Introduction
Pancreatic cancer is one of the common malignances
of the digestive system with an increasing incidence rate. Due to
its insidious onset, the diagnosis of pancreatic cancer is usually
delayed. Owing to the low resection rate, high recurrence rate, and
resistance to radiotherapy and chemotherapy (1), patients with pancreatic cancer
typically have a poor prognosis. Previous studies have identified
11 death-from-cancer signatures related with tumor metastasis and
treatment prognosis (2,3), and ubiquitin-specific protease 22
(USP22) is one of them. As a component of the transcription
regulatory histone acetylation complex SAGA, USP22 regulates gene
transcription at an epigenetic level through the deubiquitination
of histones, exerting broad biological functions, including cell
cycle progression, embryonic development and telomere homeostasis
(4–6). USP22 is expressed in numerous normal
human tissues but is overexpressed in malignant tumors, such as
colorectal, liver, breast, gastric, bladder and lung cancer, a
showing correlation with tumor progression and metastasis (7–14).
Findings of these studies have demonstrated the importance of USP22
in cancer development; however, the role of USP22 in pancreatic
cancer has not been investigated.
Autophagy is a self-degradative process regulating
cell defense and stress response in eukaryotic systems. It is
highly regulated by multiple cell signaling pathways, such as the
PI3K/AKT/mTOR and Ras/RAF1/MEK1/2/ERK1/2 pathways (15–17),
to respond sensitively to cellular cues. Autophagy is a
double-edged sword that can either prevent or promote cancer
development depending on cellular contexts. Autophagy can induce
non-apoptotic or necrotic cell death or chemotherapy-induced cell
death (18,19). It promotes cancer cell survival
under hypoxia, nutrient depletion or growth factor deprivation
(20,21). Thus, inhibition of autophagy can
increase the sensitivity to chemotherapy, leading to cancer
remission (22–24). Recent studies have shown that cancer
cells with active autophagy tend to survive longer, causing poor
prognosis of cancer (22,23,25).
Pancreatic cancer is known to have a higher level of
autophagy than other epithelial cancers (26). The expression of LC3 (a key
structural and regulatory protein for the formation of the
autophagosome) is low or absent in normal exocrine pancreas and in
low-grade pancreas intraepithelial neoplasia-1 (PanIN-1) and
PanIN-2 lesions. However, this expression is elevated in high-grade
PanIN-3 and pancreatic ductal adenocarcinoma (PDAC), suggesting the
relevance of autophagy in pancreatic cancer progression (27). Stem-like pancreatic cancer cells
also show more active autophagy than less stem-like cells (28). This evidence suggests the regulatory
role of autophagy in pancreatic cancer progression that can be
developed into a new biomarker or therapeutic target.
Although USP22 and autophagy have been relatively
well studied, their relationship in pancreatic cancer remains to be
determined. In the present study, we first identified the
overexpression of USP22 and LC3 in pancreatic cancer patient
tissues. Using a pancreatic cancer cell line, we also demonstrated
that USP22 increased LC3 processing and induced autophagy to
promote cell survival. Further analysis with a large number of
patient specimens identified a strong correlation between
USP22-induced autophagy and the poor prognosis of pancreatic
cancer.
Materials and methods
Pancreatic cancer patient samples
Pancreatic cancer tissues were collected from 68
patients during surgery at the First Affiliated Hospital of Dalian
Medical University, China between 2002 and 2006. Ten adjunct
non-cancerous tissues were also collected as the controls. All the
tissues were fixed by formalin and embedded in paraffin wax for
histological and immunohistochemical experiments. The pancreatic
cancer tissues were pancreatic ductal adenocarcinoma. The cancers
were staged according to the American Joint Committee on Cancer
(AJCC) standards (29). All the
procedures with regard to patient recruitment, informed consent,
sample collection and processing were approved by the IRB of Dalian
Medical University.
Reagents
Earle’s balanced salt solution (EBSS),
3-methyl-adenine (3-MA) and monodansylcadaverine (MDC) were
purchased from Sigma. LysoTracker Red and kinase inhibitors
(PD98059 and LY294002) were purchased from the Beyotime Institute
of Biotechnology. 2′,2′-Difluorodeoxycytidine gemcitabine (GEM) was
obtained from Dalian Melone, Biotechnology Co., Ltd. USP22-specific
shRNA, negative control shRNA (shNC), USP22 expression construct
and the blank vector were designed and synthesized by GenePharma.
USP22 antibody was purchased from Abcam, LC3 antibody from Sigma,
antibodies against BECN1, SQSTM1, Bcl-2, caspase-3, AKT1 from
Proteintech, and antibodies against phospho-AKT1 (Ser473), ERK1/2
and phospho-ERK1/2 from Bioworld.
Cell culture and transfection
The human pancreatic cell line (Panc-1) was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). Panc-1 cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; HyClone) supplemented
with 10% fetal bovine serum, 100 IU/ml penicillin and 100 μg/ml
streptomycin at 37°C with 5% CO2. Transfection of Panc-1
cells was performed using Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer’s instructions.
Immunohistochemical (IHC) staining
The general procedure of IHC was performed according
to the protocol described (30).
The USP22 antibody was used at 1:400 dilution and LC3 antibody at
1:200 dilution. Biotin-labeled secondary antibodies were used for
visualization through HRP-streptavidin with 3,3′-diaminobenzidine
(DAB) substrate. The counterstaining was carried out with
haematoxylin. Staining with pre-immune IgG was used as the
control.
Transmission electron microscopy
(TEM)
Panc-1 cells were collected by trypsin digestion
method and fixed with 2.5% glutaraldehyde. The cell pellets were
then fixed with 1% osmic acid. After a series of dehydration, cell
pellets were embedded in Embedding Medium (Sigma). Ultrathin
sections (50–70 nm) were prepared using Leica EM UC6 ultramicrotome
and stained with uranyl acetate and lead citrate, followed by
observation on a JEM-2000EX transmission electron microscope.
LysoTracker Red and MDC staining
Cells were stained with LysoTracker Red in
phosphate-buffered saline (PBS) or 0.1 mM MDC in DMEM at 37°C for
30 min in the dark. After washing three times with PBS, the cells
were examined using fluorescence microscopy.
Immunofluorescence staining
Panc-1 cells were fixed with 4% paraformaldehyde,
and then permeabilized with 0.1% Triton X. After blocking with goat
serum, the cells were incubated with USP22 or LC3 antibody
overnight at 4°C, followed by incubation with corresponding
secondary antibody for 40 min at room temperature. The cells were
counterstained with DAPI for observation by fluorescence
microscopy.
Cell proliferation assay
Cell proliferation was measured by the CCK-8 Kit
(Beyotime Institute of Biotechnology) according to the
manufacturer’s instructions. Cells in 100 μl media were reacted
with 10 μl CCK-8 reagent at 37°C for 2 h, followed by measuring the
absorbance at 450 nm on a microplate reader. The data were analyzed
using SPSS software.
Cell cycle assay
Cells were harvested and fixed with 70% ice-cold
ethanol for 24 h, and then stained with propidium iodide (PI) for
cell cycle analysis by flow cytometry. The data were analyzed using
SPSS.
Apoptosis assay
Cells were harvested and stained with the Annexin
V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology). The apoptotic cells were determined by flow
cytometry and the data were analyzed using SPSS.
Reactive oxygen species (ROS) assay
Cells grown in 96-well plates were incubated with 10
μM DCF-DA for 20 min at 37°C. The DCF fluorescence (Ex 485
nm and Em 535 nm) was measured using a multimode
plate reader. The data were analyzed by SPSS.
Western blotting
Cells were lysed using RIPA buffer [25 mM Tris-HCl
(pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS]
with protease and phosphatase inhibitors (EMD Biosciences). After
sonication for 2 min at 4°C and centrifugation for 10 min at 4°C,
the supernatant was taken as the total cell lysate. Protein
concentration was quantified using the Bradford method. Equal
amounts of total protein (50 μg) were analyzed by
SDS-polyacrylamide gel (SDS-PAGE). Following transfer to the NC
membrane, the blot was probed with primary antibodies as indicated
and then incubated with HRP-labeled secondary antibodies for
visualization using enhanced chemiluminesence reagents (Thermo).
The images were obtained by the Bio-Rad Imaging System.
Statistical analysis
The overall survival curves were generated using the
Kaplan-Meier method. The relationship between USP22 and LC3 in
pancreatic cancer tissues was analyzed by the Spearman rank
correlation analysis. The relationship between the level of LC3 or
USP22 expression and clinicopathological characteristics was
examined using the χ2 test. The differences among groups
were analyzed by one-way ANOVA and the Student-Newman-Keuls (SNK)-q
test using SPSS 17.0 software. Differences were considered to
indicate a statistically significant result with a P-value
<0.05.
Results
Overexpression of USP22 correlates with a
high level of autophagy in human pancreatic cancer
To examine whether USP22 is overexpressed in
pancreatic cancer and determine its relationship with autophagy and
pancreatic cancer prognosis, 68 pancreatic cancer tissue samples
and 10 adjacent normal pancreatic tissue samples were collected and
analyzed in the present study. IHC staining was used to detect
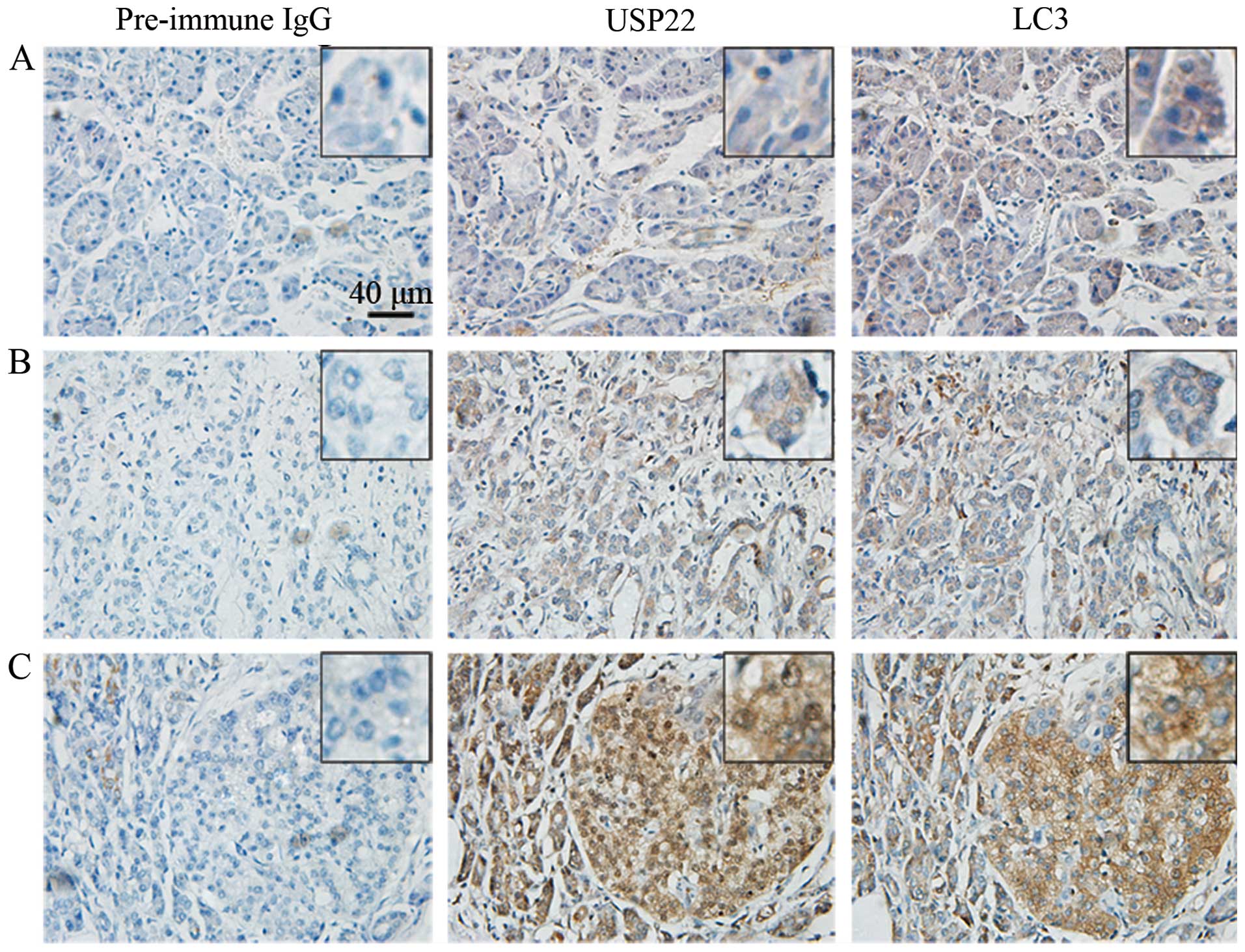
USP22 and LC3 in situ. By analyzing several slides each for
all 78 samples, it was found that both USP22 and LC3 were expressed
in these patient samples to different extents. The representative
images are shown in Fig. 1.
Notably, USP22 was not expressed in normal adjacent tissues, but
was overexpressed in advanced pancreatic cancer tissues, suggesting
that USP22 may play an important role in pancreatic cancer
progression. By contrast, LC3 was expressed at basal level in
normal pancreatic tissue and also elevated to a high level in
advanced pancreatic cancer tissue, indicating that it is required
for physiological and pathological autophagy. In addition, it was
found that USP22 was localized to the cytoplasm and nucleus,
whereas LC3 was localized to the cytoplasm only, both of which are
consistent with their physiological functions.
Quantitative analysis of all IHC results revealed
that there were 66.2% of pancreatic cancer samples expressing USP22
and 52.9% for LC3. However, in adjacent normal tissues the
expression of USP22 and LC3 was 0 and 10%, respectivley (Table I). The USP22 and LC3 proteins showed
a significant difference between pancreatic cancer and the adjacent
normal tissue (P=0.000, 0.011). Further statistical analysis
demonstrated that 44.1% of pancreatic cancer patients were USP22-
and LC3-positive, whereas the percentage for double-negative in
USP22 and LC3 was 25%. Tumors (22.1%) were USP22-positive but
LC3-negative, while 8.8% of tumors were LC3-positive but
USP22-negative (Table II). The
association analysis using SPSS indicated that the correlation
between USP22 and LC3 was strong in pancreatic cancer (ρ=0.385,
P=0.001). These results suggested that the overexpression of USP22
is highly related with autophagy in pancreatic cancer, indicating
the significance of investigating the pathological role of USP22 in
pancreatic cancer.
 | Table ISummary of USP22 and LC3 expression
status in pancreatic cancer and adjacent normal tissues analyzed in
this study. |
Table I
Summary of USP22 and LC3 expression
status in pancreatic cancer and adjacent normal tissues analyzed in
this study.
| USP22 expression
(n) | LC3 expression
(n) | |
|---|
|
|
| |
|---|
| Group | Neg. | Pos. | Neg. | Pos. | Total (n) |
|---|
| Adjacent normal
tissue | 10 | 0 | 9 | 1 | 10 |
| Pancreatic cancer
tissue | 23 | 45 | 32 | 36 | 68 |
 | Table IIThe relationship between USP22 and
LC3 expression identified by IHC experiments. |
Table II
The relationship between USP22 and
LC3 expression identified by IHC experiments.
| USP22
expression |
|---|
|
|
|---|
| LC3 expression | Negative | Positive | Total |
|---|
| Negative | 17 | 15 | 32 |
| Positive | 6 | 30 | 36 |
| Total | 23 | 45 | 68 |
Overexpression of USP22 and the high
level of autophagy correlate with the poor prognosis of pancreatic
cancer patients
To clarify the relativity of USP22 and autophagy to
pancreatic cancer prognosis, we systematically analyzed the
correlation of USP22 expression and autophagy with all
clinicopathological characterics of pancreatic cancer from the 68
patients. The cancer stages were determined according to the AJCC
system. Based on the IHC analyses of all 68 samples as above, we
identified the following relationships (Table III): i) tumor differentiation,
lymphatic vessel infiltration and cancer stage were associated with
the expression of USP22 and LC3 (P<0.05); ii) pancreatic
external invasion was associated with USP22 (P<0.05) but not
with LC3 (P>0.05); iii) age, gender, tumor location and tumor
size were not associated with the expression of USP22 and LC3
(P>0.05). These results proved the positive relationship between
the expression of USP22 and LC3 and the progression of pancreatic
cancer, suggesting that USP22 overexpression may be a causal factor
for pancreatic cancer.
 | Table IIIStatistical analysis of the
correlation between the expression of USP22 and LC3 and the
clinicopathological parameters of pancreatic cancer. |
Table III
Statistical analysis of the
correlation between the expression of USP22 and LC3 and the
clinicopathological parameters of pancreatic cancer.
| | USP22
expression | LC3 expression |
|---|
| |
|
|
|---|
| Clinicopathological
parameters | n | Neg. | Pos. | P-value | Neg. | Pos. | P-valuea |
|---|
| Age (years) | | | | 0.945 | | | 0.397 |
| <60 | 27 | 9 | 18 | | 11 | 16 | |
| ≥60 | 41 | 14 | 27 | | 21 | 20 | |
| Gender | | | | 0.783 | | | 0.931 |
| Male | 40 | 13 | 27 | | 19 | 21 | |
| Female | 28 | 10 | 18 | | 13 | 15 | |
| Tumor location | | | | 0.959 | | | 0.418 |
| Pancreatic
head | 50 | 17 | 33 | | 25 | 25 | |
| Pancreatic body
and tail | 18 | 6 | 12 | | 7 | 11 | |
| Tumor size
(cm) | | | | 0.339 | | | 0.357 |
| <3 | 30 | 12 | 18 | | 16 | 14 | |
| ≥3 | 38 | 11 | 27 | | 16 | 22 | |
| Tumor
differentiation | | | | 0.004 | | | 0.012 |
| Well | 8 | 6 | 2 | | 7 | 1 | |
| Moderate | 33 | 13 | 20 | | 17 | 16 | |
| Poor | 27 | 4 | 23 | | 8 | 19 | |
| Lymphatic vessel
infiltration | | | | 0.007 | | | 0.019 |
| Without | 41 | 19 | 22 | | 24 | 17 | |
| With | 27 | 4 | 23 | | 8 | 19 | |
| Pancreatic external
invasion | | | | 0.02 | | | 0.096 |
| Without | 31 | 15 | 16 | | 18 | 13 | |
| With | 37 | 8 | 29 | | 14 | 23 | |
| AJCC cancer
stage | | | | 0.004 | | | 0.011 |
| IA/IB/IIA | 40 | 19 | 21 | | 24 | 16 | |
| IIB/III/IV | 28 | 4 | 24 | | 8 | 20 | |
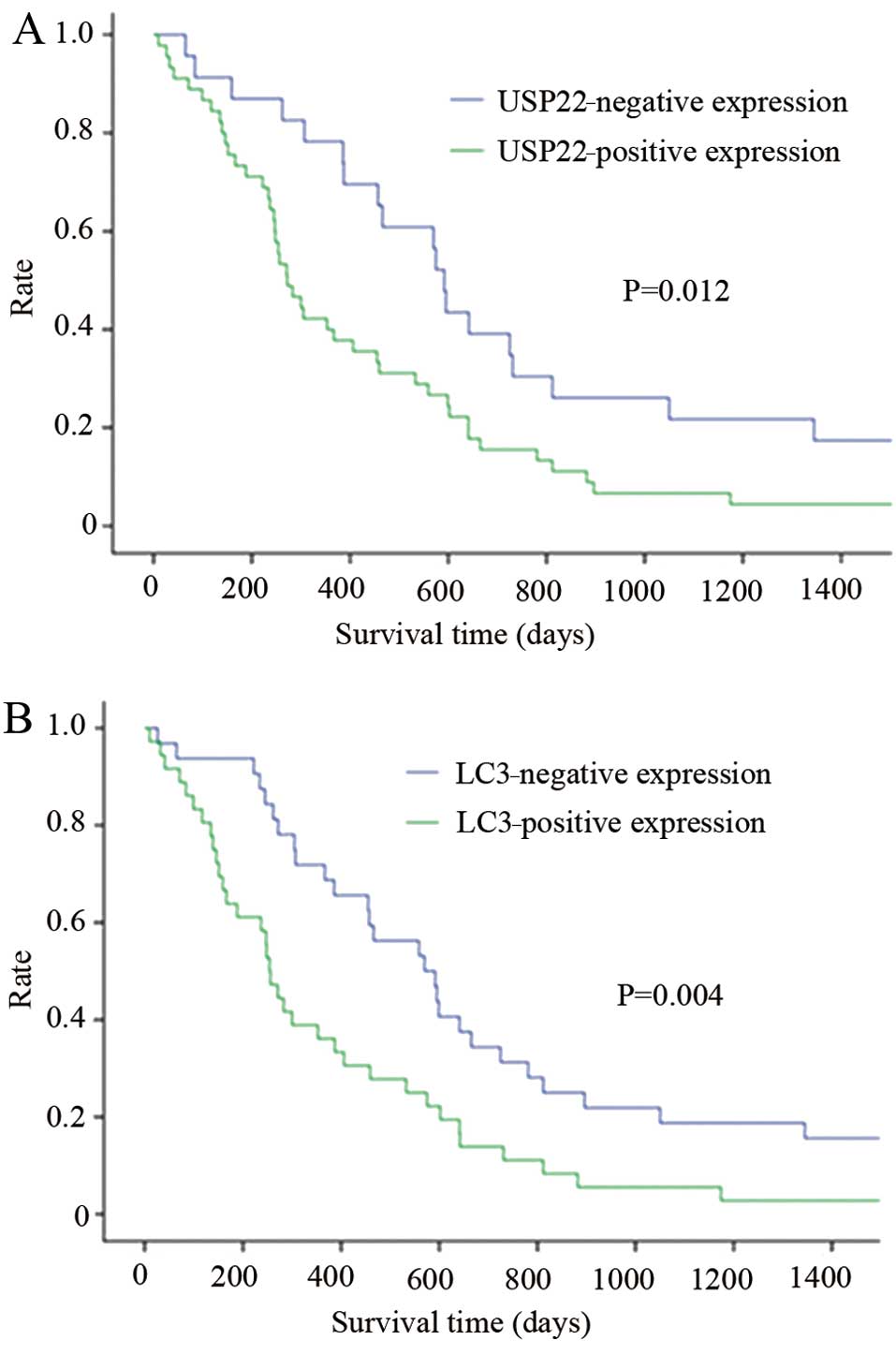
To confirm the relationship between USP22 and LC3
and the outcome of pancreatic cancer, the survival curve of
patients was analyzed using the Kaplan-Meier method. The results
demonstrated that the overexpression of USP22 and LC3 was
significantly correlated with short survival time (Fig. 2), indicating a close relationship
between USP22 overexpression and/or enhanced autophagy and the poor
prognosis of pancreatic cancer patients.
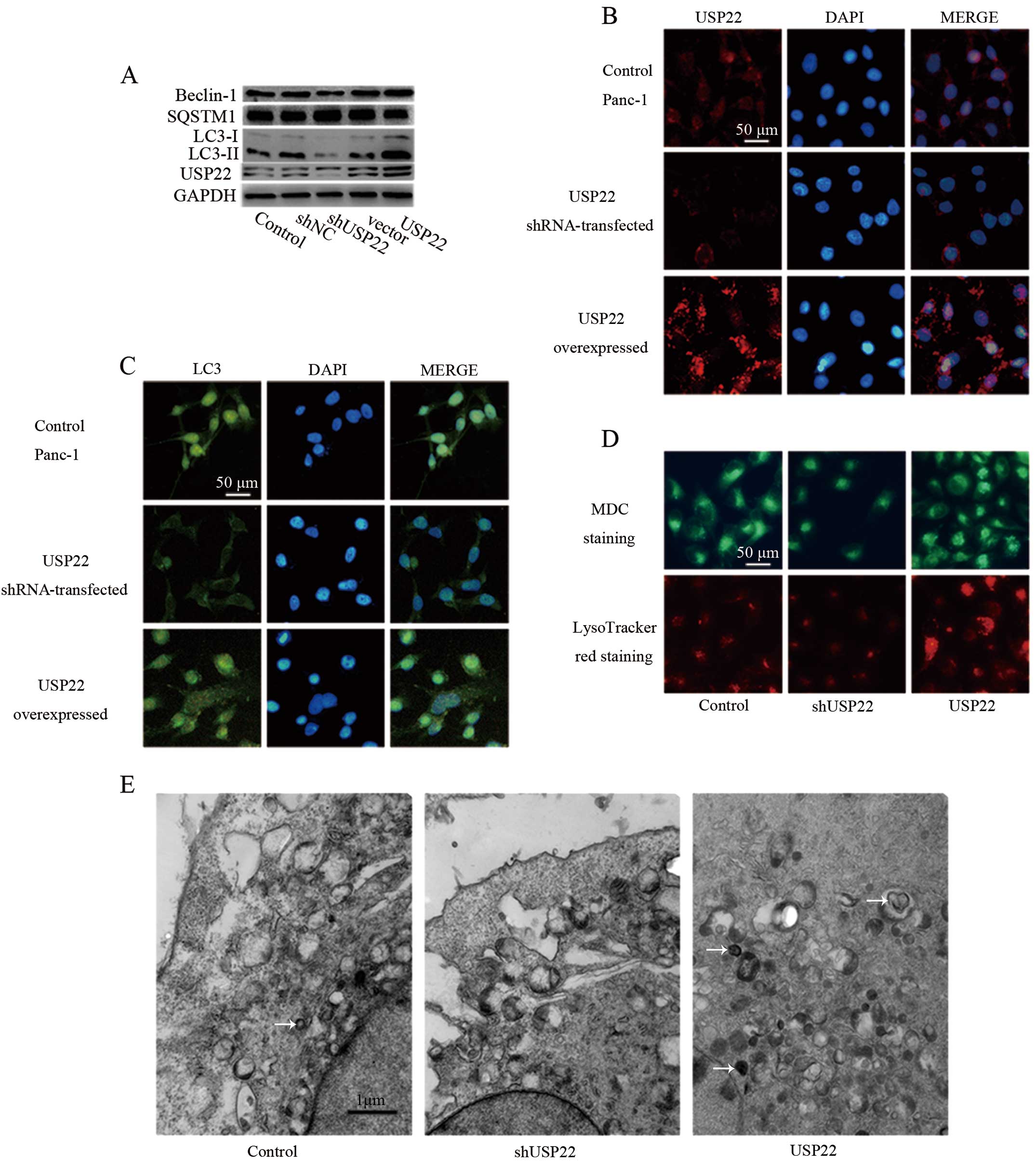
USP22 induces autophagy in Panc-1
cells
Results from the clinical samples above suggested
that USP22 was able to promote LC3 expression, therefore leading to
autophagy. To clarify this possibility, the Panc-1 pancreatic
cancer cell line was utilized for the experiments. As shown in
Fig. 3A, when USP22 was
downregulated by shRNA or overexpressed by USP22 cDNA construct,
USP22 levels were altered accordingly. USP22 shRNA was found to
markedly knock down USP22, concomitant with a notable decrease in
LC3-II, indicating that USP22 was a factor for promoting LC3
processing to generate a more active form LC3-II. SQSTM1, a
selective substrate of autophagy, was also decreased following
USP22 overexpression, demonstrating that USP22 promoted authophagy.
However, the other autophagy component beclin-1 was not affected by
USP22, suggesting the early stage of autophagy may not be the
target of USP22. Immunofluorescent staining experiments further
supported the high efficiency of USP22 knockdown or overexpression
(Fig. 3B) and the corresponding
change in LC3 (Fig. 3C).
To investigate whether autophagy flux is promoted by
USP22, LysoTracker Red staining (specific staining for lysosome)
and MDC staining (specific staining for autophagosome) were
performed. As shown in Fig. 3D, the
number of lysosome and autophagosome were increased by the
overexpression of USP22, suggesting that the formation of
autophagosome fused with lysosome was promoted by USP22.
Furthermore, TCM analysis also showed the increased number of
autophagosome following the overexpression of USP22 (Fig. 3E). Taken together, these results
demonstrated that USP22 is a factor that promotes autophagy in
pancreatic cancer cells.
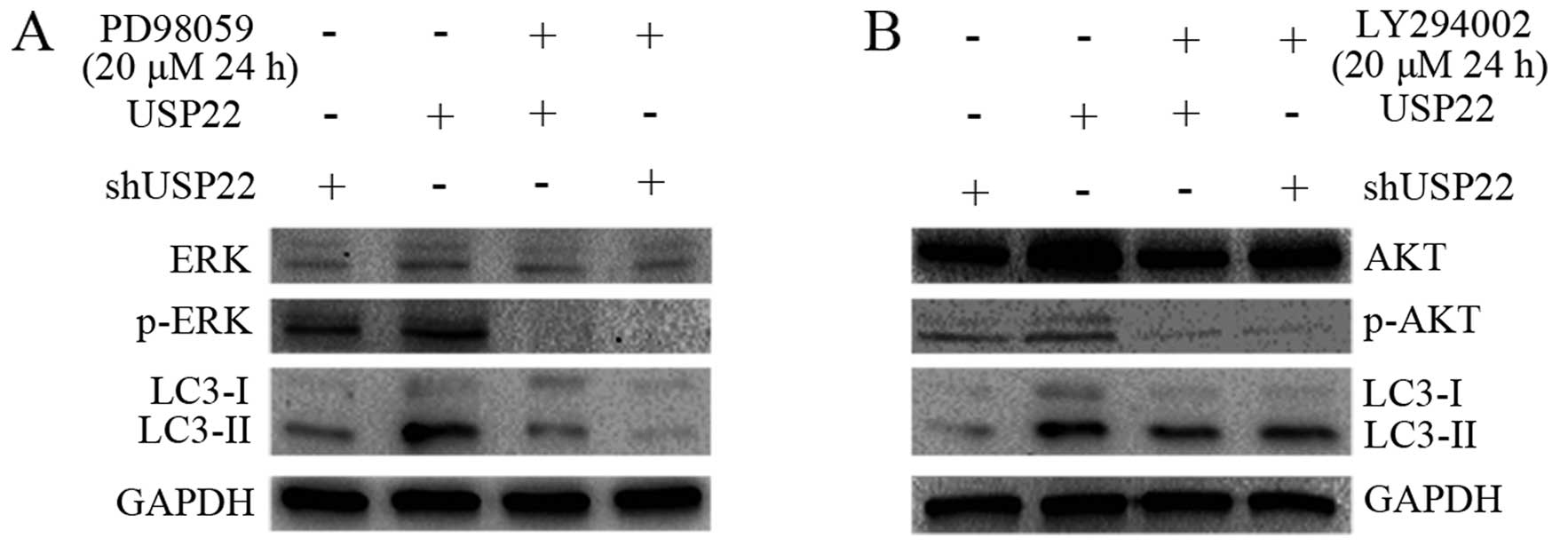
Activation of ERK is one of the
mechanisms for USP22-induced autophagy
To investigate the molecular mechanism underlying
the augmented autophagy by USP22, MAPK and PI3K/AKT pathways were
specifically analyzed in the present study. Using RNAi and gene
overexpression combined with the application of kinase inhibitors,
it was found that USP22 overexpression slightly increased ERK1/2
activity as reflected by the phosphorylated form of ERK1/2. The
level of LC3-II was concomitantly increased (Fig. 4A). In addition, inactivation of
ERK1/2 by a specific inhibitor (PS98059) abolished LC3-II
formation, suggesting that ERK1/2 is one of the kinases involved in
the promotion of LC3-II formation and subsequent autophagy, which
may occur through the phosphorylation of LC3 as observed in the
regulation of LC3 by PKA and PKC (31,32).
However, the inhibition of AKT1 by a PI3K inhibitor (LY294002) did
not alter the level of LC3-II (Fig.
4B). Thus, these results demonstrated that ERK activation is
involved in USP22-induced autophagy.
USP22-induced autophagy increases Panc-1
cell survival
To elucidate the mechanism of USP22-induced
autophagy on the prognosis of pancreatic cancer patients, cell
death and survival pathways were further examined. When an
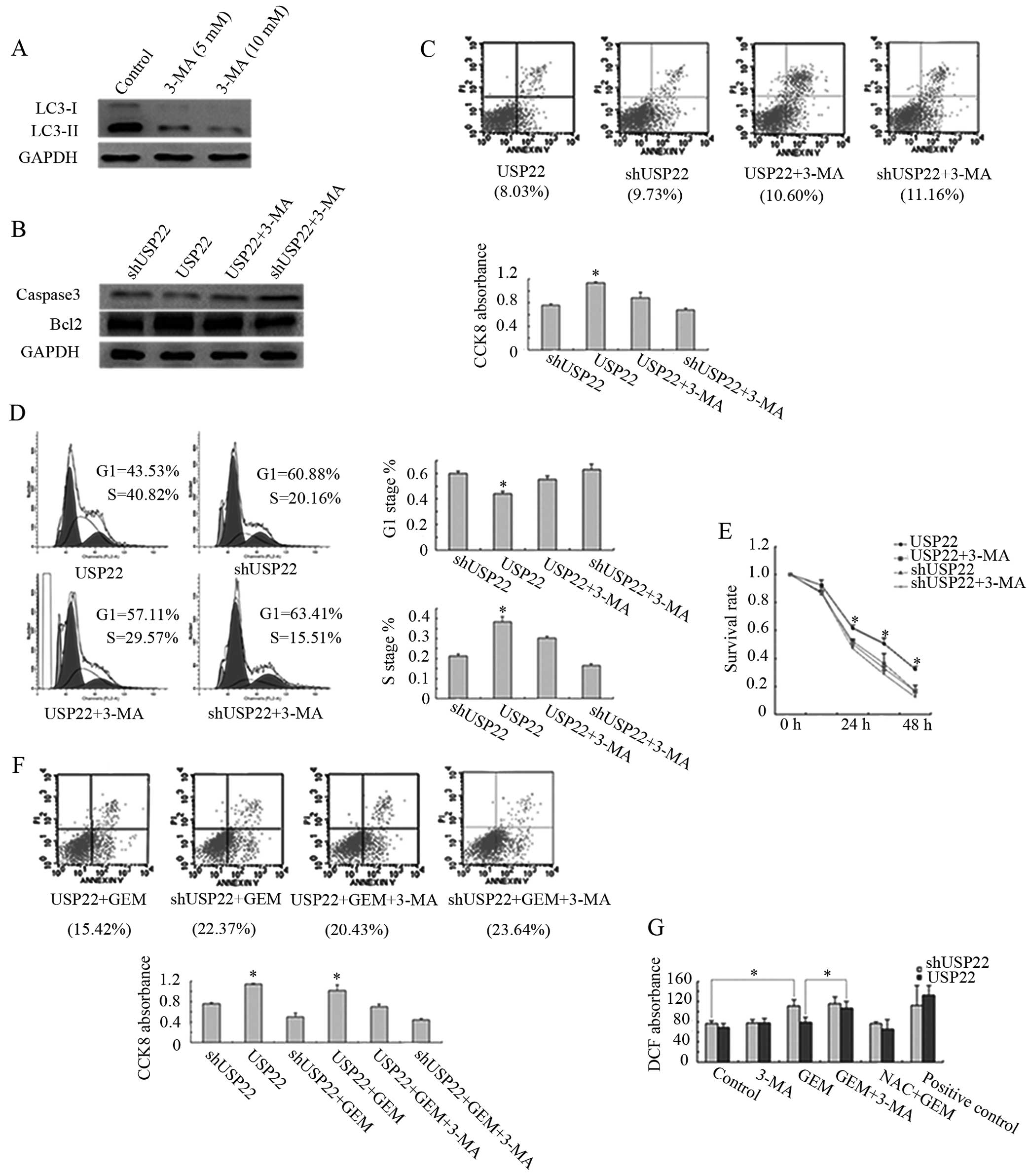
autophagy inhibitor 3-MA was used to inhibit autophagy in Panc-1
cells, it was found to be effective as indicated by the decrease of
LC3-II (Fig. 5A). The effect of
USP22-induced autophagy on apoptosis was then analyzed. Western
blotting of anti-apoptotic Bcl-2 and pro-apoptotic caspase-3 showed
almost no difference following the overexpression or knockdown of
USP22 or treatment with 3-MA (Fig.
5B). Further analysis of apoptosis with flow cytometry did not
show any obvious changes (Fig. 5C,
upper panel). These results demonstrated that USP22-induced
autophagy has no significant effect on apoptosis in Panc-1 cells.
We also examined Panc-1 cell proliferation under various levels of
USP22. It was found that the proliferation of USP22
shRNA-tranfected cells was reduced by ~30% as compared to
USP22-overexpressed cells. The 3-MA treatment reduced the
proliferation of USP22 cells by ~20%, but only by 10% for USP22
shRNA-transfected cells (Fig. 5C,
bottom panel). Flow cytometric analysis revealed that the
percentage of S phase in USP22-overexpressed cells was increased by
2-fold over the USP22 shRNA-transfected cells and by 1.4-fold over
the USP22-overexpressed and 3-MA-treated cells (Fig. 5D). These data confirm that the
function of USP22-induced autophagy promoted Panc-1 cell
proliferation.
Nutritional deficiency is a characteristic of the
pancreatic cancer microenvironment (33), which is closely related to the
development of pancreatic cancer (34,35).
To determine whether USP22-induced autophagy enhances resistance to
starvation, Panc-1 cells were starved in EBSS for various
timepoints. USP22-overexpressed cells showed enhanced resistance to
starvation as compared to USP22 shRNA-transfected cells. However,
the capacity of resistance to starvation was decreased after
treatment with 3-MA (Fig. 5E),
suggesting that USP22 conferred resistance to nutritional
starvation is mediated by autophagy.
When Panc-1 cells were treated with a chemotherapy
drug gemcitabine (36–38), it was found that the apoptotic rate
of USP22-overexpressed cells was less than that of the USP22
shRNA-transfected cells. However, the apoptotic rate was increased
by combinational treatment with gemcitabine and 3-MA (Fig. 5F, upper panel). The cell
proliferation assay demonstrated that the inhibition rate of
proliferation by USP22 overexpression and gemcitabine treatment was
less than that by the knockdown of USP22 and gemcitabine treatment.
Combined treatment with gemcitabine and 3-MA further inhibited
Panc-1 cell proliferation (Fig. 5F,
lower panel). These results demonstrated that USP22 also promoted
cell survival under chemotherapeutic condition through autophagy.
Since gemcitabine functions as a nucleoside metabolic inhibitor, it
generates cellular stresses, such as ROS. As shown in Fig. 5G, knockdown of USP22 plus
gemcitabine treatment increased the ROS level as compared to USP22
overexpression and gemcitabine treatment. Additional treatment with
3-MA increased the level of ROS, suggesting that the prevention of
ROS production by USP22 may be another mechanism for USP22-mediated
cell survival.
The aforementioned results indicated that
USP22-induced autophagy increases cell proliferation and the
resistance to stresses, such as starvation and chemotherapy,
thereby synergistically promoting the cell survival of pancreatic
cancer.
Discussion
The present study aimed to identify the correlation
of USP22 overexpression with poor prognosis of pancreatic cancer
patients, which is mediated by the autophagy mechanism. The
investigation with a large number of clinical samples ensured the
medical relevance of this study, thus establishing a foundation for
future clinical studies.
Systematic analyses on pancreatic cancer cell lines
using various techniques have defined the central identification of
this study, USP22-induced LC3 processing altering into active form
LC3-II, leading to the enhanced autophagy that increased the cell
survival and resistance to nutritional starvation and chemotherapy,
all of which synergistically resulted in the poor prognosis of
pancreatic cancer. Thus, the present study elucidates an oncogenic
role rather than a tumor suppressive function of autophagy in
pancreatic cancer progression. Activation of ERK1/2 was identified
to be one of the mechanisms underlying the promotion of LC3
processing by USP22. The detailed mechanism concerning whether
ERK1/2 phosphorylates LC3-I and increases its processing into
LC3-II remains to be determined. However, we hypothesize that other
molecular mechanisms through different signaling pathways may be
involved in the regulation of USP22 effects on autophagy. Studies
on AMPK and mTOR pathways may expand the association of
USP22-induced autophagy with cancer metabolomic regulation. Besides
LC3, other autophagy steps or components (e.g., autophagy-related
genes) may also be the targets regulated by USP22. Of note, it is
still not known how USP22 regulates autophagy components at the
gene transcriptional level, all of which are to be addressed in
future investigations.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 30870719), and the Dalian
Municipal Science and Technology Foundation (no. 2011E15SF114).
References
|
1
|
Gukovskaya AS and Pandol SJ: Cell death
pathways in pancreatitis and pancreatic cancer. Pancreatology.
4:567–586. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glinsky GV, Berezovska O and Glinskii AB:
Microarray analysis identifies a death-from-cancer signature
predicting therapy failure in patients with multiple types of
cancer. J Clin Invest. 115:1503–1521. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glinsky GV: ‘Stemness’ genomics law
governs clinical behavior of human cancer: implications for
decision making in disease management. J Clin Oncol. 26:2846–2853.
2008.
|
|
4
|
Atanassov BS, Evrard YA, Multani AS, et
al: Gcn5 and SAGA regulate shelterin protein turnover and telomere
maintenance. Mol Cell. 35:352–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XY, Varthi M, Sykes SM, et al: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Lang G, Ito S, et al: A TFTC/STAGA
module mediates histone H2A and H2B deubiquitination, coactivates
nuclear receptors, and counteracts heterochromatin silencing. Mol
Cell. 29:92–101. 2008. View Article : Google Scholar
|
|
7
|
Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM
and Baek KH: The expression patterns of deubiquitinating enzymes,
USP22 and Usp22. Gene Expr Patterns. 6:277–284. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YL, Yang YM, Xu H and Dong XS:
Increased expression of ubiquitin-specific protease 22 can promote
cancer progression and predict therapy failure in human colorectal
cancer. J Gastroenterol Hepatol. 25:1800–1805. 2010. View Article : Google Scholar
|
|
9
|
Liu YL, Yang YM, Xu H and Dong XS:
Aberrant expression of USP22 is associated with liver metastasis
and poor prognosis of colorectal cancer. J Surg Oncol. 103:283–289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang DD, Cui BB, Sun LY, et al: The
co-expression of USP22 and BMI-1 may promote cancer progression and
predict therapy failure in gastric carcinoma. Cell Biochem Biophys.
61:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Yao L, Zhang X, et al: Elevated
expression of USP22 in correlation with poor prognosis in patients
with invasive breast cancer. J Cancer Res Clin Oncol.
137:1245–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning J, Zhang J, Liu W, Lang Y, Xue Y and
Xu S: Overexpression of ubiquitin-specific protease 22 predicts
poor survival in patients with early-stage non-small cell lung
cancer. Eur J Histochem. 56:e462012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling SB, Sun DG, Tang B, et al: Knock-down
of USP22 by small interfering RNA interference inhibits HepG2 cell
proliferation and induces cell cycle arrest. Cell Mol Biol.
58(Suppl): OL1803–OL1808. 2012.PubMed/NCBI
|
|
15
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogier-Denis E and Codogno P: Autophagy: a
barrier or an adaptive response to cancer. Biochim Biophys Acta.
1603:113–128. 2003.PubMed/NCBI
|
|
17
|
Pattingre S, Bauvy C and Codogno P: Amino
acids interfere with the ERK1/2-dependent control of macroautophagy
by controlling the activation of Raf-1 in human colon cancer HT-29
cells. J Biol Chem. 278:16667–16674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pardo R, Lo Ré A, Archange C, et al:
Gemcitabine induces the VMP1-mediated autophagy pathway to promote
apoptotic death in human pancreatic cancer cells. Pancreatology.
10:19–26. 2010. View Article : Google Scholar
|
|
19
|
Tung WL and Wang Y, Gout PW, Liu DM,
Gleave M and Wang Y: Use of irinotecan for treatment of small cell
carcinoma of the prostate. Prostate. 71:675–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
21
|
Debnath J, Baehrecke EH and Kroemer G:
Does autophagy contribute to cell death? Autophagy. 1:66–74. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Liu D, Yang Y and Zhao S: Effect of
autophagy inhibition on chemotherapy-induced apoptosis in A549 lung
cancer cells. Oncol Lett. 5:1261–1265. 2013.PubMed/NCBI
|
|
24
|
Shingu T, Fujiwara K, Bögler O, et al:
Inhibition of autophagy at a late stage enhances imatinib-induced
cytotoxicity in human malignant glioma cells. Int J Cancer.
124:1060–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujii S, Mitsunaga S, Yamazaki M, et al:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
26
|
Yang S and Kimmelman AC: A critical role
for autophagy in pancreatic cancer. Autophagy. 7:912–913. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang S, Wang X, Contino G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rausch V, Liu L, Apel A, et al: Autophagy
mediates survival of pancreatic tumour-initiating cells in a
hypoxic microenvironment. J Pathol. 227:325–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Egner JR: AJCC cancer staging manual.
JAMA. 304:1726–1727. 2010. View Article : Google Scholar
|
|
30
|
Gillett CE: Immunohistochemistry. Methods
Mol Med. 120:191–200. 2006.
|
|
31
|
Cherra SR III, Kulich SM, Uechi G, et al:
Regulation of the autophagy protein LC3 by phosphorylation. J Cell
Biol. 190:533–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang H, Cheng D, Liu W, Peng J and Feng
J: Protein kinase C inhibits autophagy and phosphorylates LC3.
Biochem Biophys Res Commun. 395:471–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levine B and Klionsky DJ: Development by
self-digestion: molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guerra C, Collado M, Navas C, et al:
Pancreatitis-induced inflammation contributes to pancreatic cancer
by inhibiting oncogene-induced senescence. Cancer Cell. 19:728–739.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Neesse A, Michl P, Frese KK, et al:
Stromal biology and therapy in pancreatic cancer. Gut. 60:861–868.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burris HR, Moore MJ, Andersen J, et al:
Improvements in survival and clinical benefit with gemcitabine as
first-line therapy for patients with advanced pancreas cancer: a
randomized trial. J Clin Oncol. 15:2403–2413. 1997.PubMed/NCBI
|
|
37
|
Donadelli M, Dando I, Zaniboni T, et al:
Gemcitabine/cannabinoid combination triggers autophagy in
pancreatic cancer cells through a ROS-mediated mechanism. Cell
Death Dis. 2:e1522011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
DeNicola GM, Karreth FA, Humpton TJ, et
al: Oncogene-induced Nrf2 transcription promotes ROS detoxification
and tumorigenesis. Nature. 475:106–109. 2011. View Article : Google Scholar : PubMed/NCBI
|