kinase erbB2,
triggering specific phosphorylation of erbB2 (17). The expression level of Muc4 has
previously been reported to be markedly upregulated in human
gallbladder carcinomas (18,19)
and cholangiocarcinomas (20).
In the present study, we investigated the validity
of the hypothesis that upregulation of Muc4 and its interaction
with erbB2 in BK5.erbB2 mice are involved in the process of
gallbladder carcinogenesis through modulation of phosphorylation of
the receptor tyrosine kinase and subsequent erbB2 signaling for
cell growth promotion.
Materials and methods
Generation and identification of
transgenic mice
BK5.erbB2 mice were generated in the University of
Texas, MD Anderson Cancer Center, Science Park Research Division
(Smithville, TX, USA) as previously described (10). Transgenic animals were identified by
PCR of DNA isolated from the tails of weanlings using
oligonucleotides specific for the rabbit β-globulin cDNA as
previously described (12). The
organ specimens of BK5.erbB2 mice (gallbladder, trachea, esophagus
and forestomach) were harvested in the University of Texas, MD
Anderson Cancer Center, Science Park Research Division and supplied
to the laboratories of Cancer Biology and Molecular Immunology,
Graduate School of Pharmaceutical Sciences, University of Tokyo
(Tokyo, Japan).
Real-time quantitative polymerase chain
reaction
Steady-state mRNA levels were determined by
real-time quantitative PCR using a GeneAmp 5700 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA). Primers and
probes for mouse Muc4 (ASGP2) were designed using
Primer Express (Applied Biosystems). In each experiment, PCR was
carried out in triplicate. The PCR data were expressed relative to
the amount of rRNA present in each specimen and then
averaged. The primers and probes were designed as follows: mouse
Muc4 forward, 5′-GATGAGACAGAGTACCATGCAGATG-3′ and reverse,
5′-GAACCGGCGTCTGAGAATAGA-3′; probe
FAM5′-AACATCCCCAGAAGCGTGTACCCTGG-3′TAM.
In situ hybridization
Gallbladder tissues were immediately frozen in
liquid nitrogen and stored at −80°C. Frozen sections of 8 μm
thickness were cut on a cryostat and thaw-mounted onto MAS-coated
slides. The expression and localization of Muc4
(ASGP2) mRNA on the tissue sections were analyzed as
previously described (21). The
hybridization reaction was carried out at 52°C overnight. To detect
Muc4 gene signals, the probe was synthesized using a Roche DIG RNA
Labeling kit to utilize the following sequence:
AAACCTCAAACCACCACAACCACCGAGGTGACCACATCAACTCCTTCAGCCTCCTCACGTGACCAAATACAGACAGAGACAAGTTCTCAAAGAACAATCTCTCCTGATGGAACAACCACCTCACATGCTCCCAGTATCAGCAGCTCAGCTCCAAGTACAACACACATGTTAACCACAACATCCTCCACAGAAAGTACCTCAGTAGACTCAGGACACACAACAGCAATAACAACTCAAGGTTTAACACCTGCCACCGCACAAGTCTCACTGACACCTTCATCCCAGAATATGTCAACAGTGTCAACACCCATCACCTCAACTCTTACTCAGAGACAACACACTGGAAGCAAGCAGACCAGCAGCA
(GeneBank AF520422).
Immunoblot analysis
Whole tissue lysates and immunoprecipitates, which
were prepared as previously described (12), were electrophoresed through 7–10%
SDS/polyacrylamide gels and transferred to polyvinylidene
difluoride membranes. After blocking with 1% non-fat powdered milk
in PBST [0.05% Tween in phosphate-buffered saline (PBS)], the
protein levels of Muc4 (ASGP2), erbB2, phosphorylated (p)-erbB2,
MAPK, p-MAPK, Akt, p-Akt and Cox-2 were detected by incubating the
membrane with the corresponding anti-Muc4 (ASGP2) antibody (Ab)
(1:1,000; Zymed Laboratories, Inc. South San Francisco, CA, USA),
anti-erbB Abs (1:1,000), anti-MAPK Abs (1:1,000), anti-Akt Abs
(1:1,000) (all from Cell Signaling Technology, Beverly, MA, USA),
anti-cyclooxygenase-2 (Cox-2) (1:1,000) or anti-microsomal
prostaglandin E synthase-1 (mPGES-1) (1:500) (both from Cayman
Chemical Company, Ann Arbor, MI, USA). Protein bands were
visualized as previously described (12).
Immunohistochemical stainings
Immunohistochemistry was performed using
formalin-fixed, paraffin-embedded tissue sections. Tissue sections
were blocked with normal donkey serum and a Mouse-to-Mouse
Detection system (Chemicon International, Temecula, CA, USA) and
incubated with anti-Muc4 Ab overnight at 4°C. After three washes
with PBS, the tissue sections were incubated with the secondary
FITC-conjugated, affinity-purified F (ab′)2 fragment of anti-mouse
IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA,
USA). The tissue sections were analyzed using a FluoroView Laser
Confocal microscope (Olympus America, Melville, NY, USA). The
localization of erbB2 and p-erbB2 was determined with their Abs
(Cell Signaling Technology) by the same method as that used for the
detection of Muc4.
Assay of tissue concentration of
prostaglandin E2
Frozen tissues were homogenized in an ice-cold
buffer (pH 8.4) and then stored at −20°C. Aliquots were assayed by
a highly specific radioimmunoassay (anti-PGE2 antibody;
Amersham, London, UK) for PGE2 in duplicate and at two
dilutions. The final results are expressed as ng PGE2/mg
protein.
Statistical analysis
Values are given as means ± SE (standard error).
Means of two groups were compared with the Mann-Whitney rank sum U
test (two-tailed test), and multiple comparisons were performed by
ANOVA. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Expression status of Muc4 and ErbB2 in
the gallbladder and other organs of BK5.ErbB2 transgenic mice
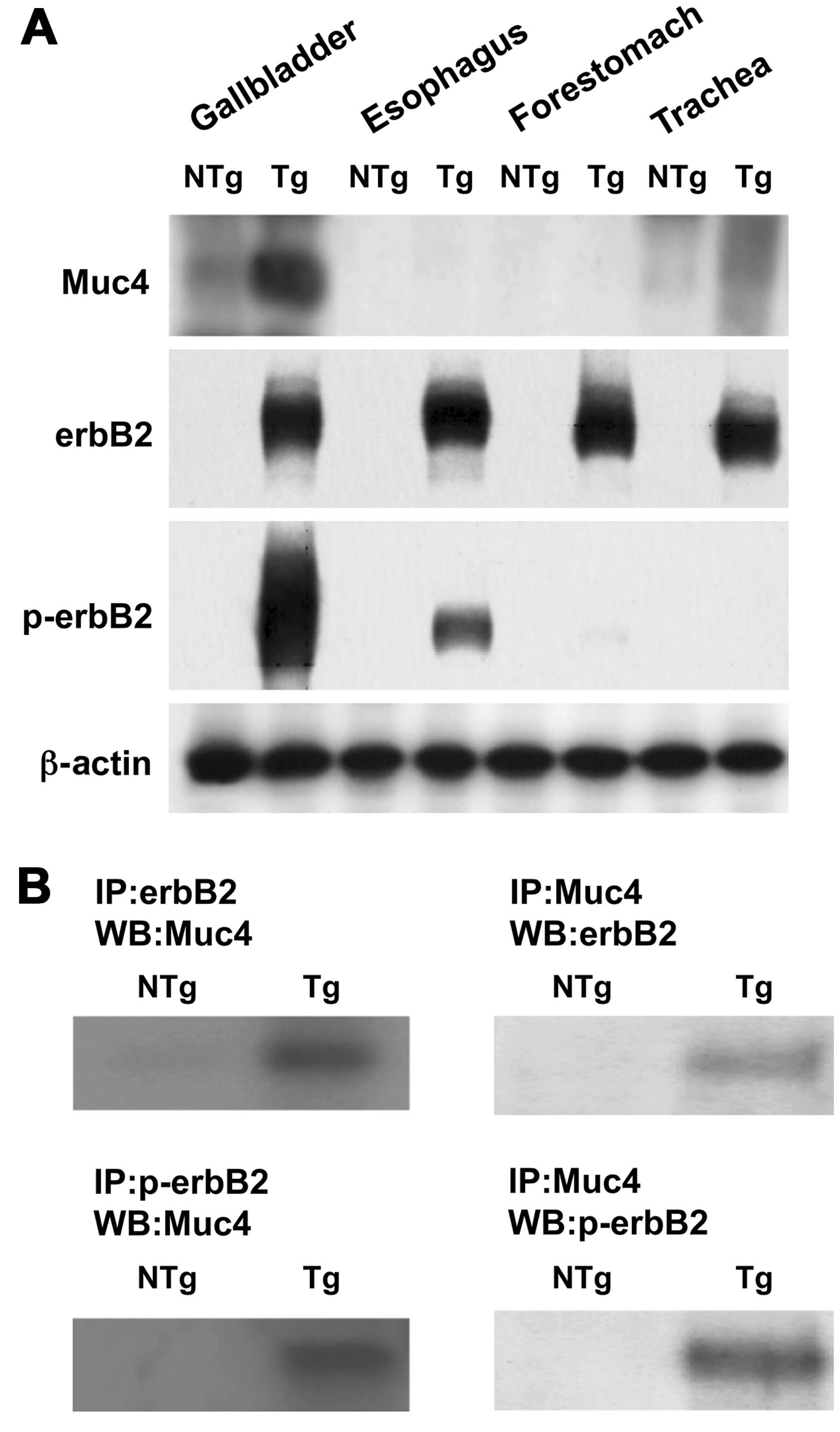
Immunoblot analysis of the gallbladder and other
organ tissues showed that, in BK5.erbB2 mice, Muc4 (ASGP2) protein
was overexpressed in the gallbladder, whereas little or no Muc4
protein was found in the trachea, esophagus and forestomach, in
each of which erbB2 is overexpressed (Fig. 1A). As expected, the expression
levels of erbB2 protein were significantly increased in the
gallbladder, trachea, esophagus and forestomach of BK5.erbB2 mice
compared to the levels in NTg mice, whereas hyperphosphorylation of
erbB2 was found only in the gallbladders (Fig. 1A).
Analysis of immunoprecipitation of gallbladder
lysate with erbB2 followed by immunoblot with Muc4 revealed that
coimmunoprecipitation of Muc4 with erbB2 as well as Muc4 with
p-erbB2 was significantly increased (>30-fold) in gallbladder
carcinoma from BK5.erbB2 mice compared to gallbladders from NTg
mice (Fig. 1B). These results were
confirmed by analysis of immunoprecipitation with Muc4 followed by
immunoblot with erbB2 (Fig.
1B).
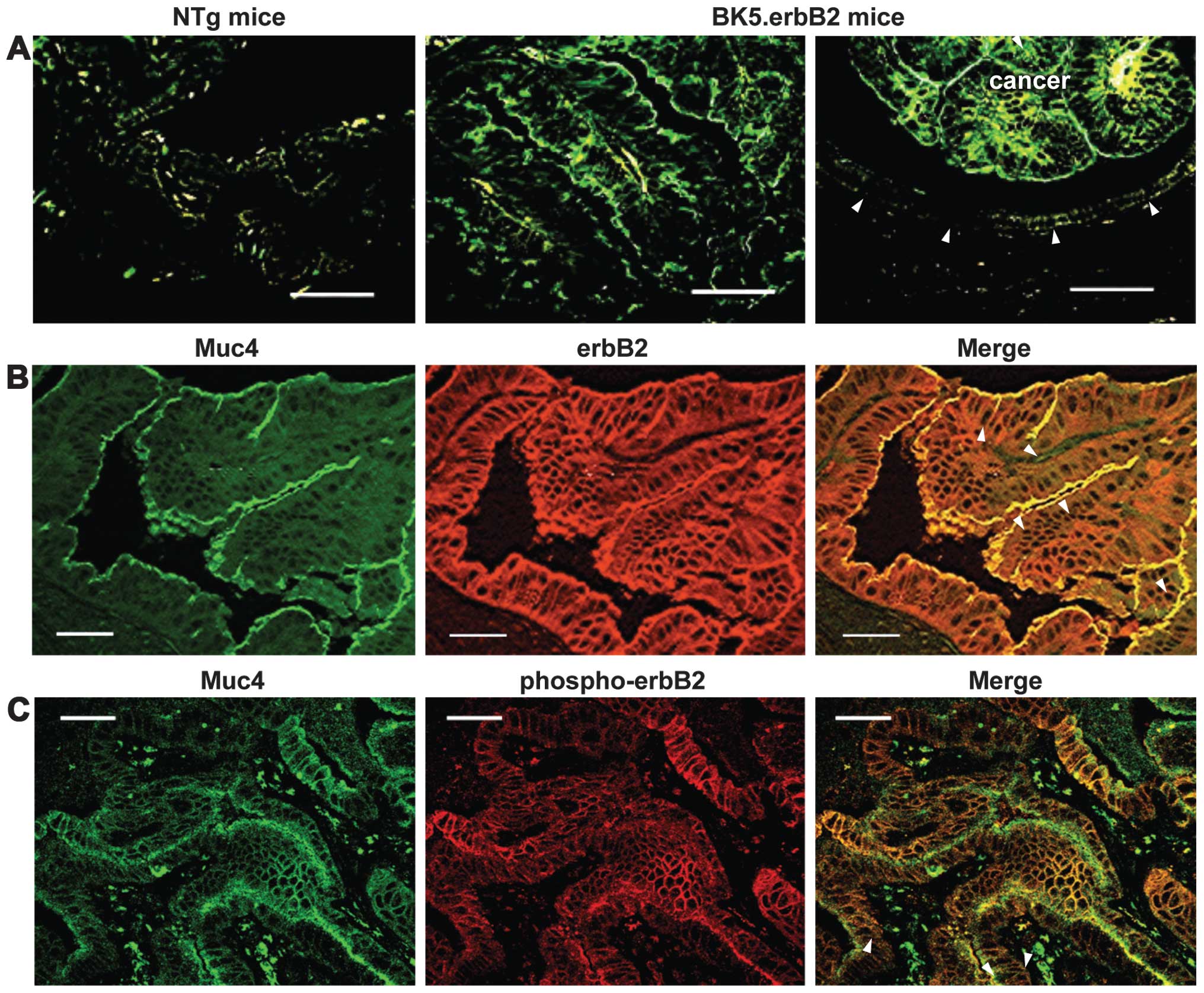
Localizations of Muc4 and ErbB2 in the
gallbladder of BK5.ErbB2 transgenic mice
The localizations of Muc4 (ASGP2) and erbB2 in the
gallbladder of 8 NTg mice and those of 10 BK5.erbB2 mice were
determined by indirect immunofluorescence staining. Strong
immunostaining with anti-Muc4 (ASGP2) Ab was observed in the
cancerous epithelia as being restricted predominantly to the apical
membranous components of BK5.erbB2 mice (Fig. 2A). A modest degree of the
immunostaining was also observed in the hyperplastic epithelia as
being a precancerous lesion (22)
(data not shown). However, no or only trace immunostaining was
observed in the epithelia of NTg mice (Fig. 2A). Table
I summarizes the results of immunohistochemistry of Muc4.
 | Table IImmunohistochemical expression of
erbB2, phosphorylated erbB2 and Muc4 in gallbladders of
non-transgenic and BK5.erbB2 Tg mice. |
Table I
Immunohistochemical expression of
erbB2, phosphorylated erbB2 and Muc4 in gallbladders of
non-transgenic and BK5.erbB2 Tg mice.
| | BK5.erbB2 mice |
|---|
| |
|
|---|
| NTg mice (n=8) | Hyperplasia
(n=8) | Carcinoma
(n=10) |
|---|
| Gallbladders | n (%) | n (%) | n (%) |
|---|
| erbB2 | 4 (50) | 8 (100) | 10 (100) |
| p-erbB2 | 0 (0) | 2 (20) | 10 (100)a |
| Muc4 | 0 (0) | 8 (100)a | 10 (100)a |
Immunostaining with anti-erbB2 Ab showed that a
strong expression of erbB2 was observed in both the apical and
basolateral membranous components of the cancerous epithelia of
BK5.erbB2 mice (Fig. 2B). A modest
degree of the immunostaining was also observed in the hyperplastic
epithelia (data not shown). However, no or only a slight degree of
the immunostaining was observed in the epithelia of NTg mice (data
not shown). For the expression status of p-erbB2, the strong
immunostaining with anti-p-erbB2 Ab was observed in the cancerous
epithelia of BK5.erbB2 mice and a modest degree of the
immunostaining was also observed in the hyperplastic epithelia of 2
BK5.erbB2 mice (data not shown). However, the immunostaining was
not observed in any epithelia of NTg mice (data not shown).
Table I summarizes the results of
immunohistochemistry of erbB2 and p-erbB2.
Of note, the immunostaining with the Muc4 Ab
overlapped (yellow, indicated by arrows) with those with the erbB2
Ab and with the p-erbB2 Ab in the apical membranous components of
the cancerous epithelia, indicating the co-localization of Muc4 and
erbB2/p-erbB2 (Fig. 2B and C).
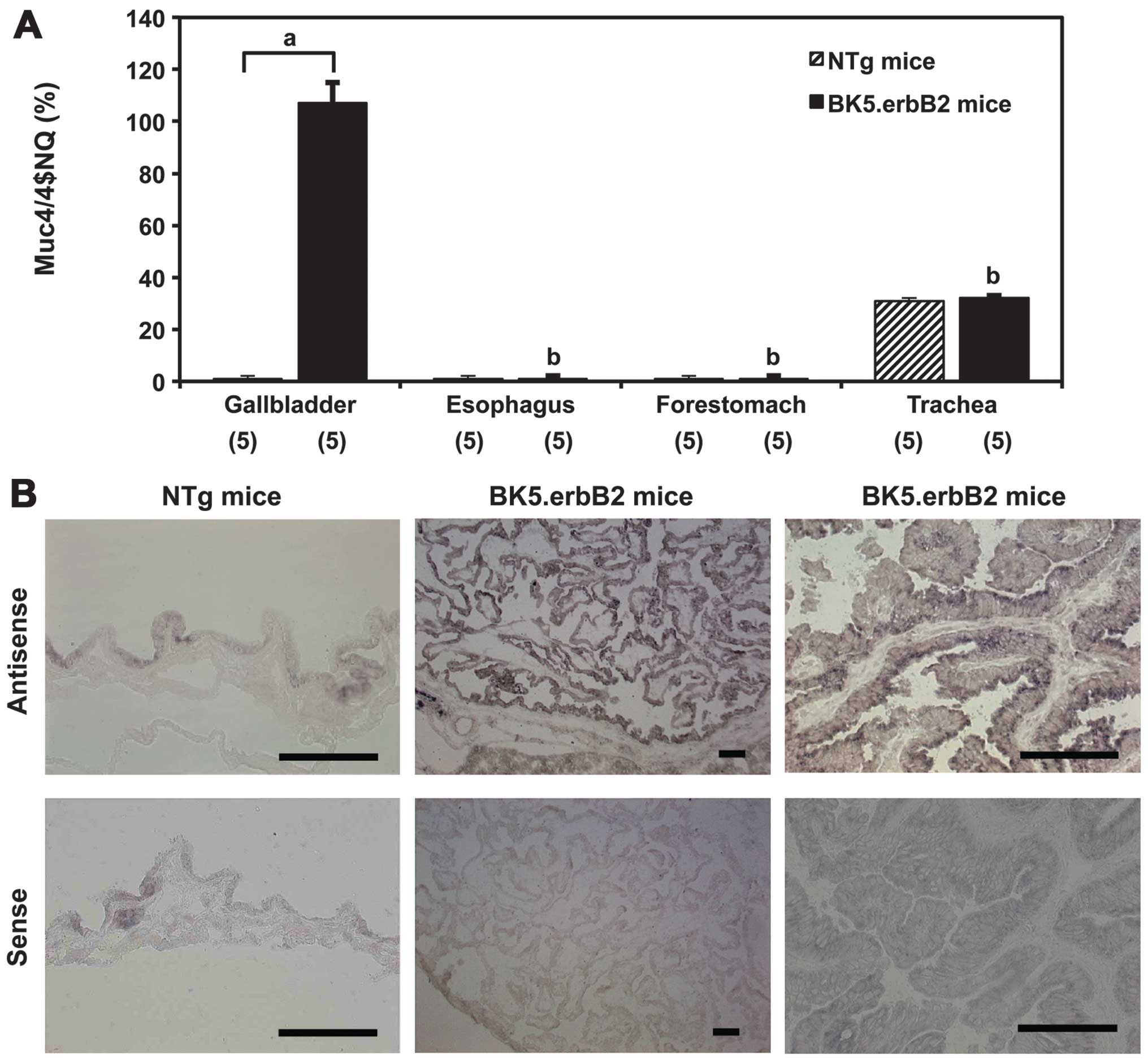
Gene expression levels of Muc4 in the
gallbladder and other organs of BK5.ErbB2 transgenic mice
The steady-state mRNA level of Muc4 (ASGP2) was
significantly higher in the specimens of gallbladders of BK5.erbB2
mice (108±7% of rRNA mRNA, means ± SE; P<0.01) than in
those of NTg mice (2±0.1%) (Fig.
3A). Analysis of in situ hybridization showed that Muc4
mRNA was expressed homogeneously in the epithelia but not in the
stroma of specimens of gallbladder carcinoma tissues (Fig. 3B). Muc4 mRNA levels were
significantly elevated in cancerous lesions of the gallbladder
compared to non-cancerous lesions of the gallbladder from BK5.erbB2
mice and normal epithelia of gallbladder from NTg mice. The mRNA
levels were not increased in the specimens of other organs, such as
the trachea, esophagus and forestomach, in each of which erbB2 is
overexpressed (data not shown).
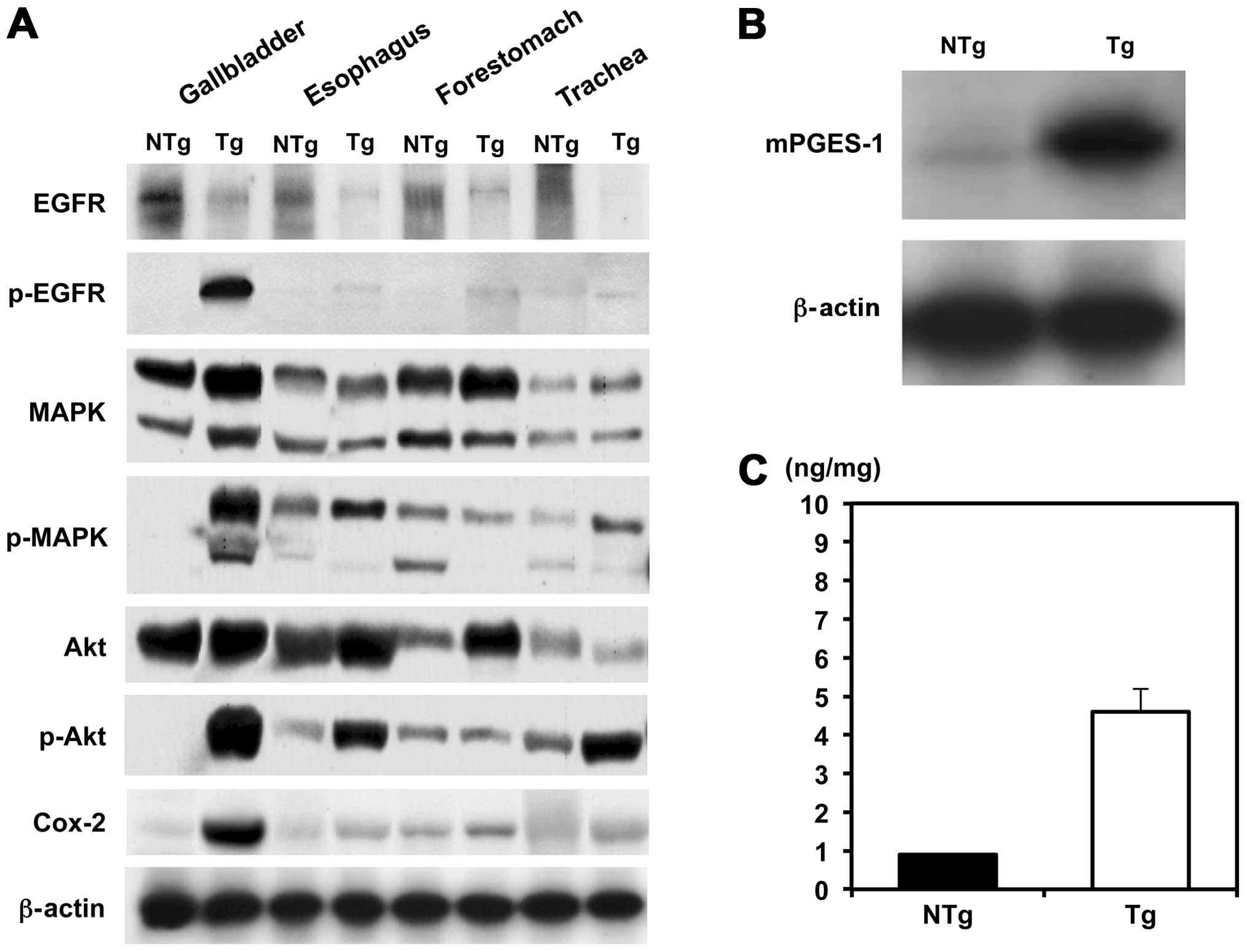
Expression status of MAPK, Akt and Cox-2
in the gallbladder and other organs of BK5.ErbB2 transgenic
mice
ErbB2 regulates Cox-2 expression via the Akt and
MAPK signaling pathways in human cancer cells (22–24).
To clarify the mechanistic basis of gallbladder carcinogenesis in
BK5.erbB2 mice, relative expression at protein levels and their
phosphorylation of erbB2-associated molecules, such as MAPK, Akt
and Cox-2, were investigated (Fig.
4A). In terms of erbB2-downstream molecules, phosphorylation
levels of MAPK and Akt were significantly elevated in the
gallbladder of BK5.erbB2 mice. Moreover, in parallel to the
activation of these molecules, Cox-2 protein levels were
significantly elevated in the gallbladder of BK5.erbB2 mice, and
this change was associated with the overexpression of mPGES-1
(Fig. 4B) and with the
overproduction of PGE2 in the gallbladder (Fig. 4C).
Discussion
Accumulating evidence suggests that constitutive
expression or activation of erbB2 may be involved in the
development of human biliary tract carcinomas, such as gallbladder
carcinoma (8,9) and cholangiocarcinoma (20,26,27).
This has been experimentally confirmed by the fact that
constitutive overexpression of erbB2 in gallbladder epithelia of
mice leads to a high incidence of adenocarcinoma (10) as well as by the results of our
previous study showing that the development of gallbladder
carcinoma is inhibited by treatment with selective EGFR/erbB2
tyrosine kinase inhibitors (28).
ErbB2 has been shown to lack a specific ligand that
affects erbB2 signaling through the formation of heterodimers with
other erbB2 family members (29,30). A
series of studies described by Carraway et al (16,17)
has implicated the involvement of Muc4 in cellular signaling. Muc4
has been shown to activate erbB2 through direct interaction with
the receptor tyrosine kinase and to potentiate tumor growth in
mammalian carcinoma cells (31,32),
and overexpression of Muc4 in the mouse mammary gland results in
hyperplasia in the developing gland (16). Also, in human digestive organs, it
should be noted that MUC4 is strongly expressed in adenocarcinomas
of the gallbladder (18) and
pancreas (33), but not in the
normal gallbladder and pancreas (33). Therefore, in the present study, we
investigated the hypothesis that upregulation of Muc4 and its
interaction with erbB2 are involved in the process of gallbladder
carcinogenesis in BK5.erbB2 mice.
In BK5.erbB2 mice, expression levels of Muc4 (ASGP2)
mRNA and protein were upregulated, to a large extent, in the
gallbladder carcinoma tissues, compared to the levels in
gallbladders of NTg mice (Figs. 1
and 3). In immunohistochemistry, a
modest degree of Muc4 protein was observed in the hyperplastic
epithelia as being a precancerous lesion (data not shown). Little
or no Muc4 protein was detected in the epithelia of the trachea,
forestomach and esophagus of BK5.erbB2 mice, in which erbB2 is
overexpressed. Hyperphosphorylated erbB2 was found in the
gallbladder carcinoma tissues, but not in the other organs
(Fig. 1). The results of
immunoprecipitation experiments (Fig.
1) and immunofluorescent double stainings (Fig. 2) revealed a direct interaction
between Muc4 and hyperphosphorylated erbB2 in the gallbladder and
their co-localization in the cancerous epithelia. Collectively, it
is likely that the expression levels of gallbladder Muc4 are
increased in connection with the phosphorylation status of the
erbB2 in the process of carcinogenesis, and that this molecular
relationship is further enhanced in the process of cancer growth,
leading to the strong expression levels of Muc4 coupled with
p-erbB2. It is also likely that an interaction between Muc4 and
phosphorylated erbB2 enhances erbB2-downstream signaling pathways
important for the carcinogenesis. Further studies are required to
elucidate the biological roles of Muc4 in carcinogenesis and/or
carcinoma progression.
Upon examination of downstream signaling pathways,
hyperphosphorylation of MAPK and Akt was observed in the
gallbladder carcinoma tissues, suggesting that MAPK and/or PI3K
signaling pathways may play a role in producing the gallbladder
phenotype in BK5.erbB2 mice. Supporting the results obtained for
BK5.erbB2 mice, in rat cholangiocyte transformants overexpressing
activated erbB2/neu (34), an
enhanced downstream signaling was observed to be p44/42 MAPK and
p60 Akt. Furthermore, a selective inhibitor of erbB2 tyrosine
kinase exerts a potent antitumor activity through suppressing the
activation of Akt, an anti-apoptotic molecule, in erbB2-positive
breast carcinoma cells, but not in erbB2-negative cells (35). The results of these in vitro
experiments (34,35) and those of the present in
vivo experiments suggest that MAPK and Akt are erbB2-downstream
molecules important for gallbladder carcinogenesis, during which
the presence of Muc4 potentiates the heregulin effects on
phosphorylation of erbB2, MAPK and Akt (14), and further enhances the tumor
growth.
Also on downstream signaling pathways, in
association with the overexpression of activated erbB2, significant
upregulation of both Cox-2 and mPGES-1, a stimulus-inducible enzyme
functioning downstream of Cox-2 in the PGE2-biosynthetic
pathway (36), was observed in the
gallbladder carcinomas of BK5.erbB2 mice (Fig. 4). A strong degree of Cox-2
expression was observed in the hyperplastic epithelia as being a
precancerous lesion (data not shown). Activated erbB2 regulates
Cox-2 expression via the Akt and MAPK signaling pathways in human
cancer cells (23–25). It is likely that the expression
levels of not only Muc4 but also Cox-2 are increased in connection
with the phosphorylation status of the erbB2 in the gallbladder.
The significant overproduction of Cox-2-derived PGE2
(Fig. 4) further emphasizes the
functional relationship between erbB2 activation and Cox-2/mPGES-1
induction in the gallbladder and likely plays an important role in
the carcinogenesis process. The association of Cox-2 with erbB2/neu
has also been considered important for rodent models of
cholangiocarcinogenesis (37,38).
In rat cholangiocyte transformants overexpressing activated
erbB2/neu (34), erbB2/neu
overexpression coupled to Cox-2 upregulation and increased
PGE2 production may act in a complementary manner to
regulate telomerase expression. Collectively, overexpression of
activated erbB2 coupled to Muc4, Cox-2 and mPGES-1 may contribute
to tumorigenesis.
A comparison was made between gallbladder carcinoma
of BK5.erbB2 mice and that of human subjects. It has been proposed
that there are two primary morphological pathways for the
development of human gallbladder carcinoma; one involves
adenoma-carcinoma development and the other involves de novo
development (39). Kawamoto et
al showed that gallbladder carcinoma of BK5.erbB2 mice, all of
which are well-differentiated lesions, arises via these two
distinct pathways, which are reminiscent of development sequences
observed in human carcinoma (22).
In our previous study (19) on
expression levels of MUC4 (ASGP2) and erbB2 in human carcinoma,
there was a strong correlation between histological grade (well-
and moderately-differentiated adenocarcinoma) and their expression
levels (our unpublished data). Co-expression of MUC4 and erbB2 is
present in approximately a quarter of the cases of well- and
moderately-differentiated adenocarcinomas. The experiments revealed
their complex formation in the carcinoma tissues (19). Moreover, overexpression of both MUC4
and erbB2 and their complex formation were associated with
hyperphosphorylation of MAPK and Akt in the carcinoma tissues
(19). Although clinical
significance of MUC4 expression or MUC4/erbB2 complex formation is
to date unknown, the relevance of BK5.erbB2 mice as a model of
human gallbladder carcinoma is underscored here by the molecular
and pathological similarities.
Collectively, the findings of the present study
summarize that BK5.erbB2 mice develop adenocarcinoma of the
gallbladder but not the other organs in which erbB2 is
overexpressed. In the gallbladder, it is likely that upregulation
of Muc4 in connection with the phosphorylation status of erbB2 and
an interaction of Muc4 with the phosphorylated erbB2 play important
biological roles in gallbladder carcinogenesis and/or cancer growth
through potentiating the receptor tyrosine kinase and alteration of
erbB2 downstream signaling pathways and also potentiating the
erbB2-Cox-2 pathway. Transgenic approaches would be invaluable in
similar situations and undoubtedly prove helpful in elucidating the
biological roles of Muc4. Therapeutic options for gallbladder
carcinoma are still limited. Based on the results of the present
study, targeting MUC4 and erbB2 could provide a new and effective
therapy for a number of patients with gallbladder carcinoma.
Acknowledgements
The authors would like to thank Dr Kaoru Kiguchi and
Professor John DiGiovanni, the University of Texas, MD Anderson
Cancer Center, Science Park Research Division, Smithville, Texas,
USA, for their kind supply of biological tissue specimens of
BK5.erbB2 transgenic mice. This study was supported by
grants-in-aid (nos. 15590618, 23390318 and 24390323) for scientific
research from the Ministry of Education, Science and Culture.
References
|
1
|
Henson DE, Albores-Saavedra J and Corle D:
Carcinoma of the gallbladder. Histologic types, stage of disease,
grade, and survival rates. Cancer. 70:1493–1497. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japan VSo, Tokyo (Japan). Japanese
Ministry of Health and Welfare. Statistics Assoc; 1998
|
|
3
|
Cubertafond P, Gainant A and Cucchiaro G:
Surgical treatment of 724 carcinomas of the gallbladder. Results of
the French Surgical Association Surgery. Ann Surg. 219:275–280.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rückert JC, Rückert RI, Gellert K, Hecker
K and Müller JM: Surgery for carcinoma of the gallbladder.
Hepatogastroenterology. 43:527–533. 1996.
|
|
5
|
Pandey M and Shukla VK: Diet and
gallbladder cancer: a case-control study. Eur J Cancer Prev.
11:365–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lowenfels AB, Lindström CG, Conway MJ and
Hastings PR: Gallstones and risk of gallbladder cancer. J Natl
Cancer Inst. 75:77–80. 1985.PubMed/NCBI
|
|
7
|
Kimura K, Ohto M, Saisho H, Unozawa T,
Tsuchiya Y, Morita M, Ebara M, Matsutani S and Okuda K: Association
of gallbladder carcinoma and anomalous pancreaticobiliary ductal
union. Gastroenterology. 89:1258–1265. 1985.PubMed/NCBI
|
|
8
|
Suzuki T, Takano Y, Kakita A and Okudaira
M: An immunohistochemical and molecular biological study of
c-erbB-2 amplification and prognostic relevance in gallbladder
cancer. Pathol Res Pract. 189:283–292. 1993. View Article : Google Scholar
|
|
9
|
Yukawa M, Fujimori T, Hirayama D, Idei Y,
Ajiki T, Kawai K, Sugiura R, Maeda S and Nagasako K: Expression of
oncogene products and growth factors in early gallbladder cancer,
advanced gallbladder cancer, and chronic cholecystitis. Hum Pathol.
24:37–40. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kiguchi K, Carbajal S, Chan K, Beltrán L,
Ruffino L, Shen J, Matsumoto T, Yoshimi N and DiGiovanni J:
Constitutive expression of ErbB-2 in gallbladder epithelium results
in development of adenocarcinoma. Cancer Res. 61:6971–6976.
2001.PubMed/NCBI
|
|
11
|
Di Marco E, Pierce JH, Knicley CL and Di
Fiore PP: Transformation of NIH 3T3 cells by overexpression of the
normal coding sequence of the rat neu gene. Mol Cell Biol.
10:3247–3252. 1990.PubMed/NCBI
|
|
12
|
Kiguchi K, Bol D, Carbajal S, Beltrán L,
Moats S, Chan K, Jorcano J and DiGiovanni J: Constitutive
expression of erbB2 in epidermis of transgenic mice results
in epidermal hyperproliferation and spontaneous skin tumor
development. Oncogene. 19:4243–4254. 2000.
|
|
13
|
Klapper LN, Kirschbaum MH, Sela M and
Yarden Y: Biochemical and clinical implications of the ErbB/HER
signaling network of growth factor receptors. Adv Cancer Res.
77:25–79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroeder JA, Thompson MC, Gardner MM and
Gendler SJ: Transgenic MUC1 interacts with epidermal growth factor
receptor and correlates with mitogen-activated protein kinase
activation in the mouse mammary gland. J Biol Chem.
276:13057–13064. 2001. View Article : Google Scholar
|
|
15
|
Jepson S1, Komatsu M, Haq B, Arango ME,
Huang D, Carraway CA and Carraway KL: Muc4/sialomucin complex, the
intramembrane ErbB2 ligand, induces specific phosphorylation of
ErbB2 and enhances expression of p27kip, but does not
activate mitogen-activated kinase or protein kinaseB/Akt pathways.
Oncogene. 21:7524–7532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carraway KL, Ramsauer VP, Haq B and
Carothers Carraway CA: Cell signaling through membrane mucins.
Bioessays. 25:66–71. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carraway KL III, Rossi EA, Komatsu M,
Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway
CA and Carraway KL: An intramembrane modulator of the ErbB2
receptor tyrosine kinase that potentiates neuregulin signaling. J
Biol Chem. 274:5263–5266. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buisine MP, Devisme L, Degand P, Dieu MC,
Gosselin B, Copin MC, Aubert JP and Porchet N: Developmental mucin
gene expression in the gastroduodenal tract and accessory digestive
glands. II Duodenum and liver, gallbladder, and pancreas. J
Histochem Cytochem. 48:1667–1676. 2000. View Article : Google Scholar
|
|
19
|
Miyahara N, Shoda J, Ishige K, Kawamoto T,
Ueda T, Taki R, Ohkohchi N, Hyodo I, Thomas MB, Krishnamurthy S,
Carraway KL and Irimura T: MUC4 interacts with ErbB2 in human
gallbladder carcinoma: potential pathobiological implications. Eur
J Cancer. 44:1048–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shibahara H, Tamada S, Higashi M, Goto M,
Batra SK, Hollingsworth MA, Imai K and Yonezawa S: MUC4 is a novel
prognostic factor of intrahepatic cholangiocarcinoma-mass forming
type. Hepatology. 39:220–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Braissant O and Wahli W: A simplified in
situ hybridization protocol using non-radioactively labelled probes
to detect abundant and rare mRNAs on tissue sections. Biochemica.
1:10–16. 1998.
|
|
22
|
Kawamoto T, Kiguchi K, Ruffino L, Dudek D,
Ajiki T, Thomas M and Digiovanni J: Role of ErbB2 in the
development of gallbladder cancer. Tando. 19:550–556. 2005.(In
Japanese).
|
|
23
|
Vadlamudi R, Mandal M, Adam L, Steinbach
G, Mendelsohn J and Kumar R: Regulation cyclooxygenase-2 pathway by
HER2 receptor. Oncogene. 18:305–314. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian X, LeVea CM, Freeman JK, Dougall WC
and Greene MI: Heterodimerization of epidermal growth factor
receptor and wild-type or kinase-deficient Neu: a mechanism of
interreceptor kinase activation and transphosphorylation. Proc Natl
Acad Sci USA. 91:1500–1504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murali R, Brennan PJ, Kieber-Emmons T and
Greene MI: Structural analysis of p185c-neu and
epidermal growth factor receptor tyrosine kinases: oligomerization
of kinase domains. Proc Natl Acad Sci USA. 93:6252–6257. 1996.
|
|
26
|
Ukita Y, Kato M and Terada T: Gene
amplification and mRNA and protein overexpression of c-erbB-2
(HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by
fluorescence in situ hybridization, in situ hybridization, and
immunohistochemistry. J Hepatol. 36:780–785. 2000. View Article : Google Scholar
|
|
27
|
Suzuki H, Isaji S, Pairojkul C and
Uttaravichien T: Comparative clinicopathological study of resected
intrahepatic cholangiocarcinoma in northeast Thailand and Japan. J
Hepatobiliary Pancreat Surg. 7:206–211. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiguchi K, Ruffino L, Kawamoto T, Ajiki T
and Digiovanni J: Chemopreventive and therapeutic efficacy of
orally active tyrosine kinase inhibitors in a transgenic mouse
model of gallbladder carcinoma. Clin Cancer Res. 11:5572–5580.
2005. View Article : Google Scholar
|
|
29
|
Lenferink AE, Pinkas-Kramarski R, van de
Poll ML, van Vugt MJ, Klapper LN, Tzahar E, Waterman H, Sela M, van
Zoelen EJ and Yarden Y: Differential endocytic routing of homo- and
hetero-dimeric ErbB tyrosine kinases confers signaling superiority
to receptor heterodimers. EMBO J. 17:3385–3397. 1998. View Article : Google Scholar
|
|
30
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signaling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Komatsu M, Jepson S, Arango ME, Carothers
Carraway CA and Carraway KL: Muc4/sialomucin complex, an
intramembrane modulator of ErbB2/HER2/Neu, potentiates primary
tumor growth and suppresses apoptosis in a xenotransplanted tumor.
Oncogene. 20:461–470. 2001. View Article : Google Scholar
|
|
32
|
Price-Schiavi SA, Jepson S, Li P, Arango
M, Rudland PS, Yee L and Carraway KL: Rat Muc4 (sialomucin complex)
reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a
potential mechanism for herceptin resistance. Int J Cancer.
99:783–791. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balagué C, Audié JP, Porchet N and Real
FX: In situ hybridization shows distinct patterns of mucin gene
expression in normal, benign, and malignant pancreas tissues.
Gastroenterology. 109:953–964. 1995.PubMed/NCBI
|
|
34
|
Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt
JL, Holt SE, Rozich RA, Hixson DC and Sirica AE: erbB-2/neu
transformed rat cholangiocytes recapitulate key cellular and
molecular features of human bile duct cancer. Gastroenterology.
129:2047–2057. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida S, Naito K, Hori A, Teratani M,
Koyama M, Tasaka A and Terashita Z: TAK-165, a selective inhibitor
of HER2 tyrosine kinase: 2. Mechanism of antitumor activity on HER2
signal transduction pathway. Proc Am Assoc Cancer Res.
43:7862003.
|
|
36
|
Kamei D, Murakami M, Nakatani Y, Ishikawa
Y, Ishii T and Kudo I: Potential role of microsomal prostaglandin E
synthase-1 in tumorigenesis. J Biol Chem. 278:19396–19405. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sirica AE, Lai GH, Endo K, Zhang Z and
Yoon BI: Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma:
potential therapeutic targets. Semin Liver Dis. 22:303–313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai GH, Zhang Z and Sirica AE: Celecoxib
acts in a cyclooxygenase-2-independent manner and in synergy with
emodin to suppress rat cholangiocarcinoma growth in vitro through a
mechanism involving enhanced Akt inactivation and increased
activation of caspases-9 and -3. Mol Cancer Ther. 2:265–271.
2003.
|
|
39
|
Itoi T, Watanabe H, Ajioka Y, Oohashi Y,
Takei K, Nishikura K, Nakamura Y, Horil A and Saito T: APC, K-ras
codon 12 mutations and p53 gene expression in carcinoma and adenoma
of the gallbladder suggest two genetic pathways in gallbladder
carcinogenesis. Pathol Int. 46:333–340. 1996. View Article : Google Scholar : PubMed/NCBI
|