Introduction
Malignant pleural mesothelioma (MPM) is an
aggressive tumor that arises from serosal cells and affected
patients exhibit poor prognosis (1). The incidence of the disease is
expected to steadily increase due to occupational asbestos exposure
and is becoming recognized as a particular societal problem in
Japan (2). Indeed, over 100,000
MPM-related deaths are predicted to occur within the next 40 years
in Japan (3). Surgical resection is
only possible in a minority of patients. In addition, the 5-year
survival rate of patients that undergo surgical resection is less
than 15% (4). Chemotherapy can be
used as a standard therapy for MPM patients with advanced disease.
In this context, a variety of drugs including cisplatin (CDDP),
gemcitabine, and pemetrexed (PEM) have been used in different
combinations. Chemotherapy with PEM and CDDP has yielded the best
effect in MPM patients to date, with a response rate of 41% and a
median survival time of 12 months (5). However, major concerns related to this
treatment revolve around the short duration of response and rapid
relapse. Therefore, new therapeutic modalities that have clinically
beneficial effects are urgently required for appropriate management
of MPM patients. Recently, the histone deacetylase (HDAC)
inhibitor, vorinostat (SAHA), significantly enhanced CDDP-induced
apoptosis in MPM cells (6). In
addition, the HDAC inhibitor, valproate, in combination with CDDP
and PEM, provided additional efficacy in respect to treatment of
MPM cell line-derived xenografts in vivo (7). A phase I clinical trial of carboplatin
and paclitaxel with SAHA including non-small cell lung cancer
(NSCLC) and MPM showed significantly improved response rates and a
trend towards enhanced survival (8). Therefore, combining platinum drugs
with SAHA might be a promising therapeutic strategy in MPM.
Tumors employ several mechanisms to avoid or
actively suppress anticancer immune responses (9). The secretion by tumor cells of soluble
factors such as IL-6, IL-18 and TGF-β can directly block T-cell
proliferation, promote T-cell apoptosis, or confer resistance to
tumor cells against T-cell attack (10). The inflammatory status of
noncancerous lung tissue surrounding a tumor may also play an
important role in promoting tumor progression and metastasis in
lung adenocarcinoma (11). Among
the tumor-specific cytokines, IL-18 plays a pivotal role in
inflammation and the immune response (12–14).
Moreover, IL-18 can also promote human cancer progression and
metastasis (15). Understanding of
the inflammatory status of human cancer may provide new insights in
respect to diagnostic and therapeutic strategies.
MicroRNAs (miRNAs) are single-stranded 18–24 nt
non-coding molecules that post-transcriptionally modulate gene
expression through binding to 3′-UTRs of target mRNAs (16). miRNAs, which usually induce gene
silencing, can function as either tumor suppressors or oncogenes
(17). Recent studies have
identified several miRNAs with diagnostic, prognostic and
therapeutic potential in pulmonary malignant diseases including
NSCLC and MPM (18–21). For example, the identification of
low levels of miR-29c in MPM has provided both a new prognostic
tool and has pointed to aberrant chromatin methylation as being
involved in progression of the disease (20). A recent study demonstrated that loss
of miR-145 affects tumorigenic properties of MPM cell lines
(21). These findings have prompted
the study of miRNA expression levels as potentially important
diagnostic and prognostic tools in MPM.
In the present study, we analyzed the correlation
between gene expression and antitumor effects of PEM and SAHA in
MPM cell lines by DNA microarray-based whole transcriptome
profiling and targeted RT-PCR analysis for 10 cytokine genes. We
focused on IL-18 for further analysis among the identified genes
associated with drug resistance in MPM cells. Furthermore, miRNAs
within the miR-379/411 cluster contributed to drug resistance
through the regulation of their target IL-18. Our research has
revealed, for the first time, that IL-18 and the miR-379/411
cluster may have dual functions in tumor invasion and drug
resistance in MPM.
Materials and methods
Cell culture
We used six MPM cell lines in the present study, as
follows: NCI-H28, NCI-H2452 and ACC-MESO4 (epithelial-type),
NCI-H2052 (sarcomatoid-type), ACC-MESO1 (fibroblast-type) and
MSTO-211H (biphasic-type). NCI-H28, NCI-H2452, NCI-H2052 and
MSTO-211H were purchased from the American Type Culture Collection
(Manassas, VA, USA). ACC-MESO1 and ACC-MESO4 were obtained from the
Riken Cell Bank (Tsukuba, Japan). MPM cells were maintained in
RPMI-1640 (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum
(FBS; Gemini Bioproducts). These cell lines were obtained from 2008
to 2009, were amplified and frozen, and one aliquot of each was
thawed for this project. All cells were routinely screened for the
absence of mycoplasma. These cell lines were maintained in
RPMI-1640 medium (Gibco) supplemented with 10% FBS.
Drugs and growth-inhibition assay
PEM was purchased from Toronto Research Chemicals
(North York, Canada). SAHA was purchased from Cayman Chemicals (Ann
Arbor, MI, USA). Growth inhibition was assessed by the MTS assay to
examine the effect of PEM and SAHA on the MPM cell lines as
previously described (22). Cell
suspensions (5,000 cells/well) were seeded into 96-well plates and
increasing concentrations of PEM and SAHA (0, 0.001, 0.01. 0.1,
1.0, 10 and 100 μM) were added. After incubation at 37°C for 72 h,
MTS was added to each well and incubated at 37°C for 2 h, after
which the absorbance was measured with a test wavelength of 490 nm
using a microplate reader (Dynatech MR7000; Dynatech, Billinghurst,
UK). The IC50 value was defined as the concentration of
PEM and SAHA needed for 50% reduction of growth and was calculated
by SigmaPlot12 (Hulinks, Inc., Tokyo, Japan). Each experiment was
performed independently three times. The corrected absorbance of
each sample was calculated and compared with that of the untreated
control.
RNA isolation and real-time quantitative
reverse transcription-PCR
Total RNA was isolated from MPM cell lines, as
previously described (11). The
expression profiles of 10 cytokine genes [i.e. interleukin 1A
(IL-1A), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 6
(IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), interleukin
12A (IL-12A), interleukin 18 (IL-18), interleukin 24 (IL-24) and
inter-leukin 27 (IL-27)] were examined by quantitative real-time
PCR (qRT-PCR) analysis using specifically designed TaqMan Human
Gene Expression Assays (Applied Biosystems, Foster City, CA, USA).
GAPDH served as an endogenous control. Gene expression data (mean ±
SD from triplicate samples) are shown as ΔCt. The qRT-PCR
assessment of gene expression was performed using the ABI Prism
7700 Sequence Detector system (Perkin Elmer/Applied
Biosystems).
GeneChip and miRNA array analysis
High-density oligonucleotide array analysis was
carried out using Affymetrix HG-U133A GeneChips (22,282 probe
sets), as previously described (23). In terms of miRNA profiling, 5 μg of
total RNA was employed for hybridization on miRNA microarray chips
containing 667 probes with the TaqMan® Array Human
MicroRNA A+B Card Set ver. 2.0 (Life Technologies, Carlsbad, CA,
USA) on a 7900 Real-Time PCR system (Applied Biosystems, Foster
City, CA, USA), as previously described (24).
Oligonucleotidetransfection
Small interfering RNAs (siRNAs) targeting IL-18 were
purchased from Dharmacon Research Inc. (Lafayette, CO, USA), and
the homologous negative controls were obtained from Invitrogen.
miR-379 and miR-411 mimics, and the control-mimic, were obtained
from Sigma-Aldrich (St. Louis, MO, USA). IL-8 siRNAs and
miR-379/411 mimics were transfected using Lipofectamine 2000
reagent 24 h after seeding, according to the manufacturer’s
instructions (Life Technologies). Transfections of siRNA and miRNA
mimic complexes were added to cells at a final concentration of 50
nM.
Luciferase assay
Luciferase reporter constructs containing portions
of the IL-18 3′ UTRs were generated by GeneCopoeia, Inc.
(Rockville, MD, USA). H28 cells were cultured in 24-well plates for
24 h and cotransfected with 100 ng/μl of IL-18 3′ UTR reporter
constructs and 50 nm of miR-379 mimic, miR-411 mimic, or
control-mimic using Lipofectamine 2000 for 24 to 48 h. After
transfection, cells were harvested, lysed and assayed with a
Dual-Luciferase Reporter assay kit (Promega, Madison, WI, USA)
according to the manufacturer’s instructions. Firefly luciferase
activity was normalized to Renilla luciferase activity for
each transfected well. Each experiment was performed in duplicate
and repeated three times.
Invasion assay
The ability of cells that either overexpressed
miR-379 or miR-411, or were silenced for IL-18 to invade through a
Transwell filter was measured using a Cytoselect 96-well cell
invasion assay (Cell Biolabs, Inc., San Diego, CA, USA). Gently,
MESO1 cell suspension (2×105 cells) with or without
either miR-379 or miR-411 mimic, or si-IL-18 in serum-free medium
was added to the membrane chamber and incubated for 24 h. The
subsequent procedures were performed according to the
manufacturer’s protocol.
Results
Effect of PEM and SAHA treatment on the
growth of MPM cells
We examined the antitumor activities of PEM and SAHA
against six MPM cell lines. The IC50 values of PEM and
SAHA against this panel of cell lines were determined by an MTS
assay (Table I). One micromole is
much lower than the mean peak plasma concentration of PEM
achievable in patients, indicating a surprisingly high in
vitro sensitivity of MPM cells to this agent (25). Based on relative sensitivity to PEM,
these cell lines were classified as either sensitive
(IC50 of ≤1 μM) or resistant (IC50 of >1
μM). The H28 and 211H cell lines exhibited IC50 values
of 0.1 μM (highly sensitive). The H2052 cell line had an
IC50 of 0.1 to 1 μM (intermediate-sensitive). The
PEM-resistant group included H2452, MESO1 and MESO4 cells. In
relation to clinical trial data, PEM was shown to be effective in
MPM patients with an epithelial histological type. However, on the
basis of this in vitro study, PEM displayed antitumor
effects against MPM independent of histological type. The antitumor
activities of SAHA were also examined against the same panel of MPM
cell lines (Table I). According to
the highest concentration of the drug (Cmax) in patients
treated with SAHA (2–5 μM), these cell lines were classified as
sensitive (IC50 of ≤5 μM) or resistant (IC50
of >5 μM). Only the 211H cell line was deemed sensitive to SAHA
(26). Based on the relative
sensitivities against the two drugs, 211H cells were recognized as
being commonly drug sensitive. The H28 and H2052 cell lines were
classified as intermediate-sensitive cells. Finally, H2452, MESO1
and MESO4 cells were deemed as being commonly resistant to both
agents.
 | Table IIC50 values in 6 MPM cell
lines responding to treatments with PEM and SAHA as determined by
the MTS assay. |
Table I
IC50 values in 6 MPM cell
lines responding to treatments with PEM and SAHA as determined by
the MTS assay.
| Cell line
(pathological type) |
|---|
|
|
|---|
| Treatment | 211H (biphasic) | H28 (sarcoma) | H2052
(epithelial) | H2452
(epithelial) | MESO1
(fibroblast) | MESO4
(epithelial) |
|---|
| PEM IC50
(μM) | 0.07 | 0.07 | 0.57 | >100 | >100 | >100 |
| SAHA IC50
(μM) | 3.3 | 9.1 | 7.2 | 7.2 | 8.1 | 9.2 |
| Drug
sensitivity | Sensitive | Intermediate | Intermediate | Resistant | Resistant | Resistant |
IL-18 as a marker of resistance to
SAHA
We next performed gene expression profiling of the
same set of six MPM cell lines by GeneChip analysis. We used the
MTS results for PEM and SAHA to develop a molecular model of drug
sensitivity. Gene expression profiles were compared between the
drug sensitive 211H cells and the five remaining cell lines
displaying either intermediate or complete resistance to both
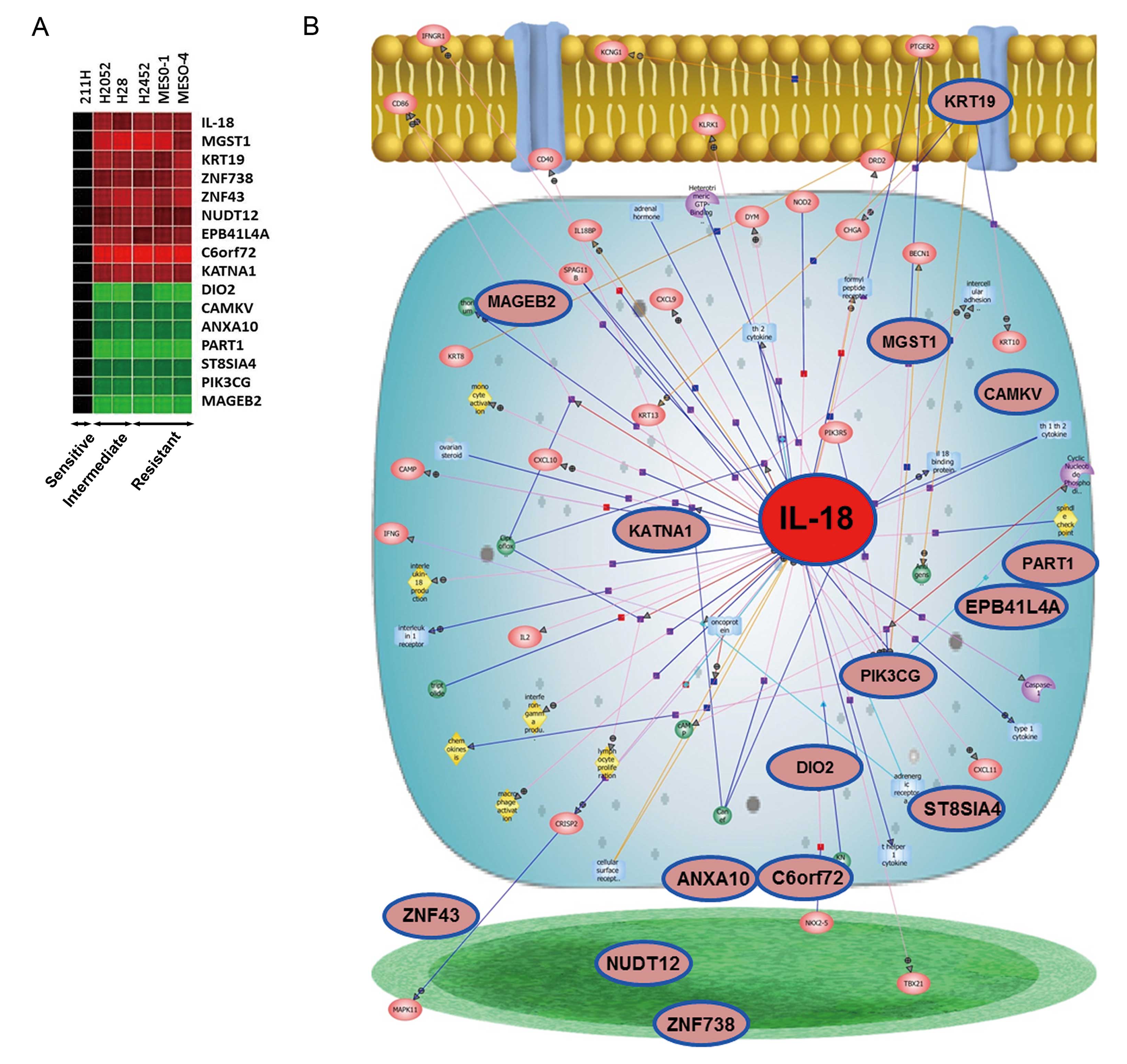
agents. Sixteen genes were identified as being significantly
correlated with the drug sensitivity (fold change >1.5;
P<0.05; Fig. 1A). Next, pathway
analysis was performed using 16 genes to provide a viewpoint of the
biological function of the identified differentially expressed
genes, as previously described (27). Sixteen genes, associated with
chemosensitivity, were identified based on the biological functions
of the altered/associated genes (Fig.
1B). Pathway analysis identified IL-18 as an important gene
associated with drug sensitivity of MPM cells (Fig. 1B). IL-18 gene expression levels in
the resistant cell lines and those of intermediate sensitivity were
significantly higher than that found in the drug-sensitive 211H
cells (Fig. 1A). Based on the
results of DNA microarray and pathway analysis, we chose IL-18 for
further analysis of drug resistance in MPM cells.
Cytokine gene expression profiles in MPM
cells
To investigate the role of IL-18 in promoting drug
resistance in the context of MPM treatment, we analyzed the
expression of IL-18 and the other 9 cytokine genes in the same
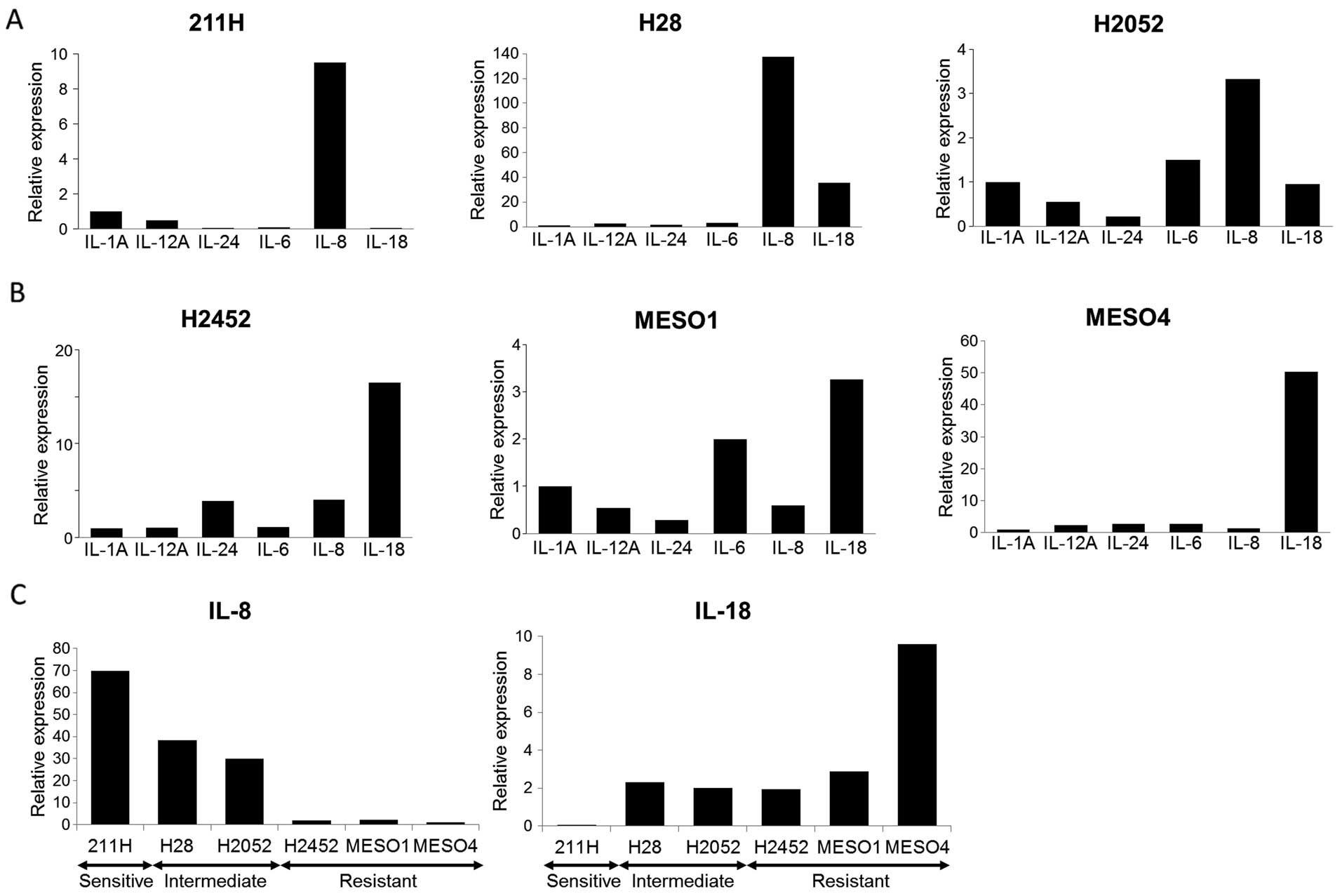
panel of cell lines. Among the cytokines evaluated, IL-2, IL-4,
IL-10 and IL-27 mRNA expression was undetectable in practically all
MPM cell lines tested. Therefore, we evaluated the correlation
between the expression of the remaining 6 cytokine genes and drug
sensitivity in the MPM cells (Fig. 2A
and B). In three drug-sensitive and intermediate MPM cell lines
including 211H, H2052 and H28 cells, IL-8 gene expression levels
were highest among the 6 cytokine genes in the three PEM-sensitive
cell lines (Fig. 2A and C). In
contrast, IL-18 was the most upregulated compared to the other 5
cytokine genes in the three drug-resistant cell lines (Fig. 2B and C). Therefore, IL-18 was
investigated further as a candidate marker of drug sensitivity.
Regulation of IL-18 expression via
miR-379 and miR-411
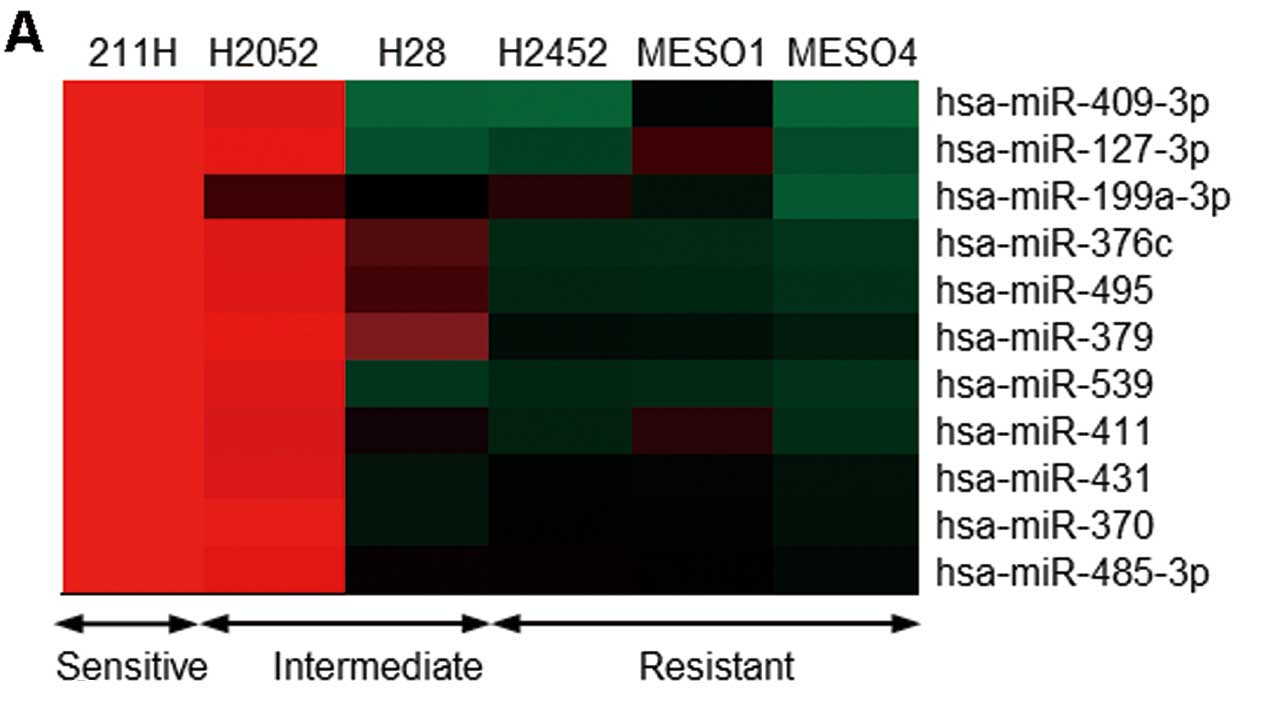
We next examined miRNA expression profiles in the
MPM cell lines to clarify the mechanism of IL-18 induction in
drug-resistant MPM cell lines. The expression levels of 11 miRNAs
in the three drug-resistant cell lines were increased significantly
more than that observed in the 211H cells (fold change >10.0;
Fig. 3A). We next proceeded to
identify potential targets using the Targetscan and miRNA.org
database, comprehensive resource of miRNA target predictions and
expression profiles. We found that miR-379 and miR-411 belonged to
the same cluster of miRNAs located on chromosome 14q32 that
commonly target IL-18. Fig.
3B shows the regions within the 3′ UTR of the IL-18 gene that
could serve as binding sites for miR-411 based on Targetscan
predictions. We validated the downregulation of miR-379 expression
in the three drug-resistant cell lines, as well as reduction in
miR-411 expression in the two drug-resistant cell lines, using
qRT-PCR analysis (Fig. 3C).
Next, we performed a luciferase reporter assay to
verify that miR-379 and miR-411 directly target IL-18. Mature
miR-379 and miR-411 in the H28 cells were significantly increased
from 24 to 48 h after transfection of the relevant mimics (Fig. 3D). We found that co-transfection of
either the miR-379 or miR-411 mimic with the IL-18 3′ UTR reporter
vector significantly decreased luciferase activity in the H28 cells
as compared with the control (Fig.
3E). In addition, qRT-PCR analysis showed that treatment with
either the miR-379 or miR-411 mimic induced downregulation of IL-18
mRNA expression in the H28 cells from 24 to 48 h (Fig. 3F). These data showed that IL-18 is a
direct target of miR-379 and miR-411.
miR-379 and miR-411 inhibit the invasive
activity in MPM cells and improve sensitivity to SAHA
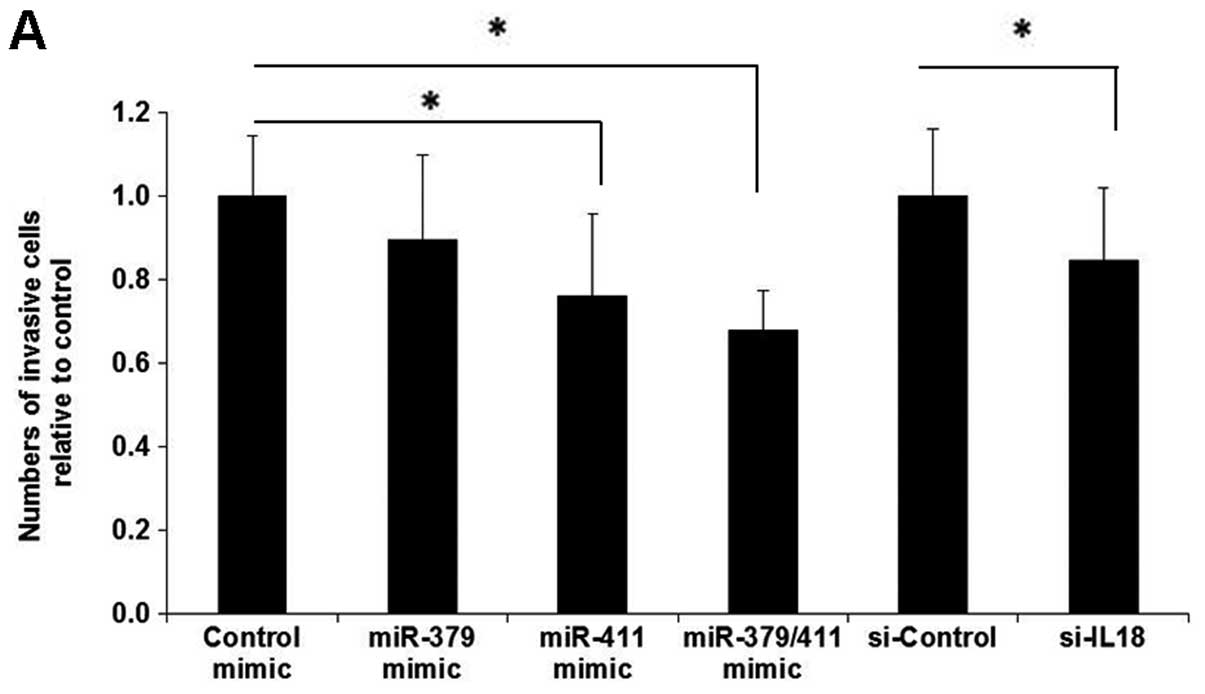
We then examined the function of miR-379 and miR-411
in an invasion assay using MESO1 cells which express these miRNAs
at a constitutively low level. Introduction of miR-411, as well as
IL-18 silencing, significantly suppressed the invasive activities
of these cells in vitro (Fig.
4A). Furthermore, the combination of both miR-379 and miR-411
mimics further inhibited the invasive capacity of these cells
(Fig. 4A). Finally, we evaluated
whether miR-379 and miR-411 elicited resistance to PEM and SAHA.
Introduction of the combined miR-379/miR-411 mimics into MESO1
cells mediated a trend towards improved sensitivity to PEM
(Fig. 4B). It is noteworthy that
the same combination of miR-379/miR-411 mimics also appeared to
increase sensitivity to SAHA (Fig.
4B). The IC50 values of miR-379/miR-411-transfected
and parental MESO1 cells were 1.0 and 8.1 μM, respectively. Indeed,
the IC50 value of the miR-379/miR-411-transfected MESO1
cells was below that observed in the drug-sensitive 211H cells (3.3
μM). These findings suggest that miR-379 and miR-411 play a key
role in the carcinogenesis of MPM cells by targeting IL-18 and
contributing to the sensitivity of MPM cells to SAHA and PEM.
Discussion
We investigated whether specific genes and miRNAs
could be useful as biomarkers of the drug response in MPM. In this
study, IL-18 was identified as a drug-resistant gene by whole
transcriptome and targeted cytokine gene expression profiling of
MPM cell lines. IL-18 was originally discovered as an
IFN-γ-inducing factor (12). IL-18
combines with IL-12 and induces IFN-γ production by T and NK cells,
mediates Th1 polarization and is involved in mediating defense to
pathogens (13). IL-18 also
combines with IL-2 and exhibits antitumor properties through the
induction of an immune response (14). In addition, IL-18 alone can promote
angiogenesis, metastasis, and escape from the immune response in
the absence of Th1-like cytokines. In several human cancers,
increased IL-18 serum levels accompany tumor progression and
contribute to a poor prognosis (28). These reports indicate that IL-18 is
an important cytokine in respect to the promotion of human cancer
progression and metastasis.
In the present study, expression levels of miR-379
and miR-411, which are located on the same cluster, were
significantly decreased in the drug-resistant MPM cell lines. A
decreased level of miR-379 has been implicated in breast cancer
(29,30). Indeed, miR-379 has been shown to
regulate cyclin B1 and TGF-β-induced IL-11 production in breast
cancer cell lines (29,30). Our results indicate that IL-18 is a
direct target of miR-379 and miR-411. Transfection of MPM cells
with miR-379 and miR-411 mimics resulted in decreased IL-18
expression, suppressed invasive capacity and contributed to
resistance to SAHA and PEM. Our results provide the first
integrated evidence for a significant role of miR-379 and miR-411
in the carcinogenesis of MPM, while also defining these as
potentially novel drug targets.
Combination chemotherapy of PEM with CDDP is
currently the best treatment available for MPM patients; however,
its effectiveness is limited (5).
New therapeutic agents are required for MPM patients. HDAC
inhibitors are emerging as candidate therapeutic agents in this
arena based on the results of in vitro and in vivo
studies (6,7). In our study, high levels of IL-18 were
found in PEM- and SAHA-resistant MPM cell lines. IL-18 silencing by
transfection of miR-379/miR-411 mimics mediated a trend towards
improved sensitivity to PEM and SAHA in MPM cells that have
constitutively high levels of IL-18. Elevated levels of IL-18 have
been shown in doxorubicin-resistant breast cancer cell lines
(31). A recent report revealed
that depletion or neutralization of IL-18 decreased NK
cell-controlled tumor metastasis in a PD-1 dependent manner, and
suggested the possibility of novel clinical development of anti-PD1
antibodies in human malignancies that produce IL-18 (32). Therefore, IL-18 targeted therapy may
be a novel potential therapeutic strategy in MPM cells with high
IL-18. Furthermore, miR-379 and miR-411 could be used as a
therapeutic intervention to regulate the expression of IL-18 and
further control tumor invasion of MPM cells.
In conclusion, we provide insight into the possible
contribution of the miR-379/411 cluster and IL-18 interaction in
MPM cells. Our data suggest that IL-18 is a key determinant of
sensitivity to PEM and SAHA in MPM cells. The miR-379/411 cluster
promoted invasion and induced resistance to PEM and SAHA by
directly targeting IL-18 in MPM cells. The miR-379/411 cluster may
represent a new therapeutic target for advanced MPM patients,
depending on the status of IL-18 expression. Further studies should
be undertaken to clarify the mechanism underlying the association
between the miR-379/411 cluster and drug resistance in MPM.
Acknowledgements
We would like to thank Ms. Shoko Tanaka for
assistance with the cytokine gene expression analysis. We would
like to thank Mrs. Haruka Isobe of MediBic for assistance with the
Pathway analysis. This study was supported in part by a
Grant-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (grant no. 24591179 to M.S.; grant
no. 25461172 to A.G.), the Clinical Rebiopsy Bank Project for
Comprehensive Cancer Therapy Development (to M.S and A.G.) and the
Smoking Research Foundation (to A.G.).
References
|
1
|
Kaufman AJ and Pass HI: Current concepts
in malignant pleural mesothelioma. Expert Rev Anticancer Ther.
8:293–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansen J, de Klerk NH, Musk AW and Hobbs
MS: Environmental exposure to crocidolite and mesothelioma:
exposure-response relationships. Am J Respir Crit Care Med.
157:69–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson BWS and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugarbaker DJ, Flores RM, Jaklitsch MT, et
al: Resection margins, extrapleural nodal status, and cell type
determine postoperative long-term survival in trimodality therapy
of malignant pleural mesothelioma: results in 183 patients. J
Thorac Cardiovasc Surg. 117:54–63. 1999. View Article : Google Scholar
|
|
5
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
et al: Phase III study of PEM in combination with cisplatin versus
cisplatin alone in patients with malignant pleural mesothelioma. J
Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurwitz JL, Stasik I, Kerr EM, et al:
Vorinostat/SAHA-induced apoptosis in malignant mesothelioma is
FLIP/caspase 8-dependent and HR23B-independent. Eur J Cancer.
48:1096–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vandermeers F, Hubert P, Delvenne P, et
al: Valproate, in combination with pemetrexed and cisplatin,
provides additional efficacy to the treatment of malignant
mesothelioma. Clin Cancer Res. 15:2818–2828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramalingam SS, Parise RA, Ramanathan RK,
et al: Phase I and pharmacokinetic study of vorinostat, a histone
deacetylase inhibitor, in combination with carboplatin and
paclitaxel for advanced solid malignancies. Clin Cancer Res.
13:3605–3610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zitvogel L, Tesniere A and Kroemer G:
Cancer despite immunosurveillance: immunoselection and
immunosubversion. Nat Rev Immunol. 6:715–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou W: Immunosuppressive networks in the
tumour environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seike M, Yanaihara N, Bowman ED, et al:
Use of a cytokine gene expression signature in lung adenocarcinoma
and the surrounding tissue as a prognostic classifier. J Natl
Cancer Inst. 99:1257–1269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamura H, Tsutsi H, Komatsu T, et al:
Cloning of a new cytokine that induces IFN-gamma production by T
cells. Nature. 378:88–91. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coughlin CM, Salhany KE, Wysocka M, et al:
Interleukin-12 and interleukin-18 synergistically induce murine
tumor regression which involves inhibition of angiogenesis. J Clin
Invest. 101:1441–1452. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Son YI, Dallal RM, Mailliard RB, Egawa S,
Jonak ZL and Lotze MT: Interleukin-18 (IL-18) synergizes with IL-2
to enhance cytotoxicity, interferon-gamma production, and expansion
of natural killer cells. Cancer Res. 61:884–888. 2001.PubMed/NCBI
|
|
15
|
Park S, Cheon S and Cho D: The dual
effects of interleukin-18 in tumor progression. Cell Mol Immunol.
4:329–335. 2007.PubMed/NCBI
|
|
16
|
Johnson SM, Grosshans H, Shingara J, et
al: Ras is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, et al: MicroRNA expression profiles classify human cancers.
Nature. 435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seike M, Goto A, Okano T, et al: MiR-21 is
an EGFR-regulated anti-apoptonic factor in lung cancer in
never-smokers. Proc Natl Acad Sci USA. 106:12085–12090. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pass HI, Goparaju C, Ivanov S, et al:
hsa-miR-29c* is linked to the prognosis of malignant pleural
mesothelioma. Cancer Res. 70:1916–1924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cioce M, Ganci F, Canu V, et al:
Protumorigenic effects of miR-145 loss in malignant pleural
mesothelioma. Oncogene. Nov 18–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
22
|
Shimokawa T, Seike M, Soeno C, et al:
Enzastaurin has anti-tumour effects in lung cancers with
overexpressed JAK pathway molecules. Br J Cancer. 106:867–875.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gemma A, Li C, Sugiyama Y, et al:
Anticancer drug clustering in lung cancer based on gene expression
profiles and sensitivity database. BMC Cancer. 6:1742006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitamura K, Seike M, Okano T, et al:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther.
13:444–453. 2014.PubMed/NCBI
|
|
25
|
Thodtmann R, Depenbrock H, Dumez H, et al:
Clinical and pharmacokinetic phase I study of multitargeted
antifolate (LY231514) in combination with cisplatin. J Clin Oncol.
17:3009–3016. 1999.PubMed/NCBI
|
|
26
|
Fakih MG, Pendyala L, Fetterly G, et al: A
phase I, pharmacokinetic and pharmacodynamic study on vorinostat in
combination with 5-fluorouracil, leucovorin, and oxaliplatin in
patients with refractory colorectal cancer. Clin Cancer Res.
15:3189–3195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyanaga A, Gemma A, Noro R, et al:
Antitumor activity of histone deacetylase inhibitors in non-small
cell lung cancer cells: development of a molecular predictive
model. Mol Cancer Ther. 7:1923–1930. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dinarello CA: The paradox of
pro-inflammatory cytokines in cancer. Cancer Metastasis Rev.
25:307–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pollari S, Leivonen SK, Perälä M, Fey V,
Käkönen SM and Kallioniemi O: Identification of microRNAs
inhibiting TGF-β-induced IL-11 production in bone metastatic breast
cancer cells. PLoS One. 7:e373612012.
|
|
30
|
Khan S, Brougham CL, Ryan J, et al:
miR-379 regulates cyclin B1 expression and is decreased in breast
cancer. PLoS One. 8:e687532013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao L, Zhang Y, Chen K, et al: Discovery
of IL-18 as a novel secreted protein contributing to doxorubicin
resistance by comparative secretome analysis of MCF-7 and
MCF-7/Dox. PLoS One. 6:e246842011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Terme M, Ullrich E, Aymeric L, et al:
IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer
Res. 71:5393–5399. 2011. View Article : Google Scholar : PubMed/NCBI
|