Introduction
Triple negative breast cancer (TNBC) is an
aggressive subtype comprising approximately 15–20% of breast cancer
cases, with a poor overall prognosis (1). In particular, the level of epidermal
growth factor receptor (EGFR) expression has been shown to be
markedly increased in TNBC patients, compared to non-TNBC patients
(1). EGFR is one of the HER family
of receptors and regulates a number of biological responses, such
as cell proliferation, invasion and maintenance of the malignant
phenotype in breast cancer (2,3).
Aberrant EGFR expression is associated with large tumor size, poor
differentiation and poor clinical outcomes in breast cancer
patients (4,5). The EGF/EGFR signaling pathway triggers
the process of epithelial-mesenchymal transition (EMT) to
facilitate tumor cell invasion via the induction of mesenchymal
markers including fibronectin (FN), Twist, and ZEB (6,7).
FN is a large adhesive glycoprotein of the
extracellular matrix and plays an important role in cell adhesion,
growth, migration, differentiation and oncogenic transformation
(8–10). It has been shown to be involved in
the development of various human cancer types (10,11).
In breast cancer patients, the level of FN expression is higher in
cases of TNBC (6). Elevated
expression of FN contributes to poor clinical outcome in breast
cancer patients (10,11). In addition, FN confers resistance to
apoptosis induced by standard chemotherapeutic agents (12). However, to date, a correlation
between the regulatory mechanism of EGFR and FN has not been fully
elucidated in breast cancer.
Silibinin, the major active constituent of
silymarin, is isolated from milk thistle seeds and exerts promising
anticancer efficacy against various cancer models, such as breast
and lung cancer (13–15). In a previous study, we also reported
that silibinin downregulates CD44 and MMP-9 expression through
inhibition of EGFR phosphorylation in SKBR3 breast cancer cells
(16). Thus, we decided to
investigate the inhibitory effect of silibinin on the induction of
FN expression through the EGF/EGFR signaling pathway in TNBC
cells.
The aim of the present study was to evaluate the
regulatory mechanism of silibinin on epidermal growth factor
(EGF)-induced FN expression in TNBC cells. EGF-induced FN
expression was suppressed by the STAT3 inhibitor, Stattic.
Silibinin also inhibited EGF-induced FN expression through the
suppression of STAT3 activity in MDA-MB468 breast cancer cells,
leading to the conclusion that silibinin has an inhibitory effect
on the EGF/STAT3 signaling pathway, causing suppression of FN
expression in TNBC cells. We demonstrated that silibinin may be a
promising therapeutic drug for the treatment of TNBC.
Materials and methods
Reagents
T47D, ZR75-1, MDA468 and BT20 human breast cancer
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). MCF7 and MDA-MB231 cells were obtained from
the Korea Cell Line Bank (Seoul, Korea). Cell culture media (DMEM
and RPMI-1640) and antibiotics were purchased from Life
Technologies (Rockville, MD, USA). Fetal bovine serum (FBS) was
purchased from HyClone (Logan, UT, USA). Rabbit monoclonal
anti-t-EGFR, p-EGFR, t-Akt, p-Akt, t-Erk, p-Erk, t-STAT3, p-STAT3,
and FN antibodies were purchased from Epitomics (Burlingame, CA,
USA). Mouse monoclonal anti-ERα and secondary horseradish
peroxidase (HRP)-conjugated antibodies were purchased from Santa
Cruz (Santa Cruz, CA, USA). Gefitinib was purchased from
Selleckchem (Houston, TX, USA). AG1478, U0126, LY294002 and Stattic
were purchased from Tocris (Ellisville, MO, USA). Silibinin was
purchased from Sigma (St. Louis, MO, USA). EGF was purchased from
R&D Systems (Minneapolis, MN, USA).
Cell cultures and chemical treatment
MCF7, MDA-MB231, MDA-MB468 and BT20 breast cancer
cells were cultured in DMEM supplemented with 10% FBS, 100 IU/ml
penicillin and 100 μg/ml streptomycin. T47D and ZR75-1 breast
cancer cells were cultured in RPMI-1640 supplemented with 10% FBS,
100 IU/ml penicillin and 100 μg/ml streptomycin.
Cells were maintained in serum-free culture medium
for 24 h, then further incubated with the indicated concentrations
of silibinin or various inhibitors, such as gefitinib, AG1478,
U0126, LY294002, and Stattic, respectively. Cells were pretreated
with silibinin or the other inhibitors for 60 min prior to
treatment with EGF, then treated with EGF for 24 h.
Western blotting
The cell lysates were used in an immunoblot analysis
for FN, EGFR, ER-α and β-actin. The proteins were boiled for 5 min
in Laemmli sample buffer, then electrophoresed in 8% sodium dodecyl
sulfate polyacrylamide (SDS-PAGE) gels. The proteins were
transferred to polyvinylidene fluoride (PVDF) membranes, and the
membranes were then blocked with 10% skim milk in Tris-buffered
saline (TBS) with 0.01% Tween-20 (TBS/T) for 15 min. The blots were
incubated with the indicated antibodies in TBS/T buffer at 4°C
overnight. The blots were washed 3 times in TBS/T and were
subsequently incubated with an anti-rabbit HRP-conjugated antibody
(1:2,000 dilution) in TBS/T buffer. After 1 h incubation at room
temperature (RT), the blots were washed 3 times in TBS/T and
ECLprime reagent was used for development.
Real-time polymerase chain reaction
(PCR)
The total RNA was extracted from the treated cells
by using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA),
according to the manufacturer’s protocol. Isolated RNA samples were
then used for RT-PCR. Samples (1 μg of total RNA) were
reverse-transcribed into cDNA in 20 μl reaction volumes using a
first-strand cDNA synthesis kit for RT-PCR, according to the
manufacturer’s instructions (MBI Fermentas; Hanover, MD, USA).
The gene expression was quantified by real-time PCR
using a SensiMix SYBR Kit (Bioline Ltd., London, England) and 100
ng of cDNA per reaction. The sequences of the primer sets used for
this analysis were: human EGFR (forward, 5′-CAT GTC GAT GGA CTT CCA
GA′ and reverse, 5′-GGG ACA GCT TGG ATC ACA CT-3′), ER-α (forward,
5′-GAA TCT GCC AAG GAG ACT CG′ and reverse, 5′-GGC AGC TCT TCC TCC
TGT TT-3′), FN (forward, 5′-CCA CCC CCA TAA GGC ATA GG′ and
reverse, 5′-GTA GGG GTC AAA GCA CGA GTC ATC-3′) and GAPDH as an
internal control (forward, 5′-ATT GTT GCC ATC AAT GAC CC-3′ and
reverse, 5′-AGT AGA GGC AGG GAT GAT GT-3′). An annealing
temperature of 60°C was used for all the primers. PCRs were
performed in a standard 384-well plate format with an ABI 7900HT
real-time PCR detection system (Foster City, CA, USA) For data
analysis, the raw threshold cycle (Ct) value was first normalized
to the housekeeping gene for each sample to get the ΔCt. The
normalized ΔCt was then calibrated to the control cell samples to
get the ΔΔCt.
Confocal microscopy
Human breast cancer MDA-MB468 and BT20 cells grown
on 4-well Lab-Tek chamber slides were allowed to adhere overnight
and were then serum-starved for 24 h before treatment with 50 ng/ml
EGF and/or silibinin for 24 h. Cells were fixed for 20 min in 4%
paraformaldehyde and incubated at 4°C overnight with an anti-FN
antibody (B3/D6 clone; DSHB, Iowa, IA, USA), then washed 3 times in
PBS. Washed slides were incubated with Alexa Fluor 488-conjugated
goat anti-mouse secondary antibody (1:50 dilution) for 60 min at
RT. Cells were then washed in PBS and slides were mounted in
Vectashield H-1200/DAPI mounting media (Vector Laboratories,
Burlingame, CA, USA). Confocal images were analyzed using an LSM700
confocal laser-scanning microscope (Carl Zeiss, Germany).
Statistical analysis
Statistical values were determined using a Student’s
t-test. The data are presented as mean values ± SEM. All quoted
P-values were two-tailed and P-values <0.05 were considered to
indicate statistically significant differences.
Results
The basal levels of FN mRNA and protein
expression are dose-dependently increased by EGF treatment in TNBC
breast cancer cells
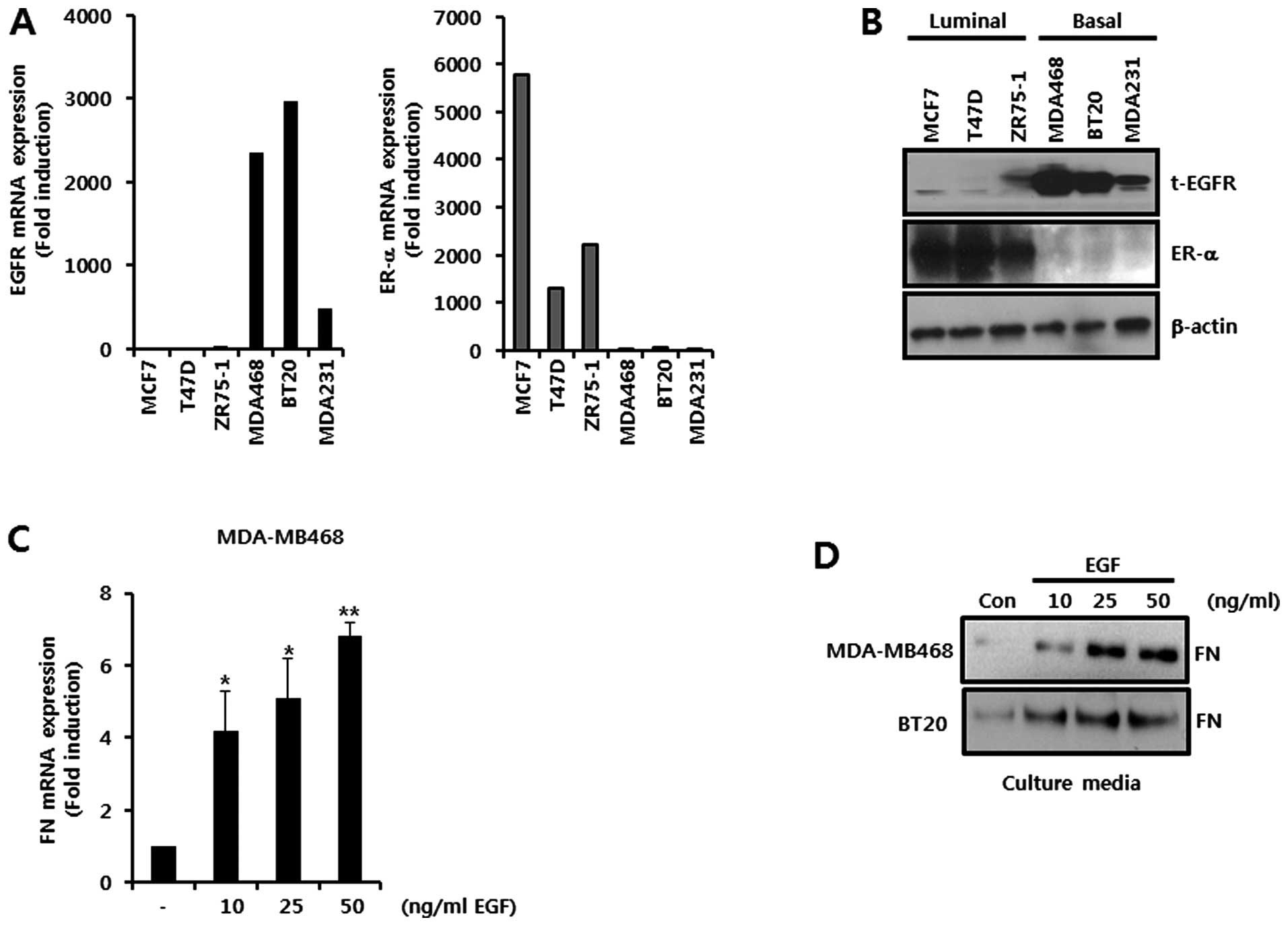
To investigate the relationship between EGFR and FN
expression, we examined the level of EGFR in various breast cancer
cells. The basal levels of EGFR and ER-α expression are shown in
breast cancer cells in Fig. 1A and
B. The basal-like breast cancer cells, MDA-MB468, BT20, and
MDA-MB231, had a high level of EGFR mRNA expression (Fig. 1A). In contrast, the luminal type
breast cancer cells, MCF7, T47D, and ZR75-1, showed overexpression
of ER-α mRNA (Fig. 1A).
Consequently, the levels of EGFR and ER-α protein expression in the
basal and luminal breast cancer cells were also high, respectively
(Fig. 1B). We selected two basal
breast cancer cell lines, MDA-MB468 and BT20, to verify the effect
of silibinin on EGF-induced FN expression. We treated the cells
with the indicated concentrations of EGF for 24 h. Our results
showed that the level of FN mRNA expression was dose-dependently
increased by EGF in MDA-MB468 cells (Fig. 1C). The level of the FN mRNA
expression was significantly increased by 4.2±1.1-fold,
5.1±1.1-fold, and 6.8±0.4-fold of the control levels with EGF
concentrations of 10, 25 and 50 ng/ml, respectively (Fig. 1C). Treated under the same
conditions, the level of FN protein expression was also shown to be
increased in both MDA-MB468 and BT20 cells (Fig. 1D). Based on these results, we
demonstrated that EGF triggers expression of both FN mRNA and FN
protein in TNBC cells.
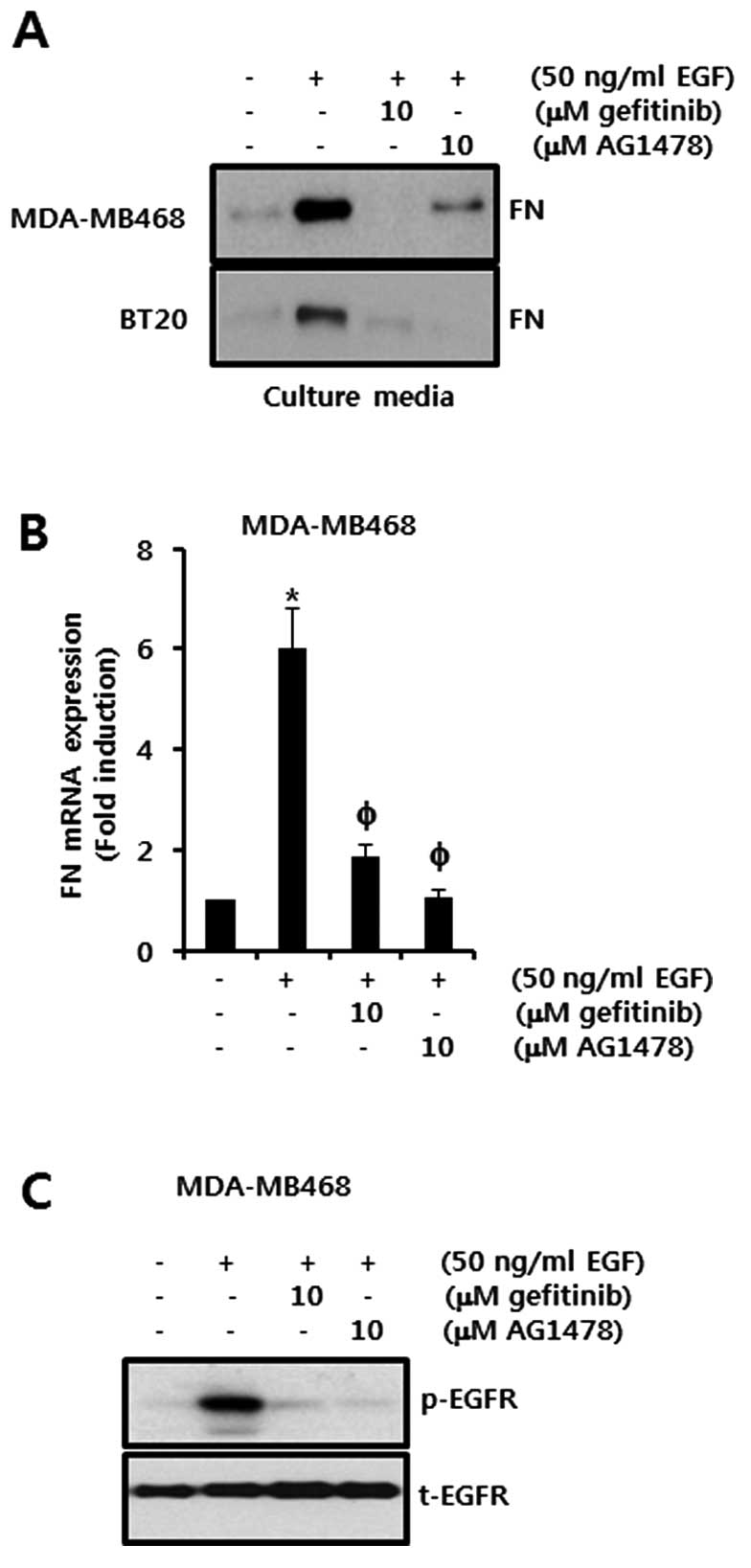
EGF-induced FN expression is decreased by
EGFR inhibitors, gefitinib and AG1478, in TNBC cells
We examined the effects of the EGFR inhibitors
gefitinib and AG1478 on EGF-induced FN expression in TNBC cells.
Cells were pretreated with either 10 μM gefitinib or 10 μM AG1478
for 60 min and were then treated with 50 ng/ml of EGF for 24 h. EGF
significantly increased the level of FN protein expression in
MDA-MB468 and BT20 cells (Fig. 2A).
However, EGF-induced FN expression was reduced by treatment with
gefitinib and AG1478, respectively, in cell culture media (Fig. 2A). Under the same conditions,
EGF-induced FN mRNA expression was significantly decreased by
treatment with gefitinib and AG1478, respectively, in MDA-MB468
cells (Fig. 2B). The FN mRNA
expression was increased to 6.0±0.8-fold that of the control level
following treatment with 50 ng/ml EGF. By contrast, EGF-induced FN
mRNA expression was markedly inhibited to 1.85±0.25-fold and
1.05±0.15-fold that of the control level in the cells pretreated
with gefitinib and AG1478, respectively (Fig. 2B).
We also examined the effects of EGFR inhibitors on
EGF-induced EGFR phosphorylation. EGFR phosphorylation peaked 15
min after EGF treatment. The phosphorylation of EGFR was
significantly reduced by the treatment with both gefitinib and
AG1478 (Fig. 2C). This shows that
the levels of FN protein and mRNA expression in TNBC cells are
upregulated by the EGF/EGFR signaling pathway.
Effect of EGFR downstream signaling
molecule inhibitors on EGF-induced FN expression in TNBC cells
Next, we investigated the effects of EGFR downstream
signaling molecule inhibitors on EGF-induced FN expression in TNBC
cells. We pretreated cells with either 10 μM UO126 (a MEK1/2
inhibitor), 10 μM LY294002 (a PI3K inhibitor), or 10 μM Stattic (a
STAT3 inhibitor), respectively, for 60 min prior to treatment with
50 ng/ml EGF. As shown in Fig. 3A,
FN protein expression was significantly increased by EGF in
inhibitor-untreated controls. However, EGF-induced FN protein
expression was suppressed in the MDA-MB468 and BT20 breast cancer
cells that were treated with any of the inhibitors (Fig. 3A). Under the same conditions, the
level of FN mRNA expression in response to the addition of EGF was
increased by 7.75 ± 1.3-fold of the control level (Fig. 3B). However, EGF-induced FN mRNA
expression was reduced to 0.95±0.2-fold, 2.0±0.19-fold and
2.0±0.5-fold of the control level by U0126, LY294002 and Stattic,
respectively (Fig. 3B).
Furthermore, we verified the effects of U0126, LY294002 and Stattic
on EGF-induced STAT3, Akt and Erk phosphorylation, respectively.
The phosphorylation of STAT3, Akt and Erk peaked 15 min after EGF
treatment. STAT3, Akt and Erk phosphorylation were shown to be
decreased by the inhibitors Stattic, LY294002 and U0126,
respectively (Fig. 3C). These
results demonstrate that EGF-induced FN mRNA and protein expression
are mediated through MEK/Erk, PI3K/Akt and STAT3-dependent
signaling pathways in TNBC cells.
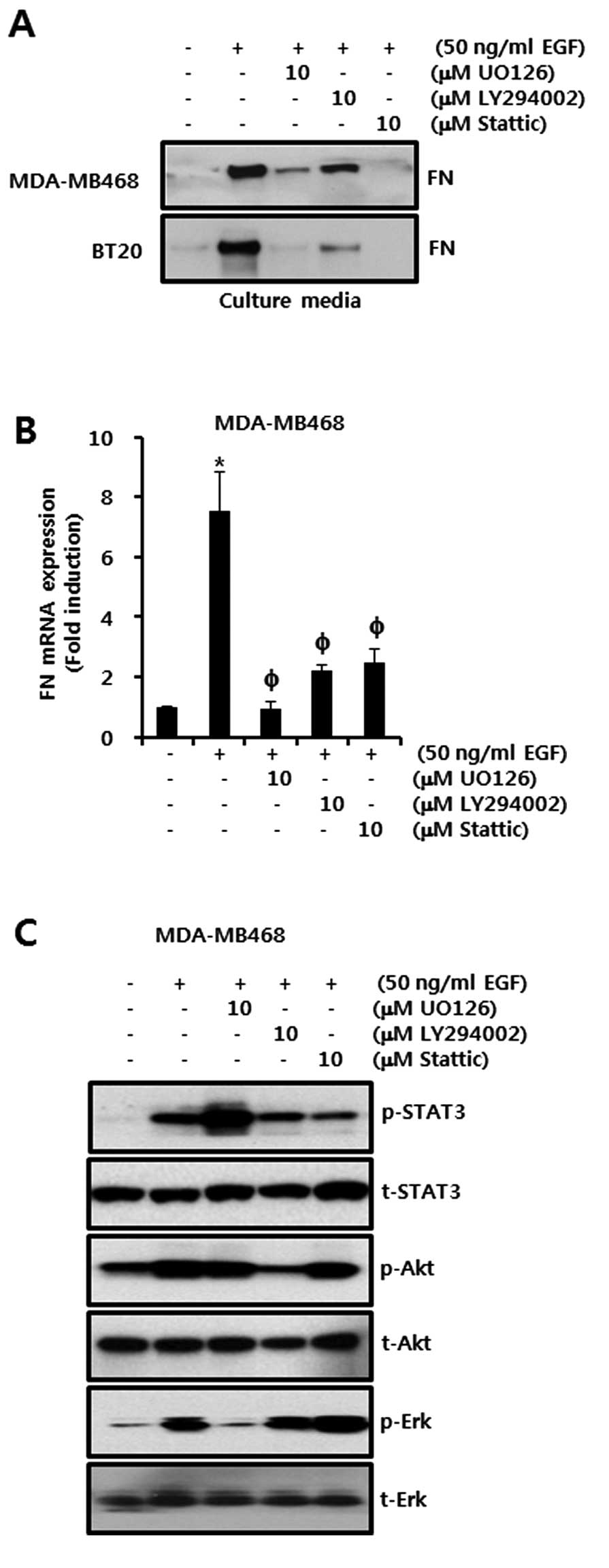 | Figure 3Effect of EGFR downstream signaling
molecule inhibitors on EGF-induced FN expression in TNBC cells. (A
and B) Levels of (A) FN protein and (B) mRNA expression in
serum-starved cells, pretreated with 10 μM UO126, 10 μM LY294002
and 10 μM Stattic, respectively, then incubated with 50 ng/ml EGF
for 24 h as analyzed by western blotting and real-time PCR,
respectively. (C) Levels of STAT3, Akt and Erk protein expression
in serum-starved cells, pretreated with U0126, LY294002 and
Stattic, respectively, then treated with EGF for 15 min as analyzed
by western blotting. These results are representative of three
independent experiments. The values shown are the means ± SEM.
*P<0.05 vs. control, P<0.05 vs. EGF-treated cells.
FN, fibronectin. |
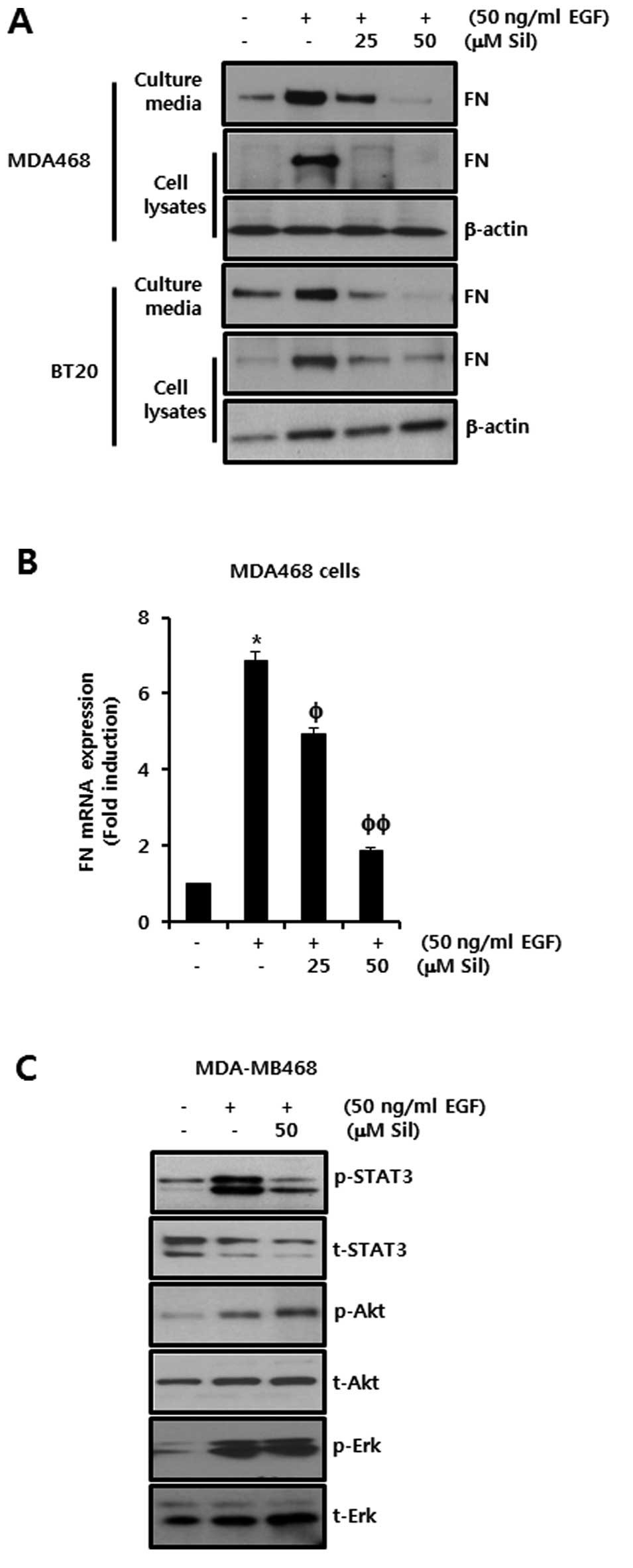
EGF-induced FN expression is decreased by
silibinin in TNBC cells
To test the effect of silibinin on EGF-induced FN
expression, we pretreated MDA-MB468 and BT20 cells with 25 and 50
μM silibinin for 60 min prior to treatment with 50 ng/ml EGF. After
24 h, we harvested the cell culture media and cell lysates. Our
results indicated that the level of FN protein expression increased
in both MDA-MB468 and BT20 breast cancer cells in response to
treatment with EGF (Fig. 4A).
However, the level of EGF-induced FN protein expression was reduced
by silibinin in a dose-dependent manner in both cell culture media
and whole cell lysates (Fig. 4A).
Under the same conditions, we also investigated the inhibitory
effects of silibinin on EGF-induced FN mRNA expression. As
expected, EGF-induced FN mRNA expression was dose-dependently
inhibited in MDA-MB468 breast cancer cells by treatment with
silibinin (Fig. 4B). The level of
FN mRNA expression was increased by 6.86-fold of the control level
by the addition of 50 ng/ml of EGF. However, the induction of FN
mRNA expression by addition of EGF was decreased to 4.92-fold and
1.88-fold of the control level by treatment with 25 and 50 μM of
silibinin, respectively (Fig.
4B).
Finally, we investigated the regulatory mechanism of
silibinin on EGF-induced FN expression in MDA-MB468 cells. Cells
were pretreated with the indicated concentration of silibinin for
60 min and then treated with EGF for 15 min. The phosphorylation of
the EGFR downstream signaling molecules, STAT3, Akt and Erk, was
increased by the addition of EGF in silibinin-untreated controls
(Fig. 4C). Notably, when we
examined the inhibitory effect of silibinin on the activation of
these three signaling pathways, we observed that only EGF-induced
STAT3 phosphorylation was markedly reduced by silibinin. The
phosphorylation of Akt and Erk was not significantly affected
(Fig. 4C). These results show that
silibinin abolished the EGF-induced FN expression in a similar
manner to the STAT3 inhibitor in MDA-MB468 and BT20 breast cancer
cells. Therefore, we demonstrated that silibinin suppresses
EGF-induced FN expression via the inhibition of STAT3
phosphorylation in TNBC cells.
Discussion
The TNBC subtype has the poorest prognosis among
breast cancer subtypes (1,17) due to limited treatment options and a
lack of clinically established targeted molecular therapies. TNBC
has a markedly increased level of EGFR expression, resulting in
aggressive metastasis and a poor prognosis, as defined by its low
five-year survival and high recurrence rates after adjuvant therapy
(1). EGFR inhibitors, such as
monoclonal antibodies (cetuximab) and small molecule inhibitors
(gefitinib), have been used in the treatment of TNBC, for which
there are currently no specific targeted therapies (18).
EGF stimulates cell growth, proliferation and
differentiation through binding to its receptor EGFR (5). High expression of EGFR within primary
tumors is associated with a poor clinical outcome in human cancers,
and EGFR is frequently overexpressed in TNBC patients (19–23).
In addition, the EGF/EGFR signaling pathway is a well-known inducer
of the EMT process in several types of human cancer (24). EGF leads to EMT-like changes,
including upregulation of mesenchymal markers and downregulation of
epithelial markers (25,26). Consistent with these reports, our
results showed that the levels of EGFR expression were
significantly increased in TNBC cells, and EGF-induced FN
expression was increased in a dose-dependent manner in TNBC
cells.
The EGF/EGFR complex mainly activates various
downstream signaling pathways, such as MEK/ERK, PI3K/Akt and
JAK/STAT3, in malignant human cancer cells (27,28).
The EGFR inhibitor AG1478, MEK1/2 inhibitor PD98059, and p38MAPK
inhibitor SB203580 completely abolished TGF-β-mediated FN
expression (29). Heparin-binding
EGF-like growth factor (HB-EGF), a heparin-binding member of the
EGF family, significantly increases the level of FN expression in
mesangial cells (30). In contrast,
neutralizing anti-HB-EGF antibody completely blocks FN expression
(30).
In accordance with these reports, our results showed
that EGF also affects the level of FN expression in TNBC cells. In
TNBC cells, EGF-induced FN expression was suppressed by UO126 (a
MEK1/2 inhibitor) and LY294002 (a PI3K inhibitor). In this study,
to the best of our knowledge, we are the first to demonstrate that
EGF-induced FN expression is significantly decreased by a STAT3
inhibitor, Stattic. These results suggest that STAT3 is a new
pathway involved in the regulation of EGF-induced FN
expression.
The level of FN expression is regulated by a variety
of growth factors, such as TGF-β, PDGF and EGF, which have been
implicated in developmental processes (29). Highly expressed FN levels in breast
tumor tissues have been correlated with tumor malignancy and the
poor prognosis of breast cancer patients (31,32).
In addition, FN expression has been shown to be involved in the
epithelial-mesenchymal transition (EMT) process, which occurs
during development and has been linked to cancer (25,26).
Growth factors, such as EGF and TGF-β, can promote the EMT
triggering of specific signaling networks (6,33–36).
Our results showed that FN expression in TNBC cells is higher than
that of other breast cancer cells (data not shown). FN expression
in response to EGF was significantly decreased by the EGFR
inhibitors, AG1478 and gefitinib, in TNBC cells.
Silibinin has multifunctional activities including
the suppression of cell invasion, inflammation, the induction of
apoptosis in various cancer cells such as breast cancer cells
(14–16,37).
In a previous study, we reported that silibinin suppresses the
level of COX-2 and VEGF through the inhibition of the Raf/MEK/ERK
pathway in breast cancer cells (14,15).
Here, we focused on the regulatory mechanism of silibinin on
EGF-induced FN expression in TNBC cells. We observed that silibinin
inhibited EGF-induced expression of FN and the phosphorylation of
STAT3 in breast cancer cells. In addition, we observed that
EGF-induced FN expression was decreased by a specific STAT3
inhibitor, Stattic. Based on these results, we conclude that
silibinin prevents EGF-induced FN expression through the inhibition
of STAT3 phosphorylation in TNBC cells.
In the present study, we conclusively verified the
regulatory mechanism of silibinin in EGF-induced FN expression in
TNBC cells. EGF-induced signaling molecules, MEK, PI3K and STAT3,
play an important role in the regulation of FN expression in TNBC
cells. However, silibinin suppressed only EGF-induced STAT3
phosphorylation, not phosphorylation in the MEK/ERK and PI3K/Akt
pathway. Hence, we demonstrated that silibinin suppresses
EGF-induced FN expression through the inhibition of STAT3 in TNBC
cells. We therefore suggest that silibinin could be used as a
potential candidate drug for the treatment of TNBC, for which there
are currently no specific targeted therapies.
Acknowledgements
This study was supported by grants from the Samsung
Biomedical Research Institute (SMR1120321), the Korea Research
Foundation, funded by the Korean Government, (NRF-2012R1A1B4000493)
and the Korea Health Technology R&D Project, through the Korea
Health Industry Development Institute(KHIDI), funded by the
Ministry of Health & Welfare, Republic of Korea
(HI09C1552).
References
|
1
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ullrich A and Schlessinger J: Signal
transduction by receptors with tyrosine kinase activity. Cell.
61:203–212. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sainsbury JR, Farndon JR, Needham GK,
Malcolm AJ and Harris AL: Epidermal-growth-factor receptor status
as predictor of early recurrence of and death from breast cancer.
Lancet. 1:1398–1402. 1987.PubMed/NCBI
|
|
5
|
Salomon DS, Brandt R, Ciardiello F and
Normanno N: Epidermal growth factor-related peptides and their
receptors in human malignancies. Crit Rev Oncol Hematol.
19:183–232. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balanis N, Wendt MK, Schiemann BJ, Wang Z,
Schiemann WP and Carlin CR: Epithelial to mesenchymal transition
promotes breast cancer progression via a fibronectin-dependent
STAT3 signaling pathway. J Biol Chem. 288:17954–17967. 2013.
View Article : Google Scholar
|
|
7
|
Wendt MK, Smith JA and Schiemann WP:
Transforming growth factor-beta-induced epithelial-mesenchymal
transition facilitates epidermal growth factor-dependent breast
cancer progression. Oncogene. 29:6485–6498. 2010. View Article : Google Scholar
|
|
8
|
Pankov R and Yamada KM: Fibronectin at a
glance. J Cell Sci. 115:3861–3863. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ritzenthaler JD, Han S and Roman J:
Stimulation of lung carcinoma cell growth by fibronectin-integrin
signalling. Mol Biosyst. 4:1160–1169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez-Garcia B, Eiro N, Marin L,
Gonzalez-Reyes S, Gonzalez LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
512–522. 2013.PubMed/NCBI
|
|
11
|
Bae YK, Kim A, Kim MK, Choi JE, Kang SH
and Lee SJ: Fibronectin expression in carcinoma cells correlates
with tumor aggressiveness and poor clinical outcome in patients
with invasive breast cancer. Hum Pathol. 44:2028–2037. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rintoul RC and Sethi T: Extracellular
matrix regulation of drug resistance in small-cell lung cancer.
Clin Sci. 102:417–424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma G, Singh RP, Chan DC and Agarwal R:
Silibinin induces growth inhibition and apoptotic cell death in
human lung carcinoma cells. Anticancer Res. 23:2649–2655.
2003.PubMed/NCBI
|
|
14
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ and Lee JE: Silibinin
prevents TPA-induced MMP-9 expression and VEGF secretion by
inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast
cancer cells. Phytomedicine. 16:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim S, Kim SH, Hur SM, Lee SK, Kim WW, Kim
JS, Kim JH, Choe JH, Nam SJ, Lee JE and Yang JH: Silibinin prevents
TPA-induced MMP-9 expression by downregulation of COX-2 in human
breast cancer cells. J Ethnopharmacol. 126:252–257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim S, Han J, Kim JS, Kim JH, Choe JH,
Yang JH, Nam SJ and Lee JE: Silibinin suppresses EGFR
ligand-induced CD44 expression through inhibition of EGFR activity
in breast cancer cells. Anticancer Res. 31:3767–3773.
2011.PubMed/NCBI
|
|
17
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferraro DA, Gaborit N, Maron R,
Cohen-Dvashi H, Porat Z, Pareja F, Lavi S, Lindzen M, Ben-Chetrit
N, Sela M and Yarden Y: Inhibition of triple-negative breast cancer
models by combinations of antibodies to EGFR. Proc Natl Acad Sci
USA. 110:1815–1820. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi J, Jung WH and Koo JS:
Clinicopathologic features of molecular subtypes of triple negative
breast cancer based on immunohistochemical markers. Histol
Histopathol. 27:1481–1493. 2012.PubMed/NCBI
|
|
20
|
Gumuskaya B, Alper M, Hucumenoglu S,
Altundag K, Uner A and Guler G: EGFR expression and gene copy
number in triple-negative breast carcinoma. Cancer Genet Cytogenet.
203:222–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rakha EA, El-Sayed ME, Green AR, Lee AH,
Robertson JF and Ellis IO: Prognostic markers in triple-negative
breast cancer. Cancer. 109:25–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martin V, Botta F, Zanellato E, Molinari
F, Crippa S, Mazzucchelli L and Frattini M: Molecular
characterization of EGFR and EGFR-downstream pathways in triple
negative breast carcinomas with basal like features. Histol and
Histopathol. 27:785–792. 2012.PubMed/NCBI
|
|
23
|
Liu D, He J, Yuan Z, Wang S, Peng R, Shi
Y, Teng X and Qin T: EGFR expression correlates with decreased
disease-free survival in triple-negative breast cancer: a
retrospective analysis based on a tissue microarray. Med Oncol.
29:401–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ackland ML, Newgreen DF, Fridman M,
Waltham MC, Arvanitis A, Minichiello J, Price JT and Thompson EW:
Epidermal growth factor-induced epithelio-mesenchymal transition in
human breast carcinoma cells. Lab Invest. 83:435–448. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun X, Fa P, Cui Z, Xia Y, Sun L, Li Z,
Tang A, Gui Y and Cai Z: The EDA-containing cellular fibronectin
induces epithelial-mesenchymal transition in lung cancer cells
through integrin α9β1-mediated activation of PI3-K/AKT and Erk1/2.
Carcinogenesis. 35:184–191. 2014.PubMed/NCBI
|
|
26
|
Sudo T, Iwaya T, Nishida N, Sawada G,
Takahashi Y, Ishibashi M, Shibata K, Fujita H, Shirouzu K, Mori M
and Mimori K: Expression of mesenchymal markers vimentin and
fibronectin: the clinical significance in esophageal squamous cell
carcinoma. Ann Surg Oncol. 20:S324–S335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim S, Choi JH, Lim HI, Lee SK, Kim WW,
Cho S, Kim JS, Kim JH, Choe JH, Nam SJ, Lee JE and Yang JH:
EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway,
but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell
line. Cell Signal. 21:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Han J, Lee SK, Koo M, Cho DH, Bae
SY, Choi MY, Kim JS, Kim JH, Choe JH, Yang JH, Nam SJ and Lee JE:
Smad7 acts as a negative regulator of the epidermal growth factor
(EGF) signaling pathway in breast cancer cells. Cancer Lett.
314:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hayashida T, Poncelet AC, Hubchak SC and
Schnaper HW: TGF-beta1 activates MAP kinase in human mesangial
cells: a possible role in collagen expression. Kidney Int.
56:1710–1720. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uchiyama-Tanaka Y, Matsubara H, Mori Y,
Kosaki A, Kishimoto N, Amano K, Higashiyama S and Iwasaka T:
Involvement of HB-EGF and EGF receptor transactivation in
TGF-beta-mediated fibronectin expression in mesangial cells. Kidney
Int. 62:799–808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Helleman J, Jansen MP, Ruigrok-Ritstier K,
van Staveren IL, Look MP, Meijer-van Gelder ME, Sieuwerts AM, Klijn
JG, Sleijfer S, Foekens JA and Berns EM: Association of an
extracellular matrix gene cluster with breast cancer prognosis and
endocrine therapy response. Clin Cancer Res. 14:5555–5564. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao ES, Zhang H, Chen YY, Lee B, Chew K,
Moore D and Park C: Increased beta1 integrin is associated with
decreased survival in invasive breast cancer. Cancer Res.
67:659–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vergara D, Valente CM, Tinelli A,
Siciliano C, Lorusso V, Acierno R, Giovinazzo G, Santino A,
Storelli C and Maffia M: Resveratrol inhibits the epidermal growth
factor-induced epithelial mesenchymal transition in MCF-7 cells.
Cancer Lett. 310:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grande M, Franzen A, Karlsson JO, Ericson
LE, Heldin NE and Nilsson M: Transforming growth factor-beta and
epidermal growth factor synergistically stimulate epithelial to
mesenchymal transition (EMT) through a MEK-dependent mechanism in
primary cultured pig thyrocytes. J Cell Sci. 115:4227–4236. 2002.
View Article : Google Scholar
|
|
35
|
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei
Y, Abbruzzese JL, Hortobagyi GN and Hung MC: Epidermal growth
factor receptor cooperates with signal transducer and activator of
transcription 3 to induce epithelial-mesenchymal transition in
cancer cells via upregulation of TWIST gene expression. Cancer Res.
67:9066–9076. 2007. View Article : Google Scholar
|
|
36
|
Kasai H, Allen JT, Mason RM, Kamimura T
and Zhang Z: TGF-beta1 induces human alveolar epithelial to
mesenchymal cell transition (EMT). Respir Res. 6:562005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheung CW, Gibbons N, Johnson DW and Nicol
DL: Silibinin - a promising new treatment for cancer. Anticancer
Agents Med Chem. 10:186–195. 2010. View Article : Google Scholar : PubMed/NCBI
|