Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide and has become the fifth most common cause
of cancer-related mortality in China (1). It is estimated that >50% of
patients succumbing to CRC had developed distant metastasis. Thus,
identifying available biomarkers and determining the mechanism
involved in the development of metastasis in CRC is necessary.
The regenerating islet-derived family members (Reg)
are a family of genes belonging to the calcium-dependent lectin
gene superfamily and a group of secretory proteins that are
essential for cell regeneration and proliferation (2). Reg4, which was isolated from a cDNA
library of ulcerative colitis (UC) tissues by Hartupee et al
(3), is the most recently
identified member of the Reg family. It has been reported that Reg4
was markedly overexpressed in several gastrointestinal types of
cancer including gastric, colorectal and pancreatic cancer
(4–8). In CRC, Oue et al (9) reported that Reg4 expression was
associated with histologic grade and metastasis and high serum Reg4
concentrations were found in patients with liver metastasis.
Furthermore, in vitro studies suggested the overexpression
of Reg4 promoted cancer cell proliferation, migration,
anti-apoptosis and resistance to chemoradiotherapy (10–13).
However, the mechanism of how Reg4 performing these functions in
CRC remains to be elucidated. Recently, it was shown that Reg4
promoted the proliferation and invasiveness of pancreatic cancer
cells through upregulation of the expression of matrix
metalloproteinase-7 (MMP-7) (14).
MMP-7, also known as matrilysin, is the smallest
member of the MMP family, playing an important role in the
degradation of extracellular matrix during cancer progression
(15). MMP-7 was elevated in
several primary types of cancer including CRC and pancreatic
carcinoma (16,17). MMP-7 is specifically expressed in
epithelial tumor cells and has proteolytic activity against a wide
spectrum of substrates such as proteoglycans, type IV collagen,
fibronectin, elastin and laminin. In addition to degrading the
extracellular matrix, MMP-7 was able to enhance angiogenesis by
degrading soluble vascular endothelial growth factor (VEGF)
receptor-1 which blocked VEGF access to endothelial cells,
potentially promoting the metastasis of colon carcinoma cells by
processing a cell surface protein(s) and thereby inducing loose and
then tight aggregation of tumor cells (18,19).
CRC cells treated with the recombinant human Reg4
(rhR4) were found to increase the expression of MMP-7 (20). However, their relationship in CRC
tissues is not fully understood. Therefore, quantitative real-time
PCR (RT-qPCR), western blot analysis, and immunohistochemistry were
applied in the present study to clarify the correlation between
Reg4 and MMP-7 in CRC from transcriptional and post-transcriptional
levels and their relationship with clinical characteristics. The
present study aimed to show that positive Reg4 expression is
associated with MMP-7 overexpression in colorectal tumors with
metastasis and is a potential prognostic factor when combined with
MMP-7 expression.
Materials and methods
Patients and specimens
Fresh primary cancer tissue and grossly visible
paired normal tissue were collected from 40 CRC patients who
underwent radical surgery at the Shanghai Jiaotong University
Affiliated First People’s Hospital. The samples were stored at
−80°C for RT-qPCR and western blot analysis. The group contained 22
male and 18 female patients with a median age of 53 (range, 32–75)
years. For the immunohistochemical study, a total of 186 patients,
who underwent curative surgery for CRC at the Shanghai Jiaotong
University Affiliated First People’s Hospital, also participated in
the present study. The group included 77 males and 109 females with
a mean age of 68 (range, 22–95) years at the time of surgery. There
were 19 cases at stage I, 76 cases at stage II, 74 cases at stage
III, and 17 cases at stage IV according to the American Joint
Committee on Cancer (AJCC) staging criteria. The patients overall
survival (OS) and disease-free survival (DFS) durations were
defined as the interval from initial surgery to death and from
initial surgery to clinically or radiologically proven recurrence
or metastasis, respectively. No patients received either
preoperative chemotherapy or radiotherapy. All the patients signed
informed consent according to protocols approved by the
Institutional Review Boards of the Shanghai Jiaotong University
Affiliated First People’s Hospital. Ethics approval for the study
was obtained from the Ethics Committee of Shanghai Jiaotong
University Affiliated First People’s Hospital.
RNA extraction and RT-qPCR
Total RNA was isolated from fresh primary tumors and
related normal mucosa of 40 CRC patients according to the
manufacturer’s instructions (TRIzol; Life Technologies, Grand
Island, NY, USA), and then reverse transcribed into complementary
DNA using the GeneAmp® PCR System 9700 (Applied
Biosystems, New York, NY, USA). Primers used in the present study
included: Reg4 sense, 5′-TTG ACTGGGACCACTGGAGA-3′ and antisense,
5′-AAGGCAA GCTTCCTCACAGG-3′; MMP-7 sense, 5′-GATGAGGATGA
ACGCTGGAC-3′ and antisense, 5′-GCTAAATGGAGTGGA GGAACAG-3′; and
GAPDH sense, 5′-TCTATAAATTGAGC CCGCAGC-3′ and antisense,
5′-CCATGGTGTCTGAGCGA TGT-3′. The amplification protocol used was:
95°C for 2 min, and then 40 cycles of 95°C for 10 sec, 60°C for 30
sec, and 72°C for 30 sec, with a final extension at 72°C for 30
sec. Crossing threshold values for individual genes were normalized
to GAPDH. Each reaction was performed in triplicate. Changes in the
mRNA expression of Reg4 and MMP-7 was calculated as fold-change
(2−ΔΔCt) relative to control following the formulae:
Reg4ΔCt = (Avg.Reg4_Ct - Avg.GAPDH_Ct), Reg4ΔΔCt = (Reg4ΔCt_tumor -
Reg4ΔCt_non-tumor); MMP-7ΔCt = (Avg.MMP-7_Ct - Avg.GAPDH_Ct),
MMP-7ΔΔCt = (MMP-7ΔCt_tumor - MMP-7ΔCt_non-tumor).
Western blot analysis
Total protein was extracted from frozen primary
colorectal tumors and adjacent normal tissues of four randomly
selected patients using the radio immunoprecipitation assay lysis
buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium
deoxycholate and 0.1% sodium dodecyl sulphate). Protein
concentrations were measured using a BCA protein assay kit
(Beyotime Biotechnology Co., Jiangsu, China). Equal amounts of
protein were subjected to 15% sodium dodecyl
sulphate-polyacrylamide gels and then transferred onto PVDF
membranes according to standard protocols. The membranes were
blocked in 5% fat-free milk solution with 0.1% Tween-20 for 1 h at
room temperature, followed by incubation with the specific primary
antibody including Reg4 (1:1,000; R&D Systems, Minneapolis, MN,
USA), MMP-7 (1:500) and GAPDH (1:1,000) (both from Abcam,
Cambridge, MA, USA) overnight at 4°C. After washing the membranes
with TBST three times, the blots were incubated with secondary
antibodies conjugated to horseradish peroxidase for 1 h at room
temperature. The bound antibodies were detected by enhanced
chemiluminescence following the manufacturer’s instructions. The
abundance of each protein was determined and normalized against
GAPDH expression.
TMA construction
For tissue microarray (TMA) construction, primary
tumors and paired normal tissues from 186 patients were retrieved
from the Department of Pathology of Shanghai Jiaotong University
Affiliated First People’s Hospital. Of these samples, 63 samples
were paired with lymph-node metastasis. Representative areas of
tissues were established and 2.0-mm diameter cores were punched
from the paraffin blocks. Two cores from each formalin-fixed,
paraffin-embedded primary tumor and matched normal tissue at a
distance of at least 2 cm from the tumor were arrayed. TMAs were
constructed using a tissue microarray. The samples were examined by
at least two investigators to prevent bias.
Immunohistochemistry
Immunohistochemical staining was performed using the
primary antibody against Reg4 (1:100; R&D Systems) or MMP-7
(1:150; Abcam), followed by incubation with secondary antibody
conjugated-HRP (DakoCytomation, Glostrup, Denmark). The slides were
counterstained with Mayer’s hematoxylin. Two independent
investigators scored the positive staining of sections without any
knowledge of the patients outcomes (double-blinded). The evaluation
was based on the staining intensity and area. The staining
intensity was graded as 0 (negative), 1 (weak) and 2 (strong).
Staining area was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3
(51–75%) and 4 (76–100%) according to the percentage of positively
stained cells. The sum of the staining score (intensity and extent)
index was designated as: 0–2 (negative), 3–6 (positive) (21).
Statistical analysis
Statistical analysis was performed with SPSS,
version 19.0 (SPSS, Inc., Chicago, IL, USA). The differences of
mRNA expression of Reg4 or MMP-7 between cancer tissues and paired
normal mucosa were calculated by the paired t-test. The two-tailed
χ2 or Fisher’s exact tests were used where appropriate,
to determine the significance of the difference among the
covariates. The correlation between Reg4 and MMP-7 protein
expression was calculated using Spearman’s test. The survival rates
were calculated by the Kaplan-Meier method. A log-rank test was
used to compare the survival curves. Cox proportional hazard models
were used to investigate the independent risk factors for death and
significant factors were selected for the final multivariate
regression model using the forward LR method. Differences at
P<0.05 were considered to indicate a statistically significant
result.
Results
Overexpression of Reg4 and MMP-7 in
primary colon cancer compared with adjacent normal mucosa
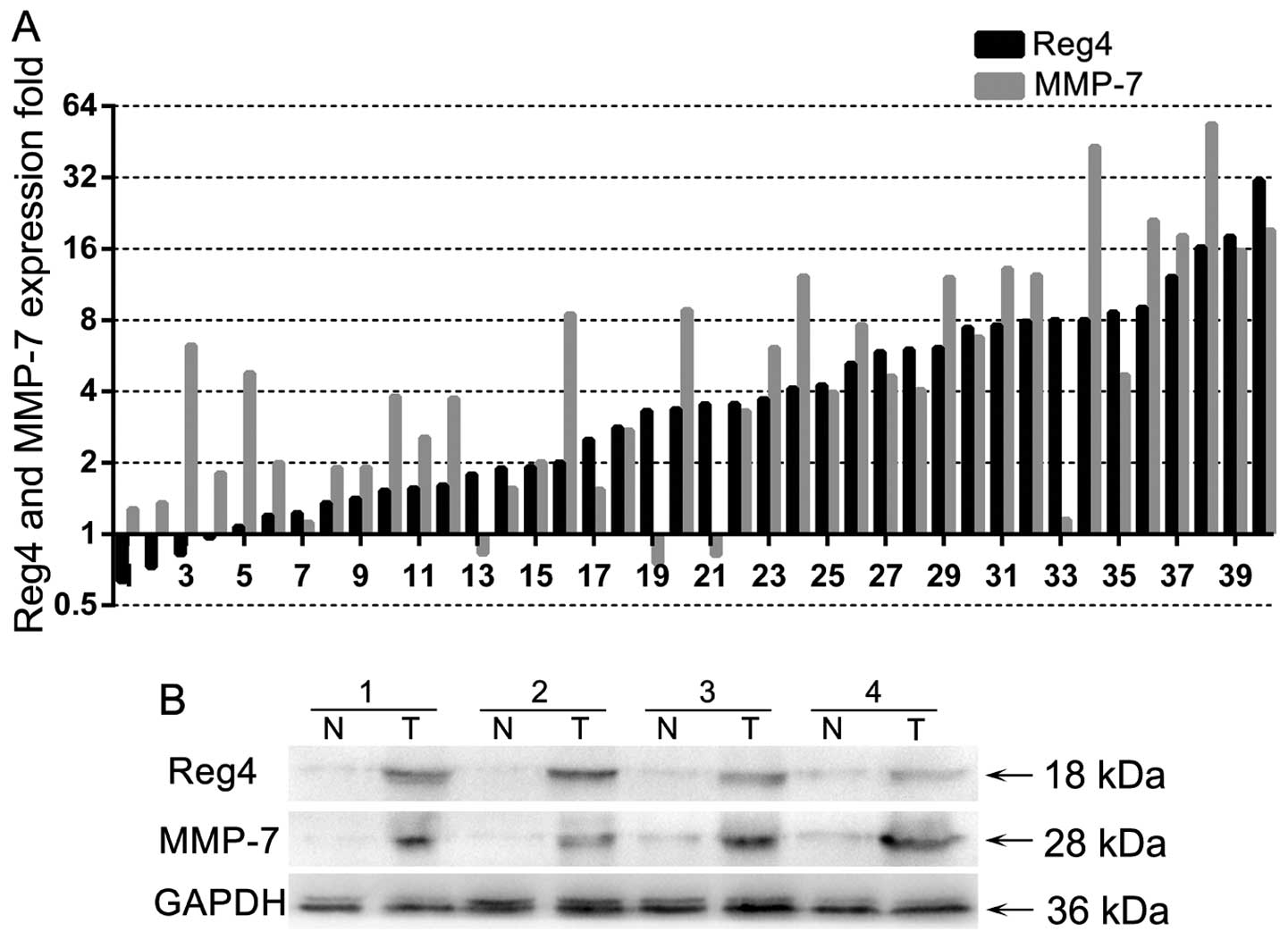
Of the 40 randomly selected, paired samples subject
to RT-qPCR analysis, 25 (62.5%) colon cancers showed a ≥2-fold
increase in Reg4 mRNA levels compared with that of the adjacent
non-cancerous tissues, while 27 (67.5%) colon cancers showed a
≥2-fold increase in MMP-7 mRNA levels (Fig. 1A). The global expression (ΔCt) of
Reg4 or MMP-7 was 6.67±1.65 or 9.13±1.35 in tumor tissues and
4.93±1.85 (P<0.001) or 7.01±2.15 (P<0.001) in normal tissues,
respectively. There were 21 (55%) colorectal cancer tissues showing
an increase in Reg4 and MMP-7 mRNA levels and a statistical
correlation of mRNA levels between Reg4 and MMP-7 was performed
(r=0.595, P<0.001). Four paired samples were randomly selected
to evaluate their protein expression by western blot analysis and
the majority of the samples showed higher levels of Reg4 and MMP-7
protein than that of the adjacent non-cancer tissues (Fig. 1B). The results suggested that Reg4
and MMP-7 were elevated in CRC at the transcriptional and
post-transcriptional levels.
Association of Reg4 and MMP-7 expression
with clinicopathological parameters
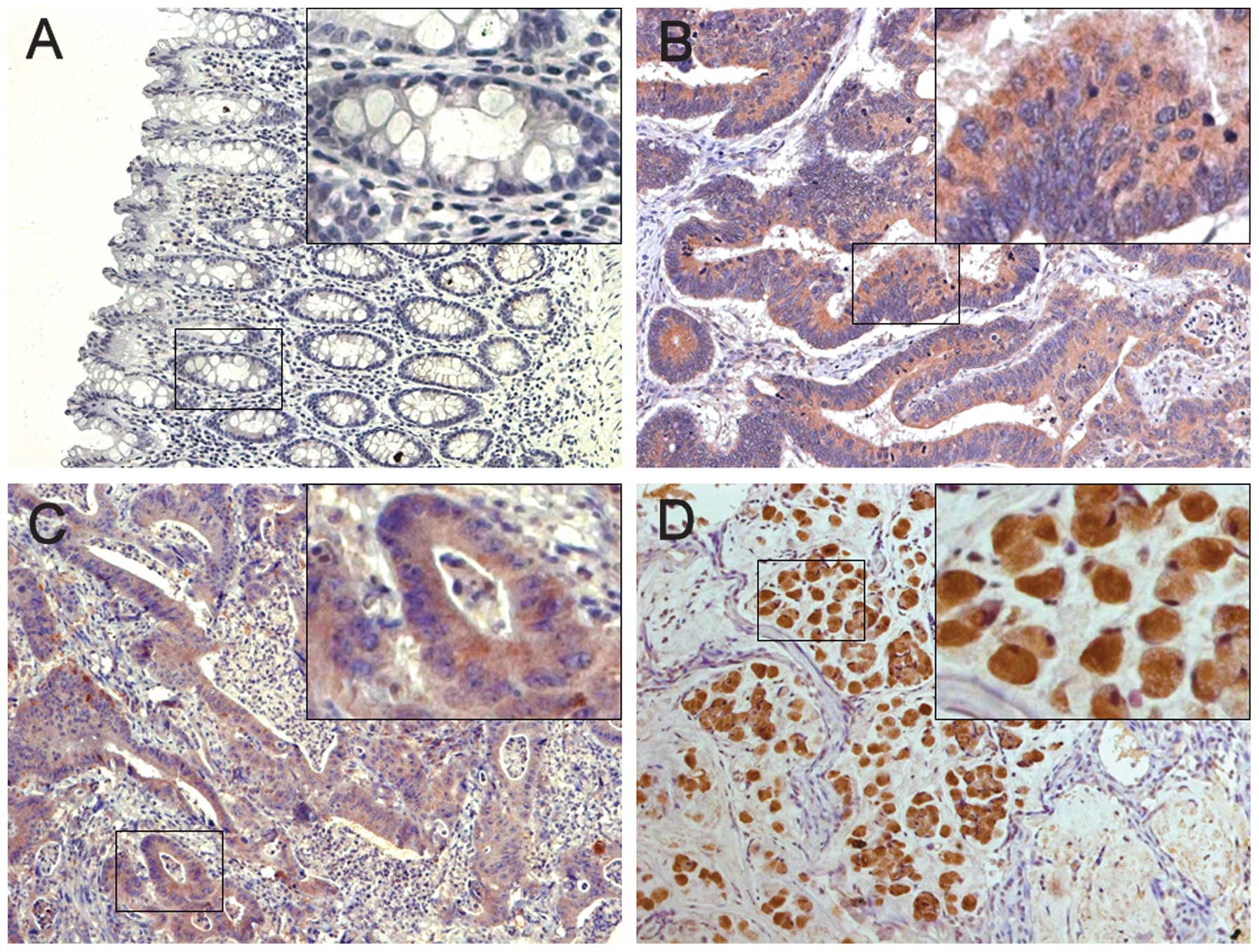
Of the 186 normal mucosa samples on the paired TMA,
168 (90.3%) cases showed negative Reg4 expression and 177 (95.2%)
showed negative MMP-7 expression while the remaining samples showed
weak cytoplasmic staining. By contrast, Reg4 and MMP-7 were
prominently expressed in colon cancer tissue specimens with 73
(39.2%) and 87 (46.8%) cases of positive staining, respectively
(Fig. 2). Of the 63 matched samples
available for analysis, both of the rates of positive Reg4 and
MMP-7 expression in CRC lymph-node metastasis (LNM) cells were
higher than that in the primary tumors (P=0.024 and P=0.003,
respectively; Table I). These data
indicate the overexpression of Reg4 and MMP-7 may correlate with
colon tumor metastasis.
 | Table IReg4 and MMP-7 immunohistochemical
staining for protein expression in normal mucosa, primary tumors
and lymph node metastasis. |
Table I
Reg4 and MMP-7 immunohistochemical
staining for protein expression in normal mucosa, primary tumors
and lymph node metastasis.
| Tissue samples | |
|---|
|
| |
|---|
| Expression of Reg4 or
MMP-7 | Normal mucosa (n=186)
(%) | CRC tissues (n=186)
(%) | LNM tissues (n=63)
(%) | P-value |
|---|
| Reg4 | | | | <0.001a |
| Positive | 18 (9.7) | 73 (39.2) | 35 (55.6) | |
| Negative | 168 (90.3) | 113 (60.8) | 28 (44.4) | |
| MMP-7 | | | | <0.001b |
| Positive | 9 (4.8) | 87 (46.8) | 43 (68.3) | |
| Negative | 177 (95.2) | 99 (53.2) | 20 (31.7) | |
| Reg4/MMP-7 | | | | <0.001c |
| Both positive | 0 (0) | 44 (23.7) | 32 (50.8) | |
| One positive | 27 (14.5) | 72 (38.7) | 14 (22.2) | |
| Both negative | 159 (85.5) | 70 (37.6) | 17 (27) | |
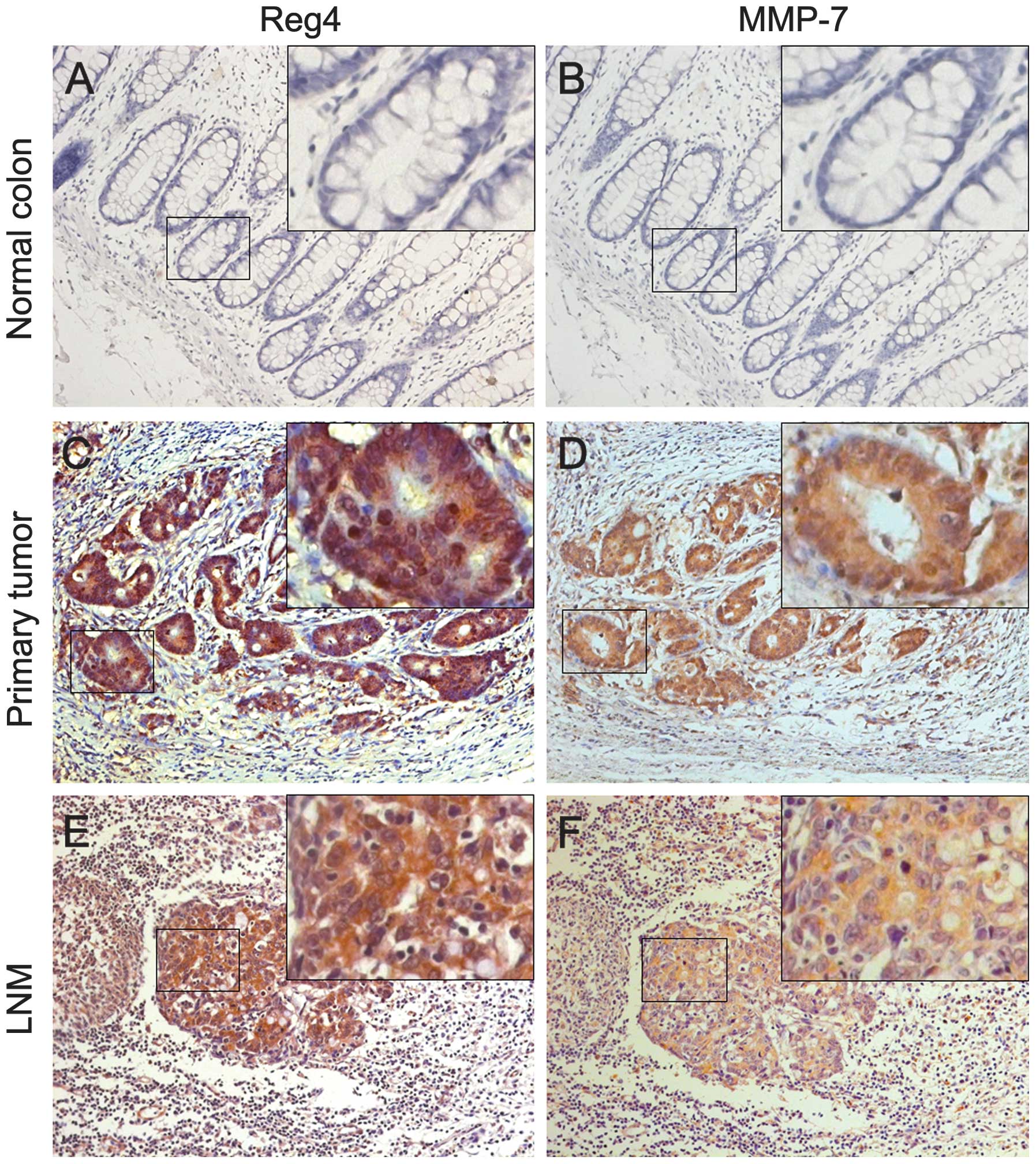
Associations of Reg4 and MMP-7 expression with
clinicopathological factors are shown in Table II. Increased Reg4 expression was
significantly associated with LNM (N stage) (P=0.003), distant
metastasis (M stage) (P=0.005), AJCC stage (P=0.004) and
differentiation (P<0.001). No correlations were found between
Reg4 expression and age, gender, T stage, tumor location or
vascular invasion. The overexpression of MMP-7 was significantly
associated with the depth of tumor invasion (T stage) (P=0.013),
LNM (N stage) (P=0.005), AJCC stage (P=0.006) and M stage
(P=0.010). No correlations were found between MMP-7 and age,
gender, tumor location, vascular invasion or differentiation.
Moreover, Reg4 was more frequently detected in samples that stained
positively for MMP-7 (Fig. 3).
Particularly in the tumors with distant metastasis, ~64.7% (11/17)
were expressed in Reg4 and MMP-7, and a statistical correlation
(r=0.555, P=0.021) was observed. Furthermore, we examined the
expression of Reg4 and MMP-7 in the metastatic lymph nodes and
found the rate of LNM with a positive expression of Reg4 and MMP-7
was higher than that in the normal mucosa and the primary cancer
(P<0.001), and the correlation of the two molecules in the
metastatic lymph nodes was statistically significant (r=0.557,
P<0.001; Table I).
 | Table IIAssociation between
clinicopathological characteristics and Reg4 or MMP-7 protein
expression. |
Table II
Association between
clinicopathological characteristics and Reg4 or MMP-7 protein
expression.
| Expression of
Reg4 | | Expression of
MMP-7 | |
|---|
|
| |
| |
|---|
| Characteristics | Positive (n=73) | Negative (n=113) | P-value | Positive (n=87) | Negative (n=99) | P-value |
|---|
| Age (years) | | | 0.432 | | | 0.746 |
| <65 | 32 | 43 | | 34 | 41 | |
| ≥65 | 41 | 70 | | 53 | 58 | |
| Gender | | | 0.198 | | | 0.547 |
| Male | 26 | 51 | | 34 | 43 | |
| Female | 47 | 62 | | 53 | 56 | |
| Location | | | 0.264 | | | 0.589 |
| Right | 35 | 46 | | 42 | 39 | |
| Transverse | 10 | 9 | | 9 | 10 | |
| Descending | 5 | 14 | | 7 | 12 | |
| Sigmoid | 23 | 44 | | 29 | 38 | |
| T stage | | | 0.276 | | | 0.013a |
| T1 | 3 | 5 | | 2 | 6 | |
| T2 | 7 | 10 | | 6 | 11 | |
| T3 | 21 | 48 | | 25 | 44 | |
| T4 | 42 | 50 | | 54 | 38 | |
| N stage | | | 0.003a | | | 0.005a |
| N0 | 30 | 66 | | 34 | 62 | |
| N1 | 22 | 36 | | 33 | 25 | |
| N2 | 21 | 11 | | 20 | 12 | |
| M stage | | | 0.005a | | | 0.010a |
| M0 | 61 | 108 | | 74 | 95 | |
| M1 | 12 | 5 | | 13 | 4 | |
| AJCC stage | | | 0.004a | | | 0.006a |
| I | 9 | 10 | | 6 | 13 | |
| II | 20 | 56 | | 28 | 48 | |
| III | 32 | 42 | | 40 | 34 | |
| IV | 12 | 5 | | 13 | 4 | |
| Vessel
invasion | | | 0.392 | | | 0.172 |
| No | 66 | 106 | | 78 | 94 | |
| Yes | 7 | 7 | | 9 | 5 | |
|
Differentiation | | | <0.001a | | | 0.762 |
| Well | 21 | 65 | | 39 | 47 | |
| Moderate | 32 | 40 | | 36 | 36 | |
| Poor | 20 | 8 | | 12 | 16 | |
| MMP-7 | | | 0.003b | | | |
| Positive | 44 | 43 | | | | |
| Negative | 29 | 70 | | | | |
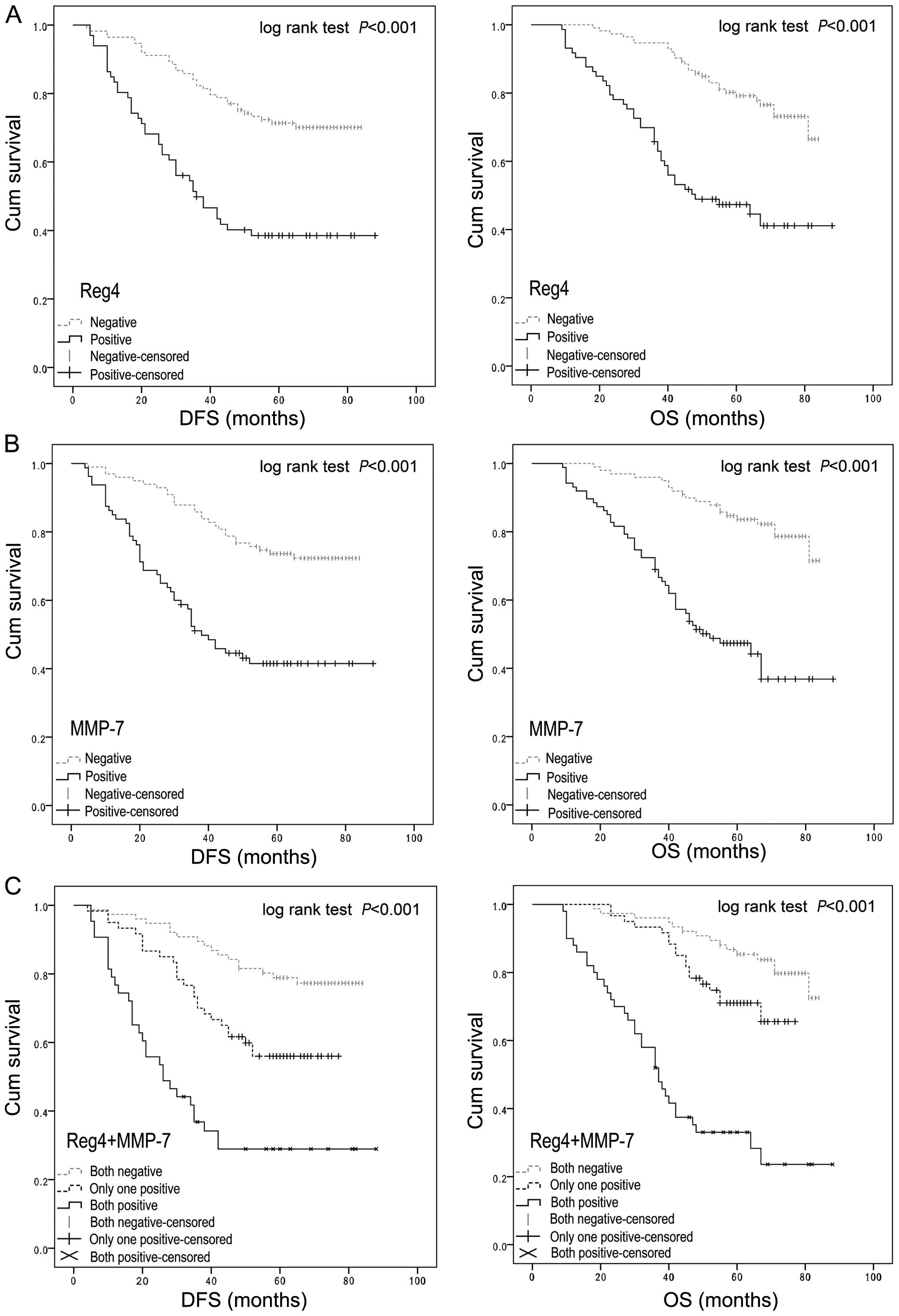
Survival analysis and prognostic
significance of Reg4 or MMP-7 expression
To assess the possible association between tumor
Reg4 or MMP-7 expression and patient survival, the Kaplan-Meier
plots with a log-rank test for OS and DFS were undertaken (Fig. 4). It showed that patients with Reg4
overexpression tumors had a poorer OS (P<0.001) and DFS
(P<0.001) than patients with Reg4-negative tumors (Fig. 4A), and positive MMP-7 expression
patients had a lower OS (P<0.001) and DFS (P<0.001) rate than
patients with a negative MMP-7 expression (Fig. 4B). Furthermore, with regard to the
concomitant expression of Reg4 and MMP-7 proteins, we divided the
samples into three groups: group 1, tumors exhibiting no expression
of Reg4 and MMP-7 (Reg4−/MMP-7−, 70
specimens); group 2, tumors with abnormal expression of only 1
protein (Reg4−/MMP-7+ or
Reg4+/MMP-7−, 72 specimens); group 3, tumors
with abnormal expression of the two proteins
(Reg4+/MMP-7+, 44 specimens). Notably, a
better OS and DFS in group 1 was observed as compared to group 2,
while group 3 showed the worst OS and DFS of the three groups
(P<0.001) (Fig. 4C).
Using a univariate analysis in the Cox proportional
hazards model, a decreased OS and increased postoperative
recurrence were associated with the depth of tumor invasion, LNM,
distant metastasis, AJCC stage, histological differentiation,
vessel invasion, Reg4 expression, MMP-7 expression and the
expression of Reg4 and MMP-7 (Table
III). The multivariate analysis revealed that the expression of
Reg4 and MMP-7 was an independent prognostic factor for OS (HR
4.63; 95% CI 2.43–8.81; P<0.001) and DFS (HR 3.88; 95% CI
2.08–7.22; P<0.001), but not the expression of Reg4 or MMP-7
alone.
 | Table IIIUnivariate and multivariate Cox
proportional hazard models for overall survival (OS) and
disease-free survival (DFS). |
Table III
Univariate and multivariate Cox
proportional hazard models for overall survival (OS) and
disease-free survival (DFS).
| OS | DFS |
|---|
|
|
|
|---|
| Variable |
Univariate
HR (95% CI) | P-value |
Multivariate
HR (95% CI) | P-value |
Univariate
HR (95% CI) | P-value |
Multivariate
HR (95% CI) | P-value |
|---|
| Age (years) |
| <65 | 1 | | | | 1 | | | |
| ≥65 | 1.08
(0.67–1.76) | 0.753 | | | 1.04
(0.65–1.67) | 0.856 | | |
| Gender |
| Male | 1 | | | | 1 | | | |
| Female | 1.52
(0.92–2.51) | 0.105 | | | 1.21
(0.75–1.93) | 0.436 | | |
| Location |
| Right | 1 | | | | 1 | | | |
| Transverse | 0.82
(0.34–1.98) | 0.654 | | | 0.83
(0.34–2.00) | 0.674 | | |
| Left | 1.04
(0.46–2.39) | 0.923 | | | 0.98
(0.43–2.23) | 0.954 | | |
| Sigmoid | 1.13
(0.67–1.92) | 0.645 | | | 1.27
(0.76–2.10) | 0.362 | | |
| T stage |
| T1 | 0.36
(0.09–1.50) | 0.161 | | | 0.34
(0.08–1.39) | 0.133 | 0.30
(0.07–1.28) | 0.103 |
| T2 | 0.08
(0.01–0.55) | 0.011a | | | 0.15
(0.04–0.60) | 0.007a | 0.26
(0.06–1.13) | 0.073a |
| T3 | 0.34
(0.20–0.61) | <0.001a | | | 0.44
(0.26–0.73) | 0.002a | 0.42
(0.24–0.73) | 0.002a |
| T4 | 1 | | | | 1 | | 1 | |
| N stage |
| N0 | 1 | | | | 1 | | 1 | |
| N1 | 5.44
(2.74–10.82) | <0.001a | | | 3.17
(1.78–5.67) | <0.001a | 2.04
(1.11–3.75) | 0.021a |
| N2 | 18.85
(9.35–38.00) | <0.001a | | | 12.19
(6.66–22.33) | <0.001a | 8.27
(4.25–16.11) | <0.001a |
| M stage |
| M0 | 1 | | | | 1 | | 1 | |
| M1 | 14.12
(7.69–25.93) | <0.001a | | | 9.68
(4.77–19.68) | <0.001a | 4.61
(2.18–9.78) | <0.001a |
| AJCC stage |
| I | 1 | | | | 1 | | | |
| II | 2.91
(0.38–22.57) | 0.306 | | | 2.42
(0.56–10.42) | 0.236 | | |
| III | 15.74
(2.16–114.86) | 0.007a | | | 8.59
(2.08–35.53) | 0.003a | | |
| IV | 110.05
(14.30–847.13) | <0.001a | | | 45.28
(9.67–211.92) | <0.001a | | |
| Vessel
invasion |
| No | 1 | | | | 1 | | | |
| Yes | 4.70
(2.54–8.70) | <0.001a | | | 4.02
(2.10–7.71) | <0.001a | | |
|
Differentiation |
| Well | 1 | | | | 1 | | | |
| Moderate | 2.54
(1.39–4.67) | 0.003a | | | 2.43
(1.41–4.17) | 0.001a | | |
| Poor | 7.51
(3.93–14.37) | <0.001a | | | 4.91
(2.58–4.76) | <0.001a | | |
| Reg4 |
| Negative | 1 | | | | 1 | | | |
| Positive | 3.40
(2.08–5.53) | <0.001a | | | 3.00
(1.89–4.93) | <0.001a | | |
| MMP-7 |
| Negative | 1 | | | | 1 | | | |
| Positive | 4.39
(2.58–7.48) | <0.001a | | | 3.05
(1.89–4.93) | <0.001a | | |
| Reg4/MMP-7 |
| Both negative | 1 | | 1 | | 1 | | 1 | |
| One positive | 1.92
(0.96–3.84) | 0.066 | 1.55
(0.77–3.12) | 0.225 | 2.31
(1.25–4.27) | 0.007a | 2.19
(1.17–4.08) | 0.014a |
| Both positive | 7.50
(4.04–13.92) | <0.001a | 4.63
(2.43–8.81) | <0.001a | 5.93
(3.25–10.84) | <0.001a | 3.88
(2.08–7.22) | <0.001a |
Discussion
Reg4 was first found from the proliferating cells in
UC which potentially developed into CRC when progression occurred
over 10 years. Findings of previous studies showed Reg4 was
markedly upregulated in UC and colorectal adenomas although
staining in the perinuclear of neuroendocrine cells in normal colon
tissues was detected (4,22,23).
In the present study, we reported that Reg4 was overexpressed in
CRC at the transcriptional and post-transcriptional levels. Further
validation by immunohistochemistry showed that 39.2% of primary
CRCs had positive Reg4 protein staining. This is consistent with
the results reported by Oue et al (9). This finding suggests that Reg4 has an
important role in the progression of CRC carcinogenesis.
In the present study, the correlations between Reg4
expression and clinicopathological characteristics were evaluated.
Overexpression of Reg4 was significantly associated with clinical
stage, T stage, lymph node metastasis (LNM) and M stage. These
correlations suggest that Reg4 overexpression may promote tumor
invasion and metastasis. Moreover, the results show that the rate
of positive Reg4 expression in LNM was higher than that in primary
tumors, and higher in patients with distant metastasis than in
patients without distant metastasis. Therefore, Reg4 may be an
effectively diagnostic biomarker of CRC patients with metastasis.
However, it is not clear how Reg4 performed its functions of
promoting metastasis of colon cancer cells.
Recently, He et al (14) reported that in pancreatic cancer
cells, the upregulation of Reg4 may lead to the overexpression of
MMP-7, thereby increasing the metastatic ability of cancer cells.
MMP-7 is important in the invasion and metastasis of carcinoma
cells by cleaving membrane proteins such as integrin β4, syndecan-1
and E-cadherin (24). Thus, we
examined the relationship between MMP-7 and clinicopathological
characteristics in CRC. In the present study, MMP-7 was more
frequently observed in lymph-node metastatic cells than primary
tumors and normal colorectal tissue, and it is indicated that MMP-7
may participate in the invasion and metastasis of CRC. Furthermore,
a statistically significant positive correlation (r=0.217, P=0.003)
between Reg4 and MMP-7 in CRC was observed. Particularly in LNM,
the correlation (r=0.557, P<0.001) between the two molecules was
more significant. These data suggest that MMP-7 may be a biomarker
predicting the metastasis of CRC when combined with Reg4.
In the present study, the patients with
overexpression of Reg4 and MMP-7 had an increased risk of tumor
recurrence and shorter survival than patients with a negative
expression of Reg4 or MMP-7. Multivariate Cox proportional hazard
model showed Reg4 combined with MMP-7 expression is an independent
prognostic factor for OS and DFS. Findings of previous studies also
suggest that the patients with positive Reg4 or MMP-7 expression
had a poor response for chemotherapy and radiotherapy than patients
with a negative Reg4 or MMP-7 expression (12,13,25).
As mentioned above, Reg4 and MMP-7 may have a strong predictive
value and serve as drug targets for CRC patients.
The mechanism of how Reg4 regulates MMP-7 in CRC is
not well elucidated. It was reported that MMP-7, which may be
considered to function as an oncogene since colorectal
tumorigenesis was suppressed in mice lacking MMP-7 (26), was a target gene of the
β-catenin/TCF-4 signaling pathway (27). Bishnupuri el al (28) recently provided evidence that Reg4
regulated CRC cell proliferation and division by the
Akt-GSK3β-β-catenin-TCF-4 signaling pathway. Therefore, Reg4 may
promote colorectal tumorigenesis and CRC metastasis by upregulating
MMP-7 through the Akt-GSK3β-β-catenin-TCF-4 signaling pathway.
To the best of our knowledge, this is the first
study to report that the expression of Reg4 was correlated with
that of MMP-7 in CRC at the transcriptional and
post-transcriptional levels. Both Reg4 and MMP-7 were significantly
upregulated in CRC and were associated with the invasion and
metastasis of tumor cells. We suggest that tumor Reg4 and MMP-7
expression is a clinically useful, prognostic indicator of cancer
metastasis, recurrence and poor patient survival. These preliminary
findings should be fully verified in CRC cells at the molecular
level and in murine models at the animal level.
References
|
1
|
Chen W, Zheng R, Zhang S, et al: Annual
report on status of cancer in China, 2010. Chin J Cancer Res.
26:48–58. 2014.PubMed/NCBI
|
|
2
|
Iovanna JL and Dagorn JC: The
multifunctional family of secreted proteins containing a C-type
lectin-like domain linked to a short N-terminal peptide. Biochim
Biophys Acta. 1723:8–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartupee JC, Zhang H, Bonaldo MF, Soares
MB and Dieckgraefe BK: Isolation and characterization of a cDNA
encoding a novel member of the human regenerating protein family:
Reg IV. Biochim Biophys Acta. 16:287–293. 2001. View Article : Google Scholar
|
|
4
|
van Beelen Granlund A, Østvik AE, Brenna
Ø, Torp SH, Gustafsson BI and Sandvik AK: REG gene expression in
inflamed and healthy colon mucosa explored by in situ
hybridisation. Cell Tissue Res. 352:639–646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamagishi H, Fukui H, Sekikawa A, et al:
Expression profile of REG family proteins REG Iα and REG IV in
advanced gastric cancer: comparison with mucin phenotype and
prognostic markers. Mod Pathol. 22:906–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Numata M, Oshima T, Yoshihara K, et al:
Relationship between Reg IV gene expression to outcomes in
colorectal cancer. J Surg Oncol. 104:205–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Xu L, Guo C, et al: Identification
of RegIV as a novel GLI1 target gene in human pancreatic cancer.
PLoS One. 6:e184342011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang L, Lan S, Liu J and Yang Z:
Expression of MK-1 and RegIV and its clinicopathological
significances in the benign and malignant lesions of gallbladder.
Diagn Pathol. 6:1002011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oue N, Kuniyasu H, Noguchi T, et al: Serum
concentration of Reg IV in patients with colorectal cancer:
overexpression and high serum levels of Reg IV are associated with
liver metastasis. Oncology. 72:371–380. 2007. View Article : Google Scholar
|
|
10
|
Rafa L, Dessein AF, Devisme L, et al: REG4
acts as a mitogenic, motility and pro-invasive factor for colon
cancer cells. Int J Oncol. 36:689–698. 2010.PubMed/NCBI
|
|
11
|
Ying LS, Yu JL, Lu XX and Ling ZQ:
Enhanced Reg IV expression predicts the intrinsic 5-fluorouracil
(5-FU) resistance in advanced gastric cancer. Dig Dis Sci.
58:414–422. 2013. View Article : Google Scholar
|
|
12
|
Bishnupuri KS, Luo Q, Sainathan SK, et al:
Reg IV regulates normal intestinal and colorectal cancer cell
susceptibility to radiation-induced apoptosis. Gastroenterology.
138:616–626. 2010. View Article : Google Scholar :
|
|
13
|
Mitani Y, Oue N, Matsumura S, et al: Reg
IV is a serum biomarker for gastric cancer patients and predicts
response to 5-fluorouracil-based chemotherapy. Oncogene.
26:4383–4393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He XJ, Jiang XT, Ma YY, et al: REG4
contributes to the invasiveness of pancreatic cancer by
upregulating MMP-7 and MMP-9. Cancer Sci. 103:2082–2091. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rucci N, Sanità P and Angelucci A: Roles
of metalloproteases in metastatic niche. Curr Mol Med. 11:609–622.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang B, Su K, Gao J and Rao Z: Expression
and prognostic value of matrix metalloproteinase-7 in colorectal
cancer. Asian Pac J Cancer Prev. 13:1049–1052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukuda A, Wang SC, Morris JP IV, et al:
Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma
initiation and progression. Cancer Cell. 19:441–455. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito TK, Ishii G, Saito S, et al:
Degradation of soluble VEGF receptor-1 by MMP-7 allows VEGF access
to endothelial cells. Blood. 113:2363–2369. 2009. View Article : Google Scholar
|
|
19
|
Kioi M, Yamamoto K, Higashi S, Koshikawa
N, Fujita K and Miyazaki K: Matrilysin (MMP-7) induces homotypic
adhesion of human colon cancer cells and enhances their metastatic
potential in nude mouse model. Oncogene. 22:8662–8670. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bishnupuri KS, Luo Q, Murmu N, Houchen CW,
Anant S and Dieckgraefe BK: Reg IV activates the epidermal growth
factor receptor/Akt/AP-1 signaling pathway in colon
adenocarcinomas. Gastroenterology. 130:137–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Tu WW, Wen YG, et al: Increased
expression of TBX2 is a novel independent prognostic biomarker of a
worse outcome in colorectal cancer patients after curative surgery
and a potential therapeutic target. Med Oncol. 30:6882013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Lai M, Lv B, et al:
Overexpression of Reg IV in colorectal adenoma. Cancer Lett.
200:69–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oue N, Mitani Y, Aung PP, et al:
Expression and localization of Reg IV in human neoplastic and
non-neoplastic tissues: Reg IV expression is associated with
intestinal and neuroendocrine differentiation in gastric
adenocarcinoma. J Pathol. 207:185–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsunezumi J, Higashi S and Miyazaki K:
Matrilysin (MMP-7) cleaves C-type lectin domain family 3 member A
(CLEC3A) on tumor cell surface and modulates its cell adhesion
activity. J Cell Biochem. 106:693–702. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Li Y, Yang L, et al: Knockdown of
MMP-7 inhibits cell proliferation and enhances sensitivity to
5-fluorouracil and X-ray irradiation in colon cancer cells. Clin
Exp Med. 14:99–106. 2014. View Article : Google Scholar
|
|
26
|
Wilson CL, Heppner KJ, Labosky PA, Hogan
BL and Matrisian LM: Intestinal tumorigenesis is suppressed in mice
lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA.
94:1402–1407. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YJ, Park HJ, Chung HJ, et al:
Wnt/β-catenin signaling mediates the antitumor activity of magnolol
in colorectal cancer cells. Mol Pharmacol. 82:168–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bishnupuri KS, Sainathan SK, Bishnupuri K,
et al: Reg4-induced mitogenesis involves Akt-GSK3β-β-Catenin-TCF-4
signaling in human colorectal cancer. Mol Carcinog. 53(Suppl 1):
E169–E180. 2014. View
Article : Google Scholar
|