Introduction
MicroRNAs of 20–25 nucleotides act as
post-transcriptional regulators of gene expression, and their
localization suggests that they primarily function in the
cytoplasm. Recently, a number of microRNAs were found in
extracellular spaces (1), and some
of these were embedded in extracellular vesicles such as exosomes
(2). However, it has been suggested
that some extracelluar microRNAs form complexes with Argonaute 2
(Ago2), high-density lipoprotein (HDL) and other RNA-binding
proteins (3–6). Therefore, microRNAs may be present in
various bound forms in extracellular spaces. Diagnostic biomarkers
were recently identified in body fluids such as serum, plasma,
urine, milk and saliva (7–11). Among these biomarkers, some
extracellular RNAs were shown to be uniquely stable in the presence
of ribonuclease (12–14). However, these data require further
validation in focused studies of extracellular microRNA
stability.
Exosomes are extracellular vesicles, ~40–200 nm in
diameter, which are secreted from epithelial (15), endothelial (16), cancer (17), dendritic (18), and mesenchymal stem cells (19), as well as B lymphocytes (20). Exosome secretion has been identified
in human and mouse cells in vitro (1). However, few studies have demonstrated
RNA secretion in other organisms.
Although the mechanisms of exosome biogenesis remain
to be adequately defined, current models suggest that exosomes are
formed within multivesicular bodies (MVBs) (21), which are formed during maturation of
early into late endosomes, with concomitant and corresponding
accumulation of intraluminal vesicles (ILVs) (22). Endosomal sorting complexes required
for transport (ESCRT) machinery are also responsible for generating
vesicles in MVBs through a process known as endosome budding
(23). In addition, ceramide is
reportedly involved in an ESCRT-independent process of exosome
generation (24). Ceramide, which
is generated from sphingomyelin by neutral sphingomyelinase 2
(nSMase2), is found in lipid components of exosome membranes
(25), and is encoded by the
sphingomyelin phosphodiesterase 3 (SMPD3) gene. Although
this enzyme has been shown to be involved in the secretion of small
RNAs such as microRNAs (26), which
small/microRNAs are released following the actions of nSMase2
remains to be determined.
In the present study, we investigated the stability
of extracellular small RNAs against external factors including
ribonuclease A (RNase A), long-term incubation, freeze-thaw, and pH
change using HuH-7 human hepatocellular cancer cells. In addition,
we examined the evolutionary conservation of SMPD3 in mammals and
determined the effects of an SMPD3 inhibitor on the release of
small/microRNAs from HuH-7 and SW480 human colorectal cancer
cells.
Materials and methods
Cell lines and culture
HuH-7 human hepatocellular cancer cells (JCRB0403)
were purchased from the Health Science Research Resources Bank
(Osaka, Japan). The human colorectal cancer cell line SW480
(CCL-228) was purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA). HuH-7 cells were cultured in Dulbecco’s
minimal essential medium (D-MEM; Wako, Tokyo, Japan) supplemented
with 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA,
USA), 100 U/ml penicillin, and 100 μg/ml streptomycin. SW480 cells
were cultured in RPMI-1640 medium (Wako) supplemented with 10% FBS,
100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were
cultured at 37°C in a 5% CO2 atmosphere.
Purification of culture supernatants
SW480 and HuH-7 cells were plated on collagen-coated
10-cm dishes at 1×106 cells/dish in culture media. After
72 h, the culture media were discarded and the cells were washed
three times in serum-free culture media. Serum-free culture media
supplemented with the SMPD3 inhibitor GW4869 (Sigma-Aldrich, St.
Louis, MO, USA) at final concentrations of 0, 1.0, 3.3, and 10.0 μM
were then added at 10 ml per dish, and the cells were cultured for
48 h. Cell culture media were then collected and centrifuged at 300
× g at 4°C for 3 min to remove floating cells. Supernatants were
centrifuged at 2,000 × g at 4°C for 15 min and were collected in
new tubes. Culture supernatants were also centrifuged at 12,000 × g
at 4°C for 35 min to remove cell debris, and the supernatants were
collected in new tubes and filtered using 0.22-μm filters.
Extracellular RNAs in the supernatants were then isolated using
Isogen II (NipponGene, Tokyo, Japan).
RNA extraction
Extracellular and intracellular RNAs from SW480 or
HuH-7 cells were isolated using Isogen II (NipponGene) according to
the manufacturer’s instructions. The sizes of extracted RNAs were
determined using an Agilent 2100 Bioanalyzer and Agilent RNA 6000
Pico kits (both from Agilent Technologies, Foster City, CA, USA)
according to the manufacturer’s instructions.
Microarray analysis
Species of extracellular microRNAs were
distinguished by labeling with Hy5 fluorescent dye using a miRCury
LNA™ microRNA Hy5 Power labeling kit (Exiqon, Copenhagen, Denmark).
Microarray analyses were then conducted using a Toray microRNA
microarray system. Toray 3D-Gene human miRNA oligo chips (Toray,
Tokyo, Japan) were hybridized with Hy5-labeled microRNAs in
hybridization solution at 32°C for 16 h using a hybridization oven.
Hybridized microarray chips were then washed in a wash buffer
according to the manufacturer’s instructions, and images of
fluorescent signals were captured using a Toray 3D-gene scanner
3000 (Toray).
Reverse transcription polymerase chain
reaction (RT-PCR) and RT quantitative PCR (RT-qPCR)
To investigate SMPD3 mRNA expression in SW480
and HuH-7 cells, cDNAs were synthesized from isolated RNAs using
High Capacity cDNA reverse transcriptase kits according to the
manufacturer’s instructions. Subsequently, qPCR for mRNAs was
performed using 2X Power SYBR-Green master mix, 10.0 μM forward and
reverse primers (Table I), and a
StepOne Plus real-time PCR system (all from Life Technologies),
under the following conditions: 10 min at 95°C, followed by 40
cycles at 95°C for 15 sec and 60°C for 60 sec. GAPDH was used as an
internal control. Expression levels were determined using the
comparative Ct method and were normalized to those from SW480
cells. Amplified fragments were then detected on 4% agarose gel
electrophoresis containing ethidium bromide using a ChemiDoc XRS
system and Quantity One software (both from Bio-Rad, Hercules, CA,
USA).
 | Table IPrimer sequences for RT-qPCR. |
Table I
Primer sequences for RT-qPCR.
| Gene name | Primer sequence | Size (nt) | Amplicon size
(bp) |
|---|
| SMPD3 | F:
5′-CGTCGTCTGTGGAGATTTCA-3′ | 20 | 76 |
| SMPD3 | R:
5′-GGTGAACAGGGAGTGTTGCT-3′ | 20 | |
| GAPDH | F:
5′-AGCCACATCGCTCAGACAC-3′ | 19 | 66 |
| GAPDH | R:
5′-GCCCAATACGACCAAATCC-3′ | 19 | |
Expression levels of extracellular and intracellular
microRNAs from SW480 and HuH-7 cells were analyzed using cDNAs that
were synthesized from microRNAs using TaqMan microRNA RT kits and
the prescribed 5X RT primer (both from Life Technologies) according
to the manufacturer’s instructions. Subsequently, qPCR for
microRNAs was performed using FastStart TaqMan probe master (Roche
Diagnostics, Basel, Switzerland), a 20X probe, and a StepOne Plus
real-time PCR system (Life Technologies) under the following
conditions: 10 min at 95°C, followed by 40 cycles at 95°C for 15
sec and 60°C for 60 sec. RNAs were isolated from 200-μl aliquots of
culture supernatants following the addition of 1 μl of 5 nM
cel-miR-39. Cel-miR-39 was used as an external control and U6 small
nuclear RNA (snRNA) was used as an internal control. Expression
levels were determined using the comparative Ct method.
Multiple alignments of SMPD3 amino acid
sequences
Amino acid sequences for Homo sapiens SMPD3,
NP_061137.1; Pan troglodytes SMPD3, XP_001167790.1; Mus
musculus SMPD3, NP_067466.1; and Bos taurus SMPD3,
NP_001179292.1, were obtained from the NCBI database (http://www.ncbi.nlm.nih.gov), and were subjected to
multiple alignment analysis using Genetyx 10 software (Genetyx,
Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM). Multiple group comparisons were performed using
one-way analysis of variance (ANOVA), followed by post hoc
pair-wise comparisons of significant differences using Dunnett’s
test. Differences were considered significant when P<0.01.
Results and Discussion
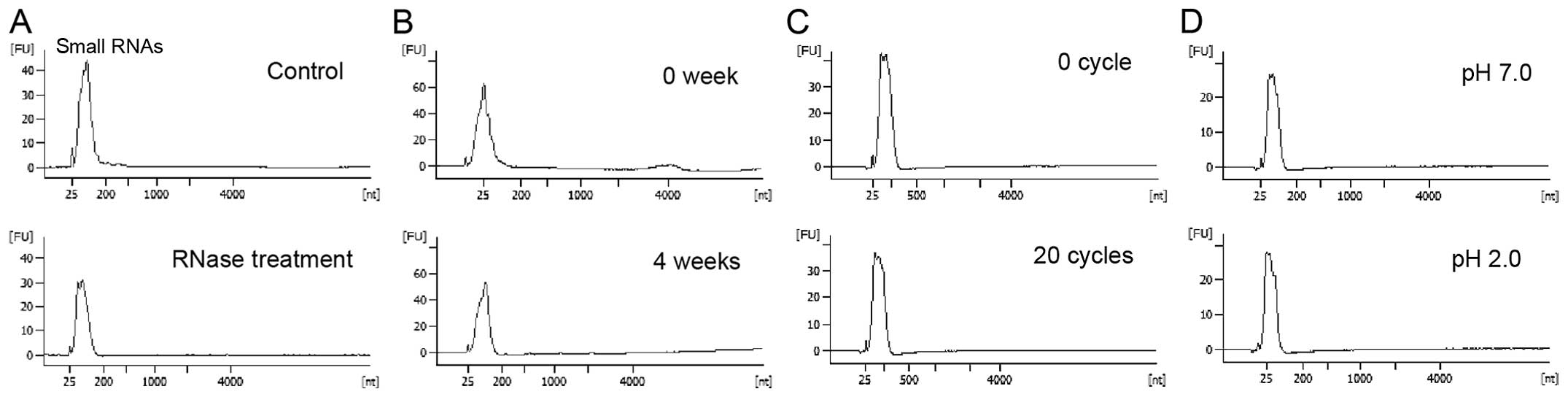
Extracellular small RNAs are stable
against changes in various conditions
Encapsulation of released cellular small RNAs in
exosomes likely allows high stability against changes in several
conditions (12–14). Accordingly, small RNAs in purified
supernatants from HuH-7 cells were stable through RNase A
treatment, long-term incubation, cycles of freezing and thawing and
pH changes.
In experiments conducted in this study, serum-free
culture supernatants from HuH-7 cells were purified by
centrifugation and filtration and were incubated with RNase A at a
final concentration of 0.4 μg/ml for 10 min at 37°C. After
extraction of total RNAs from culture supernatants, a peak for
small RNAs of 25–200 nt was detected using an Agilent bioanalyzer
(Fig. 1A). However, in culture
supernatants, small RNAs were stable after incubation at room
temperature for 4 weeks, 20 cycles of freezing and thawing (room
temperature to −80°C), and reduction of pH to 2.0 (Fig. 1B–D). These data indicate high
stability of small RNAs in culture supernatants.
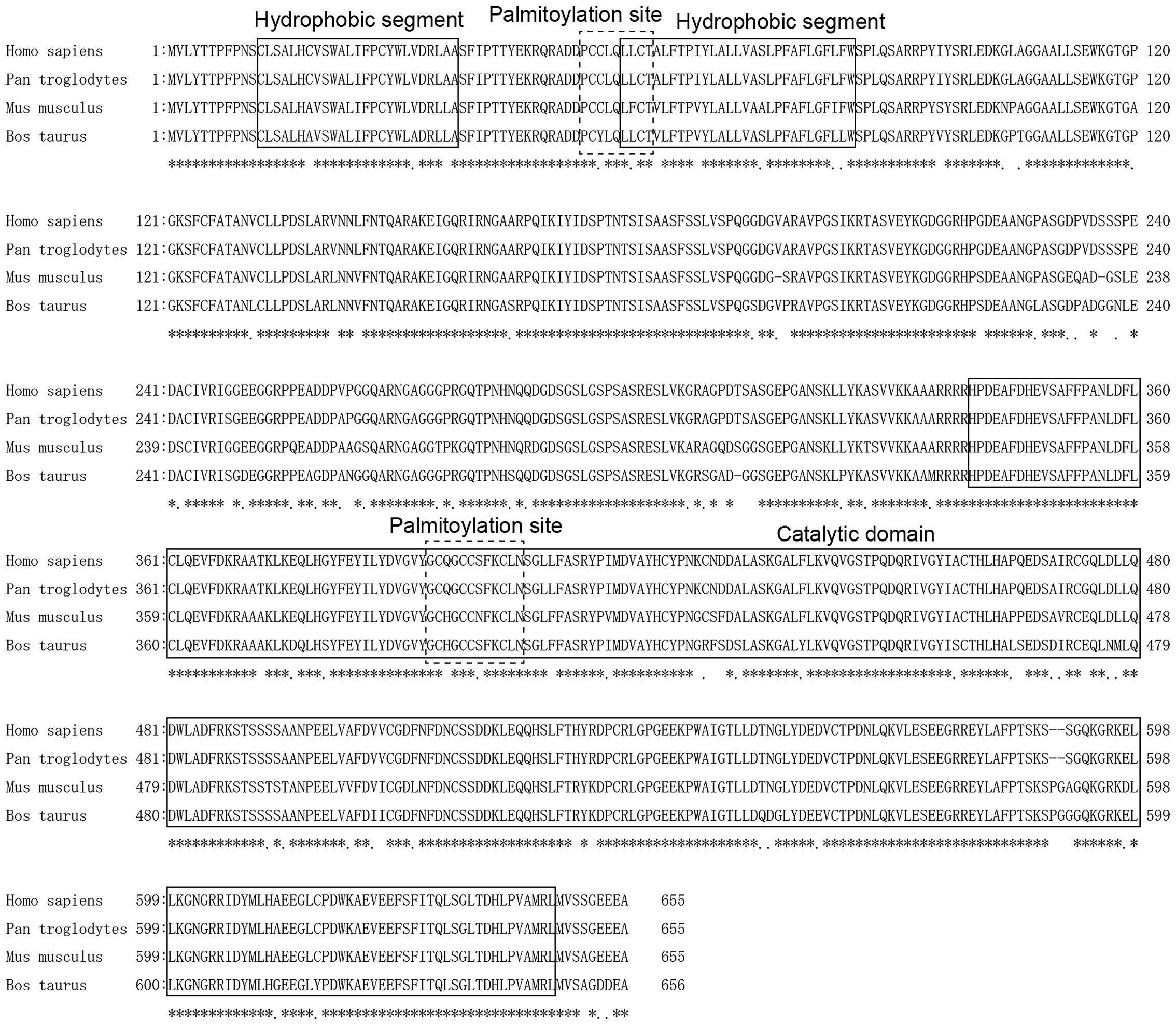
Evolutionary conservation of SMPD3 in
mammals
SMPD3 is reportedly involved in the secretion of
microRNAs (26). The present
analyses of various mammalian SMPD3 sequences (Homo sapiens,
Pan troglodytes, Mus musculus and Bos taurus)
indicated high sequence homology (Fig.
2), with an amino acid sequence identity of 99.69, 91.02 and
89.50% between Homo sapiens and Pan troglodytes,
Mus musculus, Bos Taurus, respectively. Moreover, two
hydrophobic segments, two palmitoylation sites and the catalytic
domain were highly conserved between examined mammals (Fig. 2). These analyses suggest that SMPD3
may have similar functions across these species.
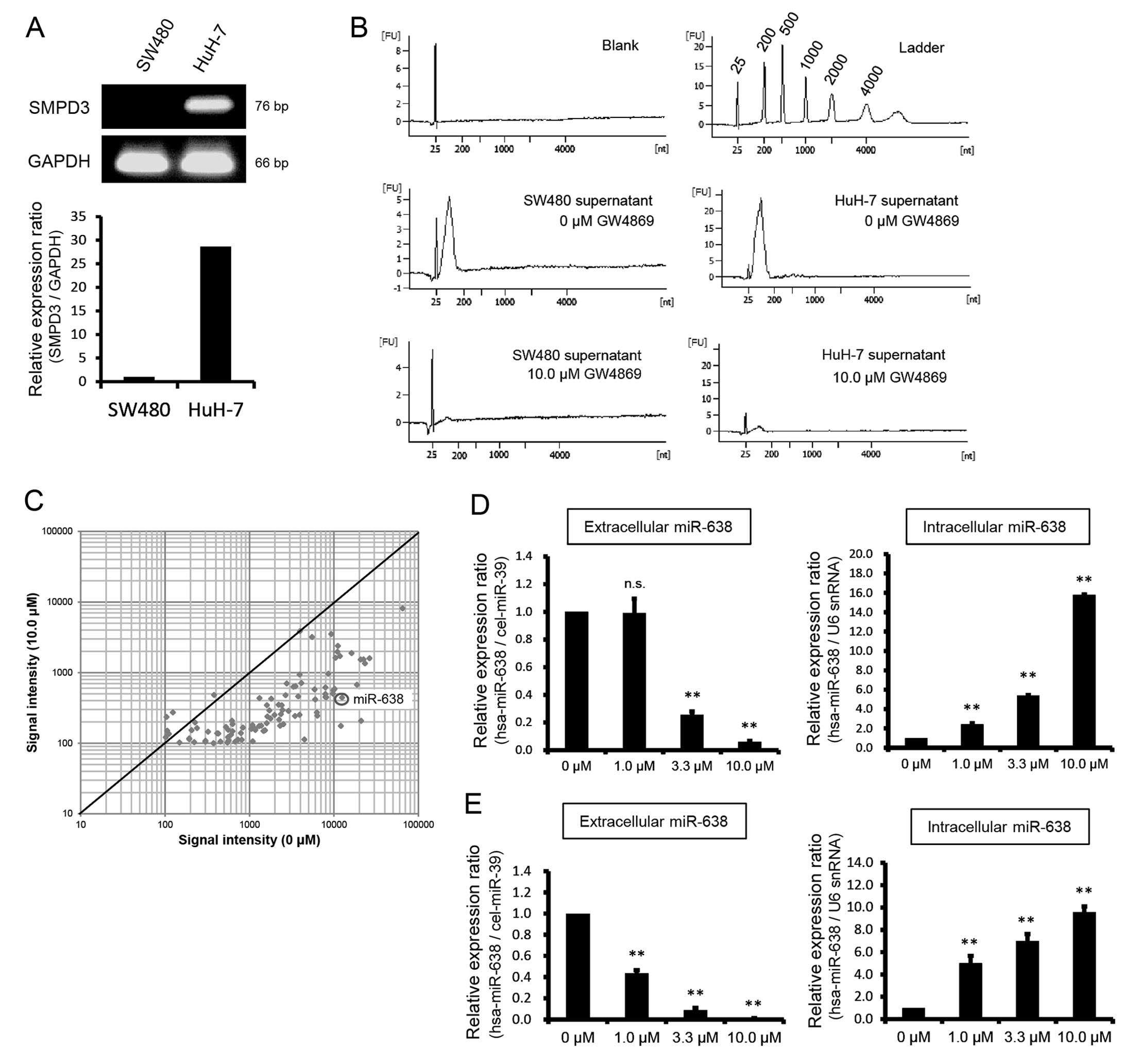
Small/microRNAs, such as miR-638, are
secreted into extracellular spaces via a ceramide-dependent
mechanism
Although nSMase2 produces ceramide from
sphingomyelin (25), it is
reportedly involved in the secretion of small RNAs such as
microRNAs (26). Thus, we
investigated the relationship between small RNA secretion and
SMPD3 mRNA expression in SW480 and HuH-7 cells. In the
RT-qPCR experiments, SMPD3 mRNA expression in HuH-7 cells
was 28.62-fold higher than that in SW480 cells (Fig. 3A). Moreover, peak RNA release from
HuH-7 cells was higher than that of SW480 cells (Fig. 3B), and corresponded with high
SMPD3 mRNA expression.
In the present study, concentrations of small RNAs
in culture supernatants were determined following treatment of
HuH-7 or SW480 cells with non-competitive SMPD3 inhibitor GW4869 at
a final concentration of 10.0 μM. In these experiments, small RNA
contents were markedly decreased after 48 h (Fig. 3B), suggesting that SMPD3 is
important in small RNA secretion.
In subsequent experiments, the amounts and species
of microRNAs in culture supernatants from GW4869-treated HuH-7
cells were analyzed using a Toray microRNA microarray system.
MicroRNA expression profiles following treatment with 0 and 10.0 μM
GW4869 for 48 h are shown in a scatter plot (Fig. 3C). Various microRNAs, including
miR-638, were decreased in culture supernatants from GW4869-treated
cells (Table II).
 | Table IIThe microRNA expression in culture
supernatants of HuH-7 cells treated with 0 or 10.0 μM GW4869 using
a Toray microRNA microarray system. |
Table II
The microRNA expression in culture
supernatants of HuH-7 cells treated with 0 or 10.0 μM GW4869 using
a Toray microRNA microarray system.
| Raw signal
intensity |
|---|
|
|
|---|
| microRNAs | 0 μM GW4869 | 10.0 μM GW4869 |
|---|
| hsa-miR-3960 | 65,098.0 | 8,112.9 |
| hsa-miR-4787-5p | 26,349.0 | 1,593.3 |
| hsa-miR-4508 | 23,348.0 | 1,349.6 |
| hsa-miR-3665 | 22,420.0 | 1,538.6 |
| hsa-miR-4484 | 21,004.0 | 207.2 |
| hsa-miR-762 | 20,776.0 | 1,527.1 |
| hsa-miR-4739 | 18,737.0 | 671.5 |
| hsa-miR-4516 | 16,181.0 | 1,884.0 |
| hsa-miR-4505 | 12,498.5 | 443.2 |
| hsa-miR-3648 | 12,080.7 | 175.5 |
| hsa-miR-4466 | 11,807.8 | 1,714.7 |
| hsa-miR-4488 | 11,136.4 | 2,385.7 |
| hsa-miR-3196 | 11,008.8 | 1,971.5 |
| hsa-miR-2861 | 10,473.1 | 1,617.8 |
| hsa-miR-638 | 10,180.6 | 587.0 |
| hsa-miR-1908 | 9,862.2 | 559.4 |
|
hsa-miR-4725-3p | 9,736.2 | 502.2 |
| hsa-miR-4294 | 9,309.6 | 3,504.9 |
| hsa-miR-3656 | 8,587.1 | 964.6 |
| hsa-miR-4467 | 8,139.1 | 446.2 |
|
hsa-miR-4745-5p | 7,940.2 | 495.4 |
| hsa-miR-4734 | 7,933.7 | 615.8 |
| hsa-miR-4497 | 7,932.5 | 451.3 |
| hsa-miR-744 | 6,356.6 | 244.6 |
| hsa-miR-4327 | 6,287.8 | 272.2 |
|
hsa-miR-4723-5p | 6,007.8 | 416.2 |
| hsa-miR-663 | 5,899.5 | 576.6 |
| hsa-miR-3621 | 5,509.6 | 3,186.3 |
| hsa-miR-4454 | 4,467.7 | 112.9 |
| hsa-miR-1268 | 4,115.5 | 711.4 |
| hsa-miR-1246 | 3,965.1 | 3,866.2 |
|
hsa-miR-3940-5p | 3,925.4 | 936.0 |
| hsa-miR-4492 | 3,916.0 | 251.9 |
| hsa-miR-3178 | 3,777.1 | 459.8 |
| hsa-miR-1469 | 3,516.2 | 639.1 |
| hsa-miR-4530 | 3,463.2 | 242.0 |
|
hsa-miR-1228* | 3,319.6 | 628.7 |
| hsa-miR-4459 | 2,894.8 | 286.1 |
|
hsa-miR-4687-3p | 2,760.3 | 700.9 |
| hsa-miR-4463 | 2,668.5 | 478.1 |
| hsa-miR-1915 | 2,516.5 | 185.4 |
| hsa-miR-1260b | 2,456.4 | 295.3 |
| hsa-miR-4281 | 2,407.2 | 344.2 |
|
hsa-miR-149* | 2,356.0 | 257.3 |
|
hsa-miR-4749-5p | 2,305.3 | 176.4 |
| hsa-miR-1275 | 2,241.6 | 215.3 |
| hsa-miR-1268b | 2,210.7 | 350.2 |
| hsa-miR-3141 | 1,979.9 | 250.6 |
| hsa-miR-4417 | 1,809.8 | 425.3 |
|
hsa-miR-4763-3p | 1,774.2 | 223.5 |
| hsa-miR-4651 | 1,719.9 | 290.3 |
| hsa-miR-4442 | 1,629.6 | 238.6 |
| hsa-miR-4741 | 1,607.0 | 345.4 |
| hsa-miR-3197 | 1,600.5 | 192.0 |
| hsa-miR-3937 | 1,528.1 | 152.4 |
| hsa-miR-4532 | 1,467.0 | 149.0 |
|
hsa-miR-4655-5p | 1,377.6 | 177.1 |
| hsa-miR-3180 | 1,312.2 | 435.1 |
|
hsa-miR-4695-5p | 1,298.0 | 174.7 |
|
hsa-miR-3180-3p | 1,172.2 | 153.8 |
|
hsa-miR-92b* | 1,163.7 | 158.8 |
| hsa-miR-671-5p | 1,091.4 | 106.2 |
| hsa-miR-1280 | 1,085.7 | 180.6 |
|
hsa-miR-4728-5p | 1,051.4 | 159.1 |
| hsa-miR-4689 | 836.7 | 166.4 |
| hsa-miR-642b | 808.9 | 111.5 |
| hsa-miR-1909 | 791.7 | 139.2 |
| hsa-miR-939 | 730.0 | 112.2 |
| hsa-miR-4656 | 677.5 | 105.1 |
| hsa-miR-3195 | 631.0 | 186.6 |
|
hsa-miR-3663-3p | 630.8 | 147.8 |
|
hsa-miR-92a-2* | 621.2 | 207.6 |
| hsa-miR-3188 | 606.1 | 132.1 |
| hsa-miR-4486 | 565.7 | 109.2 |
| hsa-miR-1202 | 559.6 | 154.5 |
| hsa-miR-4270 | 557.6 | 121.8 |
| hsa-miR-3185 | 547.7 | 102.1 |
|
hsa-miR-4649-5p | 526.5 | 169.7 |
| hsa-miR-4688 | 454.0 | 105.3 |
|
hsa-miR-4707-5p | 428.8 | 136.7 |
|
hsa-miR-4697-5p | 414.7 | 138.1 |
|
hsa-miR-4732-5p | 381.2 | 481.5 |
|
hsa-miR-4758-5p | 374.8 | 100.0 |
| hsa-miR-720 | 323.1 | 145.2 |
| hsa-miR-296-5p | 301.6 | 108.3 |
| hsa-miR-4429 | 300.4 | 105.1 |
| hsa-miR-4433 | 238.7 | 163.4 |
| hsa-miR-1290 | 226.1 | 196.4 |
| hsa-miR-483-3p | 221.0 | 103.3 |
| hsa-miR-4730 | 206.3 | 152.8 |
| hsa-miR-542-5p | 193.6 | 113.9 |
|
hsa-miR-4787-3p | 193.4 | 116.6 |
| hsa-miR-320b | 148.3 | 102.1 |
| hsa-miR-4443 | 123.7 | 272.6 |
| hsa-miR-22 | 111.2 | 135.5 |
| hsa-miR-21 | 105.8 | 149.9 |
| hsa-miR-122 | 104.1 | 234.6 |
| hsa-miR-17 | 101.8 | 120.9 |
Subsequent RT-qPCR experiments showed a significant
decrease in miR-638 expression in the SW480 and HuH-7 cells and
supernatants following treatment with GW4869 (P<0.01).
Specifically, extracellular miR-638 expression in HuH-7 cells was
decreased 3.92- and 16.67-fold in the presence of 3.3 and 10.0 μM
GW4869, respectively. In SW480 cells it was decreased 2.29-, 11.36-
and 113.14-fold in the presence of 1.0, 3.3 and 10.0 μM GW4869,
respectively (Fig. 3D–E). By
contrast, intracellular miR-638 expression was significantly
increased 2.43-, 5.41- and 15.81-fold in the presence of 1.0, 3.3,
and 10.0 μM GW4869 in HuH-7 cells, and 5.01-, 7.00-, and 9.57-fold,
respectively, in SW480 cells when compared with expression in the
presence of 0 μM GW4869 (P<0.01; Fig. 3D–E). These data suggest that SMPD3
plays an important role in the release of a number of microRNAs,
including miR-638, and that microRNAs accumulated in cells
following the inhibition of exosome membrane formation by
GW4869.
Small RNAs secretions are involved in the formation
of exosomes, the regulation of vesicle trafficking, and the plasma
membrane fusion of MVBs (28,29).
Exosome transport is considered a highly controlled process that
involves a number of Rab GTPases. Accordingly, Rab11 overexpression
has been shown to stimulate exocytosis (30), and Rab27a and Rab27b have been shown
to control exosome secretion by regulating vesicle transport of
MVBs to plasma membranes (31). In
addition, secretion of exosomes containing small RNAs requires
fusion of MVBs to plasma membranes, potentially involving soluble
N-ethylmaleimide-sensitive factor attachment protein receptor
(SNARE) protein complexes (32).
Future studies are required to clarify the mechanisms of RNA and
exosome release into extracellular spaces.
In conclusion, extracellular small RNAs are
comparatively stable due to their presence in exosomes. Moreover,
the high evolutionary conservation of SMPD3 indicates an important
role in the release of miR-638 and other microRNAs into
extracellular spaces.
Acknowledgements
This study was supported in part by the Hirosaki
University Institutional Research Grant for Young Scientists,
KAKENHI (no. 23790613), a Grant-in-Aid for Young Scientists (B), a
grant from KAKENHI (no. 25670264) for Challenging Exploratory
Research, a grant from the Suzuken Memorial Foundation (no.
11-076), a grant from the Takeda Science Foundation, and a grant
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (MEXT).
References
|
1
|
Valadi H, Ekström K, Bossios A, Sjostrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu G, Drescher KM and Chen XM: Exosomal
miRNAs: biological properties and therapeutic potential. Front
Genet. 3:562012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arroyo JD, Chevillet JR, Kroh EM, et al:
Argonaute2 complexes carry a population of circulating microRNAs
independent of vesicles in human plasma. Proc Natl Acad Sci USA.
108:5003–5008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turchinovich A, Weiz L, Langheinz A and
Burwinkel B: Characterization of extracellular circulating
microRNA. Nucleic Acids Res. 39:7223–7233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vickers KC, Palmisano BT, Shoucri BM,
Shamburek RD and Remaley AT: MicroRNAs are transported in plasma
and delivered to recipient cells by high-density lipoproteins. Nat
Cell Biol. 13:423–433. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K, Zhang S, Weber J, Baxter D and
Galas DJ: Export of microRNAs and microRNA-protective protein by
mammalian cells. Nucleic Acids Res. 38:7248–7259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brase JC, Wuttig D, Kuner R and Sultmann
H: Serum microRNAs as non-invasive biomarkers for cancer. Mol
Cancer. 9:3062010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar
|
|
9
|
Moon PG, Lee JE, You S, et al: Proteomic
analysis of urinary exosomes from patients of early IgA nephropathy
and thin basement membrane nephropathy. Proteomics. 11:2459–2475.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Michael A, Bajracharya SD, Yuen PS, et al:
Exosomes from human saliva as a source of microRNA biomarkers. Oral
Dis. 16:34–38. 2010. View Article : Google Scholar :
|
|
11
|
Lässer C, Alikhani VS, Ekström K, et al:
Human saliva, plasma and breast milk exosomes contain RNA: uptake
by macrophages. J Transl Med. 9:92011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kosaka N, Izumi H, Sekine K and Ochiya T:
microRNA as a new immune-regulatory agent in breast milk. Silence.
1:72010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge Q, Zhou Y, Lu J, Bai Y, Xie X and Lu Z:
miRNA in plasma exosome is stable under different storage
conditions. Molecules. 19:1568–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karlsson M, Lundin S, Dahlgren U, Kahu H,
Pettersson I and Telemo E: ‘Tolerosomes’ are produced by intestinal
epithelial cells. Eur J Immunol. 31:2892–2900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muturi HT, Dreesen JD, Nilewski E, et al:
Tumor and endothelial cell-derived microvesicles carry distinct
CEACAMs and influence T-cell behavior. PLoS One. 8:e746542013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King HW, Michael MZ and Gleadle JM:
Hypoxic enhancement of exosome release by breast cancer cells. BMC
Cancer. 12:4212012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montecalvo A, Larregina AT, Shufesky WJ,
et al: Mechanism of transfer of functional microRNAs between mouse
dendritic cells via exosomes. Blood. 119:756–766. 2012. View Article : Google Scholar :
|
|
19
|
Lee JK, Park SR, Jung BK, et al: Exosomes
derived from mesenchymal stem cells suppress angiogenesis by
down-regulating VEGF expression in breast cancer cells. PLoS One.
8:e842562013. View Article : Google Scholar
|
|
20
|
Escola JM, Kleijmeer MJ, Stoorvogel W,
Griffith JM, Yoshie O and Geuze HJ: Selective enrichment of
tetraspan proteins on the internal vesicles of multivesicular
endosomes and on exosomes secreted by human B-lymphocytes. J Biol
Chem. 273:20121–20127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bobrie A, Colombo M, Raposo G and Thery C:
Exosome secretion: molecular mechanisms and roles in immune
responses. Traffic. 12:1659–1668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanson PI and Cashikar A: Multivesicular
body morphogenesis. Annu Rev Cell Dev Biol. 28:337–362. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raiborg C and Stenmark H: The ESCRT
machinery in endosomal sorting of ubiquitylated membrane proteins.
Nature. 458:445–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trajkovic K, Hsu C, Chiantia S, et al:
Ceramide triggers budding of exosome vesicles into multivesicular
endosomes. Science. 319:1244–1247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marchesini N, Luberto C and Hannun YA:
Biochemical properties of mammalian neutral sphingomyelinase 2 and
its role in sphingolipid metabolism. J Biol Chem. 278:13775–13783.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kosaka N, Iguchi H, Yoshioka Y, Takeshita
F, Matsuki Y and Ochiya T: Secretory mechanisms and intercellular
transfer of microRNAs in living cells. J Biol Chem.
285:17442–17452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DH, Kim SH, Ahn KH, et al:
Identification and evaluation of neutral sphingomyelinase 2
inhibitors. Arch Pharm Res. 34:229–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rodriguez-Boulan E, Kreitzer G and Müsch
A: Organization of vesicular trafficking in epithelia. Nat Rev Mol
Cell Biol. 6:233–247. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jahn R and Fasshauer D: Molecular machines
governing exocytosis of synaptic vesicles. Nature. 490:201–207.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Savina A, Vidal M and Colombo MI: The
exosome pathway in K562 cells is regulated by Rab11. J Cell Sci.
115:2505–2515. 2002.PubMed/NCBI
|
|
31
|
Ostrowski M, Carmo NB, Krumeich S, et al:
Rab27a and Rab27b control different steps of the exosome secretion
pathway. Nat Cell Biol. 12:19–30. 2010. View Article : Google Scholar
|
|
32
|
Söllner T, Whiteheart SW, Brunner M, et
al: SNAP receptors implicated in vesicle targeting and fusion.
Nature. 362:318–324. 1993. View
Article : Google Scholar : PubMed/NCBI
|