Introduction
Colorectal cancer (CRC) is a common malignancy and
it remains the third leading cause of mortality among all human
malignancies (1–3). The risk of CRC development is
determined by genetic predisposition combined with environmental
influences, such as bacterial infections that disrupt the mucosal
barrier of the gastrointestinal tract leading to aberrant
inflammation (4). Tumor-associated
inflammation contributes to tumor growth and progression through
multiple mechanisms including increased cell proliferation and
anti-apoptotic signaling, promotion of angiogenesis, tumor immune
evasion and metastasis (5,6). Many inflammatory signals promote
tumorigenesis by activating nuclear transcription factor κB (NF-κB)
and the Janus-activated kinase (JAK)/signal transducer and
activator of transcription (STAT) signaling pathways, both in tumor
and stroma cells. Constitutively activated STAT3 and STAT5 are
expressed in a wide variety of human malignancies including
colorectal carcinomas, and often correlate with a poor prognosis
and resistance to therapies.
The JAK/STAT pathway is involved in inflammation,
proliferation and invasion/migration (7). Constitutive activation of STAT3,
resulting in an unregulated increase in cell proliferation and
reduction in cell apoptosis, is strongly correlated with the
development of numerous types of cancer including CRC (8). Therefore, inhibiting cell
proliferation and/or promoting apoptosis by the suppression of
STAT3 activation has been a major focus in the development of
anticancer drugs. Patients with CRC often exhibited elevated levels
of interleukin (IL)-6 in the serum (9) and constitutively activated STAT3,
which is expressed in the majority of colorectal tumors is
associated with a significantly higher mortality (10). Recently, it has been demonstrated
that the constitutive activation of JAK/STAT signaling is involved
in the development of CRC in cell growth, survival, invasion and
migration (11), thereby shedding
light on new therapeutic strategies for CRC treatment by
influencing the IL-6/JAK/STAT3 pathway.
AZD1480, an ATP competitive inhibitor of JAK1 and
JAK2, was recently shown to inhibit the growth of tumors including
human glioblastoma, myeloma, multiple sclerosis and lung cancer
(12–15). AZD1480 inhibited constitutive and
IL-6-induced STAT3 activation and subsequent nuclear translocation.
The ability of AZD1480 to effectively limit tumor volume was
attributed to the inhibition of STAT3. Stuart et al
conducted a study with AZD1480 to confer the therapeutic benefits
in two murine models of inflammation-associated gastrointestinal
cancer. Their results provide the first evidence that the
pharmacologic targeting of AZD1480 affords the therapeutic
suppression of inflammation-associated gastrointestinal cancer
progression in vivo (16).
In the present study, we performed an in vitro study to
examine the efficacy and potential antitumor effects of AZD1480 in
CRC, which demonstrated a therapeutic benefit for targeting
JAK/STAT signaling in CRC.
Materials and methods
Drugs
AZD1480 was provided by Selleckchem (Houston, TX,
USA). For the in vitro experiments, AZD1480 was dissolved in
100% DMSO to prepare a 10 mM stock and stored at −20°C. Recombinant
human IL-6 and tumor necrosis factor (TNF)-α (purchased from
PeproTech) were reconstituted in sterile 1X phosphate-buffered
saline (PBS) containing 0.1% BSA to prepare a 103 ng/ml
stock and stored at −20°C.
Cell lines and cell culture
conditions
Human colon carcinoma HCT116, SW480 and HT29 cell
lines were obtained from the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China) and preserved in our
institute. Cells were cultured in RPMI-1640 medium containing 10%
fetal bovine serum (Gibco, Grand Island, NY, USA) and 50 U/ml
penicillin and streptomycin at 37°C in an atmosphere of 5%
CO2, and passaged twice a week.
Western blot analysis
Tissues were homogenized and cells were lysed. Total
proteins were extracted by RIPA lysis buffer (Beyotime Inc.,
Shanghai, China) and equal amounts of protein were electrophoresed
on a 12% SDS-polyacrylamide gel and subsequently transferred to
polyvinylidene fluoride membranes. The membranes were blocked in 5%
skim milk in PBS containing 0.1% Tween-20 for 1 h at room
temperature. The membranes were incubated with the following
primary antibodies at 4°C overnight: rabbit anti-phospho-STAT3
(Tyr705), anti-phospho-JAK2 (Y1007/1008), STAT3, JAK2, PARP,
anti-phospho-NF-κB p65 and GAPDH were purchased from Cell
Signalling Technology (BSN; USA). The membranes were then washed
three times with Tris-buffered saline Tween-20 (TBST) and incubated
with horseradish peroxidase-conjugated secondary antibody (1:1,000;
Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) at
room temperature for 2 h. After three TBST washes, the membranes
were developed using ECL Plus (Millipore, Billerica, MA, USA) and
exposed to X-ray film for the visualisation of protein bands. GAPDH
was used as an internal loading control.
Quantitative PCR
Total RNAs from cells were extracted using RNAiso
Plus and reverse transcription (RT) reactions were performed using
a PrimeScript RT reagent kit (both from Takara, Dalian, China)
according to the manufacturer’s instructions. Quantitative PCR
(qPCR) was carried out in triplicate using a SYBR-Green PCR kit
(Roche, Indianapolis, IN, USA) on the StepOnePlus Real-Time PCR
System (Applied Biosystems, Foster City, CA, USA) using 1 μl
diluted cDNA as a template in a 20 μl reaction volume. PCR reaction
was carried out as follows: 95°C for 30 sec, 40 cycles of 95°C for
5 sec, 60°C for 31 sec; and the dissociation stage: 95°C for 15
sec, 60°C for 1 min and 95°C for 15 sec. The primers used for qPCR
were: cyclin D2, forward: 5′-CTGTCTCTGATCCGCAAGCAT-3′ and
reverse: 5′-GGTGGGTACATGGCAAACTTAAA-3′; c-Myc, forward:
5′-TCCCTCCACTCGGAAGGAC-3′ and reverse: 5′-CTGGTGCATTTTCGGTTGTTG-3′;
IL-6, forward: 5′-CCTGAACCTTCCAAAGATGGC-3′ and reverse:
5′-TTCACCAGGCAAGTCTCCTCA-3′; IL-8, forward:
5′-ACTGAGAGTGATTGAGAGTGGAC-3′ and reverse:
5′-AACCCTCTGCACCCAGTTTTC-3′; β-actin, forward:
5′-AGAAAATCTGGCACCACACC-3′ and reverse: 5′-TAGCACAGCCTGGATAGCAA-3′.
The 2−ΔΔCT method was used to determine relative gene
expression levels with β-actin as the endogenous control to
normalise the data.
Cell proliferation assay
Cells were plated in 96-well plates at a density of
4×103 cells/well and the Cell Counting Kit-8 (CCK-8;
Beyotime Institute of Biotechnology) was used as previously
described (?). CCK-8 (10 μl) solution was added to each well and
incubated for 1 h. The absorbance at 450 nm was calculated using a
microplate reader. Results are representative of three individual
experiments in triplicate.
Apoptosis assay by flow cytometry
Untreated and drug-treated cells were cultured in
6-well plates for 48 and 72 h. The apoptotic, dead and adherent
cells were subsequently collected and resuspended in cold PBS for
analysis. Apoptosis was examined using the Alexa Fluor®
647/7-AAD Apoptosis kit (BioLegend, San Diego, CA, USA) according
to the manufacturer’s instructions. Data were analyzed by flow
cytometry (Becton-Dickinson, San Jose, CA, USA).
Immunofluorescence assay
HT29 cells were treated with AZD1480 at different
doses. Forty-eight hours after being disposed, the cells were fixed
with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100
in PBS. Rabbit anti-phospho-STAT3 was used as a primary antibody,
and fluroescein isothiocyanate (FITC)-conjugated goat anti-rabbit
IgG (A0562; Beyotime) was used as a secondary antibody to visualize
phospho-STAT3. Nuclei were stained with DAPI.
Statistical analysis
Statistical analysis was performed with the SPSS
software 17.0. Data were presented as means ± standard deviation
(SD). The significance of the differences between groups was
estimated by one-way ANOVA. P<0.05 was considered to indicate a
statistically significant result.
Results
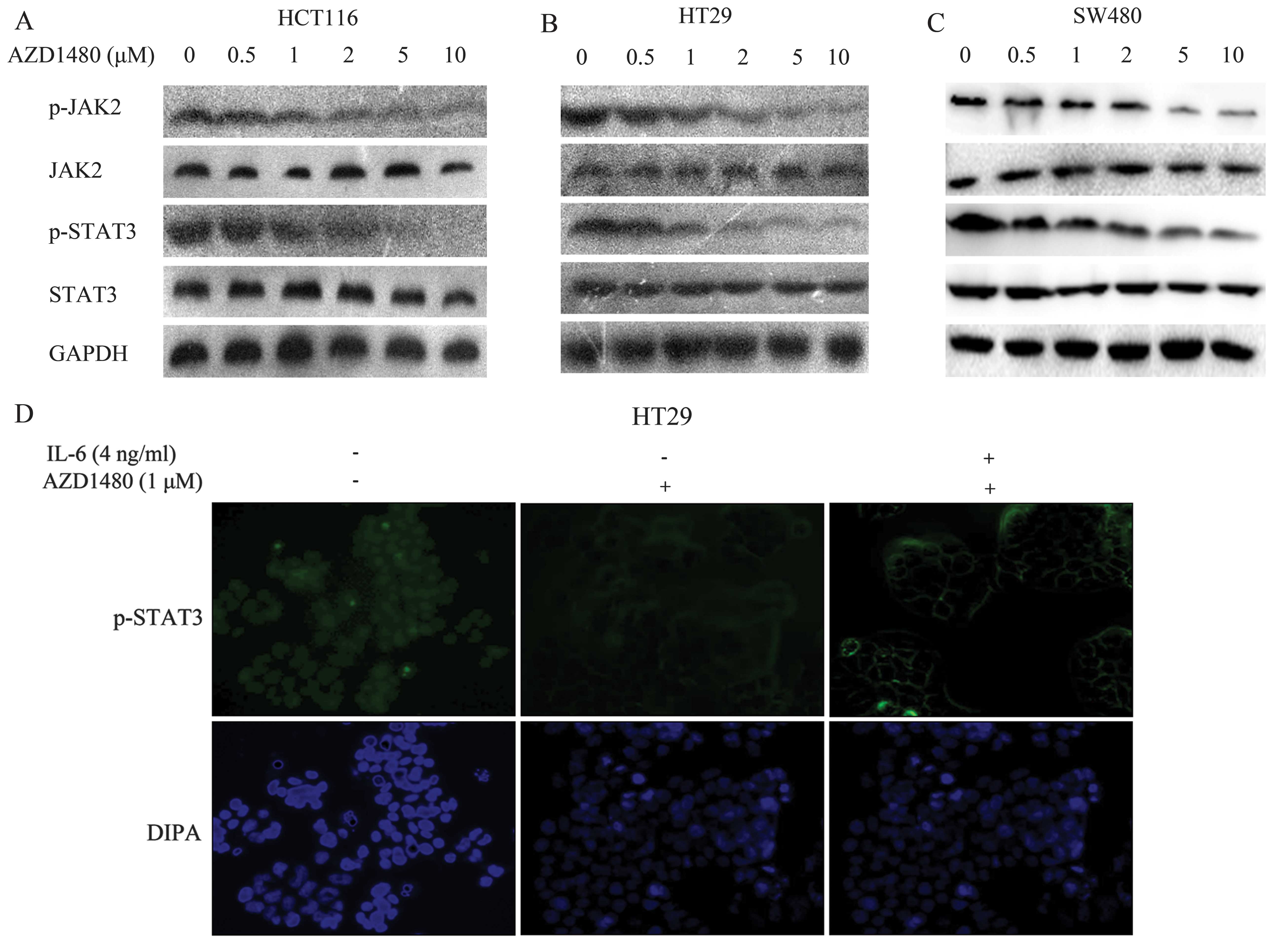
AZD1480 prevents constitutive STAT3 and
JAK2 activation in CRC cells
The inhibitory effect of AZD1480 on JAK/STAT3
signaling in three human CRC cell lines (HCT116, HT29 and SW480)
was examined. Treatment of CRC cells with AZD1480 at different
doses (0, 0.5, 1, 2, 5 and 10 μM) prevented constitutive STAT3 and
JAK2 phosphorylation in the three CRC cell lines for 2 h. Western
blot analysis showed that phosphorylated JAK2 and STAT3 were
markedly decreased from 1 μM (HCT116) (n=6, P<0.05), 2 μM (HT29)
(n=6, P<0.05), and 5 μM (SW480) (n=6, P<0.05) respectively,
and lasting for 10 μM (Fig. 1A–C).
The results showed that, AZD1480 inhibited the constitutive
activation of JAK2 and STAT3 in CRC cell lines. We also determined
the effect of AZD1480 on the signaling by immunofluorescence.
Results of recent studies have shown that
phosphorylated STAT3 translocates to the nucleus by the treatment
of IL-6 (17), thus we explored
whether AZD1480 prevented this process. HT29 cells were treated
with the indicated doses of AZD1480 for 2 h prior to the 2 h
stimulation with IL-6. The cells were fixed and stained by the
anti-phosphorylated STAT3 primary antibody and the FITC-conjugated
secondary antibody. The nucleus was stained with DAPI. Fig. 1D shows that phosphorylated STAT3 and
non-activated STAT3 are almost located in the cytoplasm instead of
the nucleus. Thus, STAT3 translocates to the nucleus when
activated.
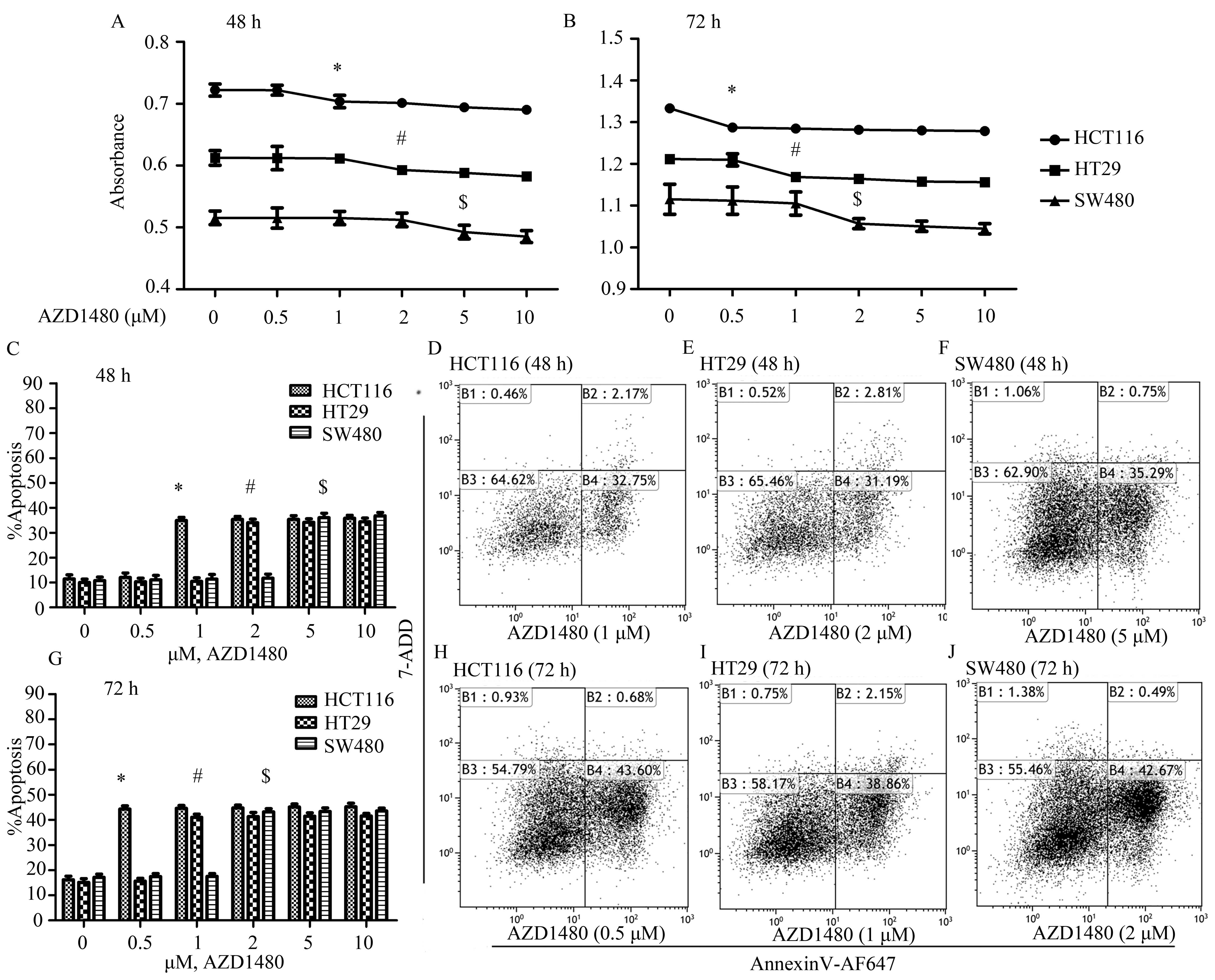
AZD1480 treatment inhibits proliferation
and induces apoptosis of human CRC cell lines
Since inhibition of JAK/STAT signaling can decrease
proliferation and induce apoptosis of CRC cells (18), we initially detected the effects of
treatment with AZD1480 on the proliferation of HCT116, HT29 and
SW480 cells. Results of the CCK-8 assays suggested that AZD1480
markedly inhibits the growth of HCT116, HT29 and SW480 cells in a
time- and dose-dependent manner (Fig.
2A and B). The concentration for inhibition of proliferation at
48 h was from ~1 μM for HCT116 cells (n=5, P<0.05) and from ~2
μM for HT29 cells (n=5, P<0.05), while in the same cell lines
the concentration at 72 h was from ~0.5 μM (n=5, P<0.05) and
from 1 μM (n=5, P<0.05), respectively. SW480 cells required
higher concentrations of AZD1480 at 72 h from ~2 μM (n=5,
P<0.05). However, these concentrations of AZD1480 did not
significantly alter the proliferation of HCT116, HT29 and SW480
cells at 24 h (data not shown).
Flow cytometry was applied to analyze the apoptotic
effect of AZD1480 in the HCT116, HT29 and SW480 cell lines. The
data suggested that AZD1480 induces the apoptosis of HCT116, SW480
and HT29 cells in a time- and dose-dependent manner (Fig. 2C–J). The percentage of apoptosis was
markedly elevated from 1 μM at 48 h (n=5, P<0.05) and 0.5 μM at
72 h (n=5, P<0.05) of HCT116 cells. In addition, the apoptotic
ratio at 48 h of HT29 and SW480 cells increased 23.93% at 2 μM
(n=5, P<0.05) and 25.29% at 5 μM (n=5, P<0.05), respectively,
while at 72 h the data increased 42% (n=5, P<0.05) and 45.89%
(n=5, P<0.05) for HT29 and SW480 cells, respectively, compared
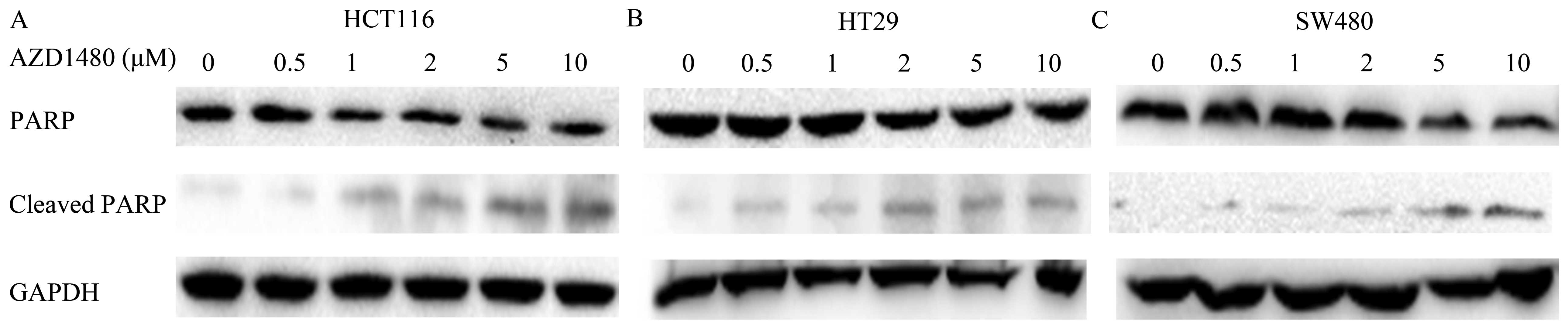
with the untreated cells. Western blot analysis was used to assess
the effect of AZD1480 inducing human CRC cell death by testing the
presence of cleaved PARP. After treatment with AZD1480 of CRC cells
for 24 h, the cleavage of PARP was significantly increased from 2
μM (HCT116) (n=6, P<0.05), 5 μM (HT29) (n=6, P<0.05), and 10
μM (SW480) (n=6, P<0.05) respectively, indicating induction of
cell death (Fig. 3A–C).
Our results also showed that treatment with AZD1480
was more effective in inhibiting proliferation and inducing
apoptosis in vitro.
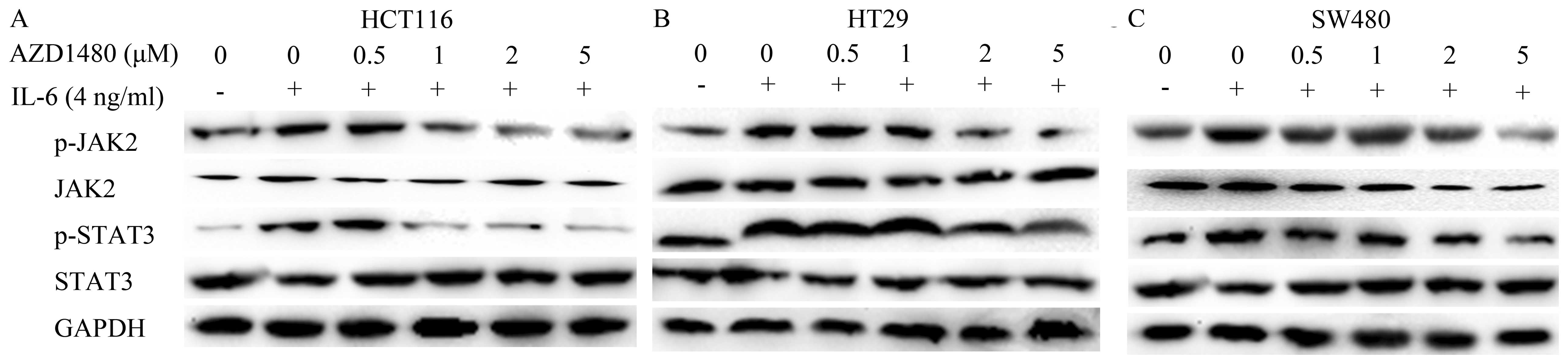
AZD1480 inhibits IL-6-inducible
JAK2/STAT3 signaling pathways
IL-6 is known as an important tumor-promoting
cytokine that enhances proliferation and anti-apoptotic effects in
tumor cells (11). Moreover,
IL-6/JAK/STAT signaling has a critical role in various aspects
including initiation, development and formation in CRC (19). Thus, we detected whether AZD1480
attenuated IL-6-induced JAK/STAT signaling by immunoblotting, and
subsequently induced antitumor effects in CRC cells. Western blot
analysis showed that treatment with AZD1480 decreased the
IL-6-induced activation of JAK/STAT in a dose-dependent manner in
the three CRC cell lines. However, the protein level of JAK2 and
STAT3 did not change after treatment with AZD1480. We also observed
that phosphorylated JAK2 and STAT3 were markedly increased
following IL-6 stimulation in HCT116 (n=6, P<0.05), HT29 (n=6,
P<0.05), and SW480 cells (n=6, P<0.05) (Fig. 4A–C).
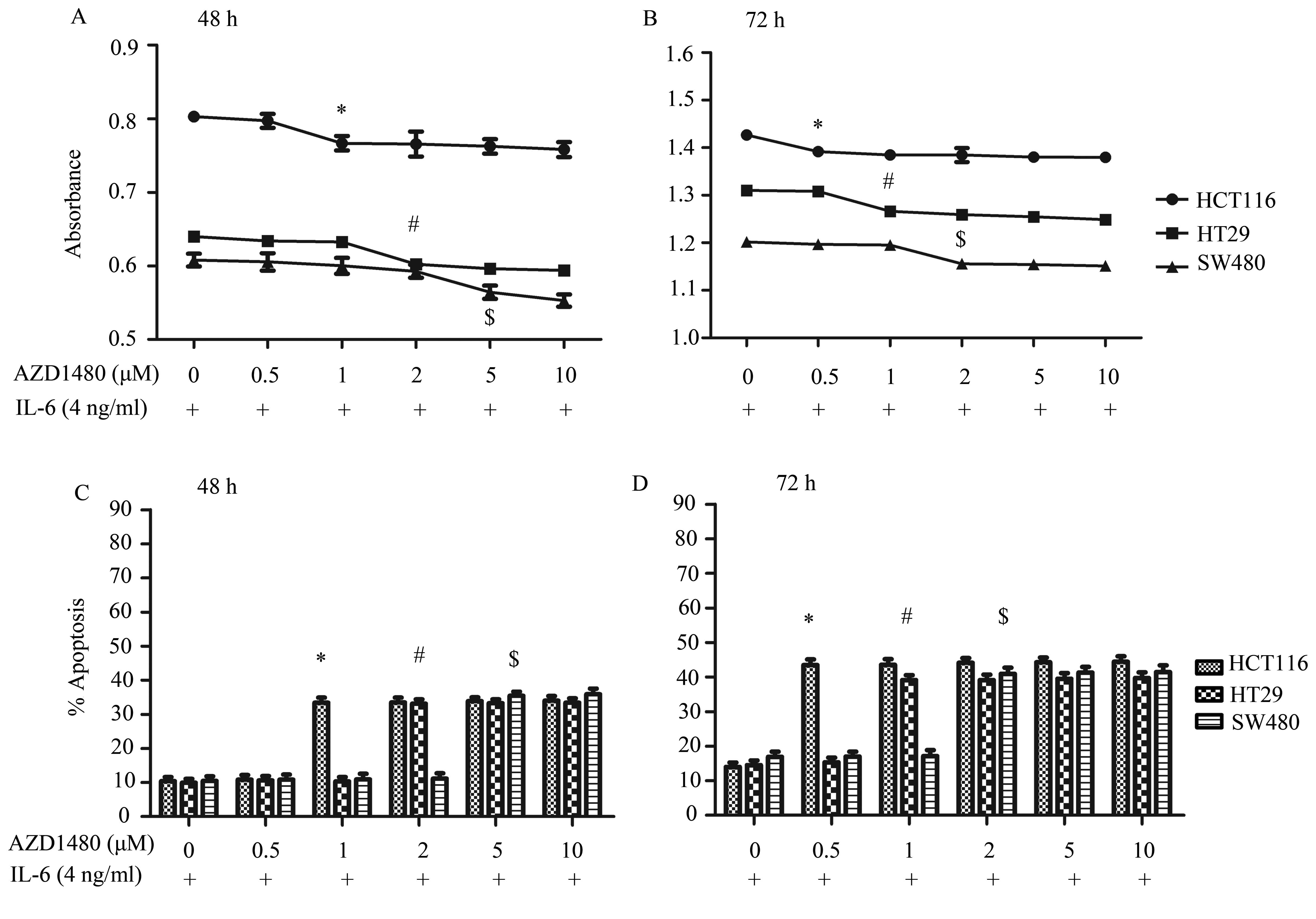
The CCK-8 assay (Fig. 5A
and B) showed that AZD1480 inhibited IL-6-induced cell
proliferation in HCT116, HT29 and SW480 cells at 48 and 72 h. The
concentration significantly changed from ~1, 2 and 5 μM for HCT116
(n=5, P<0.05), HT29 (n=5, P<0.05) and SW480 cells (n=5,
P<0.05), respectively, at 48 h, and in the same cell lines the
concentration at 72 h was changed from ~0.5 μM (n=5, P<0.05), 1
μM (n=5, P<0.05) and 2 μM (n=5, P<0.05), respectively. CRC
cells stimulated with IL-6 showed marked cell proliferation
enhancement compared with the untreated cells.
AZD1480 inhibited the survival of the three CRC cell
lines in the presence of IL-6, inducing apoptosis in a time- and
dose-dependent manner (Fig. 5C and
D). The apoptosis ratio at 48 h of HCT116, HT29 and SW480 cells
increased 24.03% at 1 μM (n=5, P<0.05), 23.11% at 2 μM (n=5,
P<0.05) and 25.01% at 5 μM (n=5, P<0.05), respectively.
However, at 72 h the results increased 39.49% at 0.5 μM (n=5,
P<0.05), 41.24% at 1 μM (n=5, P<0.05) and 44.22% at 2 μM
(n=5, P<0.05) for HCT116, HT29 and SW480 cells, respectively,
compared with the untreated cells stimulated by IL-6.
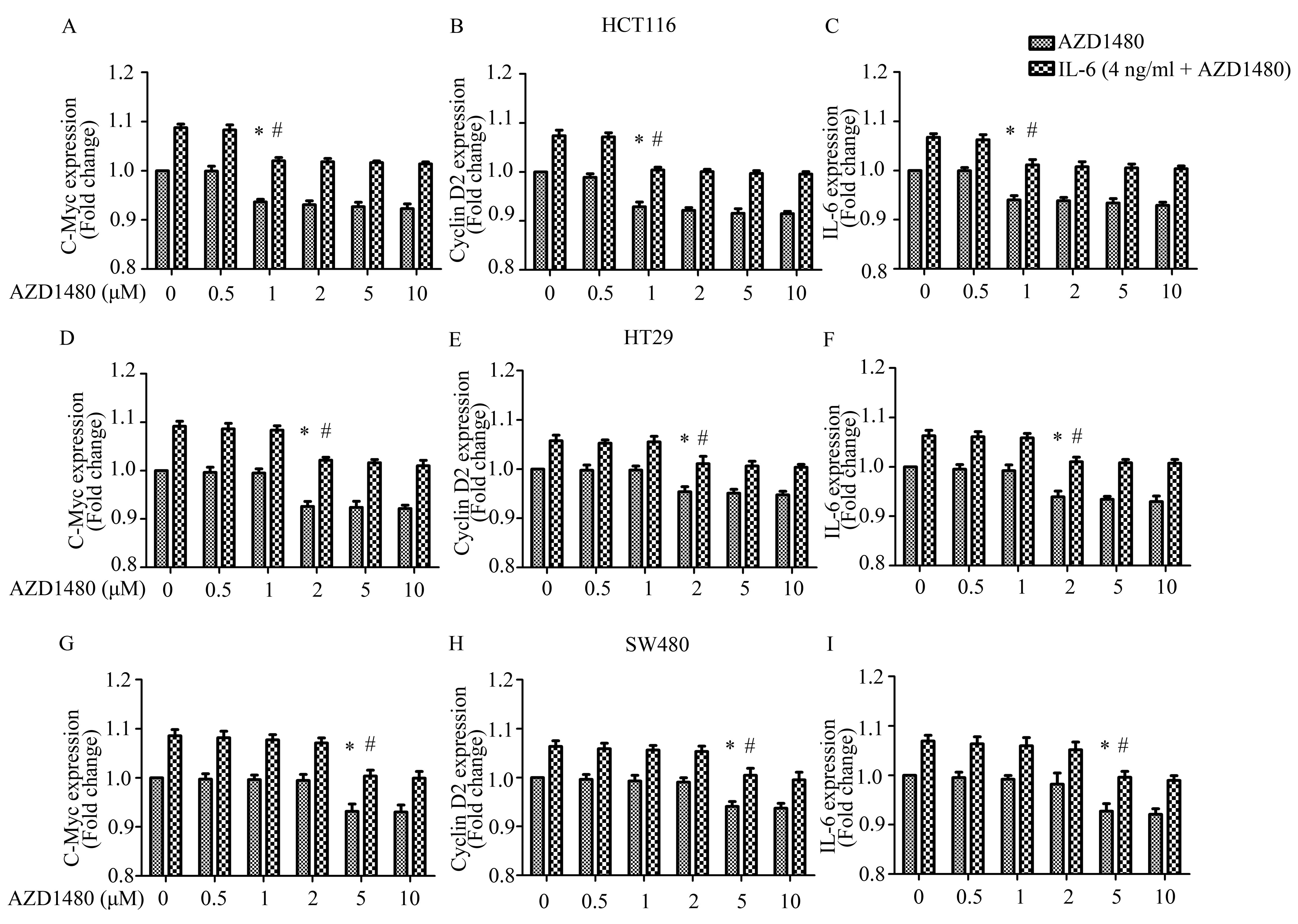
Furthermore, we investigated the effect of AZD1480
on STAT3 targets in CRC cells by qPCR to confirm whether the
inhibition of STAT3 phosphorylation associated with inhibition of
downstream gene expression. The data showed that the gene
expression of c-Myc, cyclin D2 and IL-6 was markedly decreased from
~1 μM for HCT116 cells (n=6, P<0.05), 2 μM for HT29 cells (n=6,
P<0.05) and 5 μM for HT29 cells (n=6, P<0.05). Following IL-6
stimulation, AZD1480 also significantly blocked the IL-6-induced
expression of c-Myc (n=6, P<0.05), cyclin D2 (n=6, P<0.05)
and IL-6 mRNA (n=6, P<0.05) (Fig.
6A–I). These results correlated with the changes of
phosphorylated JAK2 and STAT3.
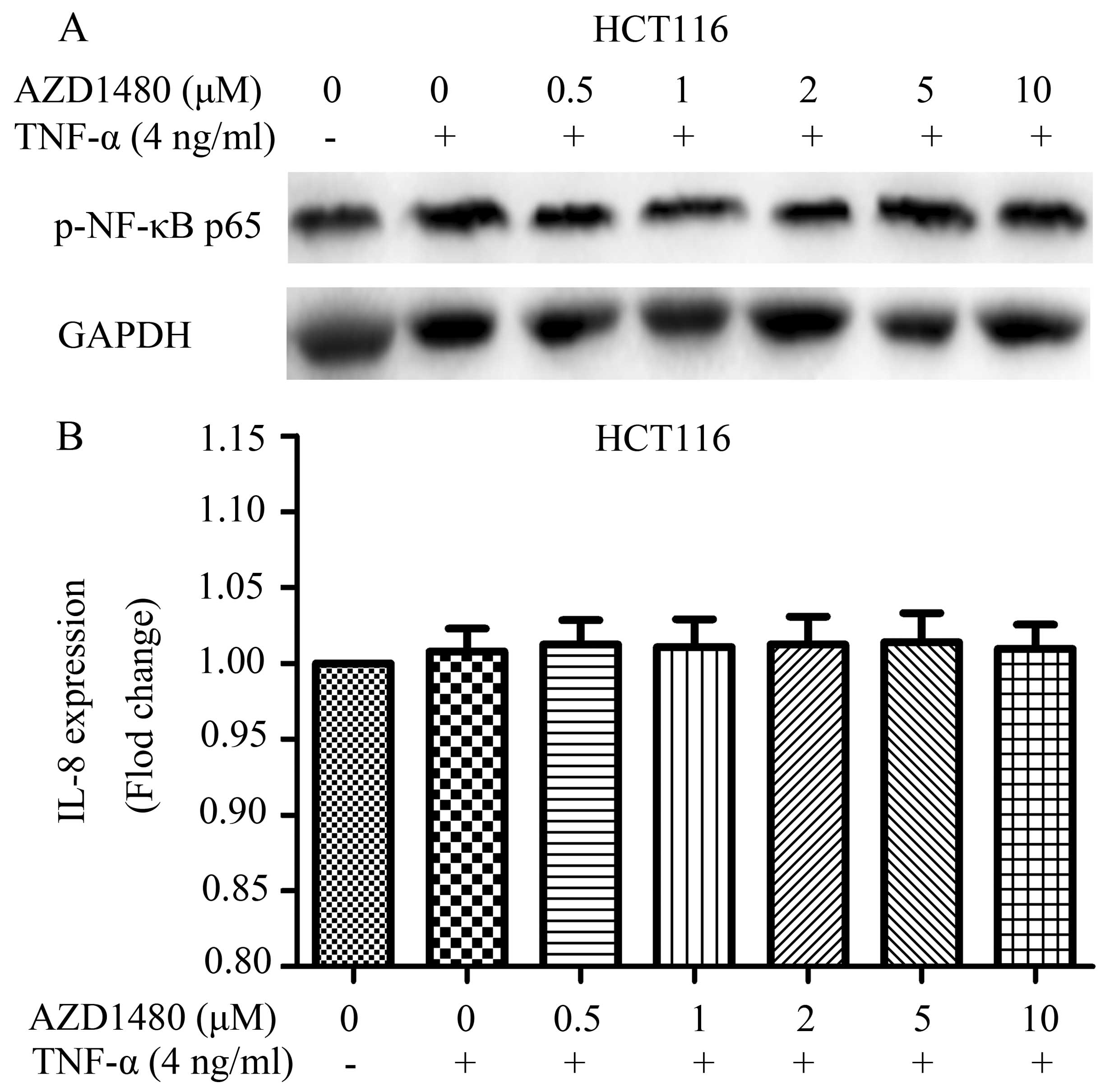
Effects of AZD1480 on NF-κB pathway
NF-κB pathway plays a crucial role in many steps of
CRC initiation and progression (20). Notably, the NF-κB pathway cooperates
with other signaling pathways such as the JAK/STAT3 pathway
(21). In the present study, we
found that AZD1480, as a JAK1/2 inhibitor, suppressed the JAK/STAT
pathway, allowing the close association with the NF-κB and JAK/STAT
pathways. We also determined the effects of AZD1480 in the NF-κB
pathway in HCT116 cells. HCT116 cells were treated with AZD1480 (1
μM) for 2 h followed by treatment with TNF-α. Our results showed
that AZD1480 does not inhibit the TNF-α-induced NF-κB p65
phosphorylation or expression of IL-8 (Fig. 7A and B), an NF-κB driven gene,
supporting the absence of pleiotropic effects of AZD1480 on
signaling pathways in CRC cells.
Discussion
In the present study, we investigated the biologic
mechanism of the novel small molecule JAK1/2 kinase inhibitor
AZD1480 on human CRC cells. AZD1480 inhibited constitutive JAK/STAT
signaling in three established CRC cell lines (HCT116, SW480 and
HT29). AZD1480 reduced the expression of several downstream gene
targets of STAT3 (c-Myc, cyclin D2 and IL-6). Additionally, AZD1480
exerted antitumor functional effects in CRC cells by a decrease in
proliferation and an increase in apoptosis. Antitumor activity of
AZD1480 was also observed in CRC cell growth stimulated by IL-6. We
found that AZD1480 prevented the IL-6-induced activation of JAK2
and phosphorylation of STAT3. In the three CRC cell lines, the
inhibition of tumorigenesis was associated with decreased
phosphorylated JAK2 and STAT3, and the decreased expression of the
targeted genes c-Myc, cyclin D2 and IL-6. Allowing for the efficacy
and potential antitumor effects of AZD1480 in CRC, we may draw the
conclusion that AZD1480 demonstrates a promising therapeutic
benefit for targeting JAK/STAT signaling in CRC.
The tumor microenvironment possesses a rich source
of inflammatory cytokines, among which the IL-6 family,
particularly IL-6 and IL-11, are markedly upregulated in many types
of cancer and regarded as one of the most important cytokine
families during tumorigenesis and progression (22). Furthermore, IL-6 drives many of the
cancer ‘hallmarks’ through the downstream activation of the
JAK/STAT signaling pathway, which is involved in a poor prognosis
in many solid cancers including CRC (16). Cytokine IL-11 also shows a strong
correlation with elevated STAT3 activation in human
gastrointestinal cancers in genetic murine models (23). Abnormalities on the level of
IL-6-driven JAK/STAT pathways are important in the processes of
hyperproliferative and invasive phenotype of CRC cells (24). The JAK/STAT signaling pathway is
associated with many types of tumors, and AZD1480, a JAK1/2 kinase
inhibitor, has been verified to suppress tumorigenesis, for
instance, in metastatic prostate cancer (25), gastrointestinal malignancy (16), hematological malignancies (26), myeloma (13), small cell lung cancer (27), pediatric sarcomas (28) and glioblastoma (12). Stuart et al found that
AZD1480 confers therapeutic benefits in two murine models of
inflammation-associated gastrointestinal cancer strictly dependent
on excessive STAT3 activation (16), which is consistent with our findings
in CRC.
The JAK/STAT signaling pathway intervenes in many
aspects of CRC development, such as cell growth, survival, invasion
and migration (29,30). Suppression of this pathway is
therefore a valuable regulative strategy for CRC. A number of
natural products such as resveratrol, flavopiridol and piceatannol
were utilized in preclinical trials indicating the ability of
inhibiting pathways involved in inflammation, whose mechanisms
include the reduction of STAT3 phosphorylation, inhibition of the
cytokine production and direct inhibition of the JAK (31). Additionally, the role of JAK
inhibition in solid tumors was tested preclinically. The JAK1/2
inhibitor AZD1480 was reported inhibiting tumor development in
models of IL-6-driven breast, ovarian and prostate cancers
(32). Thus, AZD1480 may be
considered potential material for the treatment of cancer including
CRC. The findings of the present study are in concordance with a
previous study, which suggested a key role of AZD1480 in inhibiting
JAK activity to suppress the progression of CRC in vivo
(16). The finding of key clinical
importance in the present study is that pharmacological targeting
of IL-6/JAK/STAT signaling by the JAK1/2 inhibitor AZD1480
effectively suppressed IL-6-induced CRC cell. This findding is also
important as JAK1/2 inhibitors are currently under active clinical
development for hematopoietic proliferative disorders and
malignancies (33,34). Developing therapeutic strategies for
selective STAT3 inhibition is challenging. Therefore, targeting of
upstream components reveals a pharmacologically viable alternative;
for instance, monoclonal antibodies directed towards IL-6 or its
α-chain receptor subunit or small molecule inhibitors for the JAK
family. Our results showed marked efficacy of AZD1480 in inhibiting
JAK1 and JAK2 to confer a cytostatic effect. AZD1480 inhibited
constitutive JAK/STAT signaling in three established CRC cell lines
(HCT116, SW480 and HT29). Moreover, AZD1480 suppressed the
expression of c-Myc, cyclin D2 and IL-6 which are downstream gene
targets of STAT3. Additionally, AZD1480 exerted antitumor
functional effects in CRC cells with a decrease in proliferation
and an increase in apoptosis. Notably, the antitumor activity of
AZD1480 was observed in CRC cells growth stimulated by IL-6.
Genetic alterations lead to numerous aberrant signal
transduction pathways, which are closely related to oncogenesis.
The JAK/STAT signaling pathway is a major contributor to CRC
transformation, and other pathways such as the NF-κB pathway play
critical roles in the physiological and pathological processes of
CRC. Crosstalk between the JAK/STAT and NF-κB pathways has been
verified at multiple levels, including activation of STAT3 which
was induced by IL-6 and COX-2, which are NF-κB-induced factors, and
STAT3, which accelerates NF-κB processing, leading to pro-apoptotic
responses (35) and STAT3 promoting
the nuclear translocation of NF-κB (36). Furthermore, in the context of
colitis-associated cancer, it has been demonstrated that as an
NF-κB regulated cytokine, IL-6 is a critical tumor promoter during
early colitis-associated cancer tumorigenesis, and that the
proliferative and survival effects of IL-6 are modulated by STAT3,
which also plays a vital role in JAK/STAT signaling pathway
(37). IL-6 is currently known as
an NF-κB pathway-targeted gene (38) particularly in response to TNF-α. The
elevated levels of IL-6 detected in many types of cancer have been
thought to result from activation of the NF-κB pathway. NF-κB and
STAT3 activate IL-6, as well as other genes that promote cell
survival, growth, angiogenesis, invasiveness and motility. The
complex crosstalk between the NF-κB and JAK/STAT pathways is
beginning to be elucidated, and data have shown that the
JAK/STAT/NF-κB axis is critical for tumor progression. Given the
inter-dependency of the two pathways, inhibitors such as AZD1480
may attenuate NF-κB activation in vitro in the tumor
microenvironment, as well as suppress the JAK/STAT pathway. In the
present study, we found AZD1480 does not inhibit the TNF-α-induced
NF-κB p65 phosphorylation or expression of IL-8. These results
indicate that AZD1480 shows the absence of pleiotropic effects of
AZD1480 on signaling pathways in CRC cells. Thus, AZD1480
specifically affects the JAK/STAT pathway, which is consistent with
the findings of McFarland et al (12).
In summary, to the best of our knowledge, the
present study provides the first evidence on treatment with AZD1480
through IL-6/JAK/STAT pathway in CRC cells, which confers antitumor
effects by inhibiting cancer cell proliferation, differentiation,
invasion, inflammation and immune function. Together with the
previous findings that AZD1480 inhibits progression of
gastrointestinal tumors in vivo, the present findings reveal
the underlying mechanisms by which AZD1480 inhibits growth and
survival of human CRC cells, suggesting that AZD1480 has a
practical clinical use for treating CRC.
Acknowledgements
The present study was supported by the Department of
Health of the Jiangsu Province Fund.
Abbreviations:
|
IL
|
interleukin
|
|
JAK
|
Janus-activated kinase
|
|
STAT
|
signal transducer and activator of
transcription
|
|
CRC
|
colorectal cancer
|
|
NF-κB
|
nuclear transcription factor κB
|
|
TNF
|
tumor necrosis factor
|
|
RT
|
reverse transcription
|
|
CCK-8
|
Cell Counting Kit-8
|
|
FITC
|
fluorescein isothiocyanate
|
|
SD
|
standard deviation
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Siegel RL, Ward EM and Jemal A: Trends in
colorectal cancer incidence rates in the United States by tumor
location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev.
21:411–416. 2012.PubMed/NCBI
|
|
3
|
Zanders MM, Vissers PA, Haak HR and van de
Poll-Franse LV: Colorectal cancer, diabetes and survival:
epidemiological insights. Diabetes Metab. 40:120–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jawad N, Direkze N and Leedham SJ:
Inflammatory bowel disease and colon cancer. Recent Results Cancer
Res. 185:99–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andersen NN and Jess T: Has the risk of
colorectal cancer in inflammatory bowel disease decreased? World J
Gastroenterol. 19:7561–7568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, et al: Signal transducer and activator of transcription-3,
inflammation, and cancer: how intimate is the relationship? Ann NY
Acad Sci. 1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin Q, Lai R, Chirieac LR, et al:
Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and
cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis
and cell cycle arrest of colon carcinoma cells. Am J Pathol.
167:969–980. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knüpfer H and Preiss R: Serum
interleukin-6 levels in colorectal cancer patients - a summary of
published results. Int J Colorectal Dis. 25:135–140.
2010.PubMed/NCBI
|
|
10
|
Morikawa T, Baba Y, Yamauchi M, et al:
STAT3 expression, molecular features, inflammation patterns, and
prognosis in a database of 724 colorectal cancers. Clin Cancer Res.
17:1452–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014.PubMed/NCBI
|
|
12
|
McFarland BC, Ma JY, Langford CP, et al:
Therapeutic potential of AZD1480 for the treatment of human
glioblastoma. Mol Cancer Ther. 10:2384–2393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scuto A, Krejci P, Popplewell L, et al:
The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling,
resulting in suppression of human myeloma cell growth and survival.
Leukemia. 25:538–550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Holdbrooks AT, De Sarno P, et al:
Therapeutic efficacy of suppressing the Jak/STAT pathway in
multiple models of experimental autoimmune encephalomyelitis. J
Immunol. 192:59–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murakami T, Takigawa N, Ninomiya T, et al:
Effect of AZD1480 in an epidermal growth factor receptor-driven
lung cancer model. Lung Cancer. 83:30–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stuart E, Buchert M, Putoczki T, et al:
Therapeutic inhibition of jak activity inhibits progression of
gastrointestinal tumors in mice. Mol Cancer Ther. 13:468–474. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Zhang F, Wang Y, et al: Oroxylin A
inhibits colitis-associated carcinogenesis through modulating the
IL-6/STAT3 signaling pathway. Inflamm Bowel Dis. 19:1990–2000.
2013.PubMed/NCBI
|
|
18
|
Li GH, Wei H, Lv SQ, Ji H and Wang DL:
Knockdown of STAT3 expression by RNAi suppresses growth and induces
apoptosis and differentiation in glioblastoma stem cells. Int J
Oncol. 37:103–110. 2010.PubMed/NCBI
|
|
19
|
Guthrie GJ, Roxburgh CS, Horgan PG and
McMillan DC: Does interleukin-6 link explain the link between
tumour necrosis, local and systemic inflammatory responses and
outcome in patients with colorectal cancer? Cancer Treat Rev.
39:89–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakamoto K and Maeda S: Targeting NF-κB
for colorectal cancer. Expert Opin Ther Targets. 14:593–601.
2010.
|
|
21
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12:862013.
|
|
22
|
Taniguchi K and Karin M: IL-6 and related
cytokines as the critical lynchpins between inflammation and
cancer. Semin Immunol. 26:54–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Putoczki TL, Thiem S, Loving A, et al:
Interleukin-11 is the dominant IL-6 family cytokine during
gastrointestinal tumorigenesis and can be targeted therapeutically.
Cancer Cell. 24:257–271. 2013. View Article : Google Scholar
|
|
24
|
Gordziel C, Bratsch J, Moriggl R, Knösel T
and Friedrich K: Both STAT1 and STAT3 are favourable prognostic
determinants in colorectal carcinoma. Br J Cancer. 109:138–146.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu L, Talati P, Vogiatzi P, et al:
Pharmacologic suppression of JAK1/2 by JAK1/2 inhibitor AZD1480
potently inhibits IL-6-induced experimental prostate cancer
metastases formation. Mol Cancer Ther. 13:1246–1258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furqan M, Mukhi N, Lee B and Liu D:
Dysregulation of JAK-STAT pathway in hematological malignancies and
JAK inhibitors for clinical application. Biomark Res. 1:52013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee JH, Park KS, Alberobello AT, et al:
The Janus kinases inhibitor AZD1480 attenuates growth of small cell
lung cancers in vitro and in vivo. Clin Cancer Res. 19:6777–6786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan S, Li Z and Thiele CJ: Inhibition of
STAT3 with orally active JAK inhibitor, AZD1480, decreases tumor
growth in neuroblastoma and pediatric sarcomas in vitro and in
vivo. Oncotarget. 4:433–445. 2013.PubMed/NCBI
|
|
29
|
Xiong H, Su WY, Liang QC, et al:
Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor
cell invasion in human colorectal cancer cells. Lab Invest.
89:717–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong H, Zhang ZG, Tian XQ, et al:
Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle
arrest, and reduces tumor cell invasion in colorectal cancer cells.
Neoplasia. 10:287–297. 2008.PubMed/NCBI
|
|
31
|
Fletcher S, Drewry JA, Shahani VM, Page BD
and Gunning PT: Molecular disruption of oncogenic signal transducer
and activator of transcription 3 (STAT3) protein. Biochem Cell
Biol. 87:825–833. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hedvat M, Huszar D, Herrmann A, et al: The
JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reddy MM, Deshpande A and Sattler M:
Targeting JAK2 in the therapy of myeloproliferative neoplasms.
Expert Opin Ther Targets. 16:313–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tibes R, Bogenberger JM, Geyer HL and Mesa
RA: JAK2 inhibitors in the treatment of myeloproliferative
neoplasms. Expert Opin Investig Drugs. 21:1755–1774. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nadiminty N, Lou W, Lee SO, Lin X, Trump
DL and Gao AC: Stat3 activation of NF-κB p100 processing involves
CBP/p300-mediated acetylation. Proc Natl Acad Sci USA.
103:7264–7269. 2006.
|
|
36
|
Yang J, Liao X, Agarwal MK, Barnes L,
Auron PE and Stark GR: Unphosphorylated STAT3 accumulates in
response to IL-6 and activates transcription by binding to NFκB.
Genes Dev. 21:1396–1408. 2007.PubMed/NCBI
|
|
37
|
Grivennikov S, Karin E, Terzic J, et al:
IL-6 and Stat3 are required for survival of intestinal epithelial
cells and development of colitis-associated cancer. Cancer Cell.
15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grivennikov S and Karin M: Autocrine IL-6
signaling: a key event in tumorigenesis? Cancer Cell. 13:7–9. 2008.
View Article : Google Scholar : PubMed/NCBI
|