Introduction
Human prostate cancer (PCa) is the most frequently
diagnosed non-skin cancer in the United States and is the second
leading cause of death among males (1). Although conventional therapies achieve
a high cure rate in patients presenting with localized disease, 30%
of patients develop metastatic disease (2). The lack of effective therapies for
advanced PCa is related, to a large extent, to a poor understanding
of the molecular mechanisms underlying the progression of the
disease toward invasion and metastasis (3). Therefore, identification of new
predictive biomarkers, particularly those that are indicative of
invasiveness of the disease, which could serve as targets for
establishing the effectiveness of therapeutic and chemopreventive
interventions, will improve the clinical management of PCa.
Cancer testis (CT) antigens are a unique class of
tumor antigens specifically expressed in the normal testis and show
aberrant expression in various malignancies (4). It has also been suggested that the
aberrant expression of CT antigens in tumors may contribute to
various malignant properties, such as immortality, migration,
invasion and metastatic capacity (5). Therefore, CT antigens are being
vigorously pursued as targets for therapeutic cancer vaccines
(4).
As a new member of the CT antigen family,
sperm-associated antigen 9 (SPAG9) has been reported to be involved
in the c-Jun N-terminal kinase (JNK)-signaling module and to
function as a scaffold protein for binding to JNKs, thus playing an
important role in cell survival, proliferation, apoptosis and tumor
development. SPAG9 was first suggested to be a potential target for
immunotherapy in human epithelial ovarian cancer (6). Since then, SPAG9 overexpression has
been demonstrated in various human cancers including renal, breast,
thyroid, cervical, colon and lung carcinomas. Moreover,
SPAG9-knockdown using siRNA was found to inhibit the tumor growth
of cervical cancer (7).
Importantly, SPAG9 expression was also reported to be associated
with circulating anti-SPAG9 antibodies in early stage and in low
grade breast cancer and cervical cancer patients (8,9),
suggesting its potential application in the early detection of the
disease. To date, the expression pattern and biological function of
SPAG9 in PCa remain unknown.
To evaluate the function of SPAG9 in PCa, we
examined the expression of SPAG9 in relation to clinicopathological
features using tissue microarray (TMA) of PCa tissue samples. In
addition, we further investigated the involvement of SPAG9 in PCa
cell motility, invasion and angiogenesis.
Materials and methods
Patients and samples
A PCa TMA was purchased from Shanghai Xinchao
Biotechnology (Shanghai, China). Tumors were staged according to
the 2013 revised TNM system (10)
as follows: 69 cases with stages I–II and 31 with III–IV.
Pathologic grades of tumors were defined according to the WHO
criteria (11) as follows: 58 cases
of low grade (Gleason score <6) and 42 cases of high grade
(Gleason score ≥7) PCa.
Immunohistochemistry of the TMA
Immunohistochemistry was performed according to the
streptavidin-peroxidase (SP) method using a standard SP kit
(Zhongshan Biotech, Beijing, China). The TMA slide was incubated
with monoclonal mouse anti-SPAG9 antibody (1:100) (Cell Signaling
Technology, Beverly, MA, USA) overnight at 4°C, and
diaminobenzidine (DAB; Zhongshan Biotech, Beijing, China) was used
to produce a brown precipitate.
Evaluation of the immunostaining of SPAG9
expression
We assessed the immunostaining of SPAG9 by counting
>500 cells from 5 random fields of each specimen under ×400
magnification in the best-stained tumor area of each section as
previously described (12). The
SPAG9 immunoreactivity score (IRS) was defined as the percentage of
stained prostate tissue cells. We considered a distinct cytoplasmic
positive immunoreactivity when >10% of the cancer cells stained
for the SPAG9 protein.
Cell lines and siRNA transfection
Human PCa cell lines were used as previously
described (12). Cells were grown
to 50% confluency before small interfering RNA (siRNA)
transfection. Nonspecific control siRNA (Qiagen, Mississauga, ON,
Canada) or SPAG9 siRNA (GenePharma, Shanghai, China) was
transfected by siLentFect lipid reagent (Bio-Rad, Hercules, CA,
USA) according to the manufacturer’s instructions.
Would healing assay
Wound-healing assay was employed as previously
described (13). The migration of
cells and closing of the scratch wound were observed, and
microphotographs were captured every 4 h. Within each assay the
experiments were performed in triplicate and the whole assay was
repeated three times.
Migration and invasion assays
The migration and invasion assays were performed as
described previously (14), with
the exception of 10% serum-containing medium added to the bottom
chamber. Serum-free medium was used in the top chamber in cells
that were transfected with control siRNA or SPAG9 siRNA.
HUVEC growth and tube formation
assay
The HUVEC growth and tube formation assays were used
as described previously (14). The
number of capillary-like tubes from three randomly chosen fields
was counted and photographed under a Nikon inverted microscope
(Japan).
ELISA for vascular endothelial growth
factor (VEGF)
The ELISA assay was employed as described previously
(12). The VEGF concentration was
determined using Quantikine ELISA kits according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Gelatin zymography
Gelatin zymography was carried out as previously
described (12). Sixty hours after
transfection, cells were incubated in serum-free medium for 24 h.
The proteins in the conditioned medium were concentrated with
Ultracel-30 k centrifugal filters (Millipore, Billerica, MA, USA).
Fifty micrograms of the proteins was loaded for gelatin
zymography.
Western blot analysis
Forty-eight hours after transfection, cells were
harvested from the plates, and aliquots of the cell extracts were
separated on SDS-polyacrylamide gel. The proteins were then
transferred to a nitrocellulose membrane and incubated overnight at
4°C with the following antibodies: mouse anti-SPAG9, rabbit
anti-MMP-2, -MMP-9, -c-Jun N-terminal kinase (JNK) and -p-JNK (Cell
Signaling Technology) and mouse anti-β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). Blots were washed four times
with TBST (TBS containing 0.1% Tween-20), incubated simultaneously
with IRDye® 800CW goat anti-rabbit and IRDye®
680CW goat anti-mouse secondary antibodies (1:15,000) (Li-Cor
Biosciences, Lincoln, NE, USA) for 1 h at room temperature,
followed by three washes with TBST and one wash with TBS.
Immunoblots were imaged and the bands were quantified by
densitometry using Odyssey Infrared imaging system software
(Li-Cor).
Statistical analysis
Numerical data are expressed as means ± SD.
Statistical differences between the mean values for the different
groups were evaluated with Instat 5.0 (GraphPAD Software, San
Diego, CA, USA) using one-way analysis of variance (ANOVA). For the
TMA, statistical analysis was performed using SPSS 11.5 software
(SPSS). The associations between SPAG9 staining and the
clinicopathological parameters of the PCa patients, including age,
tumor size, tumor grade and TNM stage, were evaluated by the
Chi-square test. The t-test was used for cell proliferation assays.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SPAG9 expression is overexpressed in
human PCa tissues
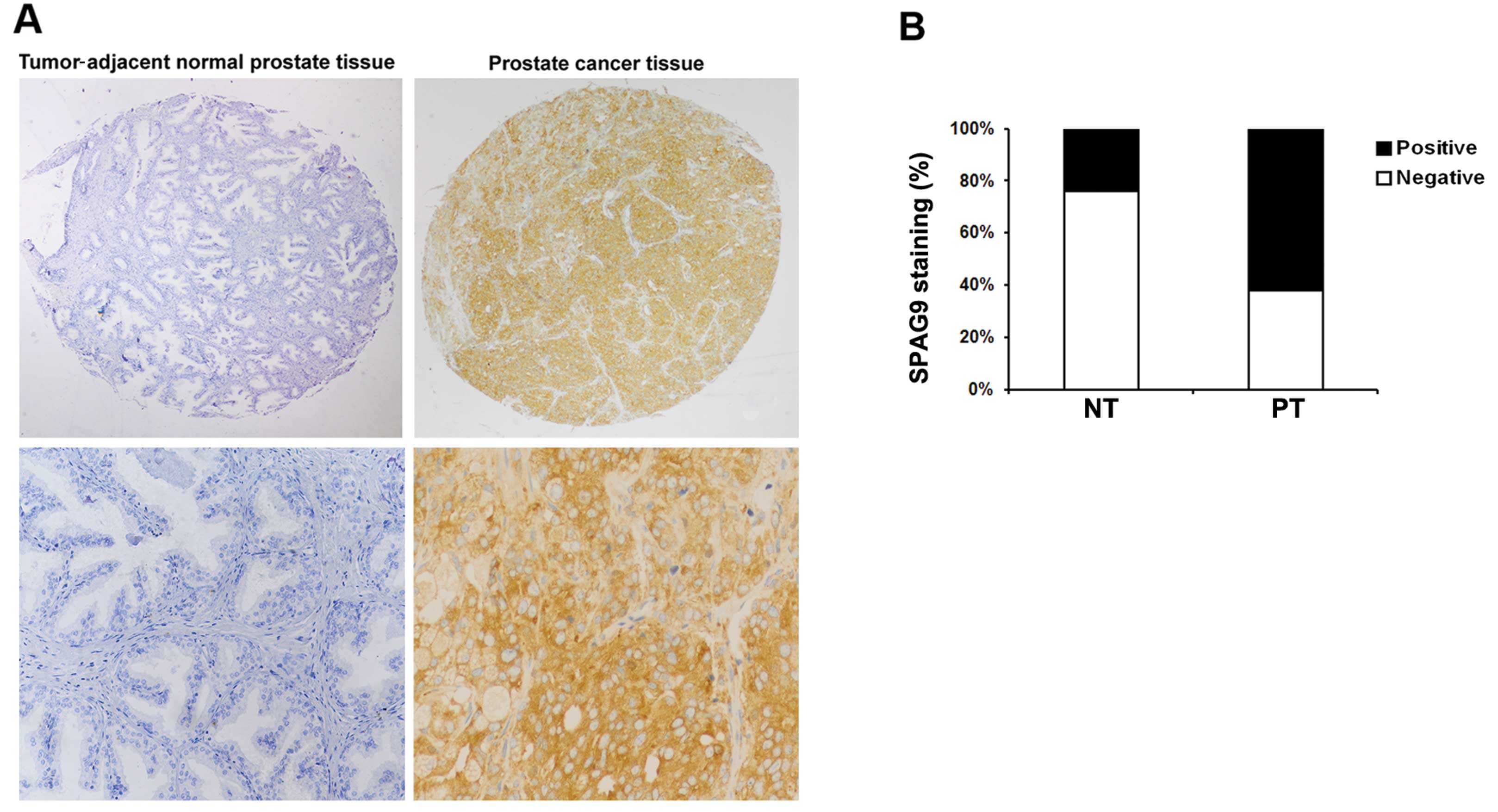
We first determined whether SPAG9 expression is
altered in human PCa. Representative images are shown in Fig. 1A. A significantly higher level of
SPAG9 expression was noted in the PCa tumor tissues than that in
the tumor adjacent normal prostate tissues (P<0.01, Fig. 1B). These findings suggest that SPAG9
is commonly expressed in PCa tissues but decreased or absent in
human prostate tissues.
SPAG9 expression is correlated with tumor
grade and TNM stage in human PCa
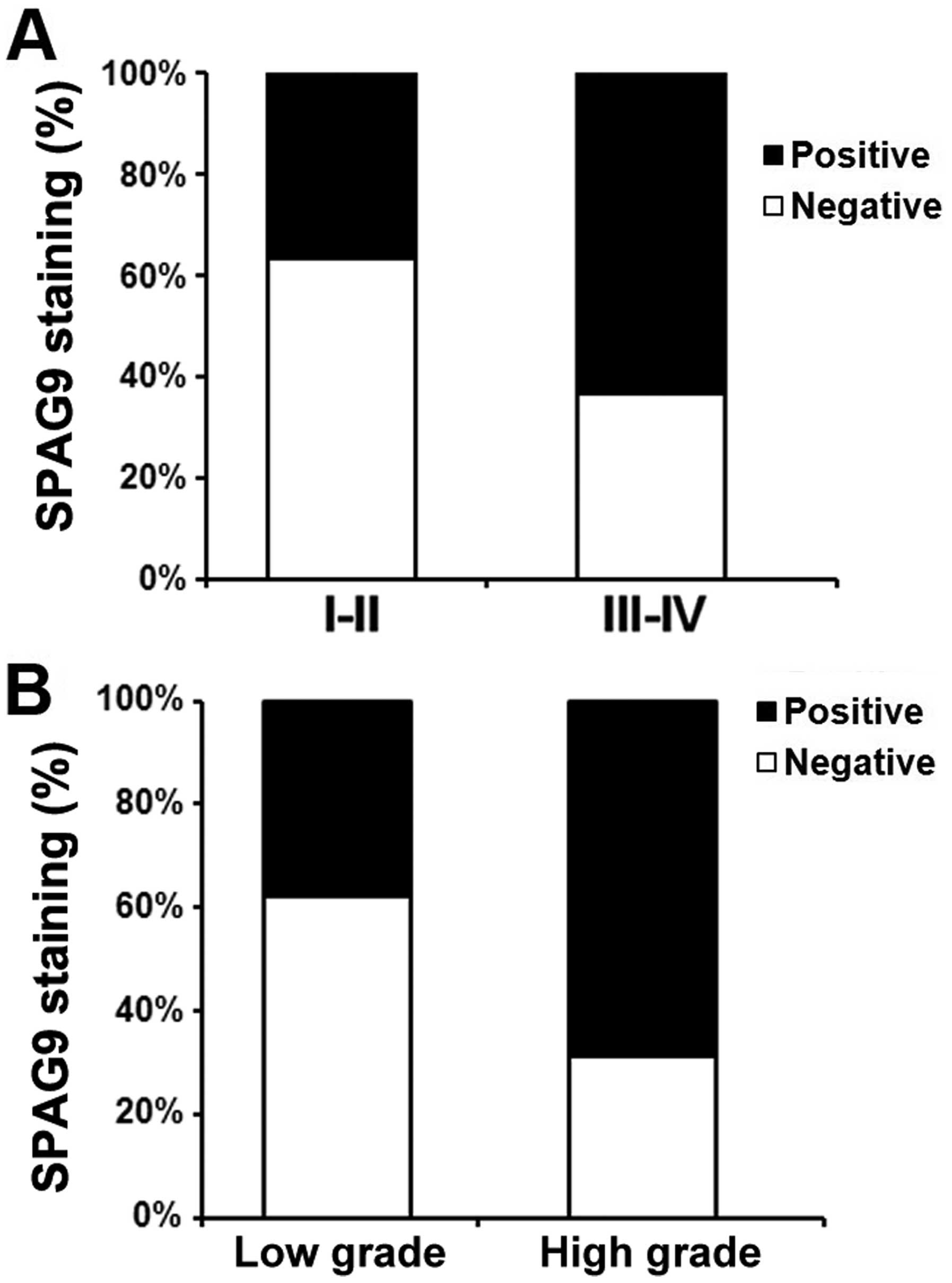
In all 100 PCa patients, the relationship between
SPAG9 expression and pathologic and clinical features is shown in
Table I. We found that SPAG9
expression was significantly correlated with tumor stage (comparing
I–II vs. III–IV) (P<0.01, Fig.
2A). In addition, SPAG9 staining was also markedly increased in
high grade when compared with low stage disease (P<0.05,
Fig. 2B). However, we did not find
significant correlations between SPAG9 expression and other
clinicopathological variables of the PCa patients, including age
(P<0.161) and tumor size (P<0.193).
 | Table IPatient characteristics and SPAG9
expression. |
Table I
Patient characteristics and SPAG9
expression.
| SPAG9 staining | | |
|---|
|
| | |
|---|
| Variables | Negative n (%) | Positive n (%) | Total | P-valuea |
|---|
| Age (years) | | | | 0.161 |
| ≤60 | 17 (35.4) | 31 (64.6) | 48 | |
| >60 | 26 (50.0) | 26 (50.0) | 52 | |
| Tumor size
(cm) | | | | 0.193 |
| ≤2.5 | 31 (64.6) | 17 (35.4) | 48 | |
| >2.5 | 40 (76.9) | 12 (23.1) | 52 | |
| TNM stage | | | | <0.01 |
| I | 29 (67.4) | 14 (32.6) | 43 | |
| II | 14 (53.8) | 12 (46.2) | 26 | |
| III | 3 (30.0) | 7 (70.0) | 10 | |
| IV | 8 (38.1) | 13 (61.8) | 21 | |
| Grade | | | | <0.05 |
| G1 | 21 (67.7) | 10 (32.3) | 31 | |
| G2 | 15 (55.6) | 12 (44.4) | 27 | |
| G3 | 9 (33.3) | 18 (66.7) | 27 | |
| G4 | 7 (46.7) | 8 (53.3) | 15 | |
Knockdown of SPAG9 by small interfering
RNA (siRNA) in DU145 and PC3 cells inhibits cell motility and
invasion
Since SPAG9 expression is related to the tumor grade
and TNM stage of PCa, SPAG9 may play important roles in one or more
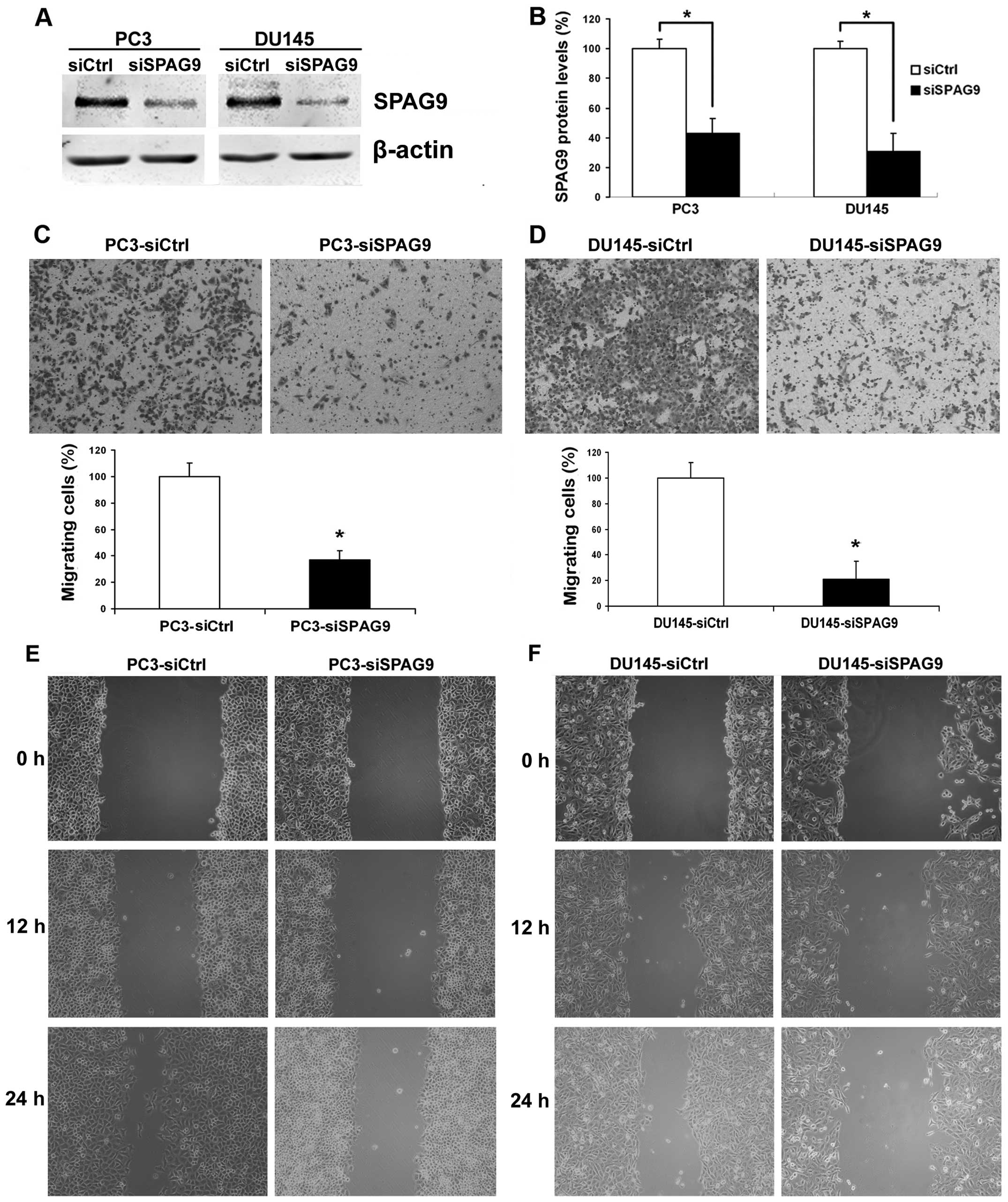
steps of PCa metastasis. We first examined the effect of siSPAG9 on
PCa cell motility. To validate whether SPAG9 functions in the
regulation of cell motility, PCa cell lines DU145 and PC3 were used
to examine changes in cellular phenotypes after SPAG9 knockdown by
RNA interference. In these experiments, treatment with SPAG9 siRNA
revealed ablation of SPAG9 protein expression (Fig. 3A and B). Therefore, the subsequent
experiments were restricted to SPAG9 siRNA in all cellular motility
experiments. In the cell migration assay, we found that
transfection of SPAG9 siRNA in DU145 and PC3 cells decreased their
ability to migrate through a Boyden chamber by 76 and 72%,
respectively (Fig. 3C and D). In
addition, in the wound healing assay, our data revealed that there
was a significant delay in wound closure after treatment with SPAG9
siRNA (Fig. 3E and F).
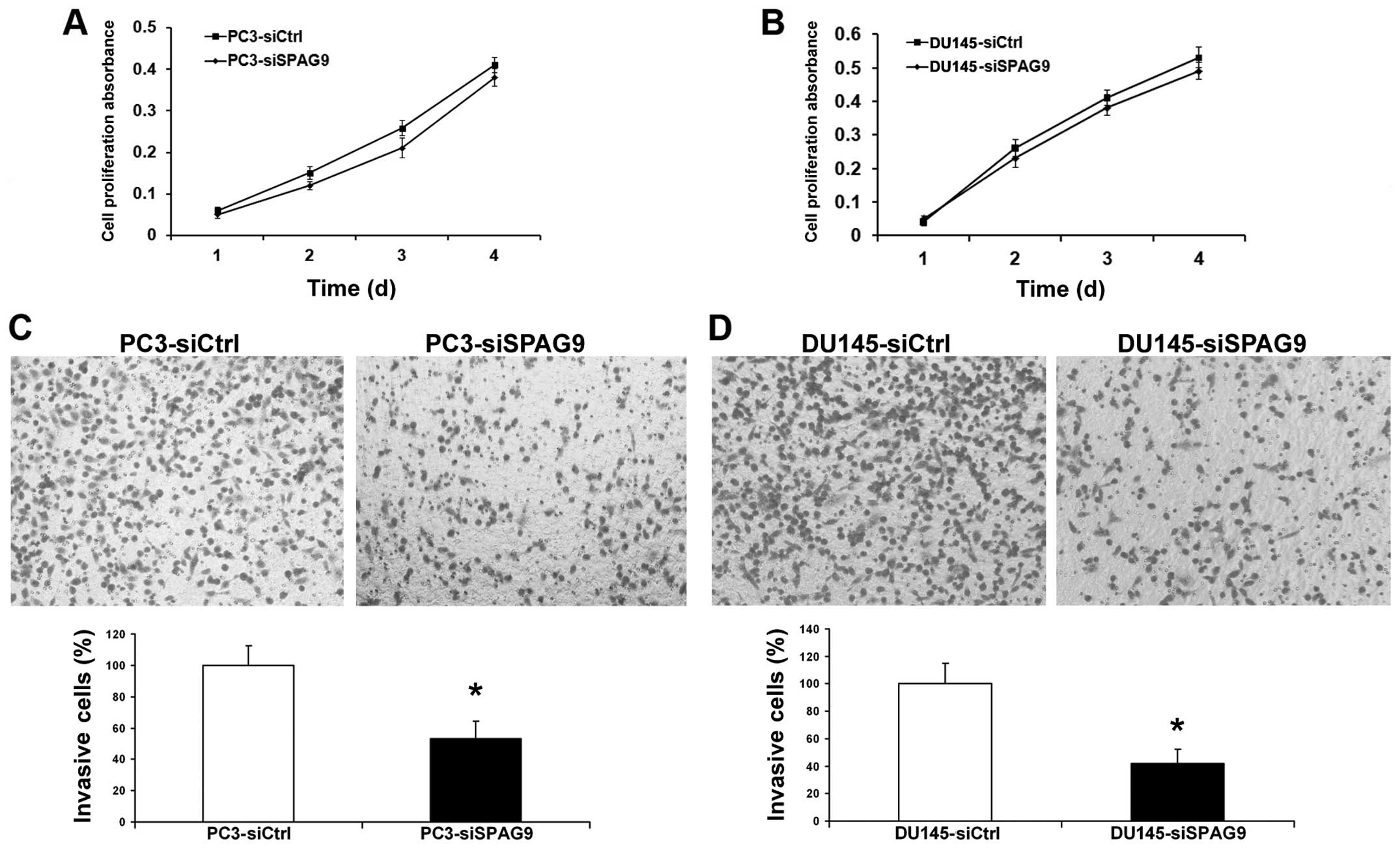
Invasion is a crucial step in the progression of
cancer metastasis. We assessed the capacity of PCa cells to invade
through Matrigel, an artificial extracellular matrix (ECM), after
transfection with control siRNA or SPAG9 siRNA. Knockdown of SPAG9
expression led to the inhibition of cell invasion by 78 and 55%
(Fig. 4C and D), and histograms
show that a significantly lower percentage of cells (P<0.05)
passed through the filters coated with the artificial ECM,
suggesting that the invasive potential of the SPAG9
siRNA-transfected prostate cancer cells was severely impaired.
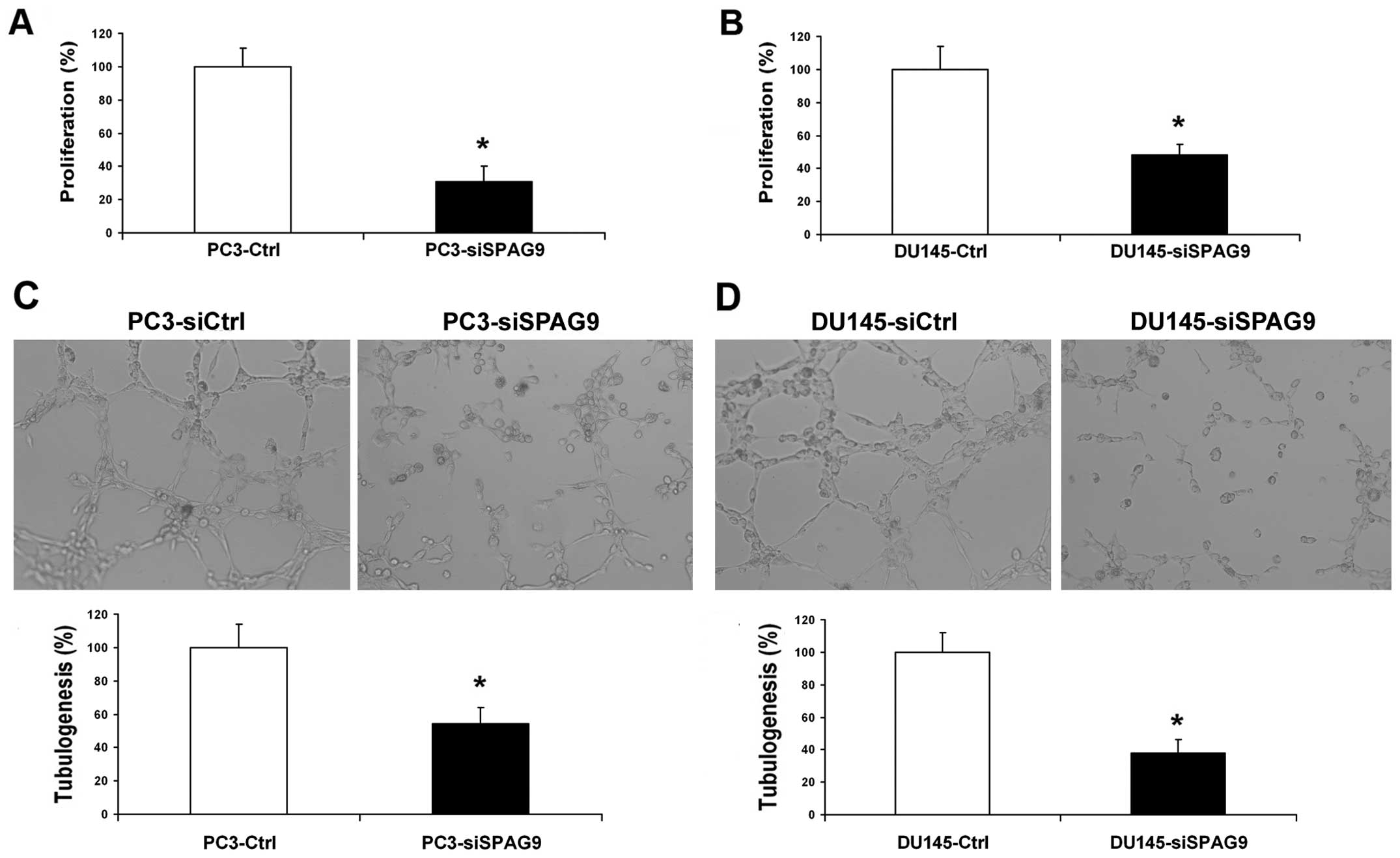
However, inhibition of SPAG9 had no effect on the proliferation of
PCa cells (Fig. 4A and B).
Silencing of SPAG9 inhibits PCa cell
angiogenesis in vitro
To further determine and study the functional role
of SPAG9 in the angiogenic potential of human PCa cells, HUVEC
growth and tube formation in vitro were investigated. The
growth of HUVECs in conditioned medium from SPAG9 siRNA-transfected
cells was inhibited by 48 and 51% compared with the corresponding
controls (Fig. 5A and B). The
average number of complete tubular structures formed by HUVECs was
significantly decreased in the conditioned medium from the
SPAG9-silenced DU145 and PC3 cells when compared with the control
group (Fig. 5C and D).
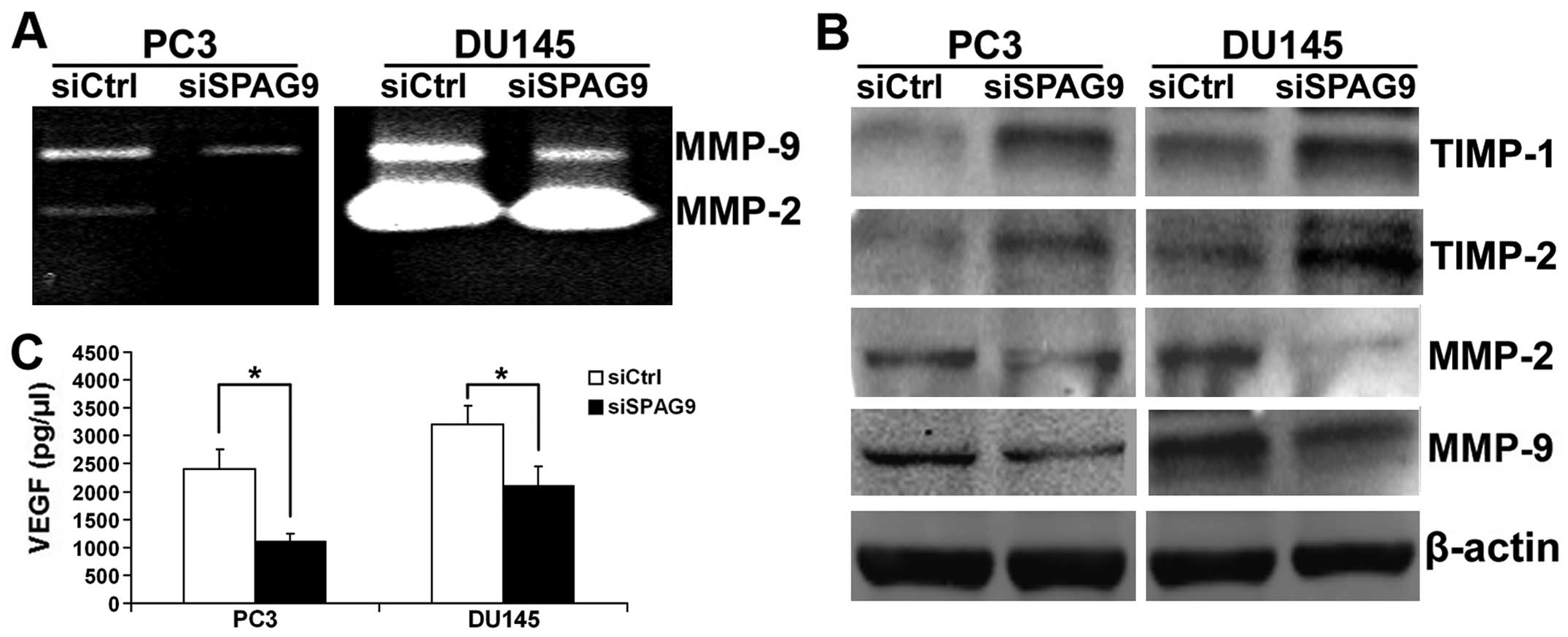
Knockdown of SPAG9 suppresses MMP-2/MMP-9
expression and activities by upregulation of TIMP-1/TIMP-2 in PCa
cells
Extensive data reveal that MMPs, in particular MMP-2
and MMP-9, play important roles in PCa cell invasion and
metastasis. Thereby, western blot and gelatin zymography analyses
were employed to determine MMP-2 and MMP-9 expression and activity
in the siSPAG9-transfected cancer cells. Our data showed that MMP-2
and MMP-9 expression and activities were significantly suppressed
after inhibition of SPAG9 expression in the DU145 and PC3 cells
(Fig. 6A and B). The activity of
MMPs is controlled by interaction with the TIMPs. We performed
western blotting and the data revealed that TIMP-1 and TIMP-2
proteins were increased after silencing SPAG9 (Fig. 6B).
SPAG9 regulates VEGF secretion in PCa
cells
VEGF is an important mitogen and survival factor for
endothelial cells. In response to angiogenic stimulation,
endothelial cells enter into an active proliferative state. To
evaluate whether depletion of SPAG9 contributes to VEGF secretion
in PCa cells, conditioned medium from DU145 and PC3 cells
transfected with SPAG9 siRNA were used to measure the secretion of
proangiogenic factor VEGF by ELISA analysis. As shown in Fig. 6C, a significant reduction in VEGF
secretion was observed in the conditioned medium from DU145 and PC3
cells transfected with SPAG9 siRNA when compared with the control
cells.
Discussion
SPAG9 is localized on the surface of sperm, and is
only expressed in haploid germ cells during spermatogenesis
(15). It may promote the process
of sperm-egg fusion which is characterized by an increase in
intracellular Ca2+ and pH1 and the tyrosine
phosphorylation of several proteins (16,17).
Recently, the role of SPAG9 in cancer has been studied due to its
high expression in cancer tissues. It is considered as a tumor
marker in several types of human cancers, such as breast cancer,
thyroid cancer, colorectal cancer and renal cell carcinoma
(6,8,9,18). In
addition, SPAG9-knockdown was found to inhibit the tumor growth of
renal cell carcinoma in vivo indicating its potential role
in regulating tumor development and metastasis (18). These observations indicate that
SPAG9 may play an important role in the tumorigenesis of human
cancer. However, the relationship between SPAG9 and PCa has not yet
been examined. To better understand the role of SPAG9 in PCa
development, we used TMA technology and in vitro cell
modeling to investigate whether SPAG9 has a function in the
development of PCa. Our clinical results showed that SPAG9
expression was upregulated in PCa and was correlated with TNM stage
and tumor grade. Our in vitro studies revealed that
knockdown of SPAG9 by siRNA in PCa cells reduced the abilities for
cell migration, invasion and angiogenesis.
It is generally accepted that surgical resection is
the most powerful tool for improving patient prognosis when early
diagnosis of PCa is successful (2).
Unfortunately, most PCa patients are diagnosed late at a locally
advanced stage (19). Thus, it is
essential to predict the risk of recurrence in order to minimize
the adverse effects and maximize the therapeutic effect of PCa
treatment. However, among the available prognostic factors for PCa,
one of the most important is the International Union Against Cancer
TNM stage as determined by the depth of invasion, involvement of
lymph nodes, and presence of distant metastasis (10). Apart from that, the Gleason grade is
also considered to be highly associated with aggressiveness and
progression of PCa; its accurate determination is crucial in
deciding the best treatment for each patient (20). In the present study, we found that
SPAG9 expression was increased in PCa tissues. Moreover, SPAG9
protein was significantly reduced in late stage and high tumor
grade disease. Our clinical evidence clearly supports the notion
that altered expression of SPAG9 contributes to PCa development and
metastasis.
Metastasis is a multistep process that involves the
detachment from the primary tumor mass, migration through the
extracellular matrix (ECM) and colonization of surrounding sites.
During this process, cell motility and invasion are essential for
metastasis (21). In the present
study, we found that SPAG9 knockdown suppressed PCa cell motility
and invasive ability through wound healing and Transwell assays.
One of the key factors in cancer invasion and metastasis is the
degradation of ECM allowing cancer cells to invade and migrate.
MMPs have been demonstrated to play important roles in this process
(22). Among the MMP family, MMP-2
and MMP-9 are postulated to promote invasion and lymph node
metastasis of PCa cells (23). To
determine how SPAG9 siRNA inhibits PCa cell motility and invasion,
we focused on elucidating the effect of SPAG9 siRNA on MMP-2 and
MMP-9 activities. Here, we found that knockdown of SPAG9 expression
significantly inhibited the bioactivities of MMP-2 and MMP-9 in
DU145 and PC3 cells. The activity of MMPs is controlled by
interaction with the TIMPs (24).
There has been evidence that the imbalance between MMPs and TIMPs
is responsible for cancer metastasis (25). Chen et al showed that
MMP-9-inhibiting activity of RUNX3 is due to the direct interaction
of RUNX3 with the TIMP-1 promoter (26). Furthermore, it has been suggested
that SPAG9 can modulate MMP-9 transcription independent of TIMP-1
and TIMP-2 (27). However, in our
study, we noted that knockdown of SPAG9 simultaneously upregulated
TIMP-1 and TIMP-2 expression, which are the negative regulators of
MMP-2 and MMP-9. The results showed that reduced cell motility and
invasive abilities were due to the low activity of MMP-2 and MMP-9
and the high level of TIMP-1 and TIMP-2 protein expression after
SPAG9 silencing. Therefore, the balance of TIMP-1/-2 and MMP-2/-9
was disrupted by SPAG9 siRNA, which eventually resulted in the
reduced cell motility and invasive abilities of the PCa cells.
Angiogenesis is essential for the growth and
metastasis of solid tumors, and inhibition of angiogenesis is
emerging as a promising strategy for cancer treatment (28). Yet, the effect of SPAG9 on
angiogenesis has not been reported. In our study, we found that
transfection of SPAG9 siRNA reduced the capacity of the PCa cell
supernatant to stimulate tube formation and proliferation of human
endothelial cells compared with this capacity in the control cells,
suggesting that knockdown of SPAG9 expression significantly
impaired the angiogenic potential of PCa cells in vitro.
However, its molecular bases are unclear. Among several potential
mechanisms, influences on the expression of various angiogenic
molecules have been shown (29,30).
Obviously, one of the most essential angiogenic factors is VEGF,
which exerts its mitogenic activity particularly on endothelial
cells. VEGF has been identified as a key mediator of tumor
angiogenesis involved in the development of tumor blood supply in
the progression of solid tumors (31). VEGF expression is suppressed by
tumor-suppressor genes p53, p75 and von Hippel-Lindau, which is
most likely due to formation of complexes with Sp1 and inhibition
of its binding to and transcriptional activation of the VEGF
promoter (32,33). We, thus, investigated whether SPAG9
regulates VEGF activity in vitro. ELISA analysis showed that
VEGF secretion was decreased by reduction of SPAG9. These results
suggest that the decrease in SPAG9 suppresses blood-vessel
formation by regulating VEGF activity. It has been reported that
SPAG9 participates in JNK pathway activation, and JNK signaling
could induce c-Jun phosphorylation at the VEGF promoter resulting
in its activation (18,34). Thus, it is possible that JNK
signaling is involved in the angiogenesis promoting function of
SPAG9.
The present study provides evidence that SPAG9 is
expressed at high levels in PCa tissues. Increased SPAG9 expression
is significantly associated with prostate progression. To the best
of our knowledge, this is the first report showing that CT protein
is involved in promoting PCa cell motility, invasion and
angiogenesis. Sliencing of SPAG9 leads to the inhibition of PCa
cell motility and invasion due to the imbalance between MMP-2/MMP-9
and TIMP-1/TIMP-2. In addition, we further demonstrated that
knockdown of SPAG9 reduced blood-vessel formation and proliferation
of HUVECs by decreasing the secretion and expression of VEGF. Thus,
these findings identify SPAG9 as a promising novel diagnostic and
therapeutic target for PCa.
Acknowledgements
This project was supported by a grant from The
National Natural Science Foundation of China (no. 81302207).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Ohlmann CH, Siemer S and Stöckle M:
Resection of metastases from prostate cancer. Urologe A.
51:363–367. 2012.(In German).
|
|
3
|
Saleem M, Adhami VM, Zhong W, et al: A
novel biomarker for staging human prostate adenocarcinoma:
overexpression of matriptase with concomitant loss of its
inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer
Epidemiol Biomarkers Prev. 15:217–227. 2006. View Article : Google Scholar
|
|
4
|
Simpson AJ, Caballero OL, Jungbluth A,
Chen YT and Old LJ: Cancer/testis antigens, gametogenesis and
cancer. Nat Rev Cancer. 5:615–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zendman AJ, Ruiter DJ and Van Muijen GN:
Cancer/testis-associated genes: identification, expression profile,
and putative function. J Cell Physiol. 194:272–288. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garg M, Chaurasiya D, Rana R, et al:
Sperm-associated antigen 9, a novel cancer testis antigen, is a
potential target for immunotherapy in epithelial ovarian cancer.
Clin Cancer Res. 13:1421–1428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg M, Kanojia D, Suri S and Suri A:
Small interfering RNA-mediated downregulation of SPAG9 inhibits
cervical tumor growth. Cancer. 115:5688–5699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanojia D, Garg M, Gupta S, Gupta A and
Suri A: Sperm-associated antigen 9, a novel biomarker for early
detection of breast cancer. Cancer Epidemiol Biomarkers Prev.
18:630–639. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garg M, Kanojia D, Salhan S, et al:
Sperm-associated antigen 9 is a biomarker for early cervical
carcinoma. Cancer. 115:2671–2683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung MS, Lee SH, Lee DH and Chung BH:
Evaluation of the 7th American Joint Committee on Cancer TNM
staging system for prostate cancer in point of classification of
bladder neck invasion. Jpn J Clin Oncol. 43:184–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van der Kwast T, Bubendorf L, Mazerolles
C, et al: Guidelines on processing and reporting of prostate
biopsies: the 2013 update of the Pathology Committee of the
European Randomized Study of Screening for Prostate Cancer (ERSPC).
Virchows Arch. 463:367–377. 2013.PubMed/NCBI
|
|
12
|
Chen F, Wang M, Bai J, et al: Role of
RUNX3 in suppressing metastasis and angiogenesis of human prostate
cancer. PLoS One. 9:e869172014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mei PJ, Bai J, Liu H, et al: RUNX3
expression is lost in glioma and its restoration causes drastic
suppression of tumor invasion and migration. J Cancer Res Clin
Oncol. 137:1823–1830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen F, Bai J, Li W, et al: RUNX3
suppresses migration, invasion and angiogenesis of human renal cell
carcinoma. PLoS One. 8:e562412013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jagadish N, Rana R, Selvi R, et al:
Characterization of a novel human sperm-associated antigen 9
(SPAG9) having structural homology with c-Jun N-terminal
kinase-interacting protein. Biochem J. 389:73–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burks DJ, Carballada R, Moore HD and
Saling PM: Interaction of a tyrosine kinase from human sperm with
the zona pellucida at fertilization. Science. 269:83–86. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luconi M, Krausz C, Forti G and Baldi E:
Extracellular calcium negatively modulates tyrosine phosphorylation
and tyrosine kinase activity during capacitation of human
spermatozoa. Biol Reprod. 55:207–216. 1996. View Article : Google Scholar
|
|
18
|
Garg M, Kanojia D, Khosla A, et al:
Sperm-associated antigen 9 is associated with tumor growth,
migration, and invasion in renal cell carcinoma. Cancer Res.
68:8240–8248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mocarska A, Staroslawska E, Iwonna ZC, et
al: Diagnostic imaging of the prostate cancer. Pol Merkur Lekarski.
33:357–363. 2012.(In Polish).
|
|
20
|
Sfoungaristos S and Perimenis P: Clinical
and pathological variables that predict changes in tumour grade
after radical prostatectomy in patients with prostate cancer. Can
Urol Assoc J. 7:E93–E97. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hynes RO: Metastatic potential: generic
predisposition of the primary tumor or rare, metastatic variants -
or both? Cell. 113:821–823. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halbersztadt A, Halon A, Pajak J,
Robaczynski J, Rabczynski J and St Gabrys M: The role of matrix
metalloproteinases in tumor invasion and metastasis. Ginekol Pol.
77:63–71. 2006.(In Polish).
|
|
23
|
Zhang XY, Hong BF, Chen GF, Lu YL and
Zhong M: Significance of MMP2 and MMP9 expression in prostate
cancer. Zhonghua Nan Ke Xue. 11:359–361. 3642005.(In Chinese).
|
|
24
|
Jinga DC, Blidaru A, Condrea I, et al:
MMP-9 and MMP-2 gelatinases and TIMP-1 and TIMP-2 inhibitors in
breast cancer: correlations with prognostic factors. J Cell Mol
Med. 10:499–510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giannelli G, Erriquez R, Fransvea E, et
al: Proteolytic imbalance is reversed after therapeutic surgery in
breast cancer patients. Int J Cancer. 109:782–785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Wei X, Guo C, et al: Runx3
suppresses gastric cancer metastasis through inactivation of MMP9
by upregulation of TIMP-1. Int J Cancer. 129:1586–1598. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi F, Ni W, Liu W, et al: SPAG9 is
overexpressed in human astro-cytoma and promotes cell proliferation
and invasion. Tumour Biol. 34:2849–2855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ellis LM, Rosen L and Gordon MS: Overview
of anti-VEGF therapy and angiogenesis. Part 1: Angiogenesis
inhibition in solid tumor malignancies. Clin Adv Hematol Oncol.
4:1–10. 2006.PubMed/NCBI
|
|
30
|
Shi Q, Le X, Abbruzzese JL, et al:
Constitutive Sp1 activity is essential for differential
constitutive expression of vascular endothelial growth factor in
human pancreatic adenocarcinoma. Cancer Res. 61:4143–4154.
2001.PubMed/NCBI
|
|
31
|
Cao Y, Guangqi E, Wang E, et al: VEGF
exerts an angiogenesis-independent function in cancer cells to
promote their malignant progression. Cancer Res. 72:3912–3918.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie K, Wei D, Shi Q and Huang S:
Constitutive and inducible expression and regulation of vascular
endothelial growth factor. Cytokine Growth Factor Rev. 15:297–324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pal S, Datta K and Mukhopadhyay D: Central
role of p53 on regulation of vascular permeability factor/vascular
endothelial growth factor (VPF/VEGF) expression in mammary
carcinoma. Cancer Res. 61:6952–6957. 2001.PubMed/NCBI
|
|
34
|
Guma M, Rius J, Duong-Polk KX, Haddad GG,
Lindsey JD and Karin M: Genetic and pharmacological inhibition of
JNK ameliorates hypoxia-induced retinopathy through interference
with VEGF expression. Proc Natl Acad Sci USA. 106:8760–8765. 2009.
View Article : Google Scholar : PubMed/NCBI
|