Introduction
Lung cancer is the most common human cancer with a
dismal prognosis, and it ranks highly in terms of cancer-related
mortality worldwide (1,2). In China, lung cancer has replaced
liver cancer as the leading cause of cancer-related deaths
(3,4). The two major types of lung cancers are
small cell lung cancer (SCLC) accounting for ~15% of primary lung
cancers and non-small cell lung cancer (NSCLC), of which the most
common subtypes are adenocarcinoma (ADC) and squamous cell
carcinoma (SCC). As generally known, lung cancer, similar to many
other cancers, arises as a result of the accumulation of genetic
and epigenetic alterations, many of which ultimately confer growth
and metastatic advantages upon the tumor cells (5,6). The
identification of genetic abnormalities of numerous key oncogenes
and tumor suppressors, such as EGFR mutations, KRAS
mutations and p53 inactivated mutations, have improved our
understanding of the molecular mechanisms involved in lung cancer
development. Moreover, rapid technological developments in genome
sequencing offer a more comprehensive and sophisticated genomic
landscape of lung cancers. Among the somatic mutations recently
identified in tumor specimens, somatic mutations in genes encoding
chromatin remodeling proteins have frequently been identified in a
variety of human cancers, including lung cancer (7,8).
Importantly, somatic mutations have been frequently detected in
genes encoding the subunits of the switch/sucrose non-fermenting
(SWI/SNF) complex in various cancers (7,9).
Inactivation mutations in several SWI/SNF subunits, such as
BRG1/SMARCA4, ARID1A and ARID2, have recently been
identified in a significant proportion of lung tumors (10–12),
indicating that SWI/SNF complexes represent a novel link between
chromatin remodeling and tumor suppression in lung cancer.
SWI/SNF chromatin remodeling complexes, which use
the energy of ATP hydrolysis to remodel nucleosomes and to mediate
transcription, play critical roles in various biological processes
including proliferation, differentiation and DNA repair (7,9).
Mammalian SWI/SNF (mSWI/SNF, also called BAF) complexes are
polymorphic assemblies of at least 13 subunits encoded by 26 genes,
making a widespread diversity of complexes with specialized
function in specific tissues (13,14).
BAF complexes, similar to known tumor-suppressor TP53, are
the most frequently and broadly mutated genes in human cancer
(14).
To date, the genomic landscape of lung cancer which
has been captured in several whole genome sequencing (WGS) projects
has contributed to the comprehensive understanding of the genetic
pathogenesis of human lung cancer. There is a large amount of
information available in the published literature concerning
genetic variants in human lung cancer. The Catalogue of Somatic
Mutations in Cancer (COSMIC) database stores somatic mutations and
associated information from human cancers extracted from the
primary literature (15,16). In the present study, we sought to
determine the role of BAF complex mutations in lung cancer through
analysis of the mutation incidence and evaluation of the mutation
rate in an established mutation reporter of lung cancer cells. We
utilized the COSMIC database to acquire the mutations of the 26
genes encoding BAF complexes in available tissue specimens and cell
lines of lung cancer. Notably, our analysis showed that BAF
complexes were mutated in 282 of 803 (35.12%) tumor samples
included, ranking second to TP53, and could contribute to
the role of genome stability or DNA repair in lung cancer.
Materials and methods
Mutation profile analysis
The mutation information including mutations of the
26 BAF genes and the top-10 frequently mutated genes in human lung
carcinoma was obtained from the COSMIC (http://www.sanger.ac.uk/cosmic) database (15,16).
Each type of simple mutation (point mutation, small insertion and
deletion) was represented by a specific mutation value, ‘0’,
without somatic mutation; ‘1’, synonymous mutation; ‘2’, missense
mutation; ‘3’, nonsense or frameshift mutation. The accumulated
mutations of the 26 BAF genes in several lung carcinoma samples
were then indicated by a defined BAF mutation index (BMI), equal to
the sum of the mutation values from the 26 BAF genes.
Construction of the slippage-luciferase
vector
For the construction of the slippage-luciferase
(slippage-Luc) vector, the luciferase-coding sequence with a
mutated initiation codon (ATG to CTG) was first PCR-amplified from
the pGL3 vector (Promega), and cloned into the BamHI and
HindIII site of the pcDNA3.1/Hygro vector (Invitrogen). An
ATG initiation codon followed with the oligonucleotides containing
CA repeats leads to out of frame luciferase expression and was
cloned into the XhoI and BamHI site of the
recombinant pcDNA3.1/Hygro-Luc construct. The oligonucleotides
containing CA repeats were synthesized as follows:
XhoI-ATG-(CA)17
(5′-TCGAGCGCCATGGAA(CA)17G-3′) and
BamHI-(CA)17
(5′-GATCC(TG)17TTCCATGGCGC-3′). The annealed
oligonucleotides were then ligated into the XhoI and
BamHI site of the modified pcDNA3.1/Hygro-Luc construct to
acquire the slippage-Luc vector.
Establishment of the mutation
reporter
The slippage-Luc vector was then linearized by
BglII digestion and transfected into Calu-3 cells using the
Lipofectamine transfection reagent (Invitrogen) according to the
manufacturer’s instructions. After 48 h, the transfected cells were
subsequently exposed to selection in medium containing 500 μg/ml
Hygromycin (Clontech). The cells were fed with selective medium
every 3–4 days until hygromycin-resistant colonies could be
visible. Expanded colonies stably expressing the slip-luciferase
transcripts were assessed by PCR.
pSUPER constructs for RNA interference
against SMARCA4
For SMARCA4 knockdown, 4 fragments of sequences
targeting against SMARCA4 were as follows: GCACCAGGAATACCTCAAT,
GGTCAATGGTGTCCTCAAA, GGAGCACAAACGCATCAAT and GGCTCTGAGTACTTCATCT,
and their corresponding oligonucleotides for transcribing short
hairpin RNA (shRNA) were synthesized. Then these annealed
phosphorylated oligos were inserted into pSUPER carrying the
neomycin-resistance gene, respectively; pSUPER carrying an
irrelevant nucleotide not matching any human gene sequences was
used as a negative control (pSUPER-shNC). RNAi knockdown efficiency
of these pSUPER constructs against SMARCA4 were evaluated 3
days after transfection in the Calu-3 cell sublines. Stable SMARCA4
knockdown was identified from double stable colonies with
hygromycin- and G418-resistance of Calu-3 cells.
Extraction of DNA and RNA
Genomic DNA was extracted from the stable cell lines
using the DNeasy Tissue kit (Qiagen), and RNA was extracted using
TRIzol® reagent (Invitrogen) according to the protocols
recommended by the manufacturer, respectively. The total RNA was
treated by RNase-free DNase I (Fermentas) to remove DNA
contamination.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The first-stand cDNA was transcribed by
PrimerScript™ RT reagent kit with gDNA Eraser (Takara). Amplified
PCR products were observed by electrophoresis on a 2% agarose gel
and visualized after staining with ethidium bromide. Real-time
RT-PCR was performed using a CFX96 system (BioRad), and relative
expression levels were calculated as 2−ΔΔCt according to
the manufacturer’s protocol. All experiments were performed in
triplicate.
SDS-PAGE and western blot assay
Total protein extracts of stable cell lines were
subjected to protein gel electrophoresis using 8% SDS-PAGE and were
then transferred onto a nitrocellulose membrane (Amersham). After
blocking with phosphate-buffered saline (PBS) containing 5% nonfat
milk, the membrane was incubated for immunoblotting assay with the
SMARCA4 antibody (1:200 dilution, Santa Cruz) at 4°C overnight. The
immunostaining signal was visualized with the Odyssey infrared
imaging system (Li-Cor Biosciences).
Quantitative analysis for mutation
rates
Luciferase activity was measured using the
Luciferase reporter assay system (Promega) according to the
instructions of the manufacturer. The experiments were performed at
least 6 times, and the data were subjected to Luria-Delbrück
fluctuation analyses (17,18).
Statistical analysis
The two-tailed t-test was used to compare mutated
genes or simple mutations, and the statistical difference between
mutation rates was determined by the Chi-square test. Pearson
correlation analysis was used for investigating the relationship
between BAF mutations and overall mutations in lung cancer samples.
The statistical analyses were performed using SPSS software. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Mutation analysis of BAF complexes in
lung carcinoma
The 26 genes encoding mammalian SWI/SNF subunits are
listed in Table I. Based on the
data from Sanger COSMIC, we first investigated the mutation type
and prevalence of these BAF genes in human lung carcinoma samples.
All BAF genes except SMARCE1 were mutated to varying degrees
(ranging 0.23–7.94%) in the lung carcinoma samples analyzed
(Table II). Of 803 lung carcinoma
samples covering all the 26 BAF genes, 282 (35.12%) exhibited at
least 1 mutated BAF gene, and the BAF-mutated frequency indicated
no significant difference between various histological types of
lung cancer (P=0.093; Table III).
These data demonstrated that BAF complexes were frequently mutated,
ranking second to TP53, and played tumor-suppressive roles
in lung cancer. To integrate more related information of the lung
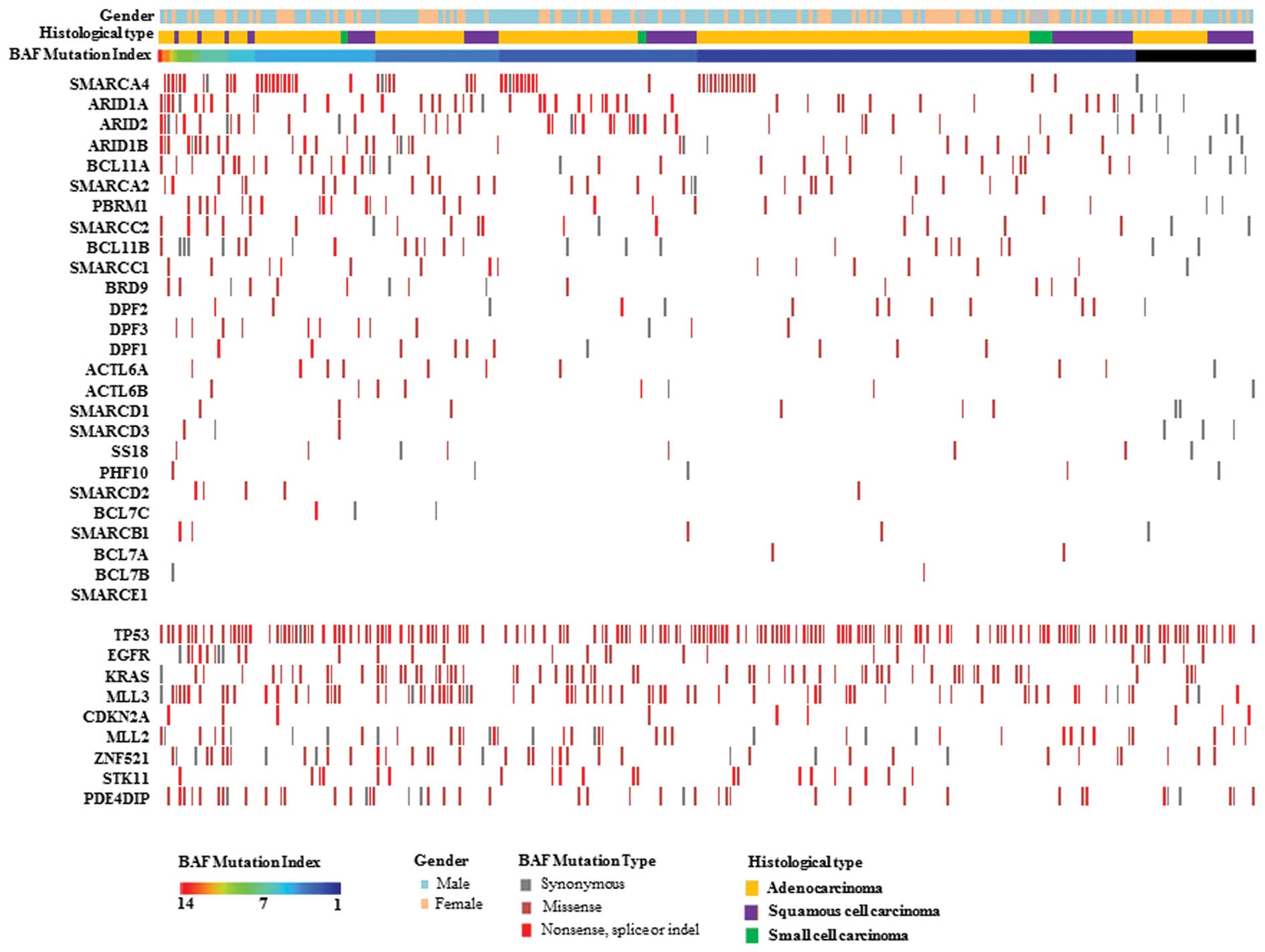
cancer samples, a mutation profile of BAF complexes was described
in these BAF-mutated lung carcinoma samples, where BAF mutation
index (BMI), gender, histological types and genotyping of driver
genes of lung cancer are shown (Fig.
1). In addition, we also observed mutation ratios of the
acknowledged driver genes with frequent mutations in lung cancer,
including TP53, EGFR, KRAS, MLL3, MLL2, ZNF521, NF1, PDE4DIP,
CDKN2A and STK11 (Table
III). Similar to BAF, the mutation frequencies of
MLL3, ZNF521, NF1 and PDE4DIP had no significant
differences between the 3 histological types of lung carcinoma,
implying that these tumor suppressors play a triggering role in the
origin of tumor cells, regardless of histological types of lung
cancer.
 | Table IMutation frequencies of driver
mutated genes in various types of lung carcinoma. |
Table I
Mutation frequencies of driver
mutated genes in various types of lung carcinoma.
| Frequency of
mutation, mutated samples/total samples (%) | |
|---|
|
| |
|---|
| | Histological
type | |
|---|
| |
| |
|---|
| Mutated genes | Lung carcinoma | ADC | SCC | SCLC | P-valuea |
|---|
| BAF | 282/803
(35.12) | 206/582
(35.40) | 67/177 (37.85) | 9/44 (20.45) | 0.093 |
| TP53 | 1,423/3,142
(45.29) | 715/1,858
(38.48) | 530/1,040
(50.96) | 178/244
(72.95) | <0.001 |
| EGFR | 9,478/30,658
(30.92) | 9,236/26,502
(34.85) | 204/3,622
(5.63) | 38/534 (7.12) | <0.001 |
| KRAS | 2,254/13,545
(16.64) | 2,131/10,778
(19.77) | 116/2,290
(5.07) | 7/477 (1.47) | <0.001 |
| MLL3 | 133/848
(15.68) | 96/609 (15.76) | 33/180 (18.33) | 4/59 (6.78) | 0.106 |
| MLL2 | 85/846 (10.05) | 44/609 (7.22) | 35/180 (19.44) | 6/57 (10.53) | <0.001 |
| ZNF521 | 83/851 (9.75) | 59/609 (9.69) | 14/180 (7.78) | 10/62 (16.13) | 0.160 |
| NF1 | 110/1,208
(9.11) | 78/855 (9.12) | 29/293 (9.90) | 3/60 (5.00) | 0.486 |
| PDE4DIP | 82/969 (8.46) | 48/666 (7.21) | 29/243 (11.93) | 5/60 (8.33) | 0.077 |
| CDKN2A | 188/2,253
(8.34) | 112/1,411
(7.94) | 70/582 (12.03) | 6/260 (2.31) | <0.001 |
| STK11 | 210/2,670
(7.87) | 191/1,964
(9.73) | 19/629 (3.02) | 0/77 (0.00) | <0.001 |
 | Table IINumber of mutations of driver mutated
genes in lung carcinoma with mutated-type and wild-type BAF. |
Table II
Number of mutations of driver mutated
genes in lung carcinoma with mutated-type and wild-type BAF.
| Mutated genes |
No. of
mutated samples |
|---|
|
|---|
| Lung carcinoma,
n=800 | P-valuea | Histological
type |
|---|
|
|---|
| ADC, n=582 | P-valuea | SCC, n=177 | P-valuea | SCLC, n=41 | P-valuea |
|---|
|
|
|
|
|---|
BAF
MT | BAF
WT | BAF
MT | BAF
WT | BAF
MT | BAF
WT | BAF
MT | BAF
WT |
|---|
| BAF | 282 | 0 | - | 206 | 0 | - | 67 | 0 | - | 9 | 0 | - |
| TP53 | 161 | 212 | <0.001 | 124 | 132 | <0.001 | 32 | 51 | 0.857 | 5 | 29 | 0.014 |
| EGFR | 29 | 66 | 0.304 | 26 | 62 | 0.213 | 3 | 4 | 0.781 | 0 | 0 | - |
| KRAS | 65 | 97 | 0.146 | 64 | 95 | 0.133 | 1 | 1 | 0.722 | 0 | 1 | 0.591 |
| MLL3 | 74 | 56 | <0.001 | 54 | 41 | <0.001 | 18 | 15 | 0.028 | 2 | 0 | 0.006 |
| MLL2 | 41 | 36 | 0.001 | 24 | 17 | 0.001 | 17 | 18 | 0.144 | 0 | 1 | 0.591 |
| ZNF521 | 41 | 37 | 0.001 | 32 | 27 | 0.001 | 8 | 6 | 0.121 | 1 | 4 | 0.910 |
| PDE4DIP | 45 | 36 | <0.001 | 29 | 19 | <0.001 | 16 | 13 | 0.036 | 0 | 4 | 0.264 |
| CDKN2A | 9 | 12 | 0.460 | 6 | 11 | 0.993 | 3 | 1 | 0.121 | 0 | 0 | - |
| STK11 | 20 | 41 | 0.675 | 20 | 40 | 0.124 | 0 | 1 | 0.434 | 0 | 0 | - |
 | Table IIIMutation frequencies of the driver
mutated genes in various types of lung carcinoma. |
Table III
Mutation frequencies of the driver
mutated genes in various types of lung carcinoma.
| Frequency of
mutation, mutated samples/total samples (%) | |
|---|
|
| |
|---|
| | Histological
type | |
|---|
| |
| |
|---|
| Mutated genes | Lung carcinoma | ADC | SCC | SCLC | P-valuea |
|---|
| BAF | 282/803
(35.12) | 206/582
(35.40) | 67/177 (37.85) | 9/44 (20.45) | 0.093 |
| TP53 | 1,423/3,142
(45.29) | 715/1,858
(38.48) | 530/1,040
(50.96) | 178/244
(72.95) | <0.001 |
| EGFR | 9,478/30,658
(30.92) | 9,236/26,502
(34.85) | 204/3,622
(5.63) | 38/534 (7.12) | <0.001 |
| KRAS | 2,254/13,545
(16.64) | 2,131/10,778
(19.77) | 116/2,290
(5.07) | 7/477 (1.47) | <0.001 |
| MLL3 | 133/848
(15.68) | 96/609 (15.76) | 33/180 (18.33) | 4/59 (6.78) | 0.106 |
| MLL2 | 85/846 (10.05) | 44/609 (7.22) | 35/180 (19.44) | 6/57 (10.53) | <0.001 |
| ZNF521 | 83/851 (9.75) | 59/609 (9.69) | 14/180 (7.78) | 10/62 (16.13) | 0.160 |
| NF1 | 110/1,208
(9.11) | 78/855 (9.12) | 29/293 (9.90) | 3/60 (5.00) | 0.486 |
| PDE4DIP | 82/969 (8.46) | 48/666 (7.21) | 29/243 (11.93) | 5/60 (8.33) | 0.077 |
| CDKN2A | 188/2,253
(8.34) | 112/1,411
(7.94) | 70/582 (12.03) | 6/260 (2.31) | <0.001 |
| STK11 | 210/2,670
(7.87) | 191/1,964
(9.73) | 19/629 (3.02) | 0/77 (0.00) | <0.001 |
Relationship between BAF complex
mutations and genome instability of the lung cancer samples
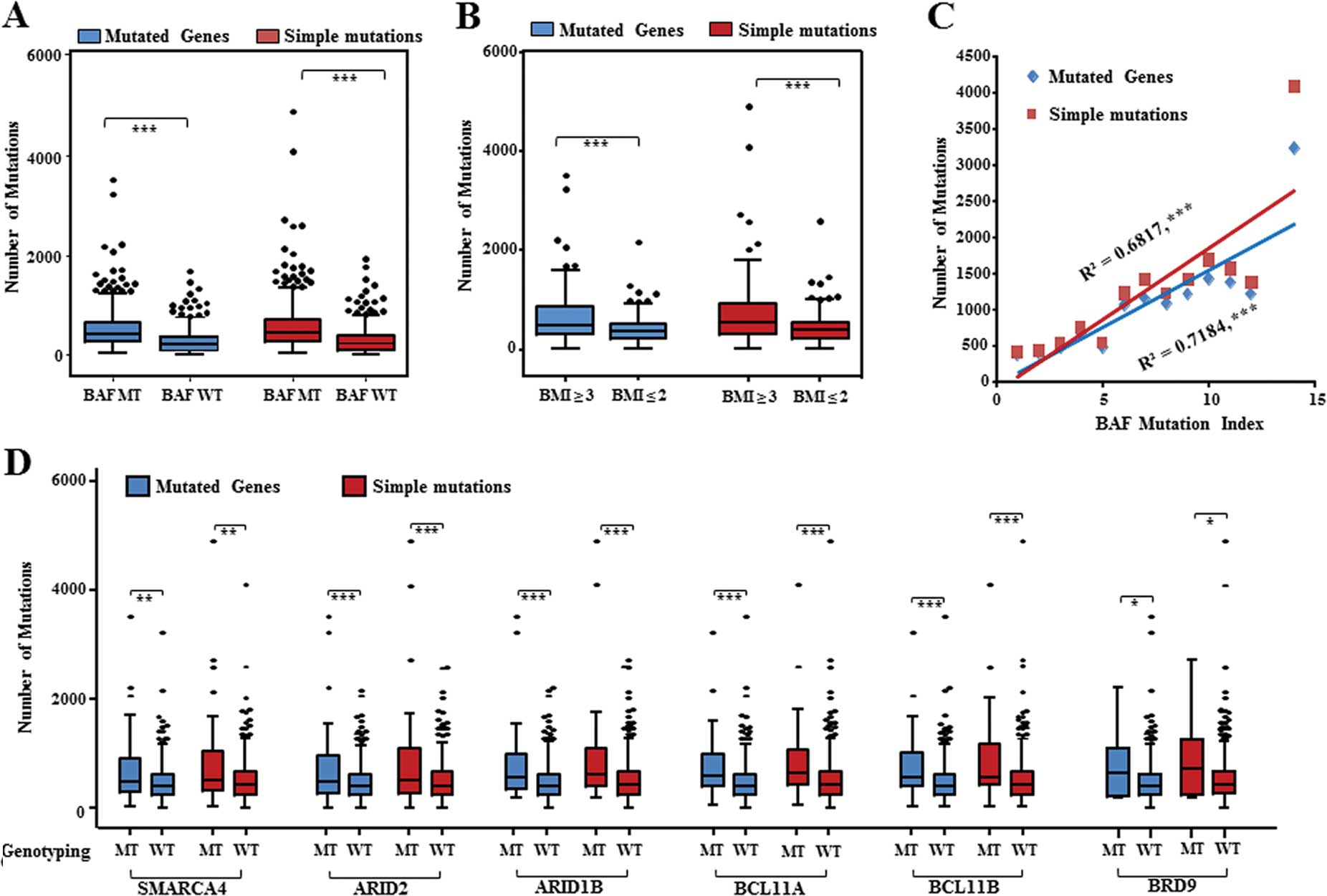
We next analyzed the numbers of mutated genes and
simple mutations occurring in the lung carcinoma samples. Among the
803 lung carcinoma samples analyzed above, 282 BAF-mutated samples
significantly exhibited more mutated genes and simple mutations
than the BAF wild-type ones (Fig.
2A), which suggests that inactivated mutations of the BAF
complexes were associated with genome instability of lung
carcinoma. Moreover, we analyzed mutation frequencies of the
acknowledged driver mutated genes in these lung carcinoma samples
with a mutated-type and wild-type BAF genotype. We analyzed the
available genotyping information of the top-10 driver mutated genes
from the 803 lung carcinoma samples, 800 of which covering 9 driver
mutated genes (no coverage with NF1) were available for further
analysis. Significantly, TP53, MLL3, MLL2, ZNF521 and
PDE4DIP were more often mutated in the BAF-mutated samples
than the BAF wild-type ones (Table
IV). According to BAF-mutated extents, these BAF-mutated lung
carcinoma samples were categorized into two groups: one group with
BMI ≥3, the other with BMI ≤2. The group with BMI ≥3 had more
significant mutated genes and simple mutations than the other group
with BMI ≤2 (Fig. 2B). To further
establish the relationship between BAF complex mutations and genome
instability, we correlated the BMI with the number of overall
mutations. Pearson correlation analysis showed that a positive
correlation existed between the BMI and the numbers of mutated
genes (R2=0.718, P<0.001) or simple mutations
(R2=0.682, P<0.001) (Fig.
2C). Collectively, these data suggest that the BAF complex
mutations are linked with genomic instability of lung cancer
types.
 | Table IVMutation numbers of driver mutated
genes in lung carcinoma with mutated-type and wild-type BAF. |
Table IV
Mutation numbers of driver mutated
genes in lung carcinoma with mutated-type and wild-type BAF.
| Mutated genes |
No. of
mutated samples |
|---|
|
|---|
| Lung carcinoma,
n=800 | P-valuea | Histological
type |
|---|
|
|---|
| ADC, n=582 | P-valuea | SCC, n=177 | P-valuea | SCLC, n=41 | P-valuea |
|---|
|
|
|
|
|---|
BAF
MT | BAF
WT | BAF
MT | BAF
WT | BAF
MT | BAF
WT | BAF
MT | BAF
WT |
|---|
| BAF | 282 | 0 | - | 206 | 0 | - | 67 | 0 | - | 9 | 0 | - |
| TP53 | 161 | 212 | <0.001 | 124 | 132 | <0.001 | 32 | 51 | 0.857 | 5 | 29 | 0.014 |
| EGFR | 29 | 66 | 0.304 | 26 | 62 | 0.213 | 3 | 4 | 0.781 | 0 | 0 | - |
| KRAS | 65 | 97 | 0.146 | 64 | 95 | 0.133 | 1 | 1 | 0.722 | 0 | 1 | 0.591 |
| MLL3 | 74 | 56 | <0.001 | 54 | 41 | <0.001 | 18 | 15 | 0.028 | 2 | 0 | 0.006 |
| MLL2 | 41 | 36 | 0.001 | 24 | 17 | 0.001 | 17 | 18 | 0.144 | 0 | 1 | 0.591 |
| ZNF521 | 41 | 37 | 0.001 | 32 | 27 | 0.001 | 8 | 6 | 0.121 | 1 | 4 | 0.910 |
| PDE4DIP | 45 | 36 | <0.001 | 29 | 19 | <0.001 | 16 | 13 | 0.036 | 0 | 4 | 0.264 |
| CDKN2A | 9 | 12 | 0.460 | 6 | 11 | 0.993 | 3 | 1 | 0.121 | 0 | 0 | - |
| STK11 | 20 | 41 | 0.675 | 20 | 40 | 0.124 | 0 | 1 | 0.434 | 0 | 0 | - |
Next, we further investigated the overall mutation
differences between the mutated-type and wild-type genotyping of
each individual BAF gene in the lung carcinoma samples,
respectively. More mutated genes or simple mutations significantly
appeared in the lung carcinoma sample with the mutated-type
genotyping of 6 genes, SMARCA4, ARID2, ARID1B, BCL11A,
BCL11B and BRD9 (Fig.
2D). There were no significant differences between the
mutated-type and wild-type genotyping of the other BAF genes in the
lung carcinoma samples (data not shown). Accordingly, the 6 BAF
genes primarily contribute to safeguard genome stability against
mutation occurrence.
Establishment of mutation reporters of
lung cancer cell lines
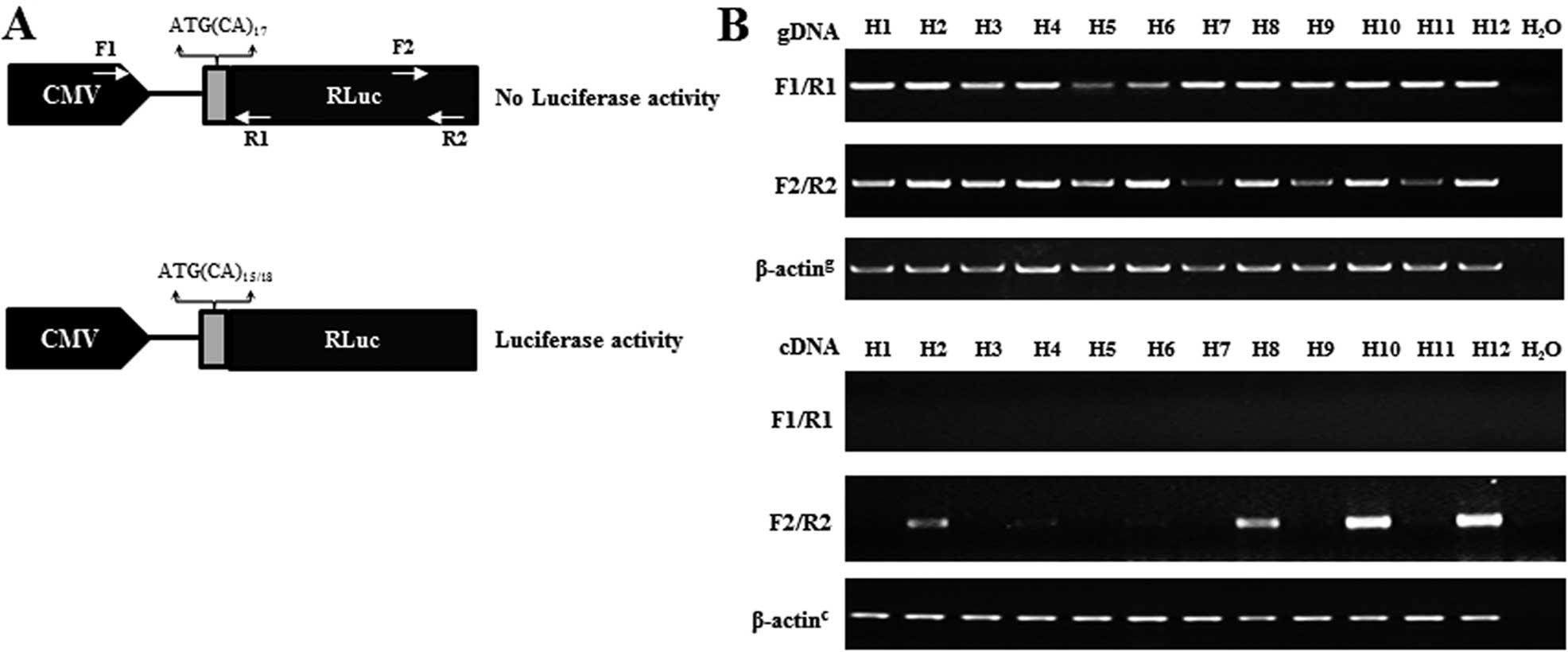
To evaluate the mutation ratio in vitro, we
developed a mutation reporter of lung cancer cells. First, a
slippage vector construct contains 17 CA-dinucleotide repeats
cloned just downstream from the initiation codon of the luciferase
gene, which leads to the luciferase-coding sequence out of frame.
However, genome instability resulting in gains of one or loss of
two CA repeats will restore the reading frame (Fig. 3A). Then the linearized slippage
vector containing the hygromycin resistance gene for selection of
stable cell lines was transfected into Calu-3 cells, a lung
adenocarcinoma cell line. We cultured Calu-3 cells with selective
medium every 3–4 days until hygromycin-resistant colonies were
visible, and the 12 colonies were selected for further
identification. The results showed that both target fragments of
the CMV promoter and Luc element on the slippage vector could be
amplified by designed primers from genomic DNA of the 12 colonies.
To evaluate the transcript levels of the slippage-Luc, the same
primers were used to amplify cDNA of the 12 cell sublines using
RT-PCR. The slippage-Luc transcripts were detected by the primers
for Luc element in 4 of the 12 cell colonies, where 2 colonies
(H10, H12) exhibiting obvious slippage-Luc transcripts were
selected for mutation reporters (Fig.
3B).
SMARCA4 knockdown leads to an increased
mutation ratio
To confirm the role of BAF inactivation in genome
stability in vitro, we selected the most frequently mutated
BAF gene, SMARCA4, for the mutation ratio assay as loss of
function in the established mutation reporters of Calu-3 cells
without SMARCA4 mutations found (data not shown). pSUPER
constructs containing shRNA (sh1, sh2, sh3 and sh4) against SMARCA4
were transfected into the two established Calu-3 cell sublines (H10
and H12), respectively. Three days after transfection, we evaluated
the RNAi knockdown efficiency of these shRNAs, using quantitative
RT-PCR assay. The result showed that the mRNA levels of SMARCA4
were efficiently and significantly decreased to <50% by sh-1 and
sh-3, as compared to shNC (Fig.
4A). Subsequently, we screened G418-resistant colonies to
establish double-stable sublines with SMARCA4 downregulation, based
upon the two Calu-3 cell reporters H10 and H12. Of these
double-stable colonies, 5 colonies (G5, G8, G19, G23 and G24) with
<50% SMARCA4 expression levels compared with their progenitor
cells and 4 colonies (G1, G3, G14 and G15) close to the original
SMARCA4 levels in their progenitor cells were used for mutation
ratio analysis (Fig. 4B).
Furthermore, we performed western blot assays to examine SMARCA4
protein expression in these selected double-stable cells. The
SMARCA4 protein level was markedly decreased in the 5 cell sublines
with stable SMARCA4 knockdown, as compared with the controls with
shNC in the two reporter cell lines (H10 and H12), respectively
(Fig. 4C). These reporter cells
were expanded for 3 weeks, where luciferase activity was measured
and Luria-Delbrück fluctuation analyses were then used to calculate
the mutation rate. Upon the pSUPER-mediated SMARCA4 knockdown, the
mutation ratios were significantly increased in the mutation
reporter cells (Fig. 4D).
Collectively, these observations suggest that SMARCA4 knockdown may
lead to the increased mutations in human lung cancer.
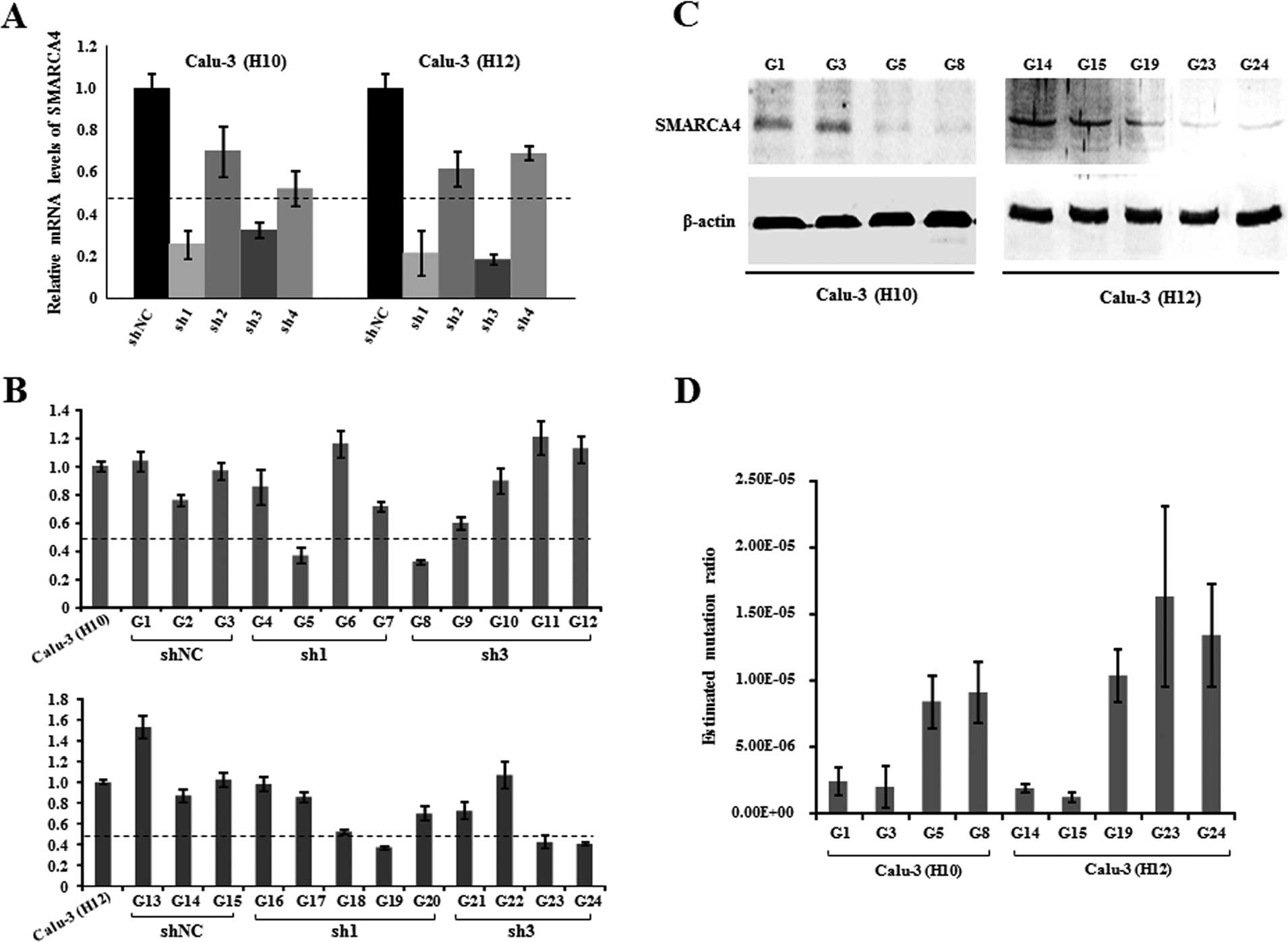 | Figure 4SMARCA4 knockdown leads to increased
mutation incidence. (A) Efficiency of the pSUPER-mediated SMARCA4
knockdown was assessed using real-time PCR in the two Calu-3 cell
sublines, H10 and H12. (B) SMARCA4 expression was evaluated by
real-time PCR in these 24 double-stable Calu-3 cell colonies stably
transfected with pSUPER constructs carrying shNC, sh1 and sh3,
respectively. (C) SMARCA4 protein expression was examined by
western blot assay in these double-stable Calu-3 cell sublines,
where β-actin was used as a loading control. (D) Mutation rates of
reporter cells with stable SMARCA4 knockdown (G5, G8, G19, G23 and
G24) were determined by Luria-Delbrück fluctuation analyses. As
controls, reporter cells were transfected with the pSUPER vector
carrying shNC (G1, G3, G14 and G15). |
Discussion
In the present study, we described the mutation
incidence of SWI/SNF chromatin remodeling genes in human lung
carcinoma, and then explored the genetic features of the
BAF-mutated lung cancer samples. As expected, our analysis showed
that all BAF genes except SMARCE1 were mutated in these lung
carcinoma samples, 35.12% of which exhibited at least 1 mutated BAF
gene, ranking second to TP53 mutation incidence. We found
that TP53, EGFR, KRAS, MLL2, CDKN2A and STK11 had
significant differences in mutation frequency among adenocarcinoma,
squamous cell carcinoma and small cell lung cancer, basically
consistent with previous reports (19–21).
However, it is worth mentioning that the mutation frequency of BAF
complexes had no significant difference between the 3 histological
types of human lung carcinoma, implying that BAF complexes
extensively underwent genetic damage in lung carcinoma, regardless
of histological types. In addition, the TP53 mutation is
associated with smoking-induced lung cancer. Smokers with lung
cancer have a higher risk of TP53 mutation than non-smokers
(22). However, our current data
shed little light on the question of whether SWI/SNF mutations are
associated with smokers in lung cancer.
The SWI/SNF chromatin remodeling complex plays
essential roles in a wide variety of cellular and biological
processes including gene expression, nuclear organization,
centromere function, chromosomal stability, differentiation,
proliferation and DNA repair (23–29).
Nevertheless, how these inactivated mutations in these complexes
contribute to tumor suppression is unclear. Notably, our analysis
revealed that BAF complex mutations were associated with genome
instability in lung carcinoma, which conforms to the biological
roles of the SWI/SNF complex in chromosomal stability and DNA
repair. Among these BAF genes, SMARCA4, ARID2, ARID1B, BCL11A,
BCL11B and BRD9 are possible major contributors to
genome stability and/or DNA repair. To elucidate this issue, we
established a mutation reporter to evaluate the mutation ratio in a
lung carcinoma cell line, Calu-3. SMARCA4, the most
frequently mutated BAF gene in lung carcinoma (8,12,30),
was used from loss of function assay in established mutation
reporter. We confirmed that the SMARCA4 gene protected the
genome against mutation occurrence, upon stable SMARCA4 in
mutation reporter cells of lung carcinoma, consistent with its
requirement during embryogenesis and its role as a tumor suppressor
to maintain genome stability (31).
In conclusion, inactivated mutations of BAF
complexes are significantly associated with more overall mutations
in lung carcinoma, and BAF complexes play an important role in
maintaining the genome stability of lung cancer.
Acknowledgements
The present study was funded by The First Affiliated
Hospital of Soochow University.
Abbreviations:
|
ADC
|
adenocarcinoma
|
|
BMI
|
BAF mutation index
|
|
COSMIC
|
Catalogue of Somatic Mutations in
Cancer
|
|
Luc
|
luciferase
|
|
NSCLC
|
non-small cell lung cancer
|
|
SCLC
|
small cell lung cancer
|
|
shRNA
|
short hairpin RNA
|
|
SCC
|
squamous cell carcinoma
|
|
WGS
|
whole genome sequencing
|
References
|
1
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.(In Japanese).
PubMed/NCBI
|
|
2
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang G, Wang Y, Zeng Y, et al: Rapid
health transition in China, 1990–2010: findings from the Global
Burden of Disease Study 2010. Lancet. 381:1987–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Son JW: Year-in-Review of Lung Cancer.
Tuberculosis and respiratory diseases. 73:137–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agullo-Ortuno MT, Lopez-Rios F and
Paz-Ares L: Lung cancer genomic signatures. J Thorac Oncol.
5:1673–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson BG and Roberts CW: SWI/SNF
nucleosome remodellers and cancer. Nat Rev Cancer. 11:481–492.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oike T, Ogiwara H, Nakano T, Yokota J and
Kohno T: Inactivating mutations in SWI/SNF chromatin remodeling
genes in human cancer. Jpn J Clin Oncol. 43:849–855. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reisman D, Glaros S and Thompson EA: The
SWI/SNF complex and cancer. Oncogene. 28:1653–1668. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manceau G, Letouze E, Guichard C, et al:
Recurrent inactivating mutations of ARID2 in non-small cell lung
carcinoma. Int J Cancer. 132:2217–2221. 2013. View Article : Google Scholar
|
|
11
|
Jones S, Li M, Parsons DW, et al: Somatic
mutations in the chromatin remodeling gene ARID1A occur in several
tumor types. Human Mutat. 33:100–103. 2012. View Article : Google Scholar
|
|
12
|
Rodriguez-Nieto S, Cañada A, Pros E, et
al: Massive parallel DNA pyrosequencing analysis of the tumor
suppressor BRG1/SMARCA4 in lung primary tumors. Hum Mutat.
32:E1999–E2017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ho L, Ronan JL, Wu J, et al: An embryonic
stem cell chromatin remodeling complex, esBAF, is essential for
embryonic stem cell self-renewal and pluripotency. Proc Natl Acad
Sci USA. 106:5181–5186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kadoch C, Hargreaves DC, Hodges C, et al:
Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes
identifies extensive roles in human malignancy. Nat Genet.
45:592–601. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forbes SA, Bindal N, Bamford S, et al:
COSMIC: mining complete cancer genomes in the Catalogue of Somatic
Mutations in Cancer. Nucleic Acids Res. 39:D945–D950. 2011.
View Article : Google Scholar :
|
|
16
|
Bamford S, Dawson E, Forbes S, et al: The
COSMIC (Catalogue of Somatic Mutations in Cancer) database and
website. Br J Cancer. 91:355–358. 2004.PubMed/NCBI
|
|
17
|
Koziol JA: A note on efficient estimation
of mutation rates using Luria-Delbrück fluctuation analysis. Mutat
Res. 249:275–280. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hall BM, Ma CX, Liang P and Singh KK:
Fluctuation analysis calculator: a web tool for the determination
of mutation rate using Luria-Delbruck fluctuation analysis.
Bioinformatics. 25:1564–1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wistuba II, Gazdar AF and Minna JD:
Molecular genetics of small cell lung carcinoma. Semin Oncol.
28:3–13. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi T, Suzuki H, Hida T, Sekido Y,
Ariyoshi Y and Ueda R: The p53 gene is very frequently mutated in
small-cell lung cancer with a distinct nucleotide substitution
pattern. Oncogene. 6:1775–1778. 1991.PubMed/NCBI
|
|
21
|
Dearden S, Stevens J, Wu YL and Blowers D:
Mutation incidence and coincidence in non small-cell lung cancer:
meta-analyses by ethnicity and histology (mutMap). Ann Oncol.
24:2371–2376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Lin XJ, Wang CP, et al: Association
between smoking and p53 mutation in lung cancer: a meta-analysis.
Clin Oncol (R Coll Radiol). 26:18–24. 2014. View Article : Google Scholar
|
|
23
|
de la Serna IL, Carlson KA and Imbalzano
AN: Mammalian SWI/SNF complexes promote MyoD-mediated muscle
differentiation. Nat Genet. 27:187–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watanabe H, Mizutani T, Haraguchi T, et
al: SWI/SNF complex is essential for NRSF-mediated suppression of
neuronal genes in human nonsmall cell lung carcinoma cell lines.
Oncogene. 25:470–479. 2006.
|
|
25
|
Vradii D, Wagner S, Doan DN, et al: Brg1,
the ATPase subunit of the SWI/SNF chromatin remodeling complex, is
required for myeloid differentiation to granulocytes. J Cell
Physiol. 206:112–118. 2006. View Article : Google Scholar
|
|
26
|
Romero OA and Sanchez-Cespedes M: The
SWI/SNF genetic blockade: effects in cell differentiation, cancer
and developmental diseases. Oncogene. 33:2681–2689. 2014.
View Article : Google Scholar
|
|
27
|
Euskirchen GM, Auerbach RK, Davidov E, et
al: Diverse roles and interactions of the SWI/SNF chromatin
remodeling complex revealed using global approaches. PLoS Genet.
7:e10020082011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kasten MM, Clapier CR and Cairns BR:
SnapShot: chromatin remodeling: SWI/SNF. Cell. 144:310.e12011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y and Price BD: Chromatin dynamics and
the repair of DNA double strand breaks. Cell Cycle. 10:261–267.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsubara D, Kishaba Y, Ishikawa S, et al:
Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal
transition phenotype and distinct histologic and genetic features.
Cancer Sci. 104:266–273. 2013. View Article : Google Scholar
|
|
31
|
Cohen SM, Chastain PD II, Rosson GB, et
al: BRG1 co-localizes with DNA replication factors and is required
for efficient replication fork progression. Nucleic Acids Res.
38:6906–6919. 2010. View Article : Google Scholar : PubMed/NCBI
|