Introduction
Cutaneous malignant melanoma (CMM) is the most
deadly form of skin neoplasm. With an estimated mortality of over
55,000 worldwide in 2012 which is increasing yearly and even with a
more rapidly growing incidence, CMM has become a major challenge
for modern oncology (1). Detailed
studies are required to provide accurate risk stratification and to
better identify the groups of patients in need of tailored
treatment strategies.
In melanomas, multiple factors with potential
prognostic value have been described over the years (2–5).
Breslow thickness still remains the most powerful prognostic factor
and is a substantial component of every CMM pathology report
(6). The presence of ulceration has
been considered as the second most important primary tumor
characteristic for the purpose of predicting patient outcome
(5). With the exception of
traumatic disruption of the epidermis, ulceration has been a key
element in the last two editions of the melanoma TNM staging
guidelines of the American Joint Committee on Cancer (AJCC) - its
presence verified in microscopic evaluation of changes in the pT
stage from pTxa to pTxb (6,7).
Recently, scientific attention has been drawn
towards another microscopic feature of the primary tumor, the
mitotic rate (MR). The inclusion of this parameter in multivariate
models confirmed its significance in risk stratification,
particularly in localized melanomas (8–10).
Additionally, several studies have indicated that MR has a higher
impact than ulceration (8,11,12).
As a valuable parameter that reflects tumor proliferative activity
and aggressiveness, MR has recently been introduced into the AJCC
melanoma staging system (6). Under
current recommendations, MR is defined as the number of mitotic
figures per square millimeter and a value ≥1 upstages the T
subcategory of the pTNM classification from a to b, but only at the
pT1 level (6). Clinically, this
translates into a recommendation for sentinel lymph node biopsy for
patients presenting with pT1b, although not for those with pT1a
stage tumors (6).
The present study aimed to examine the relationship
between key melanoma prognosticators, namely the presence of
ulceration and mitotic rate against clinicopathological
characteristics and patient survival, and to discuss the results in
the context of AJCC melanoma staging recommendations.
Materials and methods
Patients
The study group consisted of 104 patients with CMM,
who were diagnosed between 2005 and 2010 and treated at the Lower
Silesian Oncology Center in Wrocław, Poland. The group was selected
on the basis of tissue material (paraffin blocks and histopathology
slides) and the availability of medical documentation.
Comprehensive clinical data were obtained from archival medical
records. The diagnostic and therapeutic procedures utilized were
determined from medical records in the Oncology Outpatient Clinic
of the Lower Silesian Oncology Center and data provided by the
Lower Silesian Cancer Registry and Civil Register Office. The study
was approved by the Institutional Review Board of the Wrocław
Medical University, Poland.
The clinicopathological profile of the patients
included the following parameters: age and gender, primary tumor
location, tumor stratification according to AJCC, presence or
absence of nodal (pT or pN) and distant (pM) metastases,
information on disease recurrence and sentinel lymph node biopsy
(SLNB) procedures (Table I).
 | Table IClinicopathological characteristics of
the cutaneous malignant melanoma patients. |
Table I
Clinicopathological characteristics of
the cutaneous malignant melanoma patients.
| Clinicopathological
characteristics | No (%) | High mitotic rate
(≥3/mm2) | P-value | Ulceration | P-value |
|---|
| All patients | 104 (100.0) | 33 | | 49 | |
| Age (years) | | | 0.598 | | 0.205 |
| Range
(21–79)a | | | | | |
| Mean: 56.5±15.4 | | | | | |
| Median: 58.5 | | | | | |
| Genderb | | | 0.576 | | 0.313 |
| Female | 60 (57.7) | 19 | | 30 | |
| Male | 44 (42.3) | 14 | | 19 | |
| Primary tumor
locationc | | | 0.494 | | 0.056 |
| Head/neck | 15 (14.4) | 7 | | 11 | |
| Upper
extremities | 18 (17.3) | 4 | | 8 | |
| Lower
extremities | 25 (24.0) | 7 | | 9 | |
| Trunk | 42 (40.4) | 13 | | 17 | |
| Hand/foot | 4 (3.8) | 2 | | 4 | |
| Primary tumor
(pT)a | | | <0.001 | | <0.001 |
| pT1 | 34 (32.7) | 2 | | 2 | |
| pT2 | 20 (19.2) | 2 | | 6 | |
| pT3 | 27 (26.0) | 13 | | 20 | |
| pT4 | 23 (22.1) | 16 | | 21 | |
| Sentinel lymph node
biopsy status (SNLB)b | 60 (57.7) | | 0.002 | | 0.001 |
| No metastases
(SNLB−) | 48 (80.0) | 6 | | 14 | |
| Metastases present
(SNLB+) | 12 (20.0) | 7 | | 10 | |
| Regional lymph
nodes status (pN)b | | |
<0.001 | |
<0.001 |
| No metastases
(pN−) | 86 (82.7) | 20 | | 33 | |
| Metastases present
(pN+) | 18 (17.3) | 13 | | 16 | |
| Recurrenceb | | | 0.041 | | 0.093 |
| No | 87 (83.7) | 24 | | 38 | |
| Yes | 17 (16.3) | 9 | | 11 | |
| Distant
metastasesb | | | 0.034 | | 0.147 |
| No | 99 (95.2) | 29 | | 45 | |
| Yes | 5 (4.8) | 4 | | 4 | |
Tumor samples and histopathological
evaluation
Tumor specimens were fixed in 10% buffered formalin
and embedded in paraffin. All haematoxylin and eosin (H&E)
stained sections were examined by two pathologists. The parameters
of the primary tumor recorded in pathology reports included Breslow
thickness, Clark level, growth phase, histologic type, mitotic rate
(number of mitotic figures per 1 mm2), presence of
ulceration, lymphangioinvasion, microsatellitosis, intensity of
lymphocytic inflammatory infiltrate (TILs, tumor-infiltrating
lymphocytes) and microscopic evidence of regression (Table II).
 | Table IICorrelations between high mitotic
rate (hMR) and the presence of ulceration and histopathological
characteristics of the cutaneous malignant melanoma primary
tumors. |
Table II
Correlations between high mitotic
rate (hMR) and the presence of ulceration and histopathological
characteristics of the cutaneous malignant melanoma primary
tumors.
| Histopathological
characteristics | No. (%) | High mitotic rate
(≥3/mm2) | P-value | Ulceration | P-value |
|---|
| Breslow
thicknessa | | |
<0.001 | |
<0.001 |
| <1 mm | 34 (32.7) | 2 | | 2 | |
| 1.01–2.00 mm | 20 (19.2) | 2 | | 5 | |
| 2.01–4.00 mm | 27 (26.0) | 16 | | 21 | |
| >4 mm | 23 (22.1) | 13 | | 21 | |
| Clark levela | | |
<0.001 | |
<0.001 |
| I | 0 (0.0) | 0 | | 0 | |
| II | 18 (17.3) | 1 | | 0 | |
| III | 49 (47.1) | 10 | | 17 | |
| IV | 26 (25.0) | 15 | | 22 | |
| V | 11 (10.6) | 7 | | 10 | |
| Histologic
typeb | | |
<0.001 | |
<0.001 |
| Superficial
spreading melanoma (SSM) | 68 (65.4) | 11 | | 18 | |
| Nodular malignant
melanoma (NMM) | 32 (30.8) | 20 | | 27 | |
| Acral-lentiginous
melanoma (ALM) | 4 (3.8) | 2 | | 4 | |
| Mitotic
ratea | | | | |
<0.001 |
| 0 | 45 (43.3) | | | 4 | |
| 1–2 | 26 (25.0) | | | 16 | |
| ≥3 | 33 (31.7) | | | 29 | |
| Ulcerationc | | |
<0.001 | | |
| No | 55 (52.9) | 4 | | | |
| Yes | 49 (47.1) | 29 | | | |
|
Lymphangioinvasionc | | |
<0.001 | |
<0.001 |
| No | 74 (71.2) | 11 | | 25 | |
| Yes | 30 (28.8) | 22 | | 24 | |
| Growth
phasec | | | 0.314 | | 0.144 |
| Radial | 3 (2.9) | 0 | | 0 | |
| Vertical | 101 (97.1) | 33 | | 49 | |
| Tumor-infiltrating
lymphocytes (TILs)b | | | 0.010 | |
<0.001 |
| No | 18 (17.3) | 10 | | 13 | |
| Nonbrisk | 34 (32.7) | 13 | | 22 | |
| Brisk | 52 (50) | 10 | | 14 | |
|
Microsatellitosisc | | | 0.012 | | 0.078 |
| No | 98 (94.2) | 28 | | 44 | |
| Yes | 6 (5.8) | 5 | | 5 | |
| Tumor
regressionc | | | 0.495 | | 0.295 |
| No | 96 (92.3) | 30 | | 44 | |
| Yes | 8 (7.7) | 3 | | 5 | |
Statistical analysis
Statistical analysis was performed using the
Statistica 10.0 and IBM SPSS 21 software packages. Overall survival
(OS) was defined as the time between the primary surgical treatment
and death, and OS was censored at last follow-up for patients who
were still alive. Disease-free survival (DFS) was defined as the
time between the primary surgical treatment and the date of
relapse. DFS was censored at the last follow-up for patients who
survived without disease recurrence or at the date of
non-cancer-associated death. Cancer-specific overall survival
(CSOS) was defined as the time between the primary surgical
treatment and cancer-associated death, and was censored at the last
follow-up for surviving patients.
A χ2 test, exact Fisher’s test in the
case of 2×2 tables and Spearman’s rank correlation were used to
analyze the associations between mitotic rate and the presence of
ulceration and clinicopathological parameters. Differences between
the means were tested with a nonparametric test (Mann-Whitney U
test and Kruskal-Wallis test); the log-rank test was used to
compare survival in two groups. The overall survival rate was
estimated by the Kaplan-Meier method and the influence of
explanatory variables on death risk was analyzed by means of the
Cox proportional hazard regression. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
MR and the presence of ulceration in 104
melanoma patients
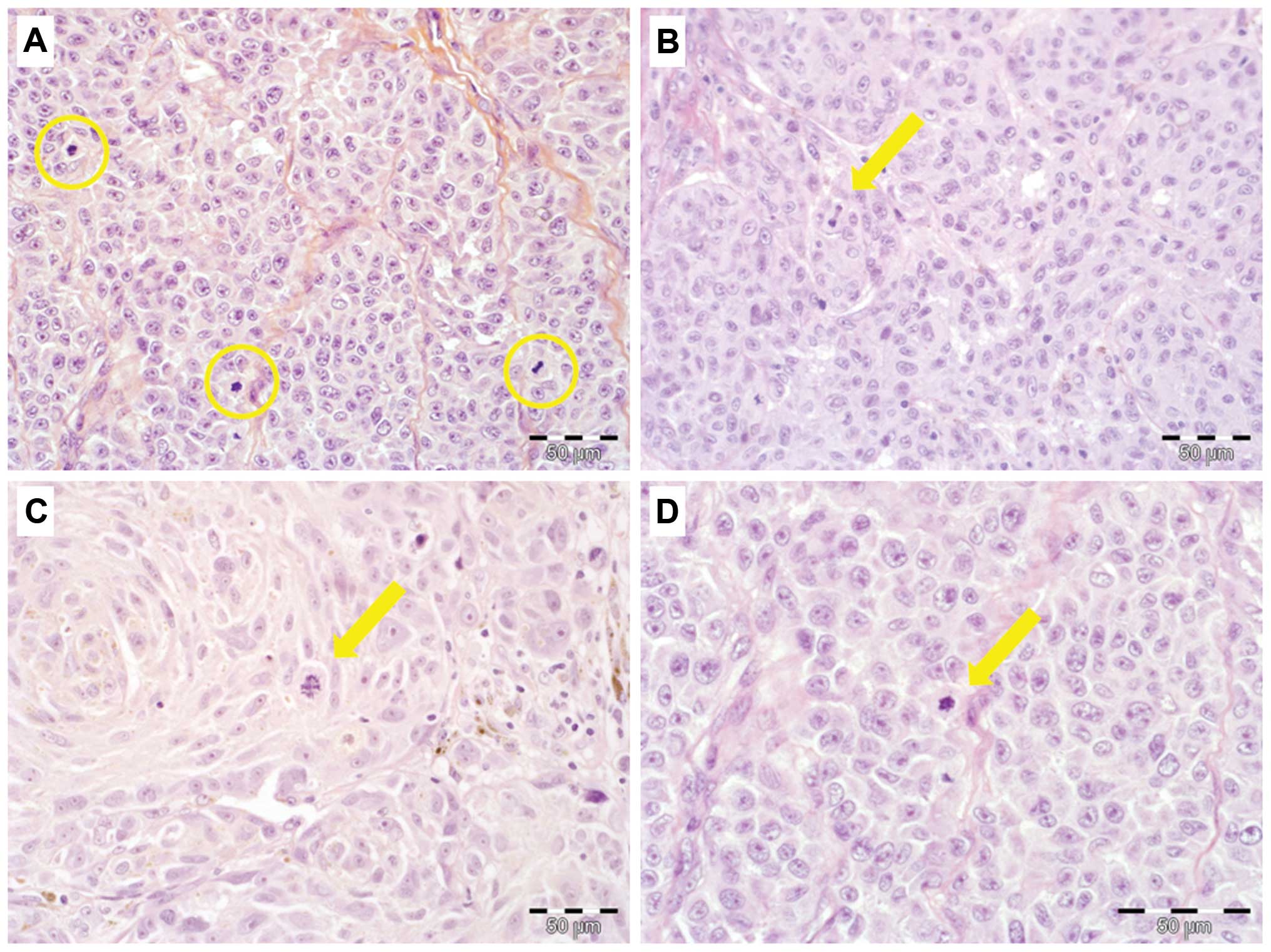
The mitotic activity of the primary tumors was
divided into three categories: no mitotic activity (0
mitoses/mm2), low activity (1–2 mitoses/mm2)
and high mitotic rate (hMR; ≥3 mitoses/mm2) (Fig. 1A–D). No mitotic activity was
detected in 45 patients (43.3%), whereas low MR was observed in 26
patients (25%). High proliferative activity was observed in 33
patients (31.7% of the study group). Analysis of MR in melanomas of
various degrees of clinical advancement with regard to pT stage of
the primary tumor revealed that only a small percentage of early
(pT1 and pT2) tumors exhibited hMR (6 and 10%, respectively). In
the advanced disease, hMR tumors accounted for 48 and 70%,
respectively in T3 and T4 melanomas (Table I).
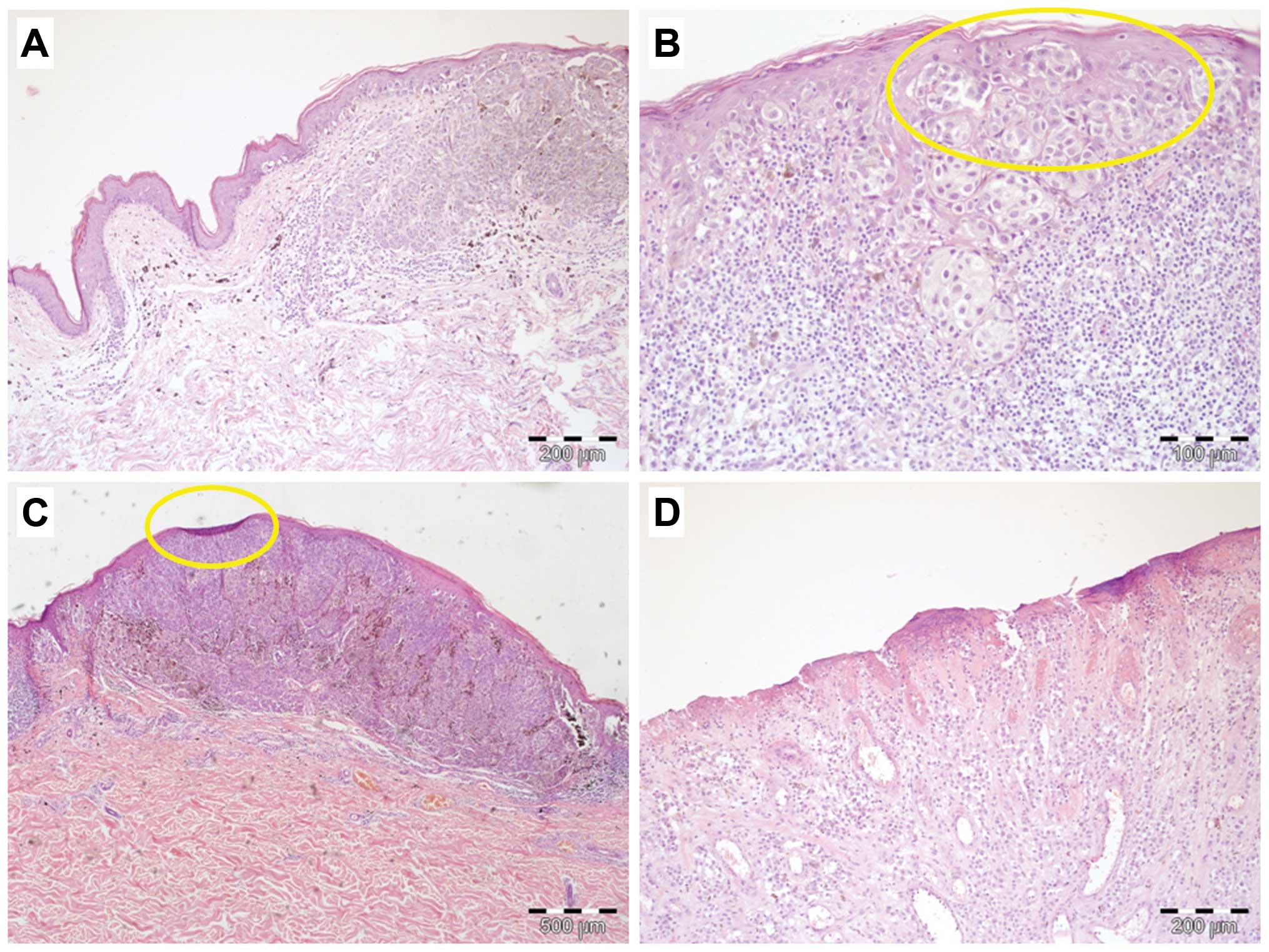
Ulceration was observed in 49% of the tumors
(Fig. 2A–D). Within the pT1 group
of tumors, only a small number (6%) of tissue specimens showed
microscopic evidence of ulceration. Interestingly, 30% of pT2
melanomas were ulcerated. Advanced (pT3 and pT4) tumors were
characterized by a significantly higher prevalence of ulceration
(74 and 91%, respectively) (Table
I).
Correlations between hMR and
clinicopathological parameters
A high mitotic rate was significantly correlated
with higher advancement of the primary tumor (pT; P<0.001), the
presence of nodal and distant metastases (P<0.001 and P=0.034,
respectively), positive status for sentinel lymph node (P=0.002)
and disease recurrence (P=0.041). Furthermore, hMR was strongly
associated with deeper infiltration according to Breslow thickness
(P<0.001) and Clark level (P<0.001), the presence of
ulceration (P<0.001), lymphangioinvasion (P<0.001),
microsatellitosis (P=0.012) and histologic type (nodular) of the
primary tumor (P<0.001). Interestingly, hMR was related to a
lower intensity of lymphocytic inflammatory infiltrate (P=0.01). No
other significant correlations were found between hMR and the other
analyzed clinicopathological parameters, including gender, age,
location and growth phase of the primary tumor and microscopic
evidence of its regression (Table
II).
Correlations between the presence of
ulceration and clinicopathological parameters
The presence of ulceration was significantly
correlated with higher advancement of the primary tumor (pT;
P<0.001) and with the presence of metastases in sentinel lymph
nodes (P=0.001) and regional lymph nodes (P<0.001). It was
further demonstrated that the presence of ulceration was associated
with deeper tumor infiltration according to Breslow thickness
(P<0.001) and Clark level (P<0.001), hMR (P<0.001),
lymphangionvasion (P<0.001) and histologic type (nodular and
acral-lentiginous) of the primary tumor (P<0.001). Another
relationship was revealed between a decrease in lymphocytic
inflammatory infiltrate intensity and ulceration of the primary
tumor (P<0.001). No further correlations were found regarding
the presence of ulceration and other analyzed clinicopathological
characteristics (Table II).
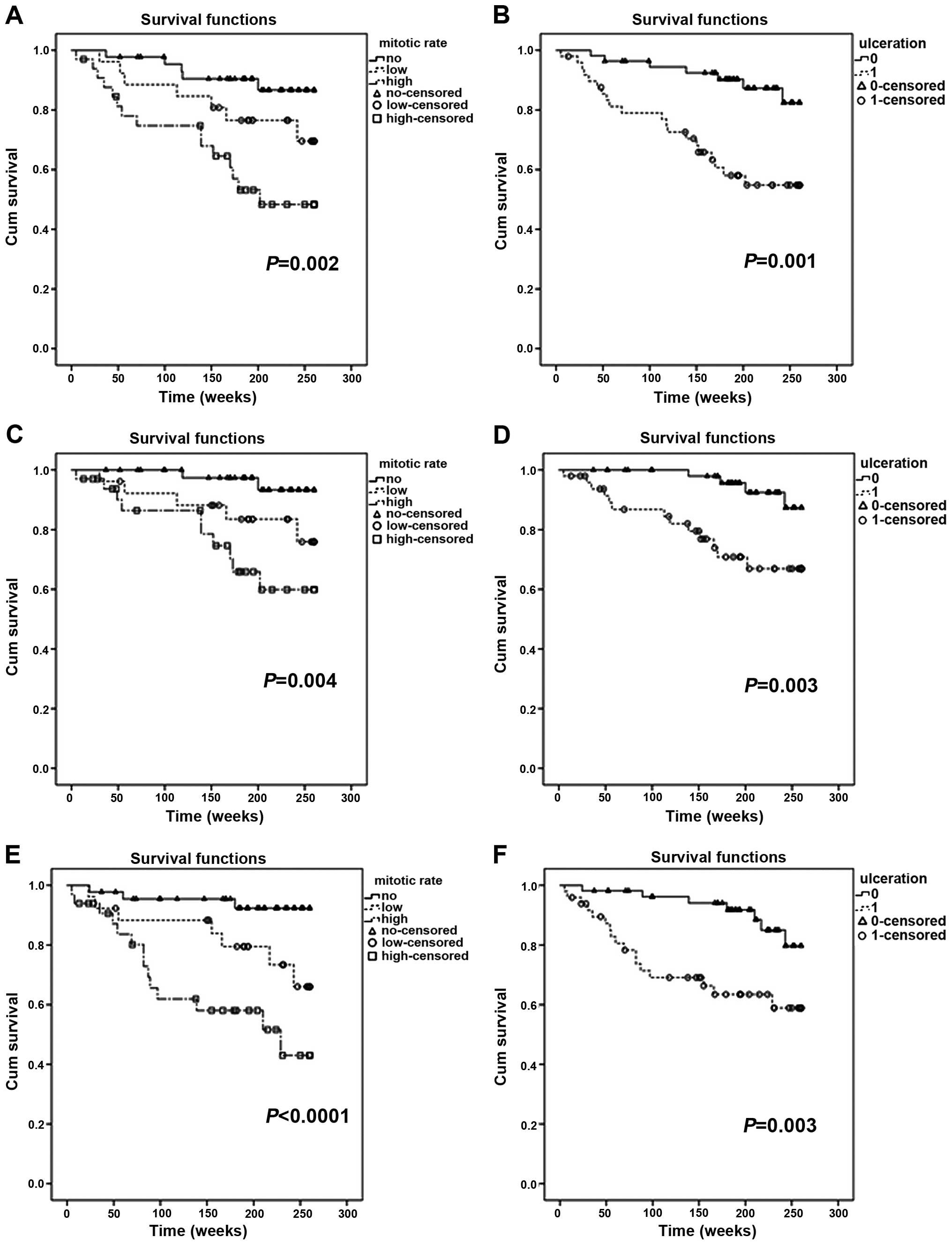
Impact of a high mitotic rate and
ulceration on the 5-year survival in melanoma patients
In the entire group of 104 patients, hMR was a
highly negative prognostic factor, and indicated considerably
shorter OS, CSOS and DFS (P=0.002, P=0.004 and P<0.001,
respectively) (Fig. 3A, C and E).
Similar relationships were observed for ulceration, which also
acted as a negative prognosticator for the entire study population
(P=0.001 for OS, P=0.003 for CSOS and P=0.003 for DFS) (Fig. 3B, D and F).
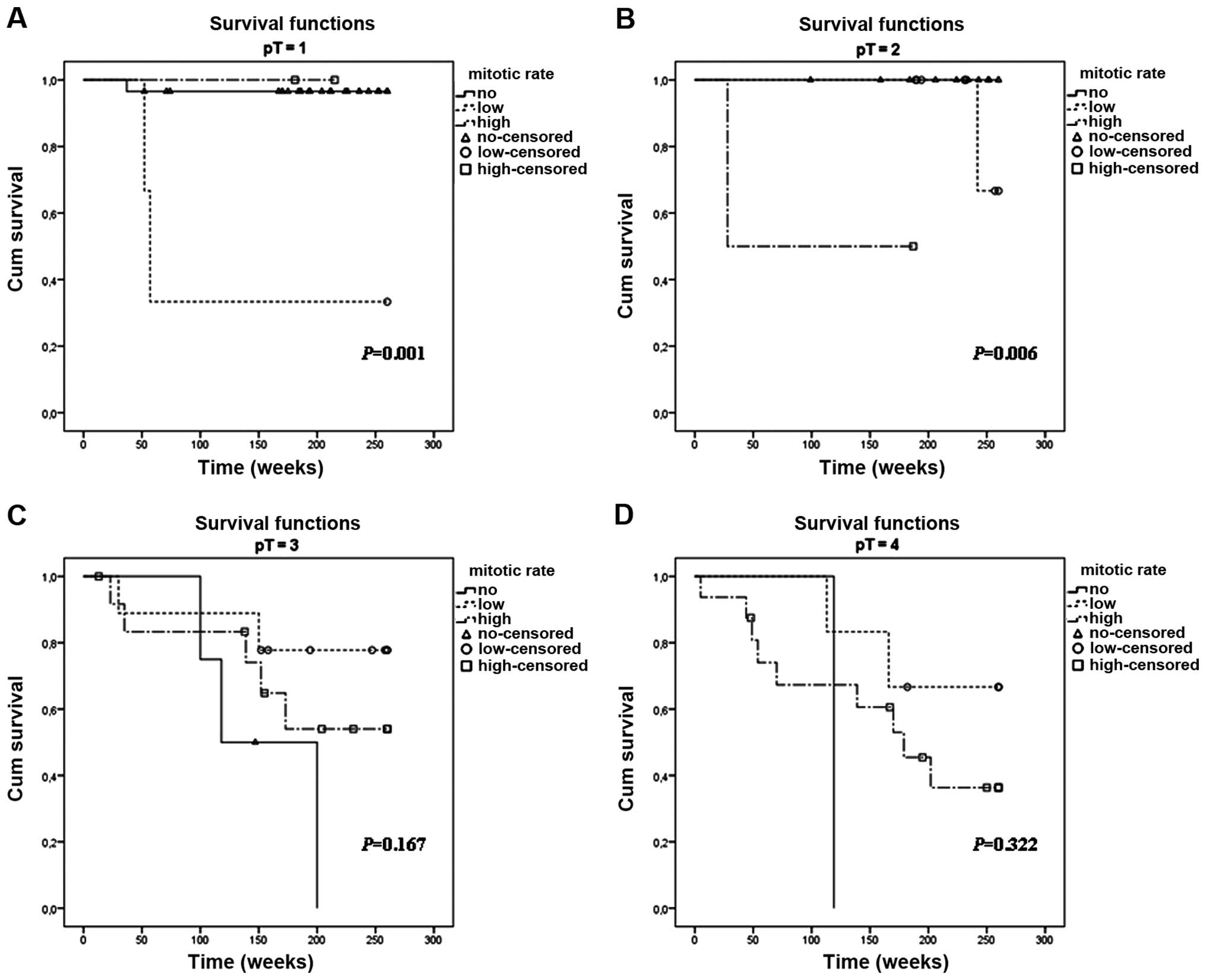
An important aspect of this study was to analyze the
prognostic significance of hMR and the presence of ulceration in
particular pT stages of primary tumor advancement. Notably, hMR
appeared to have a statistically significant negative impact on
survival in early melanomas in the pT1 (P=0.001) and pT2 subgroups
(P=0.006) (Fig. 4A and B).
Kaplan-Meier analysis of the remaining subsets (pT3 and pT4) did
not reveal any important differences in 5-year survival with regard
to MR values (Fig. 4C and D).
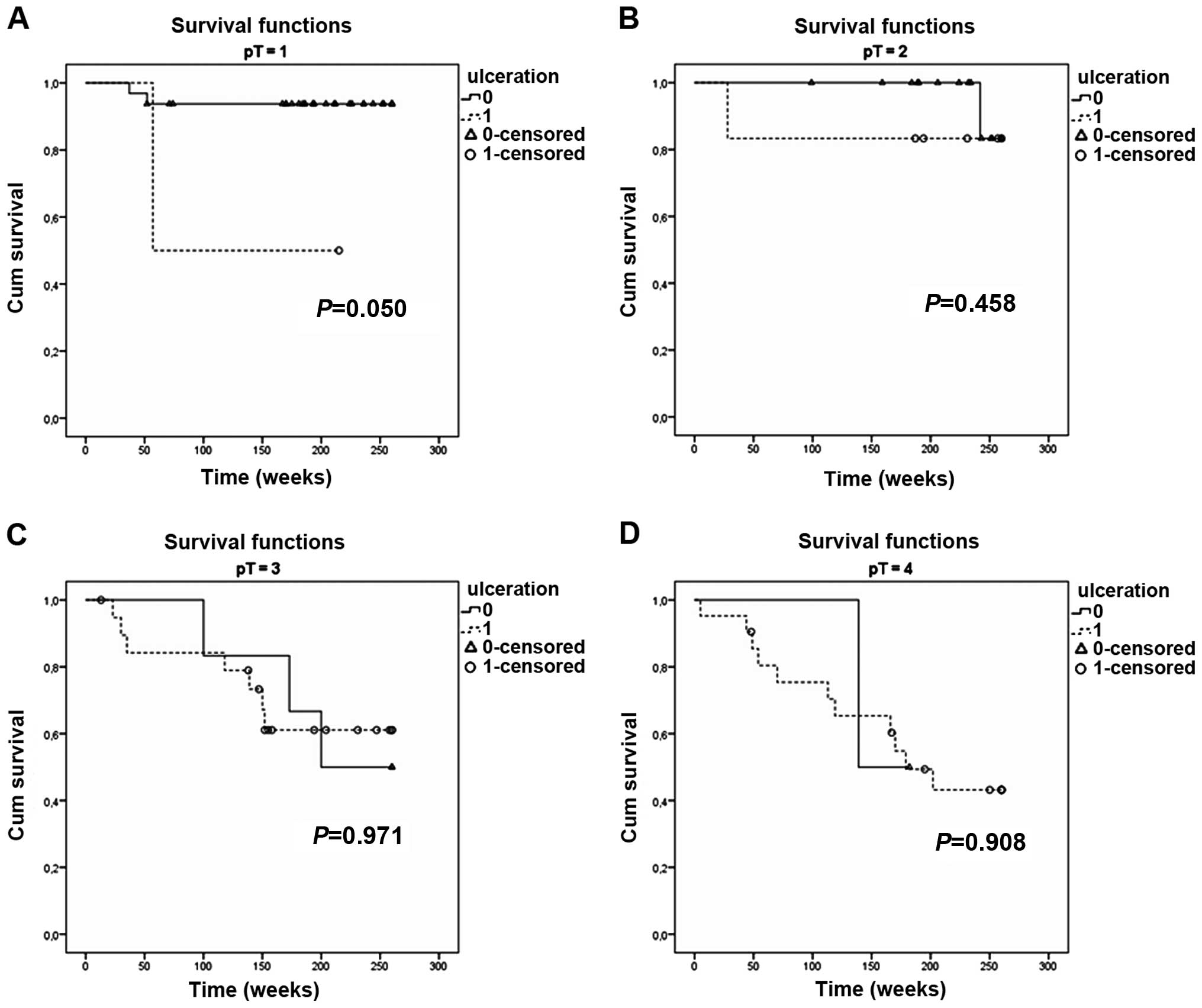
The presence of ulceration also had a prognostic
significance for early melanomas, but only for pT1 tumors (P=0.05)
(Fig. 5A). Kaplan-Meier analysis of
the other groups (pT2–T4) did not show any influence of ulceration
on the 5-year survival (Fig.
5B–D).
Multivariable Cox regression
analysis
Multivariate analysis confirmed that a high mitotic
rate and the presence of distant metastases were strongly
associated with an unfavorable prognosis (hMR: P=0.005; HR, 1.247;
95% CI, 1.069–1.456; pM: P=0.001; HR, 5.071; 95% CI, 1.883–13.656).
Ulceration had no prognostic significance in the Cox proportional
hazards model.
Discussion
The aim of the present study was to investigate the
relevance of the mitotic rate and primary tumor ulceration for the
prognosis of CMM, as well as correlations with other
clinicopathological features. In our study group of 104 patients,
hMR and the presence of ulceration were highly negative prognostic
factors, strongly correlated with shorter overall and
cancer-specific overall survival. An important aspect of the study
was to analyze the prognostic significance of hMR and the presence
of ulceration in particular pT stages of primary tumor advancement.
Notably, hMR appeared to have a statistically significant negative
impact on survival in early melanomas in the pT1 (P=0.001) and pT2
subgroups (P=0.006), whereas Kaplan-Meier analysis for pT3 and pT4
tumors did not reveal any important differences in 5-year survival
with regard to MR values. The presence of ulceration also had a
prognostic significance, but only for pT1 melanomas (P=0.05).
Kaplan-Meier analysis of other groups (pT2–T4) did not show any
influence of ulceration on 5-year survival.
The negative effect of elevated MR on patient
survival has been addressed in numerous studies and currently, this
parameter, albeit in a very limited way, affects the TNM staging of
CMM (6,8,10–14).
However, there are other examples in the literature indicating a
far greater significance of MR than that provided by the current
version of AJCC recommendations. In the study of Zettersten et
al increased MR negatively impacted overall survival in a
population of patients with thick (>4 mm) CMM (15). Nagore et al demonstrated that
in 823 localized invasive tumors, MR was the most important
prognostic factor of disease-free survival in a multivariable
analysis when Breslow thickness was considered as a continuous
variable (16).
Considering our results, the mitotic rate emerged as
a powerful parameter providing information on patient survival, and
on other crucial clinicopathological features. Thus hMR is related
to more advanced metastatic cancers and to greater risk of disease
recurrence. The relationship between hMR and distant metastases has
been recently reported by Murali et al (17). Moreover, our study confirms an
association between hMR and positivity of SNLB, which supports the
validity of the AJCC recommendation to upstage from pT1a to pT1b
based on increased MR (8,9,14). An
inverse correlation between MR and TIL grade has been noted by
Azimi et al (18), but
refuted by others (16).
Another aspect of our study was the analysis of the
prognostic significance of ulceration and investigation of
correlations between ulceration status and other
clinicopathological parameters. There are examples in the
literature which demonstrate that the impact of ulceration on
melanoma pathology is unclear. Meanwhile, according to some
studies, ulceration loses its role as an independent adverse
prognostic factor when MR is included in multivariable models
(12,17,19).
Eigentler et al reported that the presence or absence of
ulceration does not influence survival in multivariable analyses of
putative CMM prognostic factors among pT1 and pT4 tumors, while it
remains significant in intermediate (pT2 and pT3) melanomas.
However, MR was not considered in this study (20). It should be said here that
determining a universal and coherent definition of tumor-derived
(and only tumor-derived) ulceration is another problem, resulting
in inter-observer reproducibility that is not entirely satisfactory
(21).
In regards to the other correlations observed
between ulceration and clinicopathological characteristics, our
data are generally concordant with other reports. Whereas no
differences relating to the presence of ulceration were found in
regards to gender, age and primary tumor location, the findings
support the broad consensus that ulcerated lesions are thicker and
more deeply invasive (5,22,23).
An association between ulcerated melanomas and nodular histologic
type has also been previously observed (22). In accordance with our results, the
presence of ulceration has been postulated as a predictor of
sentinel lymph node involvement (14,24).
An interesting aspect reported by Balch et al and confirmed
by our study is the relationship between scanty lymphocytic
infiltrate and the presence of ulceration (25).
Considering the biology of melanoma, MR seems to be
a more reliable parameter than the presence or otherwise of
ulceration. The value of MR categorizes melanomas into tumors with
low or high proliferative potential, thus giving direct information
concerning their capacity to infiltrate deeper layers of the dermis
and, potentially, to generate regional lymph node and distant
metastases. MR is a much more objective parameter than ulceration
and its origin is never artifactual. Instead, it always reflects
the true biology of the tumor, independently of the infiltration
depth and extent of epidermal disruption. In the authors’ opinion,
ulceration is a valuable parameter that mirrors the invasive
potential of melanoma cells, albeit, primarily in moderately
advanced (pT1 and pT2) tumors. In these cases, the etiopathogenesis
of ulceration, strictly related to the destructive influence of
neoplastic melanocytes (so called consumption of the epidermis), is
doubtlessly cancer related.
In more deeply infiltrating (pT3, pT4) tumors,
ulceration loses its role as an objective parameter associated with
the nature of melanoma cells and reflecting their aggressive
behavior. In advanced melanomas, the ulceration may be
etiologically unrelated to epidermal consumption by cancer cells
and may be only a morphological manifestation of a purely
mechanical external injury. Although an analogous situation may
also occur in non-advanced tumors, it is much less probable.
To sum up, both hMR and ulceration have a very
significant influence on the outcome of patients with cutaneous
melanoma. However, as stressed above, it is hMR that is a much more
objective parameter, more accurately reflecting the biology of a
particular tumor. Because of our relatively small study population,
further larger-scale investigations are needed to more precisely
determine the pathophysiological role and prognostic significance
of hMR and ulceration, particularly in patients with early
melanoma, in whom more intensive therapy and/or more extensive
post-operative follow-up may be justified in order to improve the
prognosis.
Acknowledgements
This study was supported by Wroclaw Medical
University research grant Pbmn108.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 Cancer Incidence and Mortality Worldwide.
IARC CancerBase V1.0. (11)International Agency for Research on
Cancer; Lyon: 2013
|
|
2
|
Clark WH Jr, From L, Bernardino EA and
Mihm MC: The histogenesis and biologic behavior of primary human
malignant melanomas of the skin. Cancer Res. 29:705–727.
1969.PubMed/NCBI
|
|
3
|
Breslow A: Thickness, cross-sectional
areas and depth of invasion in the prognosis of cutaneous melanoma.
Ann Surg. 172:902–908. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balch CM, Murad TM, Soong SJ, Ingalls AL,
Halpern NB and Maddox WA: A multifactorial analysis of melanoma:
prognostic histopathological features comparing Clark’s and
Breslow’s staging methods. Ann Surg. 188:732–742. 1978.PubMed/NCBI
|
|
5
|
Balch CM, Soong SJ, Gershenwald JE,
Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross
MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond
R, Zhang Y, Liu PY, Lyman GH and Morabito A: Prognostic factors
analysis of 17,600 melanoma patients: validation of the American
Joint Committee on Cancer melanoma staging system. J Clin Oncol.
19:3622–3634. 2001.
|
|
6
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM,
McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ and Sondak
VK: Final version of 2009 AJCC melanoma staging and classification.
J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balch CM, Buzaid AC, Soong SJ, Atkins MB,
Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr,
Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross
MI, Sober A, Thompson JA and Thompson JF: Final version of the
American Joint Committee on Cancer staging system for cutaneous
melanoma. J Clin Oncol. 19:3635–3648. 2001.PubMed/NCBI
|
|
8
|
Thompson JF, Soong SJ, Balch CM,
Gershenwald JE, Ding S, Coit DG, Flaherty KT, Gimotty PA, Johnson
T, Johnson MM, Leong SP, Ross MI, Byrd DR, Cascinelli N, Cochran
AJ, Eggermont AM, McMasters KM, Mihm MC Jr, Morton DL and Sondak
VK: Prognostic significance of mitotic rate in localized primary
cutaneous melanoma: an analysis of patients in the
multi-institutional American Joint Committee on Cancer melanoma
staging database. J Clin Oncol. 29:2199–2205. 2011. View Article : Google Scholar
|
|
9
|
Kesmodel SB, Karakousis GC, Botbyl JD,
Canter RJ, Lewis RT, Wahl PM, Terhune KP, Alavi A, Elder DE, Ming
ME, Guerry D, Gimotty PA, Fraker DL, Czerniecki BJ and Spitz FR:
Mitotic rate as a predictor of sentinel lymph node positivity in
patients with thin melanomas. Ann Surg Oncol. 12:449–458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Ding S, Byrd DR, Cascinelli N, Cochran AJ, Coit DG,
Eggermont AM, Johnson T, Kirkwood JM, Leong SP, McMasters KM, Mihm
MC Jr, Morton DL, Ross MI and Sondak VK: Multivariate analysis of
prognostic factors among 2,313 patients with stage III melanoma:
comparison of nodal micrometastases versus macrometastases. J Clin
Oncol. 28:2452–2459. 2010. View Article : Google Scholar
|
|
11
|
Azzola MF, Shaw HM, Thompson JF, Soong SJ,
Scolyer RA, Watson GF, Colman MH and Zhang Y: Tumor mitotic rate is
a more powerful prognostic indicator than ulceration in patients
with primary cutaneous melanoma: an analysis of 3661 patients from
a single center. Cancer. 97:1488–1498. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnhill RL, Katzen J, Spatz A, Fine J and
Berwick M: The importance of mitotic rate as a prognostic factor
for localized cutaneous melanoma. J Cutan Pathol. 32:268–273. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Francken AB, Shaw HM, Thompson JF, Soong
SJ, Accortt NA, Azzola MF, Scolyer RA, Milton GW, McCarthy WH,
Colman MH and McGovern VJ: The prognostic importance of tumor
mitotic rate confirmed in 1317 patients with primary cutaneous
melanoma and long follow-up. Ann Surg Oncol. 11:426–433. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spatz A, Stock N, Batist G and van Kempen
LC: The biology of melanoma prognostic factors. Discov Med.
10:87–93. 2010.PubMed/NCBI
|
|
15
|
Zettersten E, Sagebiel RW, Miller JR III,
Tallapureddy S, Leong SP and Kashani-Sabet M: Prognostic factors in
patients with thick cutaneous melanoma (>4 mm). Cancer.
94:1049–1056. 2002. View Article : Google Scholar
|
|
16
|
Nagore E, Oliver V, Botella-Estrada R,
Moreno-Picot S, Insa A and Fortea JM: Prognostic factors in
localized invasive cutaneous melanoma: high value of mitotic rate,
vascular invasion and microscopic satellitosis. Melanoma Res.
15:169–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murali R, Haydu LE, Long GV, Quinn MJ, Saw
RP, Shannon K, Spillane AJ, Stretch JR, Kefford RF, Thompson JF and
Scolyer RA: Clinical and pathologic factors associated with distant
metastasis and survival in patients with thin primary cutaneous
melanoma. Ann Surg Oncol. 19:1782–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azimi F, Scolyer RA, Rumcheva P, Moncrieff
M, Murali R, McCarthy SW, Saw RP and Thompson JF:
Tumor-infiltrating lymphocyte grade is an independent predictor of
sentinel lymph node status and survival in patients with cutaneous
melanoma. J Clin Oncol. 30:2678–2683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han D, Zager JS, Shyr Y, Chen H, Berry LD,
Iyengar S, Djulbegovic M, Weber JL, Marzban SS, Sondak VK, Messina
JL, Vetto JT, White RL, Pockaj B, Mozzillo N, Charney KJ, Avisar E,
Krouse R, Kashani-Sabet M and Leong SP: Clinicopathologic
predictors of sentinel lymph node metastasis in thin melanoma. J
Clin Oncol. 31:4387–4393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eigentler TK, Buettner PG, Leiter U and
Garbe C: Impact of ulceration in stages I to III cutaneous melanoma
as staged by the American Joint Committee on Cancer Staging System:
an analysis of the German Central Malignant Melanoma Registry. J
Clin Oncol. 22:4376–4383. 2004. View Article : Google Scholar
|
|
21
|
Spatz A, Cook MG, Elder DE, Piepkorn M,
Ruiter DJ and Barnhill RL: Interobserver reproducibility of
ulceration assessment in primary cutaneous melanomas. Eur J Cancer.
39:1861–1865. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taylor RC, Patel A, Panageas KS, Busam KJ
and Brady MS: Tumor-infiltrating lymphocytes predict sentinel lymph
node positivity in patients with cutaneous melanoma. J Clin Oncol.
25:869–875. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ostmeier H, Fuchs B, Otto F, Mawick R,
Lippold A, Krieg V and Suter L: Can immunohistochemical markers and
mitotic rate improve prognostic precision in patients with primary
melanoma? Cancer. 85:2391–2399. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niakosari F, Kahn HJ, McCready D,
Ghazarian D, Rotstein LE, Marks A, Kiss A and From L: Lymphatic
invasion identified by monoclonal antibody D2–40, younger age, and
ulceration: predictors of sentinel lymph node involvement in
primary cutaneous melanoma. Arch Dermatol. 144:462–467. 2008.
|
|
25
|
Balch CM, Wilkerson JA, Murad TM, Soong
SJ, Ingalls AL and Maddox WA: The prognostic significance of
ulceration of cutaneous melanoma. Cancer. 45:3012–3017. 1980.
View Article : Google Scholar : PubMed/NCBI
|