Introduction
Glioma is one of the most common malignant primary
brain tumors. According to the 2007 World Health Organization
classification of central nervous system tumors, gliomas are
divided pathologically into four grades, which are relevant for
prognosis. Surgery, with or without radiotherapy and chemotherapy,
can relieve many symptoms in glioma patients, but clinically, tumor
recurrence often occurs (1). For
many patients, complete elimination of gliomas remains a
challenge.
In recent years, vascular-targeted therapy has
gradually been accepted. The antiangiogenic drug bevacizumab
(Avastin) has become one of the most popular vascular-targeted
therapeutic drugs for several types of tumors, including gliomas
(2). However, this traditional
antiangiogenic drug also accelerates metastasis, together with
marked hypoxia and an alternative blood supply - vasculogenic
mimicry (VM) (3). VM consists of
laminin-rich networks that can be stained with periodic acid-Schiff
(PAS) in vivo and forms extracellular matrix (ECM)-rich
tubular networks on Matrigel that mimic conventional angiogenesis
in vitro. VM is established by highly aggressive tumor cells
instead of poorly aggressive ones or endothelial cells (4). Our previous studies confirmed the
existence and clinical significance of VM in medulloblastoma and
glioblastoma, and VM might be an independent adverse prognostic
factor for overall survival (5,6). As a
novel form of blood supply, suppression of VM could be an
alternative therapeutic target for gliomas.
The mammalian target of rapamycin (mTOR) signaling
pathway is activated in the majority of human tumors. It plays an
important role in regulating angiogenesis both in normal tissues
and in tumors (7). Previous studies
have demonstrated that there is molecular crosstalk between the
mTOR and VM signaling pathways, particularly HIF-1α (8).
A recent study indicated that rapamycin acts as an
HIF-1α inhibitor and prevents VM formation of human epithelial
ovarian cancer in vivo (9).
Researchers have reached a consensus that HIF-1α is a major cause
of VM (reviewed in ref. 10).
Therefore, HIF-1α might be one of the mTOR downstream molecules
involved in VM. However, the integrated mechanism of mTOR signaling
in VM formation has not yet been investigated. In this study, we
aimed to achieve a better understanding of the role of mTOR
signaling in VM formation and to explore a novel method of
treatment targeting glioma.
Materials and methods
Patients
One hundred and twenty-seven specimens of
paraffin-embedded glioma tissues were obtained from the Department
of Pathology of Zhujiang Hospital at Southern Medical University
between 2009 and 2012. Tumor sections were reviewed by two
neuropathologists to verify the diagnosis of glioma in accordance
with the 2007 World Health Organization classification of central
nervous system tumors. Informed consent was obtained for the use of
the specimens, and the study was approved by the Research Ethics
Committee of Southern Medical University.
Cells and reagents
The human U87 malignant glioblastoma (U87-MG) cell
line was chosen for the in vitro functional test, and human
umbilical vein endothelial cells (HUVECs) were used as control
cells. Both cell lines were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, Minneapolis, MN, USA). Cells were grown
at 37°C in a humidified atmosphere of 5% CO2 and 95%
air. Serum-free culture medium was used for the cell function
assays.
Rapamycin (Sigma-Aldrich, St. Louis MO, USA) was
stored at a concentration of 10 mM in 100% DMSO at −20°C and was
diluted in serum-free medium immediately prior to use. Antibodies
against mTOR, HIF-1α, MMP-14 and MMP-2 were purchased from Abcam
(Cambridge, UK).
Hypoxia treatment
Hypoxic conditions were simulated by flushing 5%
CO2 and 95% N2 through a modified chamber
(Mitsubishi, Japan), as described previously (11), until the O2 concentration
was reduced to 1%, as measured with a Mini oxygen meter. The
culture system was sealed and incubated at 37°C.
Immunohistochemical and CD34/PAS
histochemical double-staining
Immunohistochemical and CD34/PAS histochemical
double-staining were performed as we previously described (6). For immunohistochemical staining of
each slide, a total of five random images (magnification, ×400)
were selected and examined under a microscope (Leica, Germany). The
number of stained cells and the total number of cells were counted,
and the ratio between the stained and total cells was calculated.
The following scoring was used for the stained cell ratio: <10%
was negative or weakly positive (−/+); 10–50% was strongly positive
(++); and >50% was very strongly positive (+++).
For CD34/PAS histochemical double-staining, after
immunohistochemical staining for CD34 (Zhongshan Goldenbridge
Biotechnology, Beijing, China), the sections were washed with
distilled water and slides were stained following the PAS staining
procedures before counterstaining with Mayer’s hematoxylin.
Three-dimensional (3D) culture
The in vitro study of vasculogenic mimicry
formation was assessed on Matrigel by a 3D culture. Matrigel
(Growth Factor Reduced; BD Biosciences) was thawed at 4°C, and 250
μl was quickly added to each well of a 24-well plate and allowed to
solidify for 1 h at room temperature. HUVECs or U87-MG cells were
harvested by trypsin, resuspended in medium with serum in the
presence or absence of rapamycin at the indicated concentrations,
and seeded onto the Matrigel layer at 1–5×104
cells/well. The HUVECs were used as a control. After a 6-h
incubation, the tubular network structures were visualized, and
images were captured under a phase contrast microscope. The
relative lengths of the tubes were quantified by image analysis
software (Image-Pro Plus).
Western blotting
The cells were lysed with RIPA buffer (Beyotime,
Nangtong, China) with the addition of 1% fresh protease inhibitor
cocktail 1 and/or phosphatase inhibitor cocktail 2 (Sigma) and 1 μl
100 mM phenylmethylsulfonyl fluoride (Beyotime). After removal by
scraping, the cells were placed in ice for 30 min and centrifuged
at 12,000 rpm for 10 min. The protein concentration of the samples
was determined using an Enhanced BCA protein assay kit (Beyotime).
The protein concentrations were quantified and 30 μg protein per
sample was separated on 8% SDS-PAGE. The separated proteins were
transferred onto polyvinylidene difluoride membranes (Millipore).
After blocking with 5% non-fat milk for 1 h at room temperature,
the membranes were probed with primary antibodies for 2 h at room
temperature, followed by appropriate horseradish
peroxidase-conjugated secondary antibodies (all from Abcam) for 1 h
at room temperature. The blots were detected using Pierce ECL Plus
Western Blotting Substrate (Thermo Fisher) and developed using
X-ray film.
siRNA transfection
The negative control and mTOR siRNAs were purchased
from GenePharma Biological Technology (Shanghai, China). The target
sequence of mTOR siRNA was 5′-GGCCUAUGGUCGAGAUUUATT-3′ (12). For siRNA transfection, cells at a
concentration of 2.5–5×104 cells/ml were incubated for
24 h in 6-well plates. The cells were then transfected with 200
pmol negative control (NC) and mTOR siRNA for 24–48 h in the
presence of Lipofectamine (Invitrogen) and subjected to western
blotting. We also used a positive control (GAPDH siRNA) and a
fluorescein-labeled negative control to ensure the reliability of
the method and transfection efficiency.
Cell migration assay
Cell migration was evaluated using an in
vitro wound healing assay. Cells were seeded on a 6-well plate
and incubated for 6 h to allow the formation of a cell monolayer.
Cells were scratched with the tip of a 200-μl pipette and then
incubated at 37°C under normoxic or hypoxic conditions for 24 h.
Cell motility was assessed by measuring the speed of wound closure
at specific intervals. Each experiment was conducted in
triplicate.
Statistical analysis
All experiments were repeated at least three times.
The data are expressed as mean ± standard deviation (SD) or
standard error of the mean (SEM). Statistical analysis was
performed using the Student’s t-test (two-sided). The criterion for
statistical significance was P<0.05 or P<0.01.
Results
Relationship between VM and
clinicopathological data of the glioma cases
Thirty-four cases (26.8%) with VM structures were
identified among a total of 127 glioma cases (Table I). These structures were positive
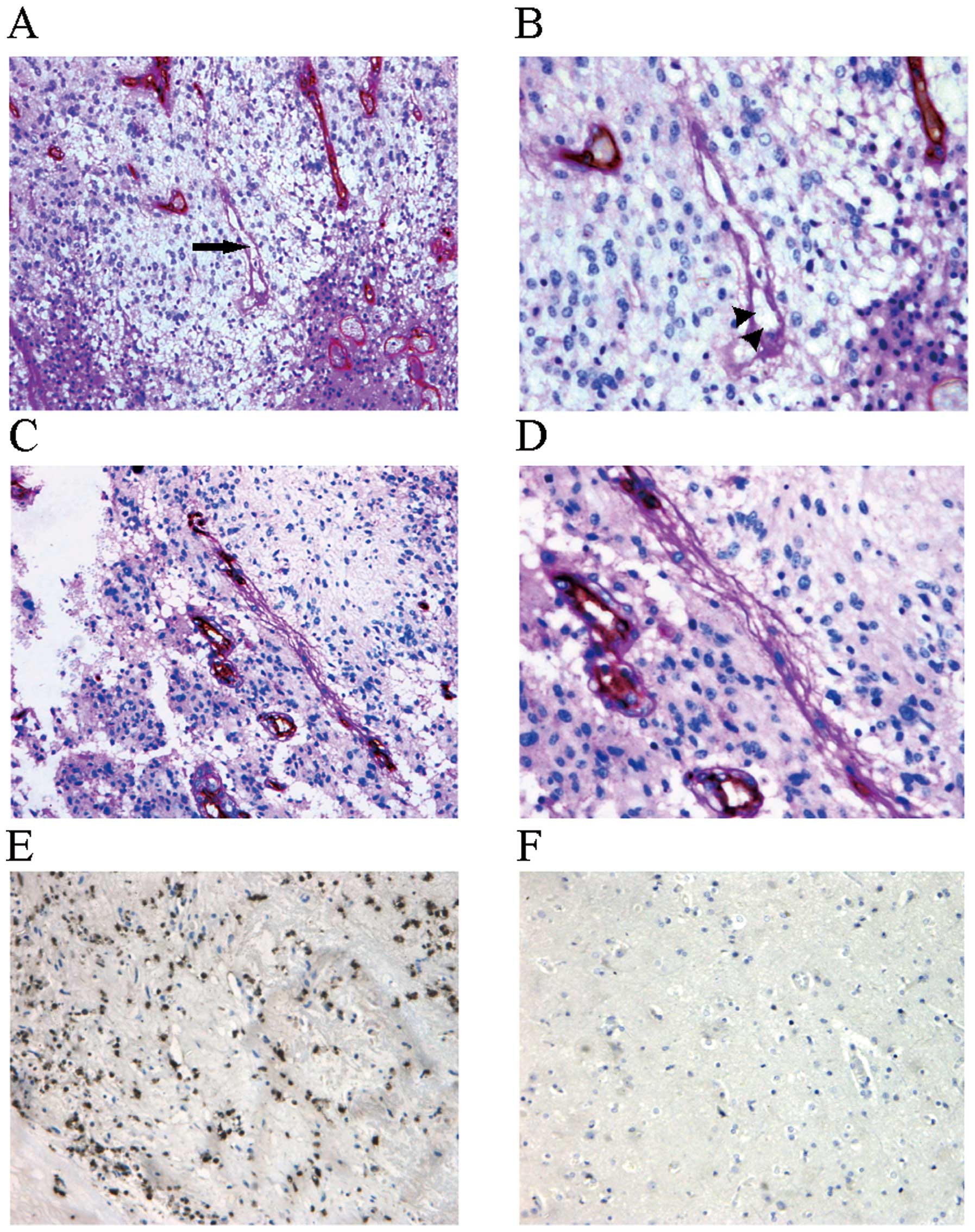
for PAS but negative for CD34 (Fig.
1A), indicating that they did not consist of endothelial cells.
Red blood cells were observed at higher magnification in the VM
structures (Fig. 1B) suggesting
that they had a blood supply function. Some VM structures were even
interlinked with CD34+ endothelial cell-lined blood
vessels (Fig. 1C and D).
 | Table IRelationship between VM and
clinicopathological data of the patients with glioma. |
Table I
Relationship between VM and
clinicopathological data of the patients with glioma.
| | VM | | |
|---|
| |
| | |
|---|
| Variables | Cases | Positive | Negative |
χ2a | P-value |
|---|
| Gender | | | | 0.486 | 0.486 |
| Male | 72 | 21 | 51 | | |
| Female | 55 | 13 | 42 | | |
| Age (years) | | | | 0.011 | 0.917 |
| <60 | 85 | 23 | 62 | | |
| ≥60 | 42 | 11 | 31 | | |
| KPS | | | | 0.026 | 0.872 |
| <60 | 36 | 10 | 26 | | |
| ≥60 | 91 | 24 | 67 | | |
| Tumor size
(cm) | | | | 0.132 | 0.717 |
| <6 | 78 | 20 | 58 | | |
| ≥6 | 49 | 14 | 35 | | |
| Pathological
grade | | | | 9.051 | 0.029b |
| I | 7 | 0 | 7 | | |
| II | 45 | 7 | 38 | | |
| III | 42 | 14 | 28 | | |
| IV | 33 | 13 | 20 | | |
| mTOR
expression | | | | 7.748 | 0.021b |
| −/+ | 10 | 1 | 9 | | |
| ++ | 83 | 18 | 65 | | |
| +++ | 34 | 15 | 19 | | |
Among all of the clinicopathological variants
compared, the pathological grade of gliomas and the mTOR expression
in the tissue sections differed significantly between the
VM-positive and VM-negative group (P<0.05). However, there was
no relationship with other clinicopathological variants such as
gender, age, Karnofsky performance score (KPS) and tumor size.
VM structures were found more frequently in highly
aggressive gliomas (33.3% of grade III and 39.4% of grade IV cases)
compared to the poorly aggressive ones (15.6% of grade II and 0.0%
of grade I cases) (χ2=9.051, P=0.029), which is
consistent with other tumors from different studies (4,13).
mTOR protein expression in the human glioma tissues
was investigated by immunohistochemistry (Fig. 1E and F). VM structures were
significantly more frequent in the tissues with a higher rate of
mTOR expression than the frequency of VM structures in tissues with
a lower rate of mTOR expression (10.0% of −/+, 21.7% of ++ and
44.1% of +++ tissues) (χ2=7.748; P=0.021). These results
strongly imply that VM is not only correlated with tumor grade but
also with mTOR signaling activity in gliomas.
Rapamycin inhibits tube structures in the
U87-MG cell line under normoxia
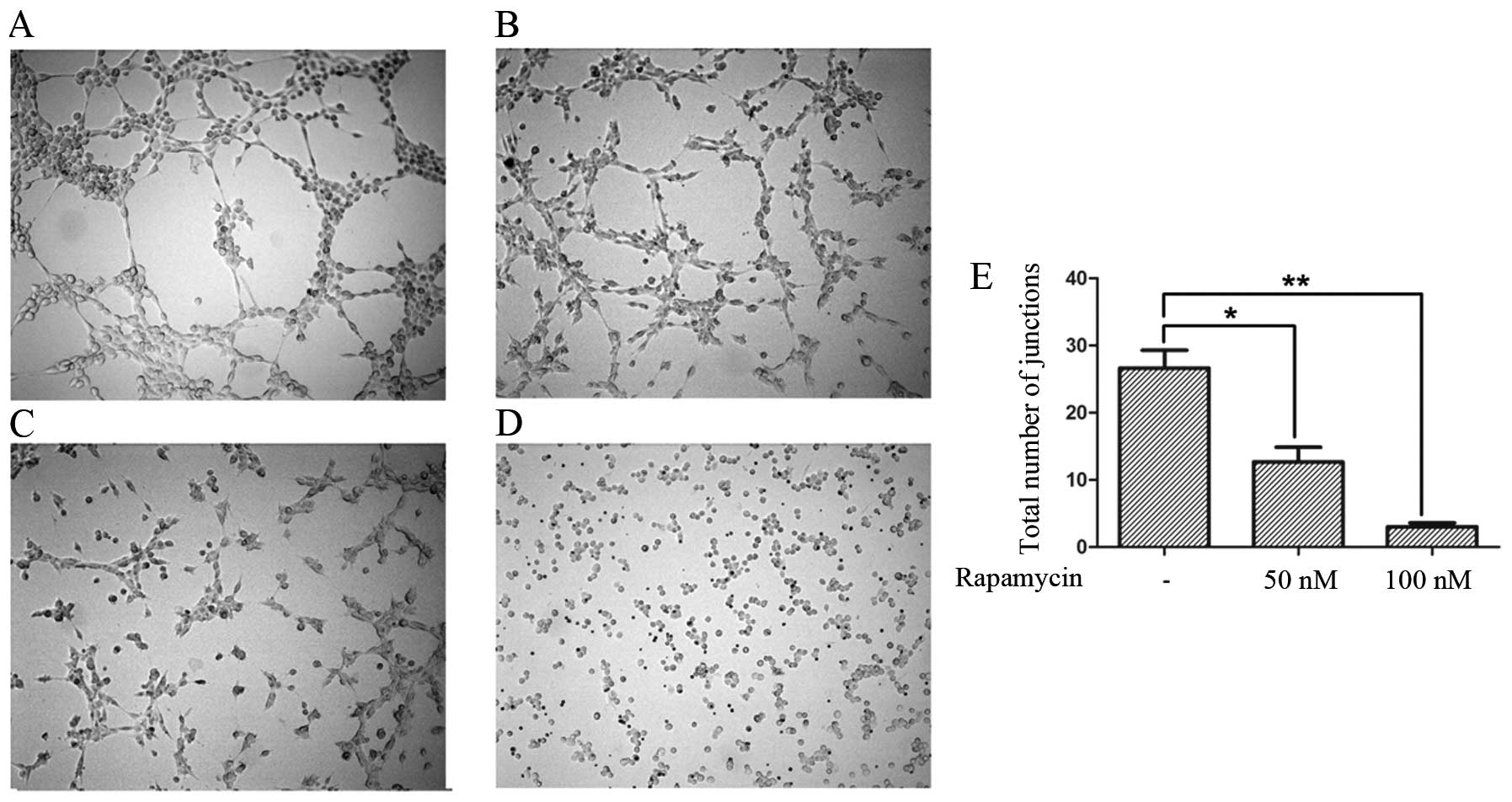
In the in vitro test, under normoxia, U87-MG
cells formed tube structures similar to HUVECs on Matrigel
(Fig. 2A and B). The tube
structures were significantly inhibited with increasing
concentrations of rapamycin (Fig. 2C
and D), and there was a significant difference between the
various degrees of inhibition (Fig.
2E).
Rapamycin inhibits stronger tube
structures in the U87-MG cell line under hypoxia
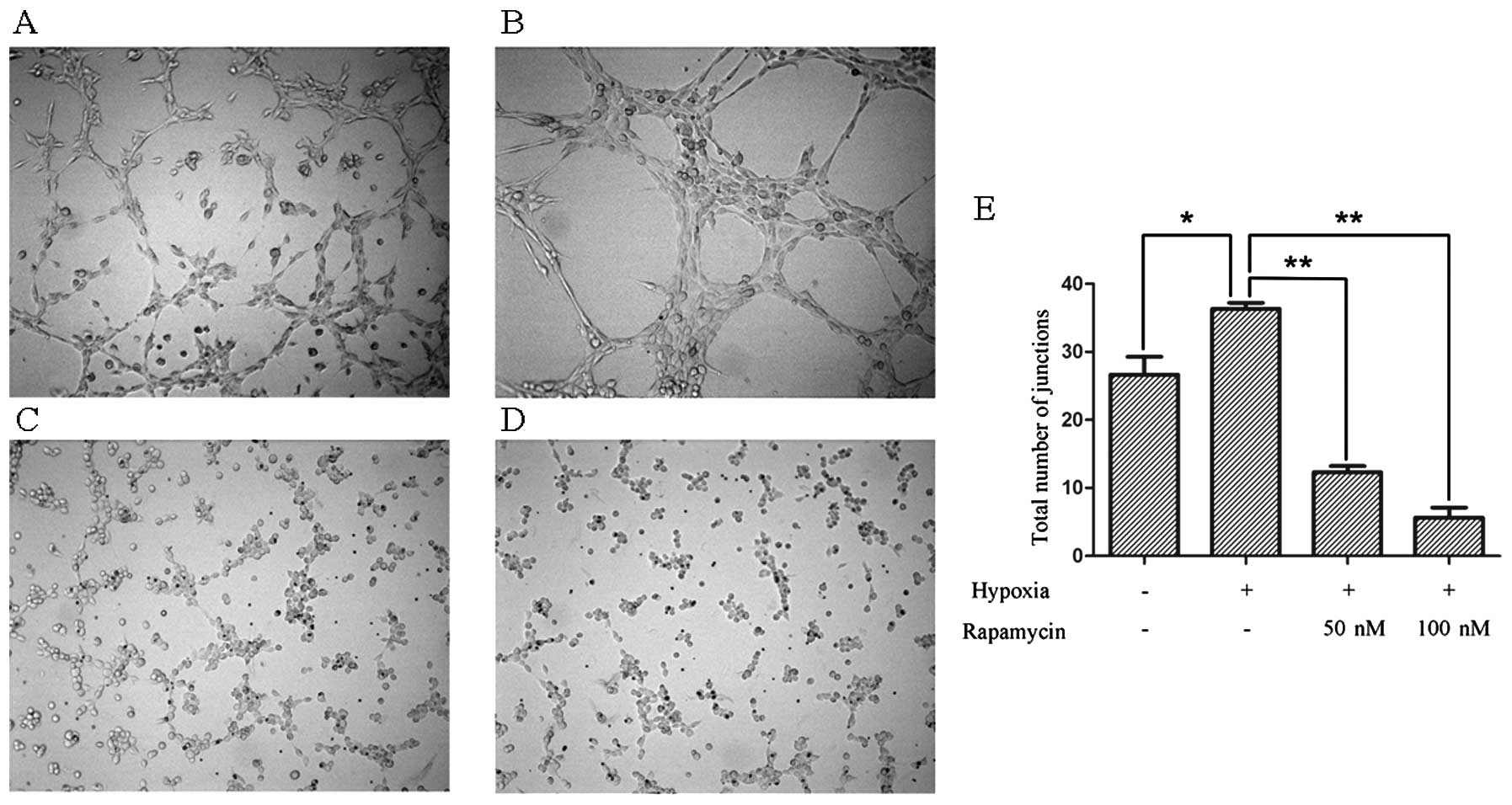
As showed in the previous experiment, the ability of
U87-MG cells to form tube structures on Matrigel appeared defective
under a normoxic condition (Fig.
3A). The tumor cells grown under hypoxic conditions showed
stronger tube structures on Matrigel (Fig. 3B). However, when treated with
rapamycin, these tube structures disappeared (Fig. 3C and D).
Rapamycin inhibits mTOR and HIF-1α
expression under normoxic or hypoxic conditions
Many studies have confirmed that intratumoral
hypoxia is closely related with the formation of VM, and HIF-1α is
often activated (14,15). We first examined the effect of
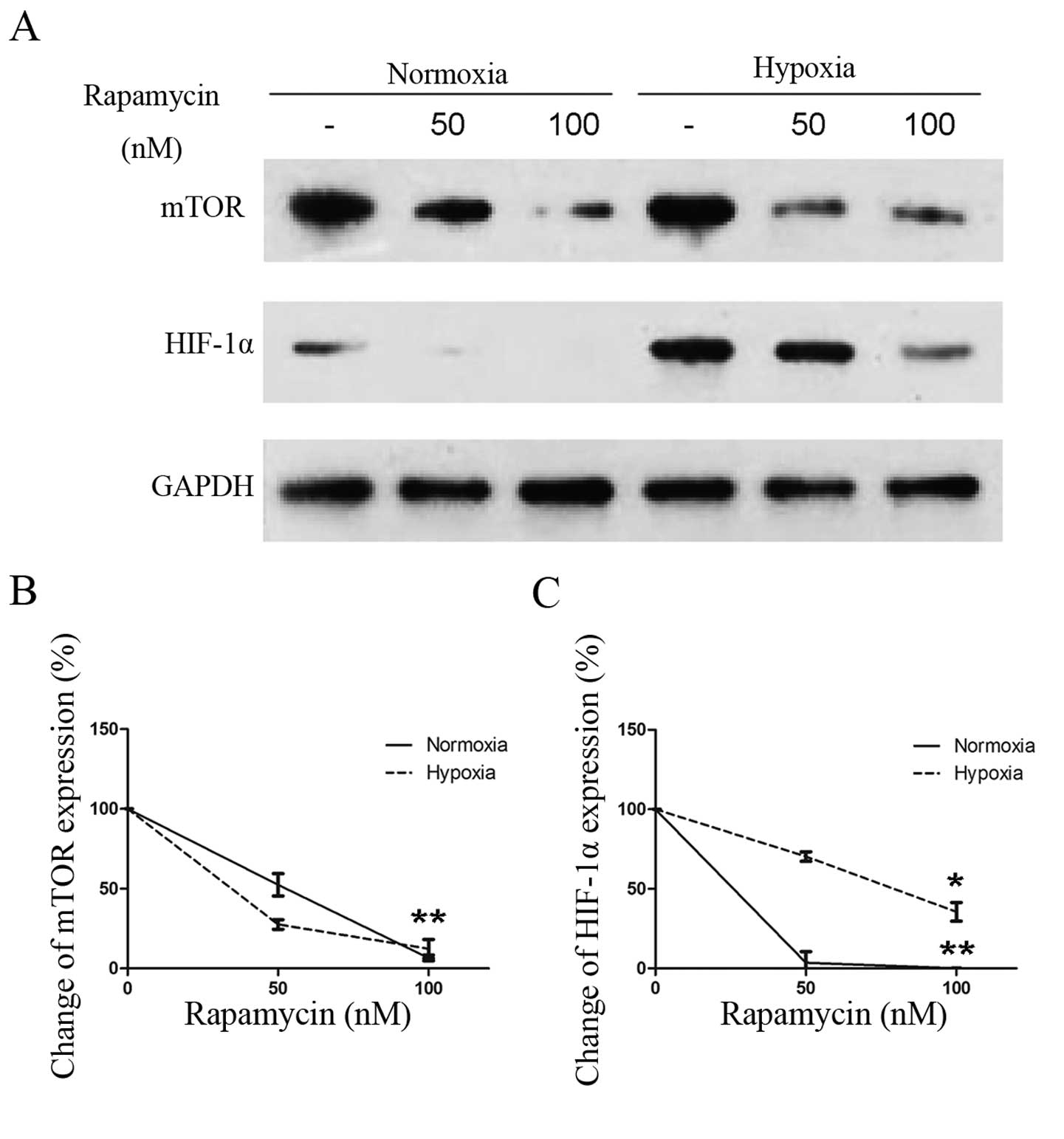
rapamycin on the activation of HIF-1α under normoxic conditions.
Western blotting revealed that rapamycin inhibited HIF-1α
expression, even when it was expressed very low under normoxia
(Fig. 4A). Quantitative analyses of
the western blotting results revealed that treatment with
increasing concentrations of rapamycin induced a dose-dependent
downregulation of HIF-1α protein in addition to inhibiting mTOR
expression (Fig. 4B). Additionally,
rapamycin induced a dose-dependent downregulation of HIF-1α
expression under hypoxia. Inhibition of HIF-1α accumulation by
rapamycin (Fig. 4C) was similar to
that in previous studies of exposure to hypoxia (8,16).
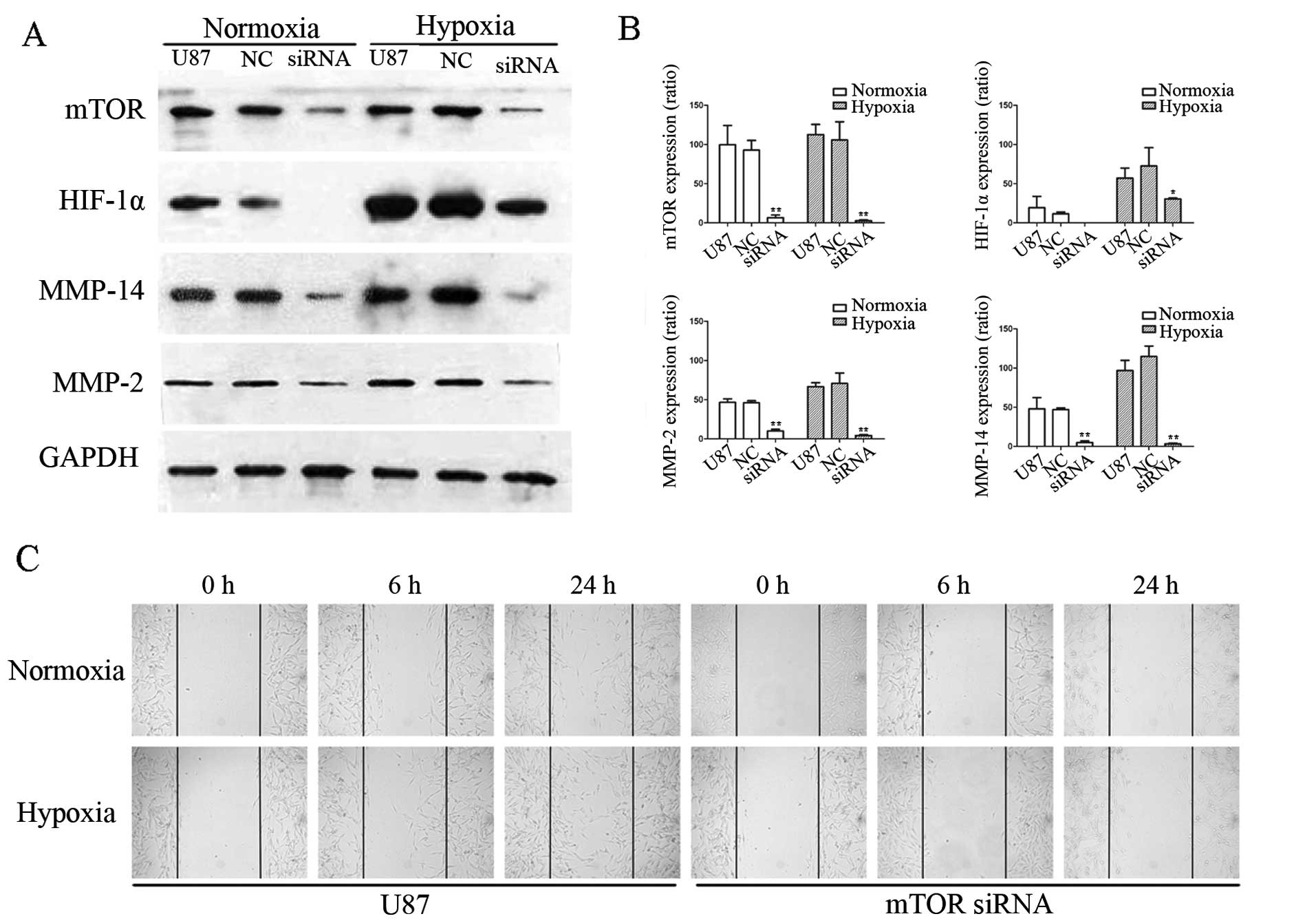
mTOR siRNA significantly decreases
downstream signaling of VM and suppresses glioma cell
migration
To verify the regulation of downstream molecules of
mTOR by rapamycin, U87-MG cells were transfected with mTOR siRNA.
The efficiency and effectiveness of transfection were identified by
western blot analysis. We designed and synthesized four mTOR
siRNAs, and chose one that maximally knocked down mTOR expression,
to screen the downstream signaling of VM. When mTOR expression was
significantly decreased by siRNA, as expected, HIF-1α expression
was also significantly downregulated, under normoxic or hypoxic
conditions (Fig. 5A). Quantitative
analyses of the western blotting results also showed the inhibitory
effects of mTOR siRNA on the expression of related molecules
(Fig. 5B).
Consequently, to establish whether the mTOR
signaling pathway influenced the final stage of VM signaling, we
investigated MMP-14 and MMP-2 expression by western blotting. As
shown in Fig. 5A and B, expression
of both MMPs was lower in the U87-MG cells transfected with mTOR
siRNA than levels in the control cells, even under hypoxia.
MMP-2 is associated with cell migration (17). We further detected the migration of
U87-MG cells when transfected with siRNA. Fig. 5C shows that cell migration increased
after hypoxia for 24 h. However, this increase did not recur after
siRNA interference.
Discussion
The initial morphologic and molecular
characterization of VM was accomplished in human melanoma. In
addition to identification in invasive melanoma, VM has also been
observed in other malignant solid tumors, including prostatic
tumors (18,19), Ewing sarcoma (20), hepatocellular carcinoma (21,22),
colorectal carcinoma (23) and
glioma (24). Our previous study
demonstrated that VM exists in glioblastomas and is a significant
prognostic factor for patient survival (5). In the present study, we confirmed that
VM was present in different grades of glioma, and the amount of VM
in the tumors increased with the grade of glioma. These results are
consistent with a previous study that showed that VM formation is
related to the invasive ability of tumors; more VM structures are
observed in highly aggressive tumors than in less aggressive ones
(4).
In addition to the malignancy of tumor cells and
tumor blood supply, the surrounding microenvironment (such as
hypoxia) is also closely related with VM formation (25,26).
However, no relationship was noted with other clinicopathological
variants such as gender, age, KPS, or tumor size. Taking all these
factors into consideration, VM formed by glioma cells is a novel
tumor microcirculation pattern, which is affected by the
characteristics inside the tumor cells and outside their
microenvironment and differs from classical angiogenesis formed by
endothelial cells. Conventional antiangiogenic therapy targeted
against tumor vasculature mainly refers to the inhibition of
endothelial cells or vascular endothelial growth factor. A previous
study found that treatment with bevacizumab (Avastin) is not
effective, but also elicits VM formation in tumors to accelerate
metastasis, with marked hypoxia (3); thus, targeted therapy for glioma
requires other modalities different from the conventional
antiangiogenic mechanism, and VM might be a suitable choice.
mTOR, a 289-kDa serine-threonine kinase, is a
therapeutic target in glioma (reviewed in ref. 27). Overactivation of the mTOR pathway
seems to play an important role in glioma (28). In the present study, the quantity of
VM structures increased with the grade of the tumor and the level
of mTOR expression. An association between the mTOR signaling
pathway and grade of malignancy of human glioma has been noted
(29). It is clear that mTOR is a
central molecule that controls initiation of protein translation.
Decreased oxygen concentration directly affects mTOR signaling and
increases the synthesis of HIF-1α (8). The present study found that rapamycin,
a special inhibitor of mTOR, significantly inhibited HIF-1α
accumulation in U87-MG cells, which is similar to the results of
other studies from different laboratories, in which hypoxia was
induced by a lower oxygen supply (8,16). All
these studies provide strong support for the conclusion that mTOR
is a positive modulator of the HIF-1α activation pathway and
influences its downstream molecules, including those involved in VM
formation.
In recent years, inhibition of signaling molecules
involved in VM has become another therapeutic approach for blocking
tumor blood supply (30,31). In this study, we found that under
normoxic or hypoxic conditions, treatment of U87-MG cells with
increasing concentrations of rapamycin induced a dose-dependent
reduction in tube structures on Matrigel, indicating that rapamycin
inhibits VM formation of U87-MG cells even when the VM structures
are defective under normoxia. This result gives a more rational
option for the clinical treatment of glioma. Some studies have
found that specific inhibitors of mTOR have a beneficial effect
against gliomas (32–34). However, these studies investigated
the roles of rapamycin or its synthetic analogs in tumor
proliferation, migration, invasion, and autophagy. In our study,
both rapamycin intervention and RNAi confirmed that mTOR is
involved in VM formation, which demonstrates a novel role of mTOR
in glioma.
Various molecules involved in VM formation have been
investigated in different tumors, including HIF-1α (14,35),
VE-cadherin (36,37), EphA2 (37,38),
MMP-14 (39), MMP-2 (39) and Ln-5-γ2 chain (40). Following the identification of the
above-described molecules involved in VM, a classical model of the
signaling cascade implicated in VM was suggested (reviewed in ref.
10). Pertinent to VM, in this
model, hypoxia is initiated to directly modulate EphA2 gene
expression (via HIF-1α) or to indirectly modulate VE-cadherin
expression (via activation of an intermediary protein),
consequently promoting the rest of the signaling cascade (41). In our study, consistent with the
proposed cascade, inhibition of mTOR was shown to abrogate glioma
VM formation. Therefore, we infer that mTOR is involved in VM
formation, and is an upstream molecule of HIF-1α. In the final
stage of the VM signaling pathway, expression and activation of
MMP-14 activates MMP-2. MMP-2 combines with MMP-14 to cleave
Ln-5-γ2 chain into promigratory fragments. Release of these
fragments into the tumor microenvironment can increase migration,
invasion, and ultimately result in VM (10). In the present study, we knocked down
the mTOR gene and found that both MMP-14 and MMP-2 were decreased
under normoxic or hypoxic conditions. The wound healing assay also
showed a significant difference between groups with or without
interference by siRNA. All of these results confirm that the
downstream molecules were influenced by mTOR siRNA. We infer that
mTOR signaling is involved in VM formation in gliomas, and
inhibition of mTOR can block expression of the downstream molecules
in the VM formation signal cascade.
A recent systematic review and meta-analysis showed
that VM-positive cancer patients had a poor 5-year overall survival
compared with VM-negative malignant tumor cases (42). Thus, treatment targeted against VM
seems essential. Given the importance of mTOR as outlined above, if
we can find an effective drug against mTOR similar to rapamycin, it
may be possible to provide a more rational and effective
vascular-targeted therapy for gliomas. In the past 10 years,
several agents have been designed to target the mTOR pathway and
many other mTOR signaling pathway inhibitors are being studied in
clinical trials. Temsirolimus, everolimus, and ridaforolimus are
rapalogs that share the same mechanism of action but differ in
pharmacokinetic properties because of different substitutions at
position C-40 of rapamycin (27,43).
The present study found and explained, for the first time, the
mechanisms of mTOR participation in the alternative form of tumor
blood supply, VM, which provides a novel potential therapeutic
target for gliomas. It is noteworthy that mTOR is located at the
top of the VM signaling cascade, which can directly sense changes
in several signals (such as energy or oxygen level). Therefore, VM
treatment targeted against mTOR may be more effective than other
downstream molecules. However, the methods we used in our study are
mainly in vitro experiments, and animal experiments are
needed to confirm the cell-based data. On the other hand, mTOR is a
macromolecule (289 kDa) and can be divided into different
components (44). Which part is
involved specifically in VM formation warrants further study, and
any further research will aid our understanding of VM
formation.
In conclusion, the present study demonstrated that
VM structures are found in highly aggressive glioma tissues by
PAS/CD34 double-staining and rapamycin can inhibit VM formation in
U87-MG cells under normoxic or hypoxic conditions. We also found
that rapamycin and mTOR siRNA can inhibit molecules involved in VM
formation via HIF-1α. All of these results indicate that the mTOR
signaling pathway is involved in the formation of VM. This study
may provide preliminary evidence for a more integrated signaling
cascade for VM formation, and mTOR is a potential therapeutic
target for gliomas.
Acknowledgements
This research was supported in part by the National
Natural Science Foundation of China (NSFC) (81272806 to Y.K.) and
(81302199 to X.S.), the Natural Science Foundation of Guangdong
Province, China (S2012010009088 to Y.K.) and the Medical Scientific
Research Foundation of Guangdong Province, China (B2013246 to
X.S.).
References
|
1
|
Ahmadloo N, Kani AA, Mohammadianpanah M,
et al: Treatment outcome and prognostic factors of adult
glioblastoma multiforme. J Egypt Natl Canc Inst. 25:21–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reardon DA, Herndon JE II, Peters K, et
al: Outcome after bevacizumab clinical trial therapy among
recurrent grade III malignant glioma patients. J Neurooncol.
107:213–221. 2012. View Article : Google Scholar
|
|
3
|
Xu Y, Li Q, Li XY, Yang QY, Xu WW and Liu
GL: Short-term anti-vascular endothelial growth factor treatment
elicits vasculogenic mimicry formation of tumors to accelerate
metastasis. J Exp Clin Cancer Res. 31:162012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maniotis AJ, Folberg R, Hess A, et al:
Vascular channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SY, Ke YQ, Lu GH, et al: Vasculogenic
mimicry is a prognostic factor for postoperative survival in
patients with glioblastoma. J Neurooncol. 112:339–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SY, Yu L, Ling GQ, et al:
Vasculogenic mimicry and its clinical significance in
medulloblastoma. Cancer Biol Ther. 13:341–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Land SC and Tee AR: Hypoxia-inducible
factor 1alpha is regulated by the mammalian target of rapamycin
(mTOR) via an mTOR signaling motif. J Biol Chem. 282:20534–20543.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su M, Feng YJ, Yao LQ, et al: Plasticity
of ovarian cancer cell SKOV3ip and vasculogenic mimicry in vivo.
Int J Gynecol Cancer. 18:476–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paulis YW, Soetekouw PM, Verheul HM,
Tjan-Heijnen VC and Griffioen AW: Signalling pathways in
vasculogenic mimicry. Biochim Biophys Acta. 1806:18–28.
2010.PubMed/NCBI
|
|
11
|
Zhu P, Ning Y, Yao L, Chen M and Xu C: The
proliferation, apoptosis, invasion of endothelial-like epithelial
ovarian cancer cells induced by hypoxia. J Exp Clin Cancer Res.
29:1242010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snijder B, Sacher R, Ramo P, et al:
Single-cell analysis of population context advances RNAi screening
at multiple levels. Mol Syst Biol. 8:5792012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu R, Yang K, Meng C, Zhang Z and Xu Y:
Vasculogenic mimicry is a marker of poor prognosis in prostate
cancer. Cancer Biol Ther. 13:527–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun W, Shen ZY, Zhang H, et al:
Overexpression of HIF-1α in primary gallbladder carcinoma and its
relation to vasculogenic mimicry and unfavourable prognosis. Oncol
Rep. 27:1990–2002. 2012.
|
|
15
|
Comito G, Calvani M, Giannoni E, et al:
HIF-1α stabilization by mitochondrial ROS promotes Met-dependent
invasive growth and vasculogenic mimicry in melanoma cells. Free
Radic Biol Med. 51:893–904. 2011.
|
|
16
|
Abraham RT: mTOR as a positive regulator
of tumor cell responses to hypoxia. Curr Top Microbiol Immunol.
279:299–319. 2004.PubMed/NCBI
|
|
17
|
Wang L, Zhang ZG, Zhang RL, et al: Matrix
metalloproteinase 2 (MMP2) and MMP9 secreted by
erythropoietin-activated endothelial cells promote neural
progenitor cell migration. J Neurosci. 26:5996–6003. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmadi SA, Moinfar M, Gohari Moghaddam K
and Bahadori M: Practical application of angiogenesis and
vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med.
13:498–503. 2010.PubMed/NCBI
|
|
19
|
Sharma N, Seftor RE, Seftor EA, et al:
Prostatic tumor cell plasticity involves cooperative interactions
of distinct phenotypic subpopulations: role in vasculogenic
mimicry. Prostate. 50:189–201. 2002. View Article : Google Scholar
|
|
20
|
van der Schaft DW, Hillen F, Pauwels P, et
al: Tumor cell plasticity in Ewing sarcoma, an alternative
circulatory system stimulated by hypoxia. Cancer Res.
65:11520–11528. 2005.PubMed/NCBI
|
|
21
|
Liu WB, Xu GL, Jia WD, et al: Prognostic
significance and mechanisms of patterned matrix vasculogenic
mimicry in hepatocellular carcinoma. Med Oncol. 28:S228–S238. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun T, Sun BC, Zhao XL, et al: Promotion
of tumor cell metastasis and vasculogenic mimicry by way of
transcription coactivation by Bcl-2 and Twist1: a study of
hepatocellular carcinoma. Hepatology. 54:1690–1706. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baeten CI, Hillen F, Pauwels P, de Bruine
AP and Baeten CG: Prognostic role of vasculogenic mimicry in
colorectal cancer. Dis Colon Rectum. 52:2028–2035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue WY and Chen ZP: Does vasculogenic
mimicry exist in astrocytoma? J Histochem Cytochem. 53:997–1002.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun B, Zhang D, Zhang S, Zhang W, Guo H
and Zhao X: Hypoxia influences vasculogenic mimicry channel
formation and tumor invasion-related protein expression in
melanoma. Cancer Lett. 249:188–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao N, Sun BC, Sun T, et al:
Hypoxia-induced vasculogenic mimicry formation via VE-cadherin
regulation by Bcl-2. Med Oncol. 29:3599–3607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
29
|
Li XY, Zhang LQ, Zhang XG, et al:
Association between AKT/mTOR signalling pathway and malignancy
grade of human gliomas. J Neurooncol. 103:453–458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itzhaki O, Greenberg E, Shalmon B, et al:
Nicotinamide inhibits vasculogenic mimicry, an alternative
vascularization pathway observed in highly aggressive melanoma.
PLoS One. 8:e571602013. View Article : Google Scholar
|
|
31
|
Serwe A, Rudolph K, Anke T and Erkel G:
Inhibition of TGF-β signaling, vasculogenic mimicry and
proinflammatory gene expression by isoxanthohumol. Invest New
Drugs. 30:898–915. 2012.
|
|
32
|
Iwamaru A, Kondo Y, Iwado E, et al:
Silencing mammalian target of rapamycin signaling by small
interfering RNA enhances rapamycin-induced autophagy in malignant
glioma cells. Oncogene. 26:1840–1851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Liu Y, Liu J, et al: Rapamycin
inhibits human glioma cell proliferation through down-regulating
mammalian target of rapamycin pathway and up-regulating
microRNA-143. Head Neck Oncol. 4:662012.
|
|
34
|
Heimberger AB, Wang E, McGary EC, et al:
Mechanisms of action of rapamycin in gliomas. Neuro Oncol. 7:1–11.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Misra RM, Bajaj MS and Kale VP:
Vasculogenic mimicry of HT1080 tumour cells in vivo: critical role
of HIF-1α-neuropilin-1 axis. PLoS One. 7:e501532012.PubMed/NCBI
|
|
36
|
Hendrix MJ, Seftor EA, Meltzer PS, et al:
Expression and functional significance of VE-cadherin in aggressive
human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad
Sci USA. 98:8018–8023. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hess AR, Seftor EA, Gruman LM, Kinch MS,
Seftor RE and Hendrix MJ: VE-cadherin regulates EphA2 in aggressive
melanoma cells through a novel signaling pathway: implications for
vasculogenic mimicry. Cancer Biol Ther. 5:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen LX, He YJ, Zhao SZ, et al: Inhibition
of tumor growth and vasculogenic mimicry by curcumin through
down-regulation of the EphA2/PI3K/MMP pathway in a murine choroidal
melanoma model. Cancer Biol Ther. 11:229–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane Type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
40
|
Seftor RE, Seftor EA, Kirschmann DA and
Hendrix MJ: Targeting the tumor microenvironment with chemically
modified tetracyclines: inhibition of laminin 5 gamma2 chain
promigratory fragments and vasculogenic mimicry. Mol Cancer Ther.
1:1173–1179. 2002.
|
|
41
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: vasculogenic mimicry in
tumor cells: diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z
and Zhou Q: Tumour vasculogenic mimicry is associated with poor
prognosis of human cancer patients: a systemic review and
meta-analysis. Eur J Cancer. 49:3914–3923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ballou LM and Lin RZ: Rapamycin and mTOR
kinase inhibitors. J Chem Biol. 1:27–36. 2008. View Article : Google Scholar
|
|
44
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|