Introduction
Epithelial ovarian cancer (EOC) is the most lethal
gynecological malignancy worldwide (1,2). As
the result of advances in surgical management and chemotherapeutic
options over the last three decades, the median survival for
ovarian cancer patients has improved (3,4).
However, progression-free and overall survival have not been
significantly altered due to the fact that the origin and
pathogenesis of EOC are poorly understood (4). In addition, most patients present with
advanced disease, for which effective therapy is currently
unavailable (5). Therefore,
identification of new molecular targets and therapeutic strategies
for EOC patients is urgently needed.
Ubiquitin specific protease 22 (USP22), one of the
11 polycomb/cancer stem cell signature genes that are critical in
controlling cell growth and death, is involved in the regulation of
pathological processes, including oncogenesis, cell proliferation
and cell cycle progression (6–8).
Increasing evidence indicates that an oncogenic role of USP22
activation may contribute to tumor progression and predict the
prognosis in a variety of human malignancies, including non-small
cell lung cancer (9), oral squamous
cell carcinoma (10), breast cancer
(11), duct (12) and gastric carcinoma (13), and colorectal cancer (14). However, the molecular mechanisms of
USP22 in EOC remain to be clarified.
Herein, we evaluated the expression of USP22 in
human EOC tissues, and analyzed its correlation with
clinicopathological characteristics and the possible prognostic
significance. The effects of USP22 on cell proliferation and tumor
growth were assessed in vitro as well as in vivo.
Materials and methods
Patients and follow-up
Paraffin-embedded tissue samples from 86 patients
with epithelial ovarian tumors and 30 normal ovaries from
hysterectomy specimens resected for non-ovarian disease were
obtained from the archives of the Department of Pathology, The
First Affiliated Hospital of Zhengzhou University between 2006 and
2012. The tumor cases were histologically confirmed ovarian serous
cystadenocarcinoma. The stage of the tumors was assessed according
to the International Federation of Gynecology and Obstetrics
(FIGO).
The follow-up was completed in 86 patients, and the
median follow-up period was 45 months. Follow-up studies included
laboratory analysis, physical examination and computed tomography
if necessary. Excluding criteria were as follows: i) patients who
had undergone chemotherapy or radiotherapy prior to surgery; ii)
patients who died within 3 months after surgery; iii) patients
whose cause of death remained unknown. The present study was
approved by the Ethics Committee of the First Affiliated Hospital
of Zhengzhou University. Informed consent was obtained from each
participant.
Cell cultures
Ovarian cancer cell lines SKOV3 and OVCAR3 were
purchased from the American Type Culture Collection (ATCC). Cells
were cultured in RPMI-1640 supplemented with 10% FBS (HyClone) and
1% penicillin streptomycin (Invitrogen) at 37°C with 5%
CO2.
Immunohistochemistry
Immunohistochemistry was performed using the
avidin-biotin immunoperoxidase technique with an
immunohistochemistry kit (ab64261; Abcam) according to the
manufacturer’s instructions. The primary antibody for the
immunohistochemistry was USP22 rabbit polyclonal antibody (1:100;
ab4812; Abcam). Paraffin-embedded samples were sectioned at a
thickness of 4 mm.
RNA extraction and quantitative real-time
PCR analysis
Total RNA was extracted using TRIzol reagent. cDNA
was synthesized with the PrimeScript RT reagent kit (Takara).
Quantitative real-time PCR analysis was carried out to detect mRNA
expression using SYBR Premix Ex Taq (Takara), and GAPDH was
used as an internal control. The primers for USP22 (115 bp) were
5′-CTA CCA GGA GTC CAC AAA GCAG-3′ (forward) and 5′-CAC ATA CGT GGT
GAT CTT CCGC-3′ (reverse). Primes for oncogenic transforming growth
factor-β1 (TGFB1) (102 bp) were 5′-CGC GTG CTA ATG GTG GAA A-3′
(forward) and 5′-CGC TTC TCG GAG CTC TGA TG-3′ (reverse). The
primers for GAPDH (138 bp) were 5′-GCA CCG TCA AGG CTG AGA AC-3′
(forward) and 5′-TGG TGA AGA CGC CAG TGGA-3′ (reverse). All
reactions were run in triplicate. The cycle threshold (Ct) values
did not differ by >0.5 among the triplicates. The USP22 and
TGFB1 levels were normalized to GAPDH to permit calculations of the
2−ΔΔCt value.
Lentivirus
USP22 and TGFB1 cDNA was amplified by PCR and
subcloned into GV115 vectors (GeneChem, China), designated as
pUSP22 and pTGFB1. Lentiviral plasmid vectors encoding short
hairpin RNAs (shRNAs) targeting USP22 and TGFB1 were generated by
GeneChem (PIEL115080513, PIEL115072998).
Western blotting
Protein lysates were prepared, subjected to
SDS-PAGE, transferred onto NC membranes, and blotted according to
the standard methods using the USP22 antibody (1:1,000; ab4812;
Abcam), TGFB1 antibody (1:1,000; #8915), cyclin D2 antibody
(1:1,000; #2924) (both from Cell Signaling Technology), anti-Cdk4
antibody (1:1,500; ab137818), anti-Cdk6 antibody (1:1,500;
ab151247) and anti-p27kip1 antibody (1:1,500; ab137736) (all from
Abcam). The β-actin antibody (1:1,500; #4967; Cell Signaling
Technology) was used as an internal control.
Cell cycle assay
Cells were transfected with the lentivirus.
Nocodazole (100 ng/ml; Sigma-Aldrich) was added 48 h after
transfection, and cells were further incubated for 20 h. Floating
and adherent cells were harvested, combined, washed once in
phosphate-buffered saline (PBS), and fixed in 70% ethanol
overnight. Staining for DNA content was performed with 50 mg/ml
propidium iodide and 1 mg/ml RNase A for 30 min. Analysis was
performed on a FACScalibur flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) with Cell Quest Pro software. Cell cycle
modeling was performed with ModFit 3.0 software (Verity Software
House, Topsham, ME, USA).
Cell proliferation assay
The cell proliferation assay was performed with
WST-8 Cell Counting Kit-8 (Beyotime, Jiangsu, China) according to
the manufacturer’s instructions. Transfected cells were seeded in
96-well plates and cultured overnight. CCK-8 solution (10 μl) was
added to 96-well plates, and the cultures were incubated for 2 h at
37°C. Cell proliferation was documented every 24 h by measuring the
absorbance at 450 nm in an automatic microplate reader (Bio-Rad,
Hercules, CA, USA). The results presented are averages from 3
independent experiments.
In vivo tumor growth assay
Female athymic BALB/c nude mice were purchased from
Vital River, a Charles River Company (Beijing, China) and were
maintained in specific pathogen-free conditions. Animal care and
experimental protocols were conducted in accordance with the
guidelines of Zhengzhou University Medical Experimental Animal Care
Commission. OVCAR3 cells (5×107) infected with the
lentivirus encoding shUSP22 [at a multiplicity of infection (MOI)
of 50] were subcutaneously injected into the flank of the nude mice
(6 in each group, female BALB/c nu/nu, 4–5 weeks), and OVCAR3 cells
treated with the lentivirus encoding shNon were used as negative
control (NC). Tumor formation in nude mice was monitored over a
6-week period. Tumor volume (V) was measured twice weekly using a
caliper and calculated as V = (tumor length ×
width2)/2.
Statistical analysis
Overall survival (OS) was defined as the interval
between surgery and death or between surgery and the last
observation time point. For surviving patients, the data were
censored at the last follow-up. Relapse-free survival (RFS) was
defined as the interval between the date of surgery and the date of
diagnosis of first recurrence. Kaplan-Meier plots and the Cox
proportional hazard regression analysis, which were applied to
identify the prognostic factors, were performed with SPSS version
13.0 (SPSS, Inc., Chicago, IL, USA). Associations between the OS
and the molecular changes or clinical characteristics were analyzed
initially by a univariate Cox proportional hazards regression
analysis. Significant prognostic factors found in the univariate
analysis were evaluated further by multivariate Cox regression
analysis.
The data are expressed as the means ± standard error
of the mean (SEM) from at least 3 independent experiments. The
differences between groups were analyzed by Student’s t-test when
two groups were compared or by one-way ANOVA when more than two
groups were compared. Analyses were performed with GraphPad Prism
version 5 (GraphPad Software, Inc., San Diego, CA, USA). All
statistical tests were two-sided and P<0.05 was considered to
indicate a statistically significant result.
Results
USP22 is overexpressed in primary EOC and
is involved in EOC development
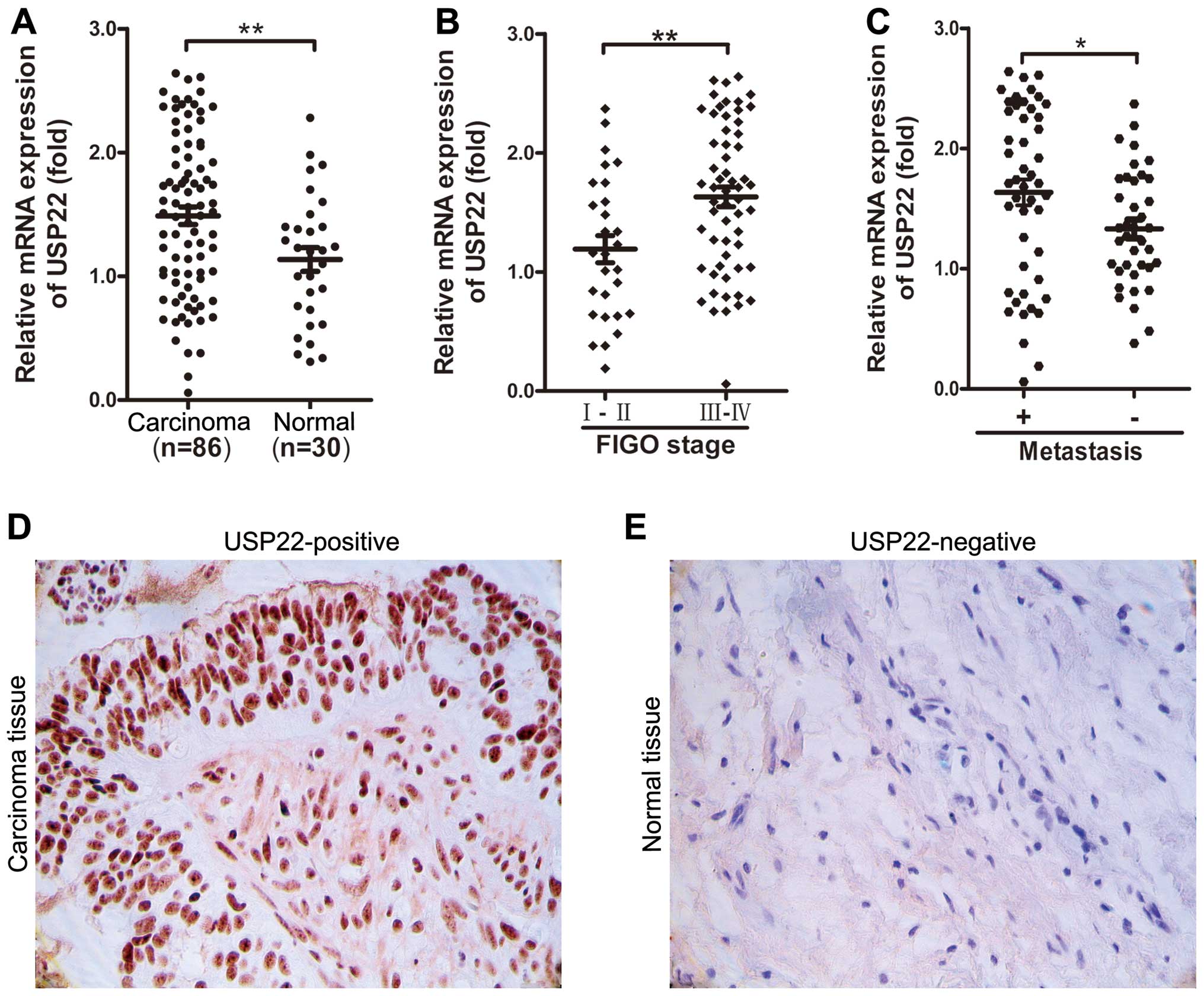
We examined the expression levels of USP22 in 86
human EOC tissues and 30 normal ovarian tissues by quantitative
real-time PCR analysis, as well as immunohistochemistry. Our data
showed that USP22 expression in the EOC tissues was overexpressed
in comparison with that in the normal ovarian tissues (P=0.0085;
Fig. 1A, D and E). We further
analyzed the association of USP22 expression with
clinicopathological parameters in EOC. As shown in Table I, the USP22 expression in EOC
tissues was significantly correlated with advanced clinical FIGO
stage (P=0.0028; Table I, Fig. 1B) and lymph node metastasis
(P=0.0313; Table I, Fig. 1C). However, no significant
associations were found between USP22 expression and patient age,
histological grade or tumor size (P>0.05; Table I). These results suggest that a
higher level of USP22 expression may be involved in EOC
progression.
 | Table ICorrelation between USP22 expression
and clinicopathologic features of EOC tissues. |
Table I
Correlation between USP22 expression
and clinicopathologic features of EOC tissues.
| Clinicopathological
features | Total cases | USP22 expression | P-value |
|---|
|
|---|
| High | Low |
|---|
| Group | | | | 0.0085b |
| Normal tissue | 30 | 19 | 11 | |
| Carcinoma
tissue | 86 | 45 | 41 | |
| Age (years) | | | | 0.8828 |
| ≤60 | 37 | 18 | 19 | |
| >60 | 49 | 27 | 22 | |
| TNM stage | | | |
0.0028b |
| I–II | 28 | 9 | 19 | |
| III–IV | 58 | 36 | 22 | |
|
Differentiation | | | | 0.8892 |
| G1 | 25 | 10 | 15 | |
| G2 | 23 | 13 | 10 | |
| G3 | 38 | 22 | 16 | |
| Lymph node
metastasis | | | |
0.0313a |
| Yes | 48 | 31 | 17 | |
| No | 38 | 14 | 24 | |
| Residual tumor size
(cm) | | | | 0.0774 |
| ≤1 | 49 | 28 | 21 | |
| >1 | 37 | 17 | 20 | |
High levels of USP22 expression are
associated with worse prognosis in EOC patients
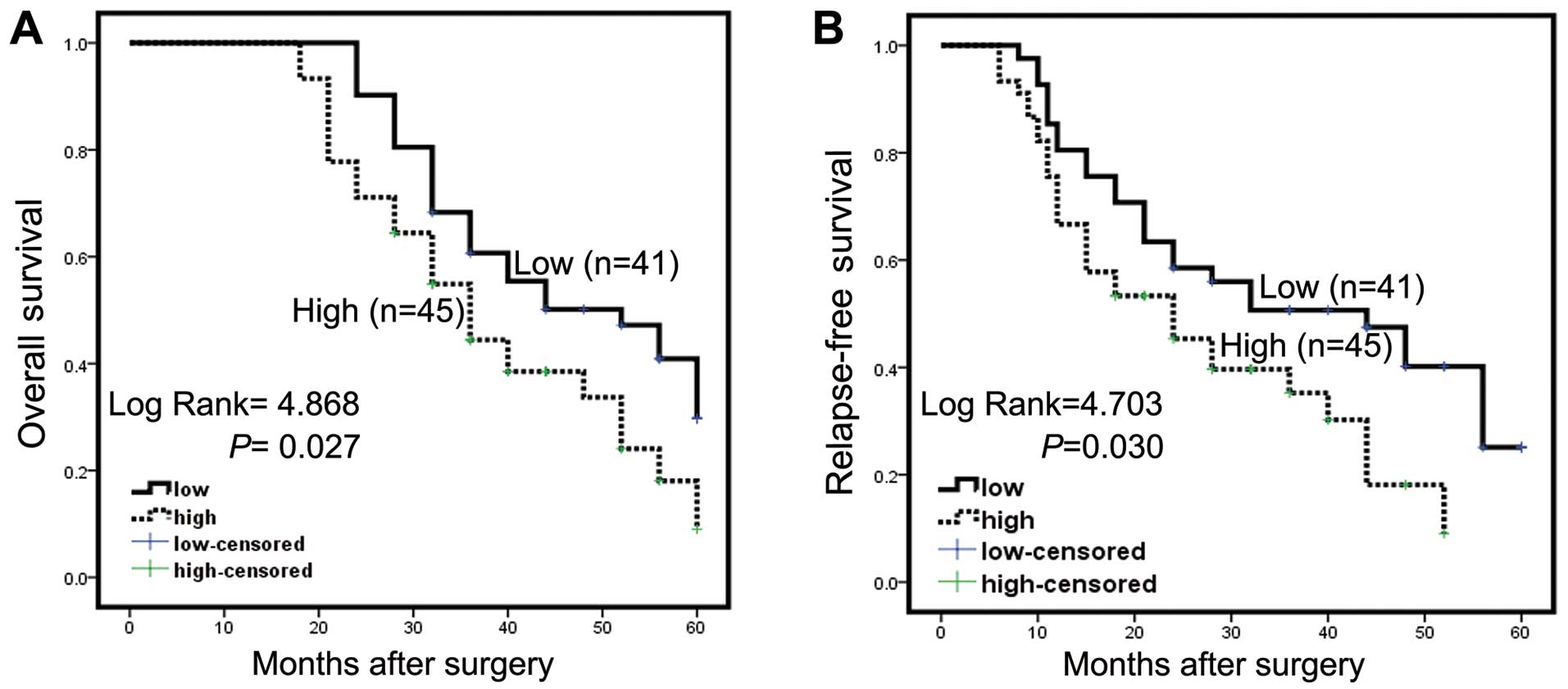
The potential association between the USP22
expression level and RFS or OS was evaluated. Kaplan-Meier analysis
plots revealed that patients with higher USP22 levels (n=45) had a
mean OS of 38.6 months, whereas patients with lower USP22 levels
(n=41) had a mean OS of 45.7 months. (P=0.027; Fig. 2A, Table
II). We also found that patients with higher USP22 levels had a
mean RFS of 27.2 months, whereas patients with lower USP22 levels
had a mean RFS of 37.2 months (P=0.030; Fig. 2B, Table
II). However, multivariate Cox regression analysis showed that
USP22 was not an independent risk factor for RFS and OS.
 | Table IIUnivariate survival analysis of RFS
and OS in patients with epithelial ovarian cancer. |
Table II
Univariate survival analysis of RFS
and OS in patients with epithelial ovarian cancer.
| | RFS (months) | | OS (months) | |
|---|
| |
| |
| |
|---|
| Variables | Cases | Mean | Median | P-value | Mean | Median | P-value |
|---|
| Expression
group |
| USP22 low | 41 | 37.218 | 44 |
0.030a | 45.742 | 52 |
0.027a |
| USP22 high | 45 | 27.212 | 24 | | 38.643 | 36 | |
| Age (years) |
| ≤60 | 37 | 34.481 | 24 | 0.403 | 42.625 | 44 | 0.684 |
| >60 | 49 | 31.992 | 28 | | 41.876 | 36 | |
| TNM stage |
| I–II | 28 | 41.107 | - |
0.008b | 46.282 | - |
0.011a |
| III–IV | 58 | 29.453 | 24 | | 40.637 | 36 | |
|
Differentiation |
| G1 | 25 | 37.189 | 48 | 0.393 | 44.284 | 52 | 0.585 |
| G2 | 23 | 33.853 | 32 | | 42.383 | 52 | |
| G3 | 38 | 28.616 | 24 | | 40.566 | 36 | |
| Lymph node
metastasis |
| Yes | 48 | 27.738 | 18 |
0.017a | 38.914 | 36 |
0.049a |
| No | 38 | 38.980 | 48 | | 46.440 | 48 | |
| Residual tumor size
(cm) |
| ≤1 | 49 | 37.442 | 44 | 0.055 | 43.980 | 40 |
0.045a |
| >1 | 37 | 29.516 | 24 | | 40.243 | 36 | |
Inhibition of USP22 suppresses the
proliferation and tumorigenesis of ovarian cancer cells in vitro
and in vivo
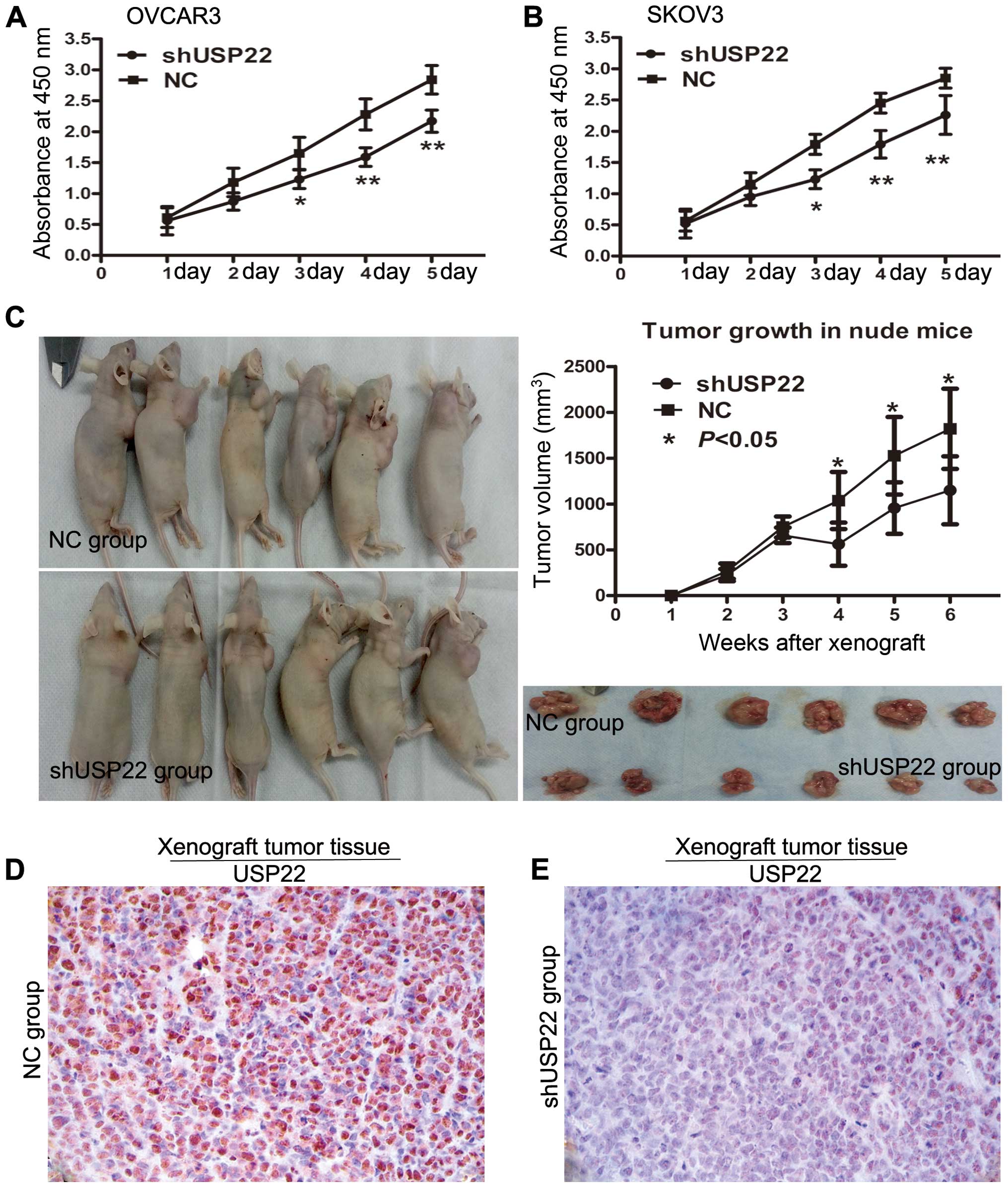
We transfected the ovarian cancer cell lines SKOV3
and OVCAR3 with the USP22 lentivirus encoding shRNA to knock down
USP22, and examined the effects on cellular proliferation. CCK-8
assays revealed that depletion of USP22 significantly decreased the
growth rate of both ovarian cancer cell lines, compared to the
negative control transfected empty vector cells (Fig. 3A and B).
The effect of USP22 on cell growth was further
confirmed by in vivo assay in xenografts. As shown in
Fig. 3C, the tumors in the
OVCAR3/NC (empty vector control) group grew more rapidly than the
tumors in the USP22-deficient OVCAR3/shUSP22 group. Significant
differences in average tumor size were observed on day 28 and at
the end of the observation post injection (tumor volume on day 35,
0.955 vs. 1.526 mm3, P=0.021; tumor volume on day 42,
1.149 vs. 1.820 mm3, P=0.017; Fig. 3C). Immunohistochemistry staining
confirmed that the tumors of the USP22-deficient OVCAR3/shUSP22
group displayed much lower USP22 levels than the tumors from the NC
control group (Fig. 3D and E).
Collectively, both the in vitro and in
vivo studies suggest that USP22 promotes EOC progression by
promoting cellular proliferation and tumor growth.
Inhibition of USP22 suppresses ovarian
cell proliferation by inducing G1 phase cell cycle arrest
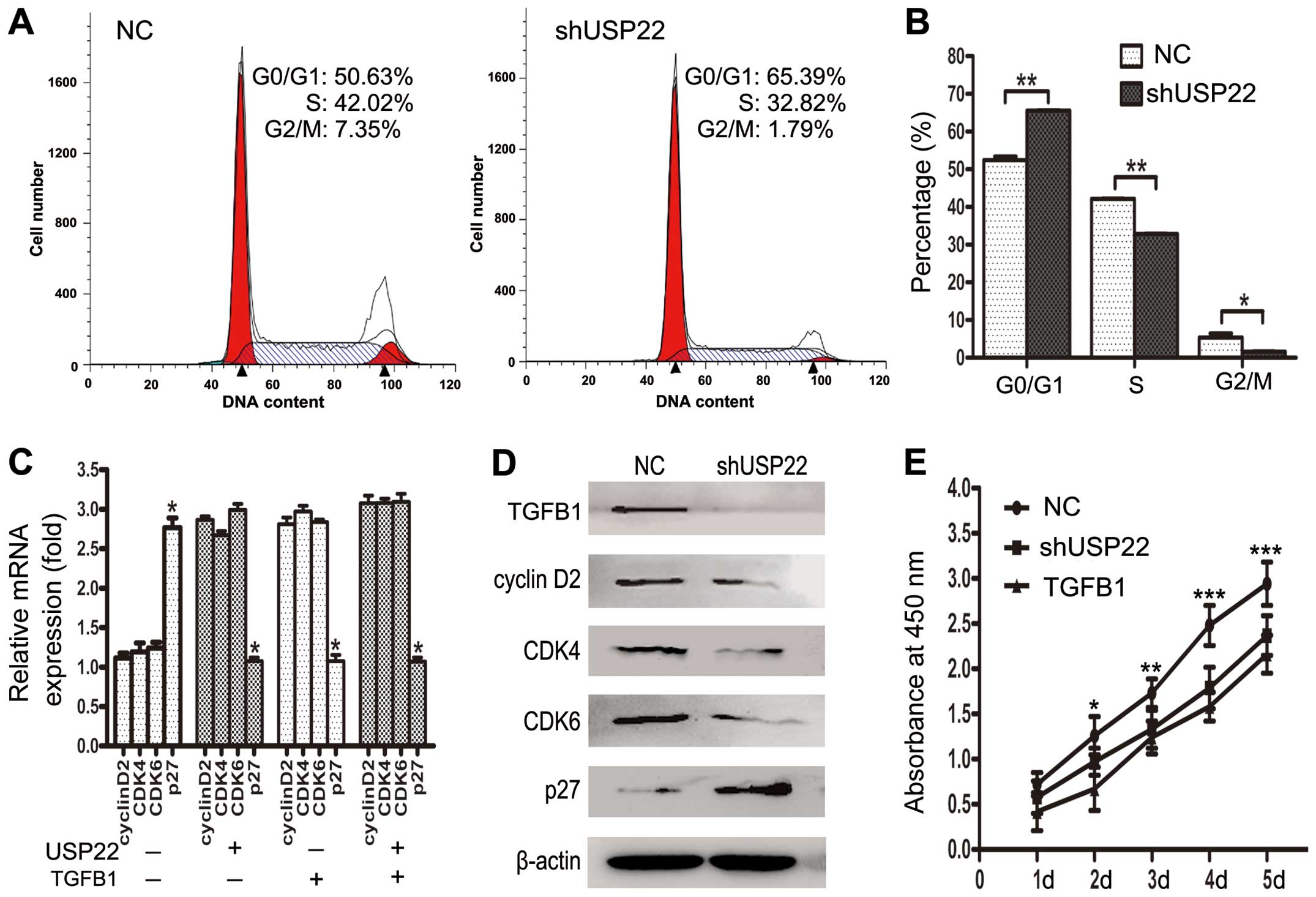
USP22 is known to play a crucial role in cell cycle
regulation (8). We further verified
the cell cycle distribution of the USP22-deficient OVCAR3 cells by
flow cytometry, to explore the possible mechanism by which USP22
regulates ovarian cancer cell proliferation. Flow cytometric
analysis of cells transfected with USP22 shRNA showed a consistent
significant decrease in the percentage of cells in the S phase and
an increase in the G1 phase compared with the negative control
cells (Fig. 4A and B). These
results indicate that depletion of USP22 results in G1 phase cell
cycle arrest in ovarian cancer cells.
TGFB1 is known as a multifunctional growth factor,
which participates in the regulation of cell proliferation,
differentiation, cell cycle arrest and other functions in many cell
types (15–17). Many cells have TGFB1 receptors
(18), and TGFB1 is frequently
upregulated in ovarian cancer cells (19,20).
Thus, we hypothesized that USP22 may synergize TGFB1 to promote
tumor cell growth via regulating TGFB1 downstream cell cycle
signaling pathways. We determined the mRNA levels of several TGFB1
target genes that are involved in cell cycle signaling in OVCAR3
cells by qPCR and western blotting including cyclin D2,
cyclin-dependent kinase 4 and 6 (CDK4 and CDK6), and cell cycle
inhibitor p27kip1. We found that the levels of cyclin D2, CDK4,
CDK6 were decreased by >1.5-fold, whereas the levels of tumor
suppressor p27kip1 were increased at least 2-fold after depletion
of USP22 or TGFB1 expression (Fig. 4C
and D). We also found that the protein levels of TGFB1 were
decreased after depletion of USP22 expression (Fig. 4D). In addition, CCK-8 assays
indicated that depletion of TGFB1 significantly reduced the growth
rate of OVCAR3 cells (Fig. 4E).
These results suggest that USP22 may accelerate ovarian cancer cell
cycle progression via synergizing with TGFB1 to regulate the TGFB1
downstream cell cycle pathway, which in turn, promotes ovarian
cancer cell proliferation.
Discussion
Deubiquitination is an important process for
numerous cellular mechanisms (21,22),
and the imbalance of this process can cause severe diseases
including cancer (23,24). USP22, a novel deubiquitinating
enzyme, is involved in the regulation of numerous pathological
processes. Based on in vitro and in vivo evidence, we
propose that USP22 is capable of suppressing EOC cell proliferation
and tumor growth, and overexpression of USP22 may facilitate EOC
progression.
Recently, increasing experimental and clinical
observations reveal that USP22 plays a crucial role in tumor
progression (25) and influences
clinical prognosis of several human malignancies (26). In human invasive breast cancer,
elevated expression of USP22 was found to be positively related to
lymph node metastasis and patient recurrence (11). In human non-small cell lung cancer,
positive expression of USP22 is significantly correlated to tumor
stage and poor overall survival (9). In human colorectal cancer, increased
mRNA expression of USP22 was associated with advanced tumor stage
and the high likelihood of therapy failure after radical resection
(27). In esophageal squamous cell
carcinoma, USP22 is an independent prognosticator for unfavorable
disease-specific survival (28). In
oral squamous cell carcinoma, USP22 is associated with recurrence
and prognosis (10). However, to
our knowledge, the detailed mechanism surrounding any role that
USP22 may play in EOC development has not been reported
previously.
In the present study, we identified that the
expression level of USP22 was significantly overexpressed in EOC
tissues compared to normal ovarian tissues. Moreover, increased
USP22 expression was associated with advanced clinical FIGO stage
and lymph node metastasis. These findings strongly suggest that
USP22 activation may play an oncogenic role in promoting tumor
progression of EOC. To further validate the potential clinical
utility of USP22, we evaluated the prognostic value of USP22 in EOC
patients. Univariate analysis revealed that cancer patients with
USP22 overexpression had a significantly worse RFS and OS after
radical surgery, compared with the patients in the low expression
group. However, multivariate analysis showed that the expression
level of USP22 was not an independent prognostic factor in EOC
patients. The lack of large-scale clinical samples may have
influenced the results. Further studies are needed to expand the
large-scale study to evaluate the prognostic impact of USP22
expression in ovarian cancer patients.
Previous studies have demonstrated that USP22 is
involved in the regulation of tumor growth and cell cycle
progression. USP22 is a dedicated subunit of the hSAGA complex, and
is required for the transcription of target genes regulated by the
c-Myc oncoprotein (8). c-Myc is a
downstream target gene of the TGFB1 signaling pathway. TGFB1
encodes the transforming growth factor β family of cytokines, which
are multifunctional peptides that regulate proliferation,
differentiation, migration, cell cycle and other functions in many
cell types (16–18). Many cells have TGFB1 receptors, and
the protein positively and negatively regulates many other growth
factors (29–31). We found that depletion of USP22
could inhibit cell growth, induce G1 cell cycle arrest in ovarian
cancer cells, and suppress tumorigenesis in a nude mouse model of
EOC xenografts. We observed a weak but significant correlation
between high TGFB1 and high USP22 expression in tumor cells. We
also examined the expression of a panel of cell cycle regulators on
TGFB1 downstream pathways. We found that the expression of cyclin
D2, CDK4, CDK6 and p27kip1 was indirectly regulated by USP22
dependent on TGFB1. Depletion of TGFB1 significantly reduced the
growth rate of ovarian cancer cells indicating that a similar
connection may be present in the cancer cells. USP22 may stimulate
cell proliferation and cell cycle through TGFB1 release from tumor
cells. Supporting our findings, USP22 was also found to influence
the cell cycle of human colorectal cancer cell line HCT116a by
downregulation of MUP expression (14). In human bladder cancer cell line EJ
(32), knockdown of USP22
expression by siRNA downregulated the expression of Mdm2 and cyclin
E, resulting in the upregulated expression of p53 and p21 leading
to cell cycle arrest and inhibition of cell proliferation.
The present study, together with the findinds from
other groups, has revealed a mechanism involving the regulation of
cell proliferation and tumor growth by USP22 through synergy with
the oncoprotein TGFB1. Further study is warranted to confirm that
this novel deubiquitinating enzyme has clinical implication as an
individualized treatment strategy for EOC patients.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81202070), and the Basic and
Advanced Technology Research Foundation from the Science and
Technology Department of Henan Province (grant no.
112102310105).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Trimbos JB, Vergote I, Bolis G, et al:
Impact of adjuvant chemotherapy and surgical staging in early-stage
ovarian carcinoma: European Organisation for Research and Treatment
of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl
Cancer Inst. 95:113–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurman RJ and Shih IeM: Molecular
pathogenesis and extraovarian origin of epithelial ovarian cancer -
shifting the paradigm. Hum Pathol. 42:918–931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hess LM, Rong N, Monahan PO, Gupta P,
Thomaskutty C and Matei D: Continued chemotherapy after complete
response to primary therapy among women with advanced ovarian
cancer: a meta-analysis. Cancer. 116:5251–5260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Z, Yang H, Kong Q, et al: USP22
antagonizes p53 transcriptional activation by deubiquitinating
Sirt1 to suppress cell apoptosis and is required for mouse
embryonic development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YL, Jiang SX, Yang YM, Xu H, Liu JL
and Wang XS: USP22 acts as an oncogene by the activation of
BMI-1-mediated INK4a/ARF pathway and Akt pathway. Cell Biochem
Biophys. 62:229–235. 2012. View Article : Google Scholar
|
|
8
|
Zhang XY, Varthi M, Sykes SM, et al: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu J, Liu YL, Piao SL, Yang DD, Yang YM
and Cai L: Expression patterns of USP22 and potential targets
BMI-1, PTEN, p-AKT in non-small-cell lung cancer. Lung Cancer.
77:593–599. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piao S, Liu Y, Hu J, et al: USP22 is
useful as a novel molecular marker for predicting disease
progression and patient prognosis of oral squamous cell carcinoma.
PLoS One. 7:e425402012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Yao L, Zhang X, et al: Elevated
expression of USP22 in correlation with poor prognosis in patients
with invasive breast cancer. J Cancer Res Clin Oncol.
137:1245–1253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piao S, Ma J, Wang W, et al: Increased
expression of USP22 is associated with disease progression and
patient prognosis of salivary duct carcinoma. Oral Oncol.
49:796–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang DD, Cui BB, Sun LY, et al: The
co-expression of USP22 and BMI-1 may promote cancer progression and
predict therapy failure in gastric carcinoma. Cell Biochem Biophys.
61:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu H, Liu YL, Yang YM and Dong XS:
Knock-down of ubiquitin-specific protease 22 by micro-RNA
interference inhibits colorectal cancer growth. Int J Colorectal
Dis. 27:21–30. 2012. View Article : Google Scholar
|
|
15
|
Tian M and Schiemann WP: The TGF-β paradox
in human cancer: an update. Future Oncol. 5:259–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bierie B and Moses HL: Tumour
microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wenner CE and Yan S: Biphasic role of
TGF-β1 in signal transduction and crosstalk. J Cell Physiol.
196:42–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Principe DR, Doll JA, Bauer J, et al:
TGF-β: duality of function between tumor prevention and
carcinogenesis. J Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar
|
|
19
|
Cho MS, Bottsford-Miller J, Vasquez HG, et
al: Platelets increase the proliferation of ovarian cancer cells.
Blood. 120:4869–4872. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai J, Tang H, Xu L, et al: Fibroblasts in
omentum activated by tumor cells promote ovarian cancer growth,
adhesion and invasiveness. Carcinogenesis. 33:20–29. 2012.
View Article : Google Scholar
|
|
21
|
Yang Y, Kitagaki J, Wang H, Hou DX and
Perantoni AO: Targeting the ubiquitin-proteasome system for cancer
therapy. Cancer Sci. 100:24–28. 2009. View Article : Google Scholar :
|
|
22
|
Song L and Rape M: Reverse the curse - the
role of deubiquitination in cell cycle control. Curr Opin Cell
Biol. 20:156–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen M, Schmitt S, Buac D and Dou QP:
Targeting the ubiquitin-proteasome system for cancer therapy.
Expert Opin Ther Targets. 17:1091–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hussain S, Zhang Y and Galardy PJ: DUBs
and cancer: the role of deubiquitinating enzymes as oncogenes,
non-oncogenes and tumor suppressors. Cell Cycle. 8:1688–1697. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kapoor S: Usp22 and its evolving role in
systemic carcinogenesis. Lung Cancer. 79:1912013. View Article : Google Scholar
|
|
26
|
Schrecengost RS, Dean JL, Goodwin JF, et
al: USP22 regulates oncogenic signaling pathways to drive lethal
cancer progression. Cancer Res. 74:272–286. 2014. View Article : Google Scholar
|
|
27
|
Liu YL, Yang YM, Xu H and Dong XS:
Increased expression of ubiquitin-specific protease 22 can promote
cancer progression and predict therapy failure in human colorectal
cancer. J Gastroenterol Hepatol. 25:1800–1805. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Wang Z and Li Y: USP22 nuclear
expression is significantly associated with progression and
unfavorable clinical outcome in human esophageal squamous cell
carcinoma. J Cancer Res Clin Oncol. 138:1291–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudla B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar
|
|
30
|
Moses H and Barcellos-Hoff MH: TGF-β
biology in mammary development and breast cancer. Cold Spring Harb
Perspect Biol. 3:a0032772011. View Article : Google Scholar
|
|
31
|
Anscher MS: Targeting the TGF-β1 pathway
to prevent normal tissue injury after cancer therapy. Oncologist.
15:350–359. 2010. View Article : Google Scholar
|
|
32
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar
|