Introduction
Fine needle aspiration cytology (FNAC) has great
value in determining the histogenesis of suspected liver nodules
(1–3). The main role of diagnostic cytology is
the differentiation between primary and secondary liver tumours as
well as to confirm malignancy by exclusion of inflammatory nodules
including simple cysts and granulomatous nodules such as
tuberculosis or sarcoidosis (4–6). The
only contraindication of FNAC is improper coagulation by a low
number of platelets (7).
FNAC has the advantage of being a non-aggressive
method with a low risk for complications (2,8).
Successful FNAC provides cells and tissues that are extremely
useful for the diagnosis conducted by biopsy specimens (9).
The sensitivity and specificity of FNAC from liver
nodules in the retrospective study was ~90% after examining more
than 4,000 cases at the Institute of Pathology at Hannover Medical
School (MHH).
The aim of this retrospective study was to detect
the validity of FNAC in determining the malignancy of liver nodules
and to differentiate between primary tumours and metastasis.
Overview of the aetiology of liver
nodules
The aetiology of liver nodules vary and two main
types, solid tumours and cystic liver lesions, are observed. Solid
tumours include i) tumours with abnormal contrast-enhanced
ultrasound (CEUS); ii) malignant tumours; iii) metastasis in the
liver by the presence of primary tumours in other organs; and iv)
tumours after adjuvant therapy to detect vitality and regression.
Cystic liver lesions include: i) histogenesis (abscess, simple
cyst, haemorrhage); ii) cystic degeneration in a malignant tumour
(mucin); and iii) parasites (hydatid cyst), bacteria, hyphae or
actinomycosis.
Contraindication of FNAC of the liver includes: i)
improper coagulation/coagulopathy (quick <50%, PTT >50%,
thrombocytes <50.000/μl; ii) therapy with heparin, coumarin or
aspirin; iii) ascitis; iv) lack of consent from the patient; v)
eating a meal before the procedure; and vi) absence of family or
medical history.
Cytological appearance of normal liver
cells
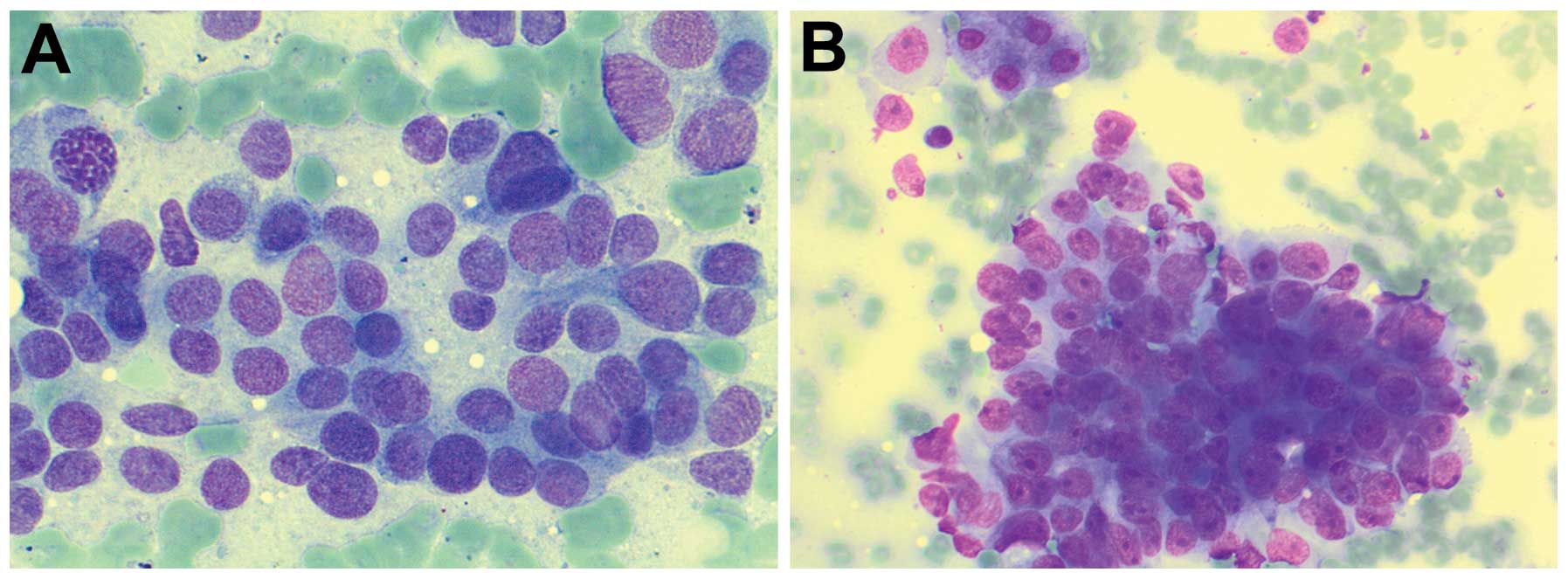
The cytological appearance of normal cells in smear
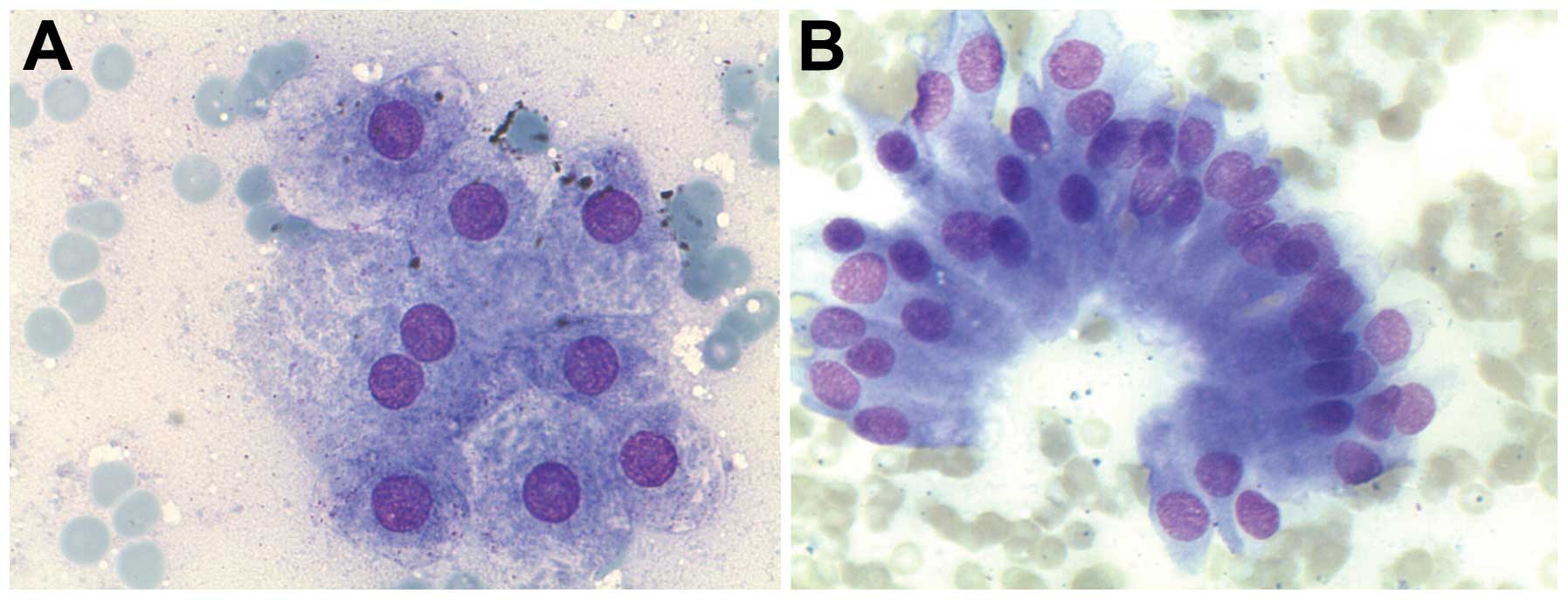
slides are as follows. Normal liver cells are polygonal cells
either isolated or in groups of cells (6–8 cells). Focal lipofuscin
is detected in older patients. The cytoplasm is distinct, wide and
stained red in haematoxylin and eosin (H&E) preparation by the
presence of many mitochondria. The nucleus is central and round.
The chromatin is finely clumped but regular. The nucleolus is
central and stained red in H&E. In ~25% of liver cells, there
are two nuclei. Nuclear inclusions are found in liver cells of
older patients or patients with diabetes mellitus (Fig. 1A).
Bile duct cells are cubical or columnar cells,
isolated or in groups. The cytoplasm is narrow and stained
bright-red in H&E smears (Fig.
1B). The nucleus is in the periphery and oval. The chromatin is
fine and granular. The nucleolus is indistinct. The endothelial
cells of sinusoids are arranged in groups of spindle cells. The
cytoplasm is narrow. The nucleus is oval and the chromatin is fine
and granular. Kupffer cells are rare in FNAC. They lie
superficially in the sinusoidal side of the liver cells. The
cytoplasm is narrow with vacuoles. The nucleus is oval with
fine-granular chromatin.
In benign, non-neoplastic nodular lesions of the
liver in FNAC, the liver cells show cytoplasmic changes as well as
the accumulation of metabolic products can be detected in metabolic
intolerance or toxic disturbances as well as by enzymatic inborn
diseases. For example, the detection of fat globules enhances the
diagnosis of fatty liver; the detection of iron in the cytoplasm
enhances the diagnosis of siderosis or hemochromatosis as well as
possible after blood-transfusion. The detection of accumulated
copper in the cytoplasm may be indicative of Wilson syndrome.
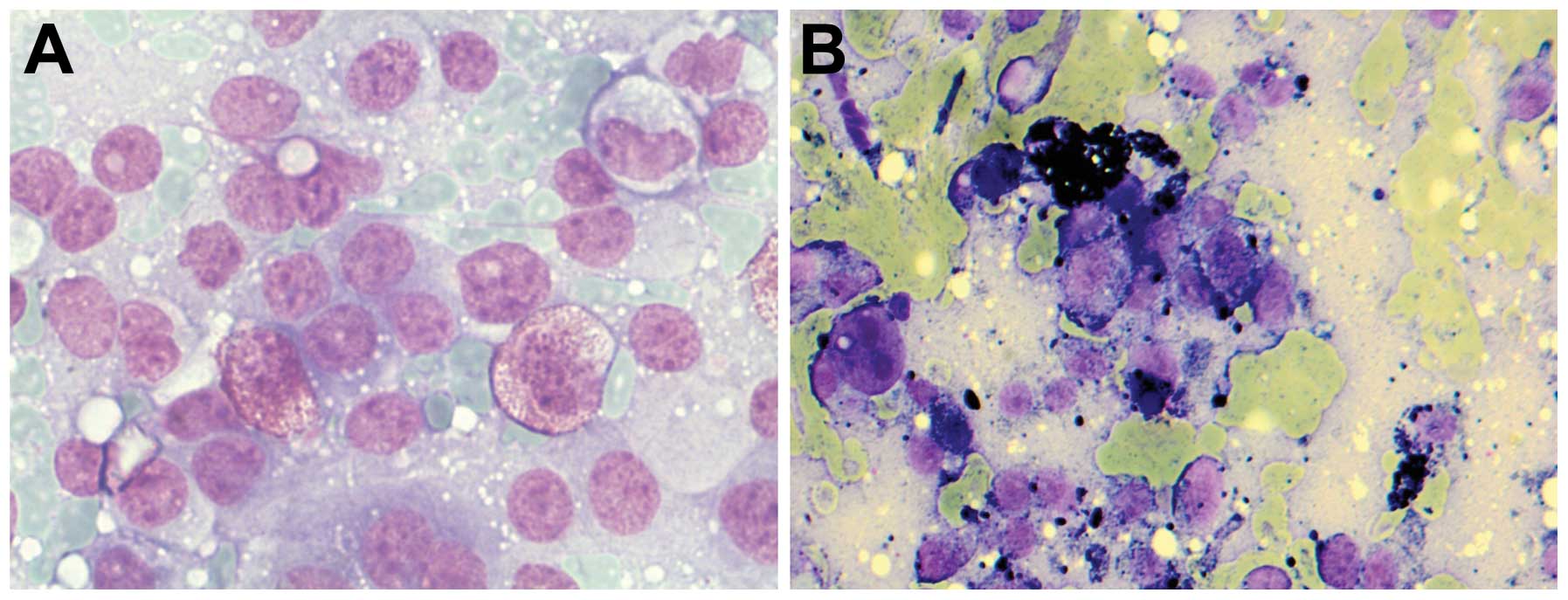
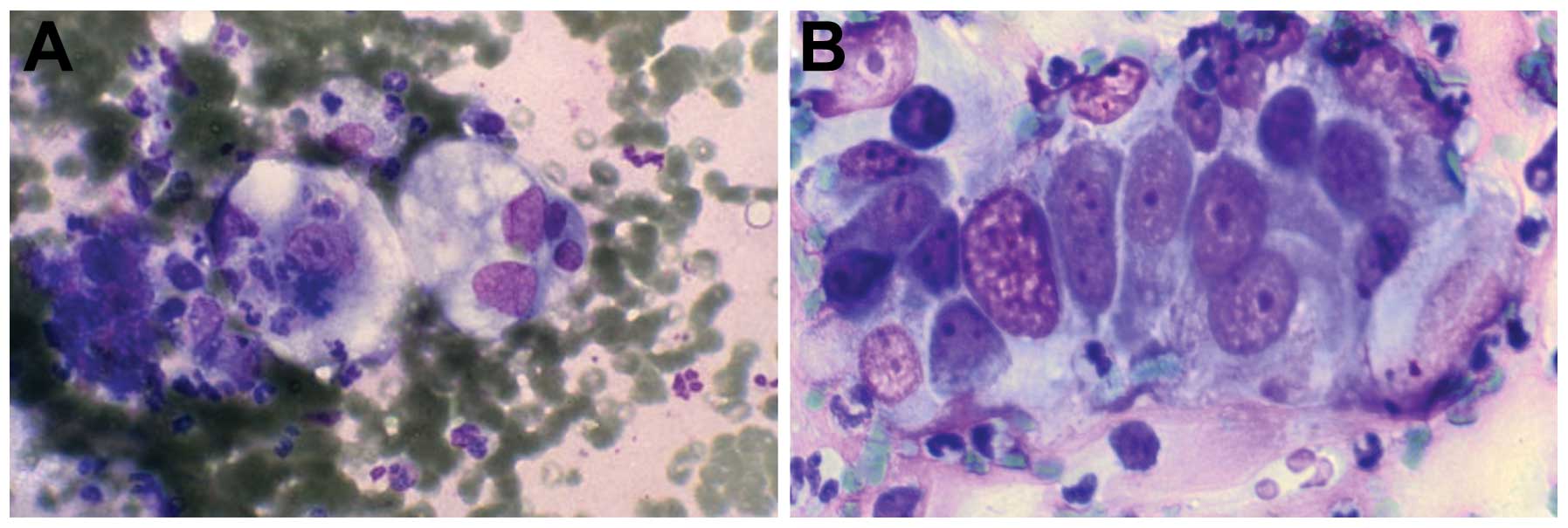
FNAC is of limited value to detect the aetiology of
liver cirrhosis, yet of value to document the presence of fibrosis
(Fig. 2A–D). The role of special
pigments is important in detecting accumulated materials: iron
(Perls’ Prussian blue) appears as intracytoplasmic dark blue,
lipofuscin (H&E) appears as perinuclear yellow brown pigment,
melanin appears as dark blue to black pigment, and bile pigment
appears as dark green or black.
Fatty liver is due to accumulation of triglycerides.
Cytoplasmic vacuoles are observed. The aetiology is primarily an
error in the metabolism of fat, for example hypertriglyceridemia, a
high-level of cholesterol and triglycerides due to alcohol,
diabetes mellitus, hypoxia or bad nutrition. In the cytoplasm, fine
to coarse vacuoles can be noted that push the nucleus to the
periphery. It is not a special indication for FNA as it can be
diagnosed either by cytology or by biopsy.
Cholestasis is due to intracellular accumulation of
bilirubin and appears as red green pigmentation either in the liver
cells or in the sinusoids. This is caused by bile duct stones,
metabolic inborn error, malignant tumours (obstructive
cholestasis), or by infection as well as medications. The
aetiological detection of different types of cholestasis is
cytologically impossible.
Inflammation of the liver leads to unspecific
cellular changes. Therefore, FNAC is helpful in detecting the
aetiology yet not in cases of viral hepatitis. In abscesses, there
are neutrophils, macrophages, necrosis or apoptosis and liver cells
with regenerative changes. In granuloma, this is an incidental
diagnosis in FNAC, particularly if thick needles are used. There is
cellular accumulation of macrophages, lymphocytes and plasma cells.
In cysts, there is a detection of a cavity in the liver. The cause
of cystic changes is congenital and simple (Fig. 8), echinococcus and secondary changes
in the tumour. In congenital and simple cysts, there are
macrophages, fluid in the background and metaplasia in the cells,
bile duct cells, possibly mucus in cases of cystic mucoid tumour or
parasitic cysts. In echinococcosis, dirty background, inflammatory
cells, spikes and PAS-positive material are noted. In secondary
cysts, due to degeneration of tumours they appear cytologically
with dirty background and atypical cells. In liver cirrhosis, FNAC
is of no value in detecting the aetiology of cirrhosis but may be
of value in the differentiation between regenerative nodules and
early phase of well-differentiated liver cell carcinoma. There are
many cells in groups or rosettes. The cells show regenerative
changes with nuclear pleomorphism and anisokaryosis. Many cells
have two nucleoli. The chromatin is regular. The cells also show a
broad cytoplasm and distinct cell membrane. Cholestasis appears in
the sinusoids or in the liver cells. In toxic aetiology, the cells
show many fat droplets. In the background, there are many stroma or
endothelial cells. Bile duct cells may also present either in
groups or in rosettes. For neoplastic liver lesions (WHO, 2010)
refer to Table V and for secondary
tumours: lung, breast, pancreas, colon and stomach refer to
Table III.
 | Table VLiver nodules (WHO, 2010). |
Table V
Liver nodules (WHO, 2010).
| Structure | Benign lesions | Malignant
lesions |
|---|
| Liver cells | Liver cell adenoma,
nodular regenerative hyperplasia, focal nodular hyperplasia and
dysplastic nodules | Liver cell carcinoma:
micro/macronodular, fibrolamellar, acinar or adenoid, clear cells,
solid subtypes |
| Bile duct cells | Simple cysts, bile
duct adenoma, bile duct cystadenoma | Bile duct carcinoma
and cystadenocarcinoma |
| Endothelial
cells | Cavernous
haemangioma |
Haemangiosarcoma/haemangioendothelioma |
| Others | | HCC-CCC,
hepatoblastoma |
 | Table IIIPrimary tumours of the liver
metastasis. |
Table III
Primary tumours of the liver
metastasis.
| Known primary tumour
of liver metastasis | No. (%) |
|---|
| Colon/rectum | 188 (27.6) |
| Pancreas | 113 (16.5) |
| Breast (Fig. 10B) | 67 (9.8) |
| Neuroendocrine
tumours (NET) | 77 (11.3) |
| CUP (carcinoma of
unknown primary) | 66 (9.6) |
| Lung (non-small cell
carcinoma) | 30 (4.4) |
| Stomach and NHL
(each) | 19 (2.8) |
| Sarcoma (Fig. 3D) and kidney (each) | 15 (2.2) |
| Lung (small cell
carcinoma) | 12 (1.7) |
| Prostate | 9 (1.3) |
| Urinary bladder | 7 (0.8) |
| Gall bladder,
oesophagus, malignant melanoma (Fig.
8B), thyroid gland (each) | 6 (0.8) |
| Vagina/cervix,
pharynx, testis (each) | 3 (0.4) |
| Plasmacytoma and
ovary (each) | 2 (0.3) |
| AML, salivary gland,
adrenal gland and undifferentiated carcinoma (each) | 1 (0.1) |
| Total | 681 |
Cytological characteristics of benign
liver cell tumours
Liver cell adenoma
Here there is no cirrhosis. There are no
parenchymatous changes in the surrounding tissue. Ultrasonography
appears as cystic degeneration in the tumour. FNAC is
contraindicated due to haemorrhagic risk. Cytologically, there are
monomorphic cells arranged in pseudoacinar formations. The cells
show minimal nuclear atypia with granular chromatin and a large
nucleolus. The cytoplasm is light and distinct with vacuoles. In
the background of the smear there is excessive necrosis, blood,
macrophages with bile pigment and inflammatory cells. Liver cell
adenomas are classified into 4 groups according to genetic changes
(Bordeaux Classification) (10,11):
i) liver cell adenoma with HNF1α inactivation; ii) liver cell
adenoma with β-catenin activation (high risk of progression to
carcinoma); iii) liver cell adenoma with inflammatory changes; and
iv) liver cell adenoma, not otherwise specified.
Focal nodular hyperplasia (FNH)
Focal nodular hyperplasia is cytologically
impossible.
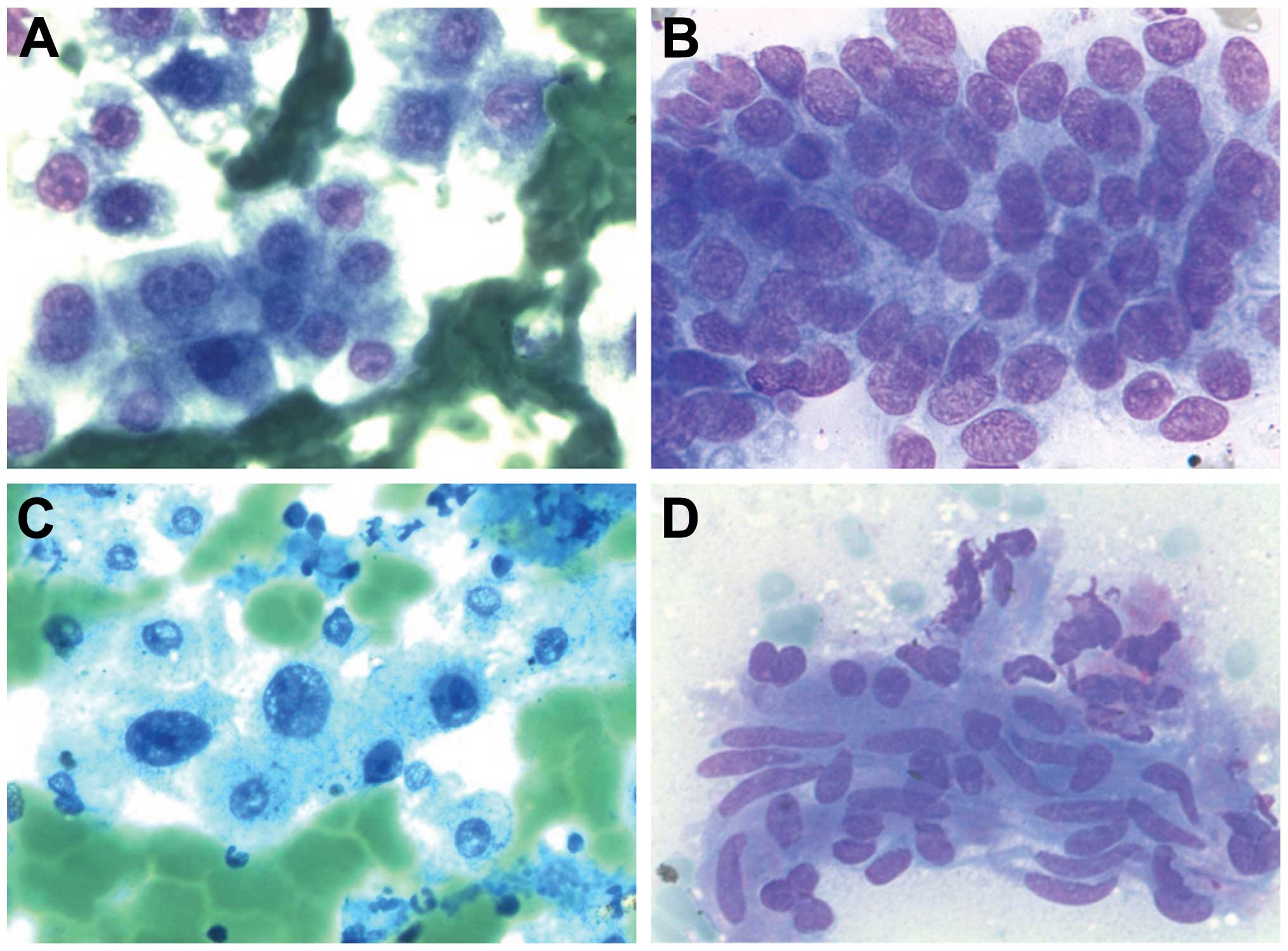
Cavernous haemangioma of the
liver
Cavernous haemangioma is an incidental diagnosis due
to the use of thick needles in FNAC or in autopsy specimens.
Cytologically (Fig. 3A–C), there is
a background with blood and endothelial cells. Many liver cells are
also present. The diagnosis should be made concomitantly with
radiologic diagnosis.
Liver cell dysplasia
Liver cells show nuclear pleomorphism, and atypia is
common in cases with liver cell cirrhosis. Cases (5–40%) with liver
cell cirrhosis are complicated by liver cell dysplasia and
carcinomas. Investigation with FISH is useful to detect aneuploidy
which may be a criterion to detect hepatocellular carcinoma (HCC)
(12).
Cytological features of malignant liver
cell tumours [grading according to Edmondson and Steiner (13)] Hepatocellular carcinoma
HCC presents widely in Asia and Africa in comparison
with Europe. It is associated with hepatitis B and C and is more
common in men than in women (4:1). Cases (90%) are preceded by
liver cirrhosis. Different grades of liver cell carcinoma have
different cytological features. In HCC (G1), the smears show many
cells and a clean background. The cells are arranged in rosettes.
The atypical cells are smaller than normal cells. The nuclei are
round with a prominent nucleolus. The cytoplasm is stained light
red with a disturbed nuclear/cytoplasmic ratio. In HCC, the
fibrolamellar type appears cytologically as abnormal strands of
liver cells with fragmentation of the stroma and cytoplasm stained
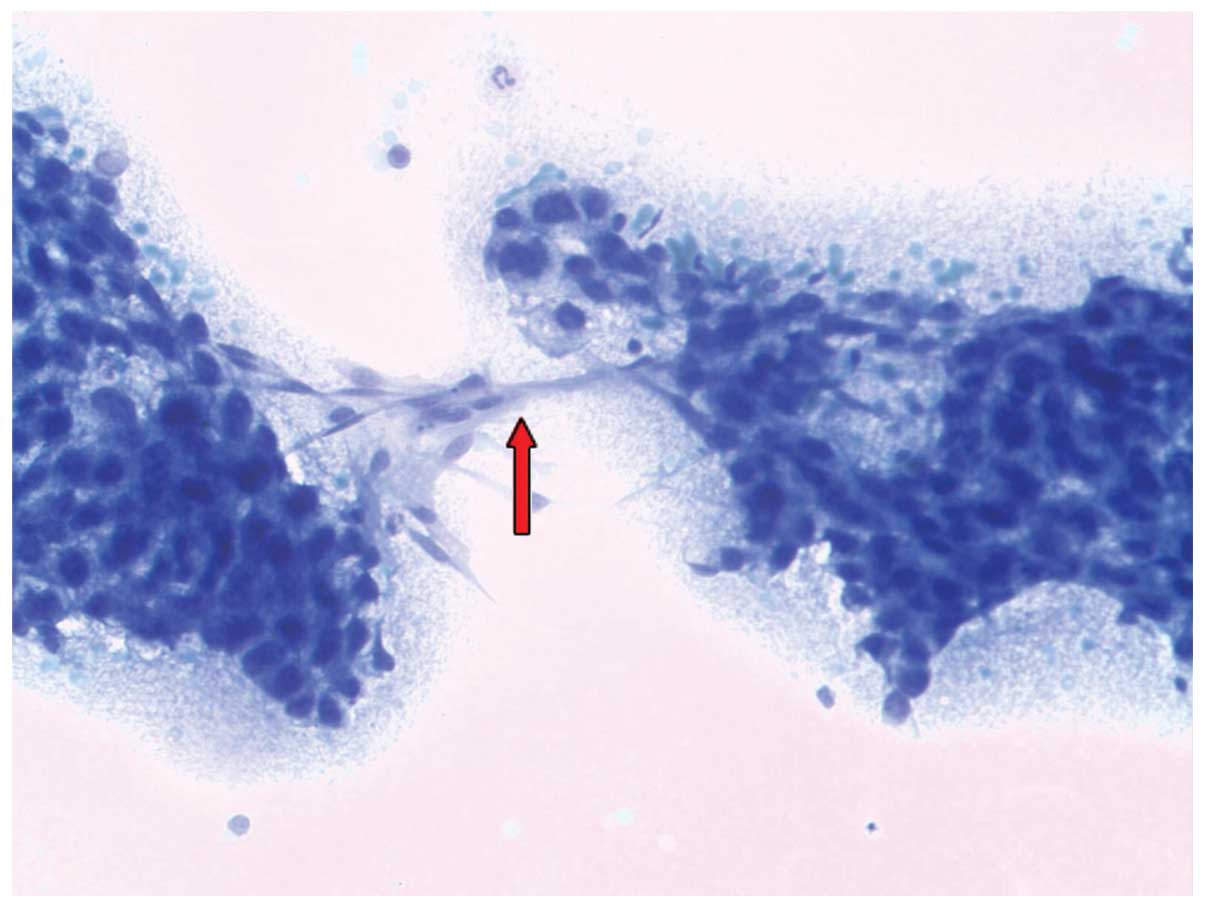
red due to mitochondria. The diagnostic feature is the formation of
bridges of endothelial cells (Fig.
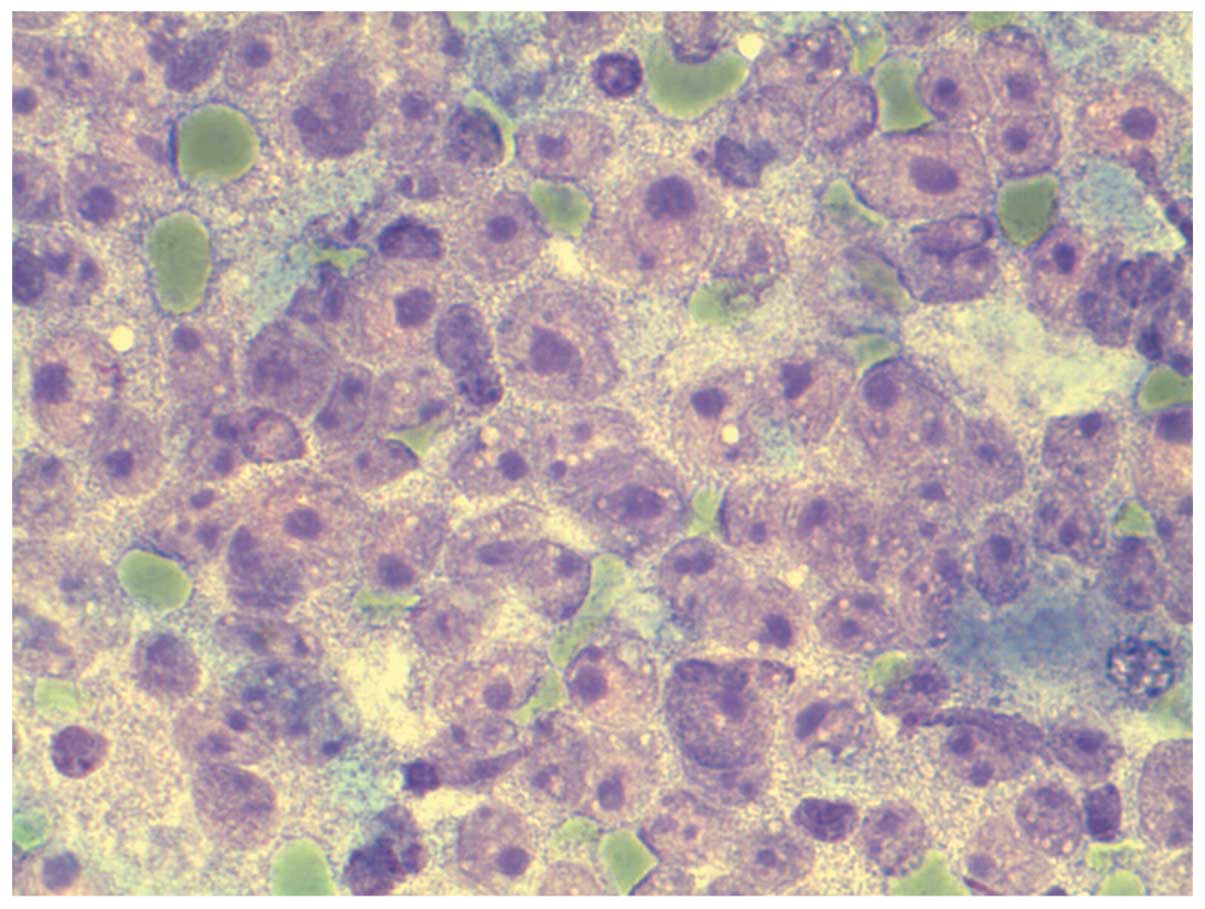
4). In HCC (G2), the smears are rich with abnormal strands of
cells. The liver cells show atypical changes such as increased
nuclear/ cytoplasmic ratio. The nucleus appears large with clumped
chromatin and irregular nuclear membrane. The nucleolus is large
and prominent. The cytoplasm is narrow and irregular. Some show
mitotic images. Clear cell type shows positive glycogen with PAS
staining (Fig. 5). In HCC (G3), the
former criteria of atypical changes are in the liver cells with
aggressive fragmentation of the stroma as well as formation of
bridges of endothelial cells. Irregular cell and nuclear membranes
are present. Necrotic changes in the background (Fig. 6) are noted. In HCC (G4), there is
prominence of the atypical changes in the liver cells, appearing
not only in groups yet also in separate cells. Smears show a dirty
background and necrosis. Indistinct cells and cytoplasmic membranes
are present. The prominence of the nucleolus is always present. In
the clear cell type, the neoplastic liver cells show clear
cytoplasm with fine vacuoles. Cytologically, it is difficult to
differentiate between this subtype and metastasis from adrenal
gland carcinoma or clear cell carcinoma of the kidney.
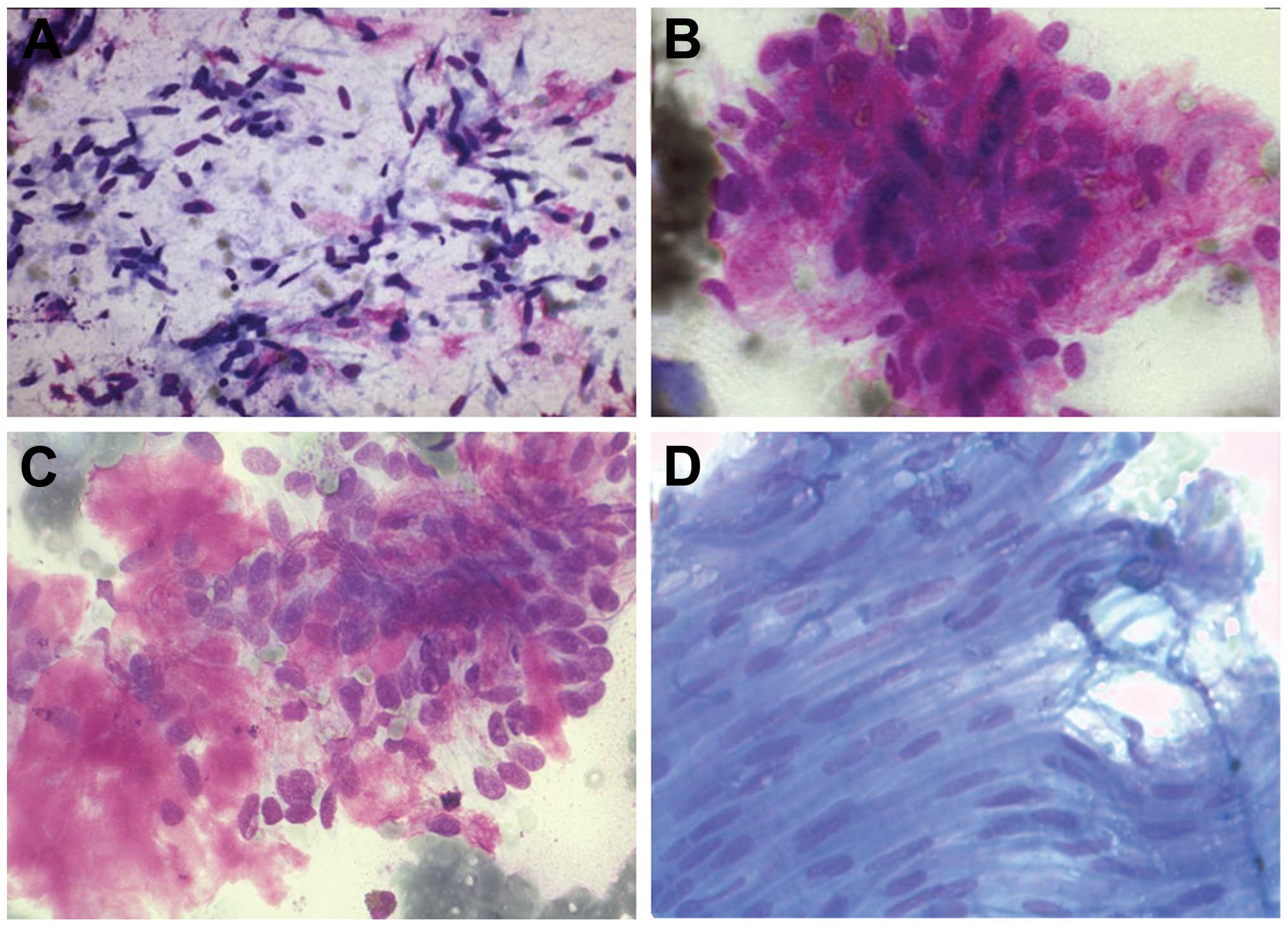
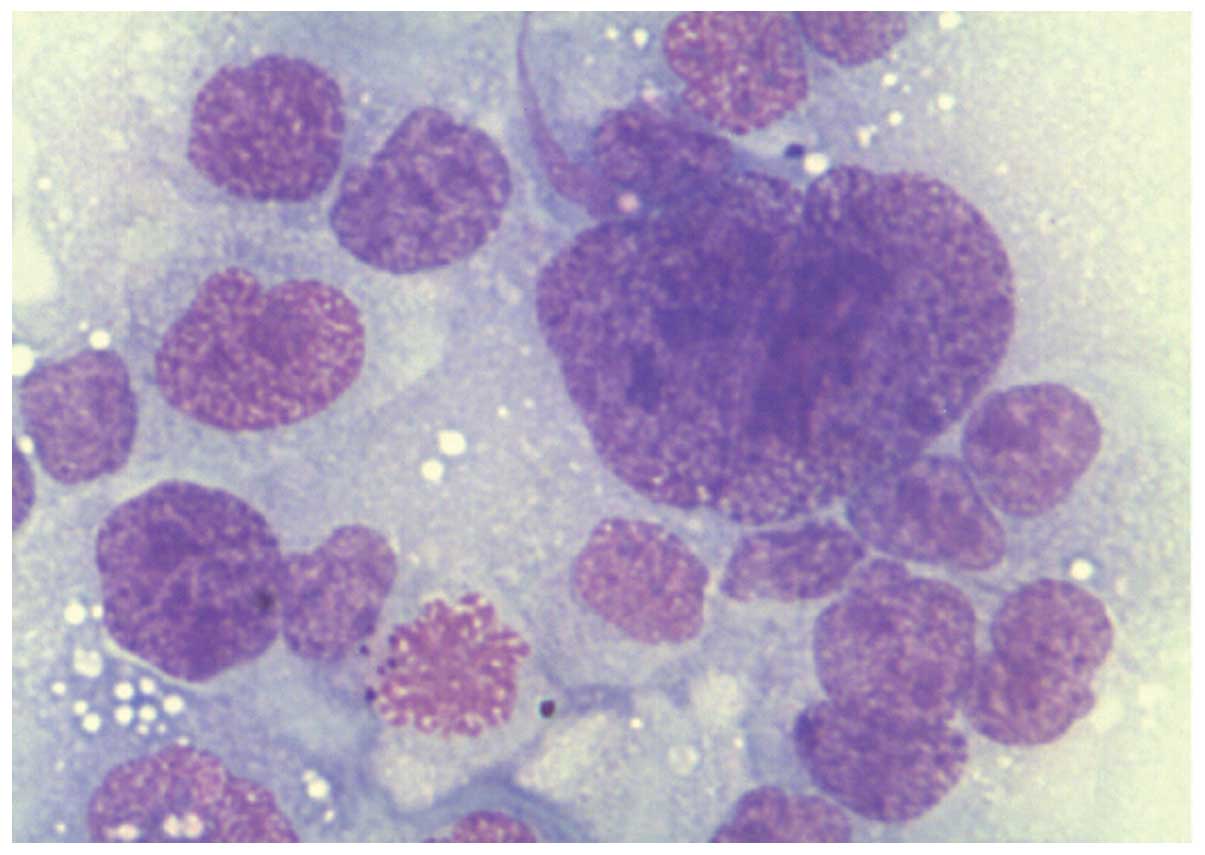
Bile duct carcinoma
In bile duct carcinoma, due to marked desmoplasia
that accompanies adenocarcinoma of the bile tract, the smears show
a small number of cells. The cells are arranged in groups or in
glands. They show peripheral nuclei with polymorphic changes. The
cytoplasm is lightly stained with vacuoles. PAS staining shows
positive vacuoles (Fig. 7).
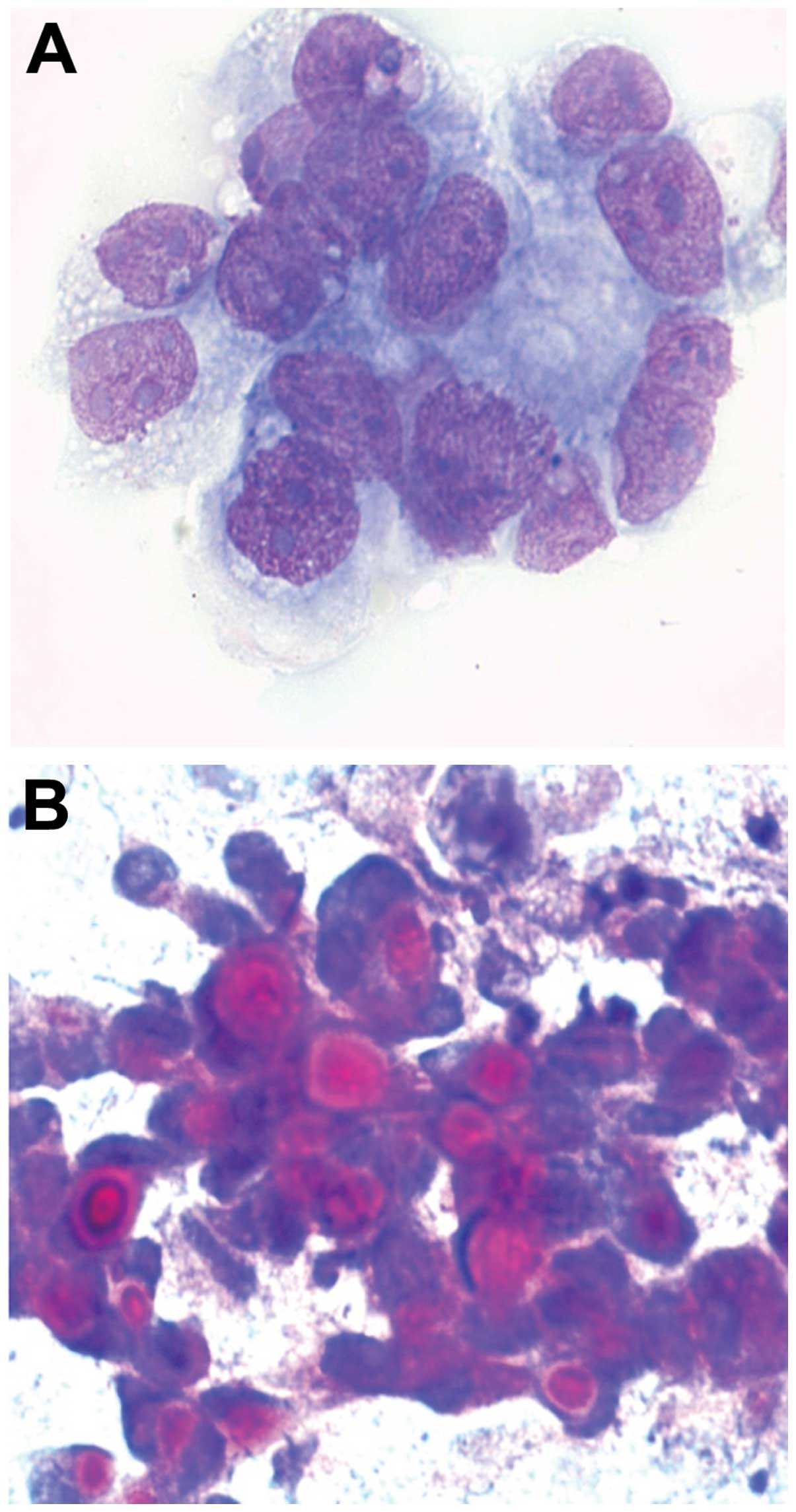
Hepatoblastoma
In hepatoblastoma, the smears are rich with cells
and necrotic debris. The cytoplasm is narrow. The cells show
indistinct cell and nuclear membranes. There are many prominent
nucleoli with atypical mitosis and increased nuclear/ cytoplasmic
ratio. Stromal cells and squamous metaplasia are present.
Extramedullary haematopoiesis is sometimes present (Fig. 8A).
Metastasis
In regards to metastasis in the liver,
cytomorphologic, immunohistochemical and clinical data are
important for the diagnosis. FNAC is a helpful method in the
differentiation between primary tumours and metastasis (Figs. 8B and 10B). For example, i) in colorectal
carcinoma, groups of cells with necrosis and inflammatory cells are
observed. Cubical cells with atypical changes (Fig. 9A) are noted. ii) In squamous cell
carcinoma, there are large cells with a wide cytoplasm and tail.
iii) In neuroendocrine carcinoma, there are rosettes of cells with
monomorphism (Fig. 10A); and iv)
in lymphoma, isolated cells with little cytoplasm and pleomorphism
are observed. The immunocytochemical (IH) (Table IV) or fluorescence in situ
hybridisation (FISH) investigation of the smears is helpful yet due
to few cells, may be difficult to use. To enhance the use of
ancillary techniques such as IH or FISH, it is recommended to
process the samples as a cell block. Some metastases, for example
from pancreas adenocarcinoma (Fig.
9B), are difficult to diagnose accurately even after use of
ancillary techniques. This is also the case in the equivalent
biopsy (Fig. 9B). Alkaline
phosphatase is positive in all cases of HCC and negative in all
cases of metastasis.
 | Table IVImmunocytochemical markers (14). |
Table IV
Immunocytochemical markers (14).
| Types of cancer | Markers |
|---|
| HCC | Hep-par-1, CEA,
glypican-3, glutamine synthetase, HSP-70, β-catenin, arginase-1,
CD-10, AFP, CK7/CK20 |
| CCC | CK7/CK19,
CK7/CK20 |
| NET | Chromogranin,
synaptophysin, NSE, CD56 |
| Pancreas | CK7/CK20 |
| Colon/rectum | CK20, CDX-2 |
|
Haemangioendothelioma | CD31, factor
VIII |
| M. lymphoma | CD45, CD20 and KL-1
(−ve) |
Materials and methods
All samples were processed in our institute and
stained with Giemsa and PAS stains as conventional methods at the
Institute of Pathology at the Hannover Medical School (MHH). They
were compared with the histological specimens that were processed
at the same institute. The patients were informed that probable
studies may be performed and they provided consent. The
retrospective analysis of the present study was approved by the
local ethics committee.
Results
Cytological examination of 4,136 (FNAC) specimens
from 1998 to 2012 found that ~39.6% of the cases were malignant,
57.5% were benign and ~2.8% had unclear cytology. Approximately
1.1% of the specimens were not sufficient for diagnosis (Table I). In the malignant cases, 40% were
liver cell carcinoma, 10.4% were bile duct carcinoma (CCC) and
~48.5% were metastasis (Table
III).
 | Table IFNAC of the liver. |
Table I
FNAC of the liver.
| Diagnosis | No. (%) |
|---|
| Benign lesion | 2,353 (56.8) |
| Malignant lesion | 1,620 (39.1) |
| Unclear/not
defined | 114 (2.7) |
| Not sufficient to
make a diagnosis | 49 (1.1) |
| Total | 4,136 |
Discussion
FNAC is the method of choice to detect the aetiology
of the majority of abnormal nodules of the liver. It is a sensitive
and non-aggressive, cost effective and rapid method and with proper
processing and experience, the nodules can be easily diagnosed. For
unclear tumours, it is preferable to process samples by cell block
techniques to enhance the use of ancillary techniques such as
immunocytochemistry or FISH.
In this retrospective study (1998–2012), 4,136 FNAC
were carried out from patients at the MHH, and diagnosed at the
Institute of Pathology. The majority (97.1%) were correctly
diagnosed in comparison to the corresponding histopathology. The
remaining cases could not be accurately diagnosed due to few
samples or the inability to perform immunocytochemistry.
In conclusion, the detection of bridges from
endothelial cells is the pathognomonic feature of the diagnosis of
HCC.
References
|
1
|
Islam T, Hossain F, Rumpa AP, Sikder NH,
Bhuiyan MA, Karim E and Hossain A: Ultrasound guided fine needle
aspiration cytology: a sensitive diagnostic tool for diagnosis of
intra-abdominal lesions. Bangladesh Med Res Counc Bull. 1:14–17.
2013.
|
|
2
|
Mueller M, Kratzer W, Oeztuerk S, Wilhelm
M, Mason RA, Mao R and Haenle MM: Percutaneous ultrasonographically
guided liver punctures: an analysis of 1961 patients over a period
of ten years. BMC Gastroenterol. 12:1732012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crowe DR, Eloubeidi MA, Chhieng DC, Jhala
NC, Jhala D and Eltoum IA: Fine-needle aspiration biopsy of hepatic
lesions: computerized tomograpic-guided versus endoscopic
ultrasound-guided FNA. Cancer. 108:180–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balani S, Malik R, Malik R and Kapoor N:
Cytomorphological variables of hepatic malignancies in fine needle
aspiration smears with special reference to grading of
hepatocellular carcinoma. J Cytol. 2:116–120. 2013. View Article : Google Scholar
|
|
5
|
Wee A: Fine needle aspiration biopsy of
malignant mass lesions in the liver: a revisit of diagnostic
profiles and challenges. J Gastrointest Oncol. 1:5–7. 2013.
|
|
6
|
Geier A, Gartung C, Staatz G, Nguyen HN
and Matem S: Moderne diagnostik benigner und maligner
raumforderungen der leber. Dtsch Ärztebl. 47:A-3120/B-2647/C-2453.
2001.(In German).
|
|
7
|
Mrzljak A, Kardum-Skelin I, Cvrlje VC,
Filipec-Kanizaj T, Sustercić D and Skegro D: Role of fine needle
aspiration cytology in management of hepatocellular carcinoma: a
single centre experience. Coll Antropol. 34:381–385.
2010.PubMed/NCBI
|
|
8
|
Swamy MC, Arathi C and Kodandaswamy C:
Value of ultrasonography-guided fine needle aspiration cytology in
the investigative sequence of hepatic lesions with an emphasis on
hepatocellular carcinoma. J Cytol. 28:178–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan S, Omar T and Michelow P:
Effectiveness of the cell blocks technique in diagnostic
cytopathology. J Cytol. 3:177–182. 2012. View Article : Google Scholar
|
|
10
|
Farkas S, Lang SA, Scherer M, Kirchner GI,
Loss M and Schlitt HJ: Leberadenome: wann muss wirklich therapiert
werden? Z Gastroenterol. 49:3–13. 2011.(In German). View Article : Google Scholar
|
|
11
|
Bioulac-Sage P, Laumonier H, Couchy G, Le
Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G,
Trillaud H, Zucman-Rossi J, Balabaud C and Saric J: Hepatocellular
adenoma management and phenotypic classification: the Bordeaux
experience. Hepatology. 50:481–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wilkens L, Flemming P, Gebel M, Bleck J,
Terkamp C, Wingen L, Kreipe H and Schlegelberger B: Induction of
aneuploidy by increasing chromosomal instability during
differentiation of hepatocellular carcinoma. Proc Natl Acad Sci
USA. 101:1309–1314. 2004. View Article : Google Scholar
|
|
13
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–504. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dabbs DJ: Diagnostic immunohistochemistry.
2nd edition. 2006
|